Abstract
The UL49.5 gene of most herpesviruses is conserved and encodes glycoprotein N. However, the UL49.5 protein of duck enteritis virus (DEV) (pUL49.5) has not been reported. In the current study, the DEV pUL49.5 gene was first subjected to molecular characterization. To verify the predicted intracellular localization of gene expression, the recombinant plasmid pEGFP-C1/pUL49.5 was constructed and used to transfect duck embryo fibroblasts. Next, the recombinant plasmid pDsRed1-N1/glycoprotein M (gM) was produced and used for co-transfection with the pEGFP-C1/pUL49.5 plasmid to determine whether DEV pUL49.5 and gM (a conserved protein in herpesviruses) colocalize. DEV pUL49.5 was thought to be an envelope glycoprotein with a signal peptide and two transmembrane domains. This protein was also predicted to localize in the cytoplasm and endoplasmic reticulum with a probability of 66.7%. Images taken by a fluorescence microscope at different time points revealed that the DEV pUL49.5 and gM proteins were both expressed in the cytoplasm. Overlap of the two different fluorescence signals appeared 12 h after transfection and continued to persist until the end of the experiment. These data indicate a possible interaction between DEV pUL49.5 and gM.
Keywords: colocalization, duck enteritis virus, intracellular localization, molecular characterization, UL49.5 protein
Introduction
Duck enteritis virus (DEV) is an alphaherpesvirus that causes duck viral enteritis (DVE) or duck plague (DP) and an acute contagious disease in waterfowl. Due to its high morbidity and mortality rate, DEV is associated with heavy economic losses in the commercial duck industry [16]. In the present study, we determined that glycoprotein N (gN), encoded by the UL49.5 gene, was conserved among herpesviruses based on genomic sequence analysis. The DEV UL49.5 gene is 288 bp in size (GenBank Accession No. EU195112), and located between the UL49 and UL50 genes. The complete sequence of DEV UL49.5 protein (pUL49.5) includes 95 amino acids (aa) with a high number of alanine, serine, and valine residues but no asparagine.
Recent studies have revealed that gN of the herpes simplex virus (HSV-1) [29], human cytomegalovirus (HCMV) [26], bovine herpesvirus 1 (BHV-1) [17], and pseudorabies virus (PRV) [20], is an envelope glycoprotein. This protein influences viral replication and localizes primarily to the cytoplasm [5]. PRV gN was reported to be an O-glycosylated structural protein of the viral envelope [7]. However, the number of amino acids is not completely consistent between each herpesvirus, and it is not yet entirely clear whether the functions of this protein are affected by this difference. In some reported herpesviruses, gN has a specific function as a single protein [11,14,18]. However, in most cases gN typically forms a protein complex with glycoprotein M (gM) through disulfide bonds [13].
Genes encoding gM and gN are conserved among herpesviruses and form a functional complex. Both proteins are located in the viral envelope, and help facilitate viral entry and egress [13]. Aside from functioning as a viral structural protein, gN has an additional role in immune evasion [14,18]. In several types of varicellovirus, gN prevents peptide transport through transporter associated with antigen processing (TAP) protein [12]. BHV-1 gN inhibits TAP by inducing degradation of the TAP complex and preventing conformational changes essential for peptide transport [17].
While the intracellular localization of most alphaherpesvirus gN, such as that in HSV-1, BHV-1, and PRV, has been well characterized [17], little is known about the location to where DEV pUL49.5 is targeted. We conducted the current investigation to determine the intracellular localization and colocalization of DEV pUL49.5 and DEV gM in living cells (DEFs) based on previous bioinformatics analysis of these two proteins [16]. Two recombinant plasmids, pEGFP-C1/pUL49.5 and pDsRed1-N1/gM, were constructed to transfect duck embryo fibroblasts (DEFs). At the same time, the plasmids pEGFP-C1 and pDsRed1-N1 were used as negative controls. The two negative control plasmids contained genes that encoded enhanced green fluorescent protein (EGFP) or red fluorescent protein (RFP), respectively. Our study provided information that helped elucidate DEV pUL49.5 and gM functions, and will be useful for further understanding the interaction of these two proteins.
Materials and Methods
Virus, cells, and viral DNA extraction
The DEV CHv strain isolated in our laboratory was grown in DEFs. Monolayer cultures of DEFs were incubated in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% newborn calf serum (NBCS) at 37℃. When the cytopathic effect (CPE) was about 80%, viral DNA was extracted from the DEV-infected DEFs with phenol-chloroform and precipitated with ethanol. The DNA was stored in TE buffer (10 mM Tris-HCl; 1 mM EDTA, pH 8.0) at -20℃.
PCR amplification and plasmid construction
A pair of primers was designed by Primer premier 5.0 and synthesized by Invitrogen (USA). To facilitate cloning, HindIII or BamHI restriction sites (underlined) were incorporated into the forward primer (5'-CCAAGCTTCGATGGCTTCTATGGAGACAGC-3') and reverse primer (5'-CGGGATCCTTACCAATCTACCCTAAACATG-3'), respectively. Primers for amplifying DEV gM (forward: 5'-CTCGAGATGGGATCCATGAATGGGC-3' and reverse: (5'-CCGCGGTTCACTATCCGACGAGAAATGAGT-3') containing XhoI or SacII restriction sites (underlined), respectively, were also designed by Primer premier 5.0 and synthesized by Invitrogen (USA). Using DEV CHv DNA as the template, the PCR was performed with reactions (20 µL/tube) containing 10 µL PrimeSTAR (premix) DNA polymerase, 0.4 µL of each primer (10 pmol each), 0.3 µL DNA template, and 8.9 µL ultrapure water.
DEV UL49.5 PCR products and pEGFP-C1 were digested by HindIII or BamHI, respectively, and then ligated with T4 ligase to produce the pEGFP-C1/pUL49.5 plasmid (Fig. 1A). The pDsRed1-N1/gM plasmid was constructed in the same way following digestion by XhoI and SacII (Fig. 1B). Successful production of the pEGFP-C1/pUL49.5 and pDsRed1-N1/gM plasmids was subsequently confirmed by PCR, restriction enzyme digestion, and sequencing as previously described [6].
Fig. 1.
Construction of the recombinant plasmids. (A) Construction of the pEGFP-C1/pUL49.5 plasmid. (B) Construction of the pDsRed1-N1/gM plasmid.
Bioinformatic analysis of DEV pUL49.5
Based on the results of NCBI BLASTN, and using the Clustal method, the phylogenetic analyses between DEV pUL49.5 and gN sequences of 15 herpesviruses were performed (Table 1). The predicted DEV pUL49.5 protein structure was analyzed with PSORT II Prediction [8], NetPhos2.0 [3], NetNGlyc1.0, NCBI Conserved Domains (National Center for Biotechnology Information, USA), and SignalP 3.0 [1]. The secondary structure was assessed by TMHMM (Center for Biological Sequence Analysis, Denmark). In addition, hydrophobicity analysis and primary structure evaluation were conducted with BioEdit 7.0 and BioEdit 7.0 and DNAstar 7.10 (DNASTAR company, USA), respectively.
Table 1.
Accession numbers of herpesvirus gN sequences used in this study

*These herpesviruses have been reported and their glycoprotein N (gN) sequences have some similarities with DEV pUL49.5 according to NCBI BLAST analyses. †The accession numbers correspond to gN sequences of different herpesviruses.
DEF culture and transfection
The pEGFP-C1/pUL49.5 plasmid and Lipofectamine 2000 (Invitrogen, USA) were mixed at a ratio ranging from 1 : 0.5 to 1 : 5 and incubated at room temperature for 20 min. The resulting complexes were used to transfect in triplicate DEFs. Cells were greater than 90% confluent before being subjected to transfection. The cells were washed with phosphate buffered saline (PBS) and incubated with 500 µL of complexes per well at 37℃ for at least 5 h. The cells were also transfected with pDsRed1-N1/gM in the same manner. The pDsRed1-N1/gM and pEGFP-C1/pUL49.5 plasmids were mixed at a ratio 1 : 1 for co-transfection and used to transfect the DEFs as described above. The cells were also transfected with pEGFP-C1 and pDsRed1-N1 as negative controls, and a mock transfection was performed with DMEM as a blank control. All negative and blank control transfections were performed in triplicate. At different time points (0, 6, 12, 18, 24, 36, 48, 60, and 72 h) after transfection, the cells were washed with PBS. The cell nuclei were then stained with 4',6-diamidino-2-phenylindole (DAPI, 5 µg/mL; Beyotime Institute of Biotechnology, China). The cells were viewed after staining with fluorescence microscopy (Nikon, Japan).
Images acquisition and processing
Images were captured with fluorescence microscopy (Nikon, Japan).
Results
Construction of the recombinant plasmids
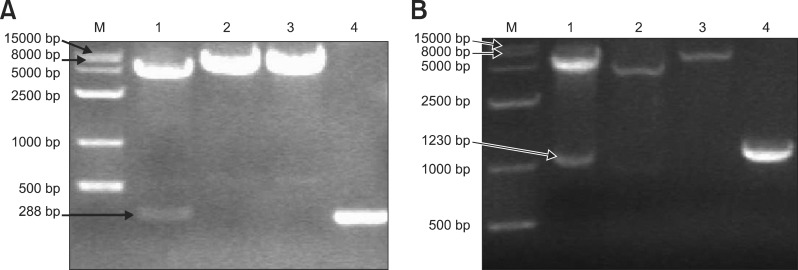
Construction of the pEGFP-C1/pUL49.5 plasmid was confirmed by PCR, restriction enzyme digestion (Fig. 2A), and sequencing. The pDsRed1-N1/gM plasmid was also successfully constructed and characterized (Fig. 2B). Sequences of the DEV UL49.5 gene and UL10 gene (encoding gM) in the plasmids were identical to those published in PubMed.
Fig. 2.
Identification of the recombinant plasmids using PCR and restriction enzyme digestion. (A) Identification of the pEGFP-C1/pUL49.5 plasmid. M, DNA marker; Lane 1, products produced by BamHI and HindIII; Lane 2, product produced by BamHI; Lane 3, product produced by HindIII; Lane 4, PCR product. (B) Identification of the pDsRed1-N1/gM plasmid. M, DNA marker; Lane 1, products produced by XhoI and SacII; Lane 2, undigested pDsRed1-N1/gM plasmid; Lane 3, product produced by SacII; Lane 4, PCR product.
Phylogenetic and structure prediction analyses of DEV pUL49.5
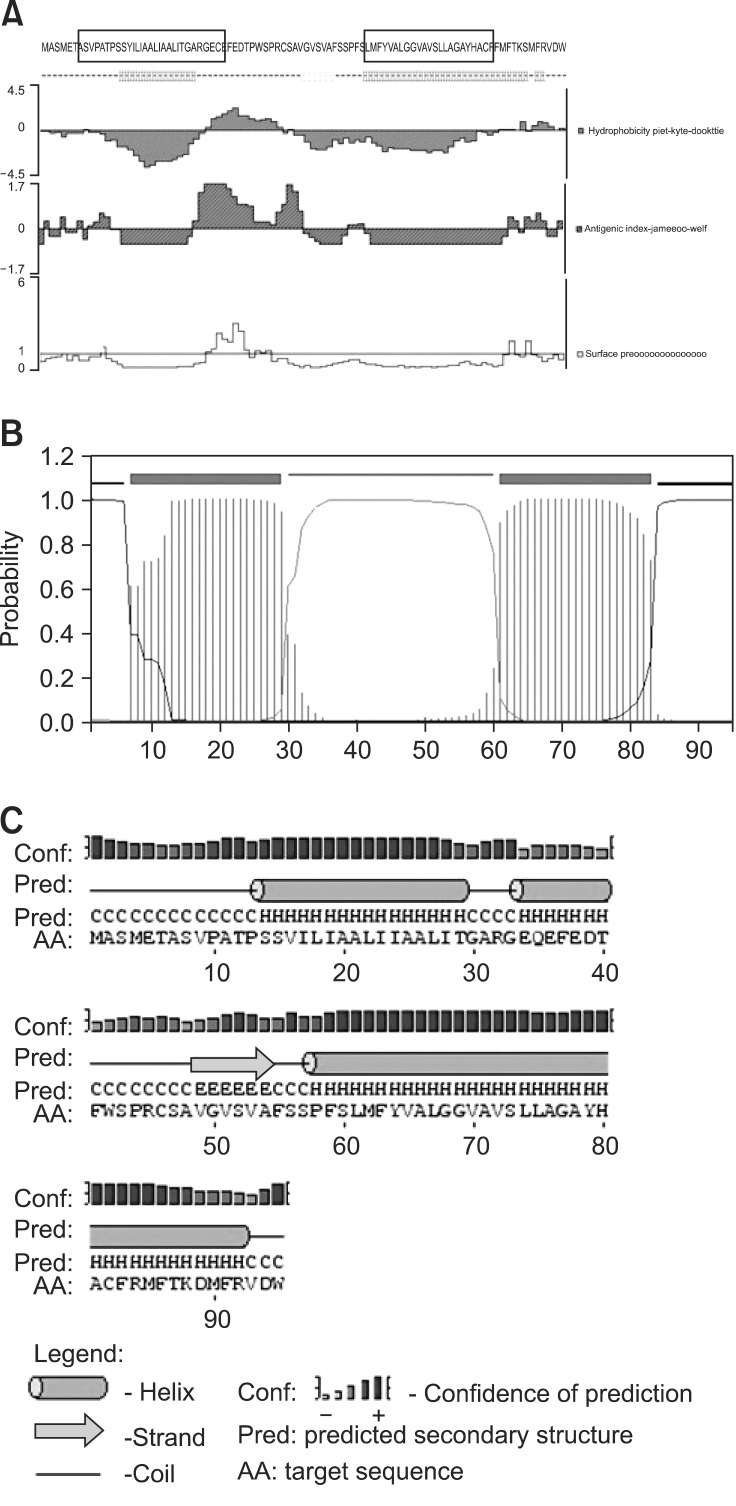
Phylogenetic analysis based on gN protein sequences of different herpesvirus strains clearly demonstrated that DEV pUL49.5 was more homologous to gN of alphaherpesvirus than with those of the beta and gamma subfamilies (Fig. 3A). Consequently, DEV pUL49.5 should be designated as Alphaherpesvirinae. Similarity comparison of gN sequences of the DEV CHv strain to those of 16 known herpesviruses clearly demonstrated that the DEV pUL49.5 most closely resembled meleagrid herpesvirus 1 (MeHV-1) gN, and both gene sequences belonged to the herpesvirus UL49.5 superfamily. DEV pUL49.5 was most closely related to MeHV-1 gN and formed a single cluster. However, the protein sequences were seldom consistent with each other (Fig. 3B).
Fig. 3.
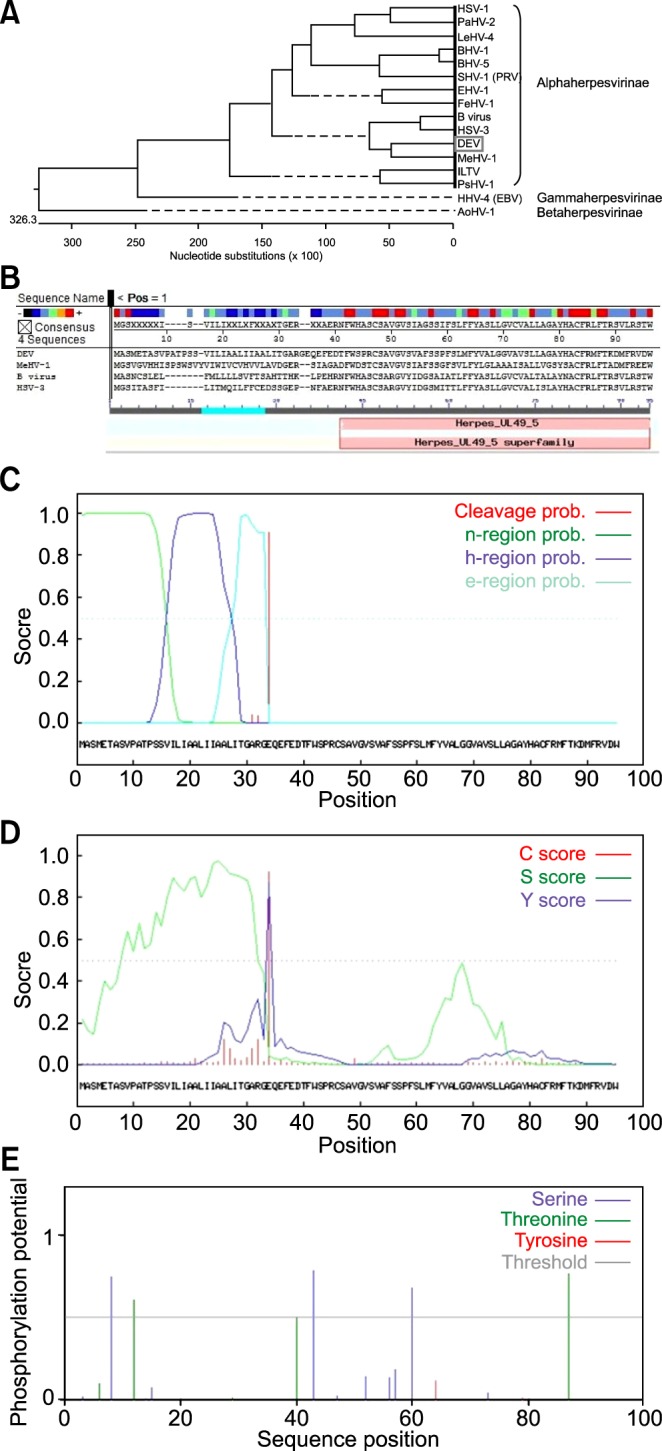
Phylogenetic analysis and prediction of functional sites in duck enteritis virus (DEV) pUL49.5. (A) The phylogenetic tree generated based on DEV pUL49.5 and gN sequences of 15 reported herpesviruses. (B) Sequence alignment showing evolutionary relationships. Red indicates similarities while blue indicates differences. (C) Predicted signal peptide in DEV pUL49.5 according to SignalP-HMM. (D) Predicted signal peptide in DEV pUL49.5 according to SignalP-NN. "C score" indicates the cleavage site probability, "S score" indicates the possible region of the signal peptide, and the "Y score" is a derivative of the C score representing the cleavage site probability. (E) Predicted phosphorylation sites in DEV pUL49.5.
Primary structure analysis showed that DEV pUL49.5 has 95 aa with a molecular weight of 10.15 kDa, theoretical isoelectric point (pI) of 5.05, and 0.760 grand average of hydropathicity (GRAVY). Using hidden Markov models (HMM) and neural networks (NN) of SignalP 3.0 [1], the most likely signal peptide cleavage site was found between 33 aa and 34 aa. The probability of SignalP-HMM was 0.907 (Fig. 3C) and that for SignalP-NN was 0.991 (Fig. 3D). Additionally, DEV pUL49.5 (Fig. 3E) appears to contain six phosphorylation sites, continued serine predictions (at aa 8, 43, and 60), and threonine predictions (at aa 12, 40, and 87). Data indicated that DEV pUL49.5 has two predicted hydrophobic domains (Fig. 4A). The predicted transmembrane domain of DEV pUL49.5 may span aa 7~29 and aa 61~83. Moreover, an ectodomain span (aa 30~60) and cytoplasmic span (aa 1~6 and aa 84~95) were all identified. These findings suggested that DEV pUL49.5 is an envelope glycoprotein with two transmembrane domains with a probability of 0.995 (Fig. 4B). Secondary structure analysis (Fig. 4C) indicated that the alpha helix (H), extended strand (E), and random coil (C) occupied 57.89% (55 aa), 6.32% (6 aa), and 35.79% (34 aa), respectively. In addition, the predicted subcellular localization demonstrated that 66.7% of the DEV pUL49.5 is in endoplasmic reticulum, 11.1% is in vacuoles, 11.1% is in the Golgi, and 11.1% is extracellular. Thus, the DEV pUL49.5 is mostly distributed in the cytoplasm.
Fig. 4.
Prediction of the DEV pUL49.5 structure. (A) Prediction of the primary DEV pUL49.5 structure. The black wire frame represents the transmembrane domain, the hydrophobicity plot is displayed in purple, and the surface probability plot is shown in yellow. (B) Transmembrane domain profile of gN. The extracellular domain is pink, the transmembrane domains are red, and the cytoplasmic domains are blue. (C) Prediction of the DEV pUL49.5 secondary structure. "H" represents the alpha helix, "E" represents the extended strand, and "C" represents the random coil.
Intracellular localization of DEV pUL49.5 in DEFs
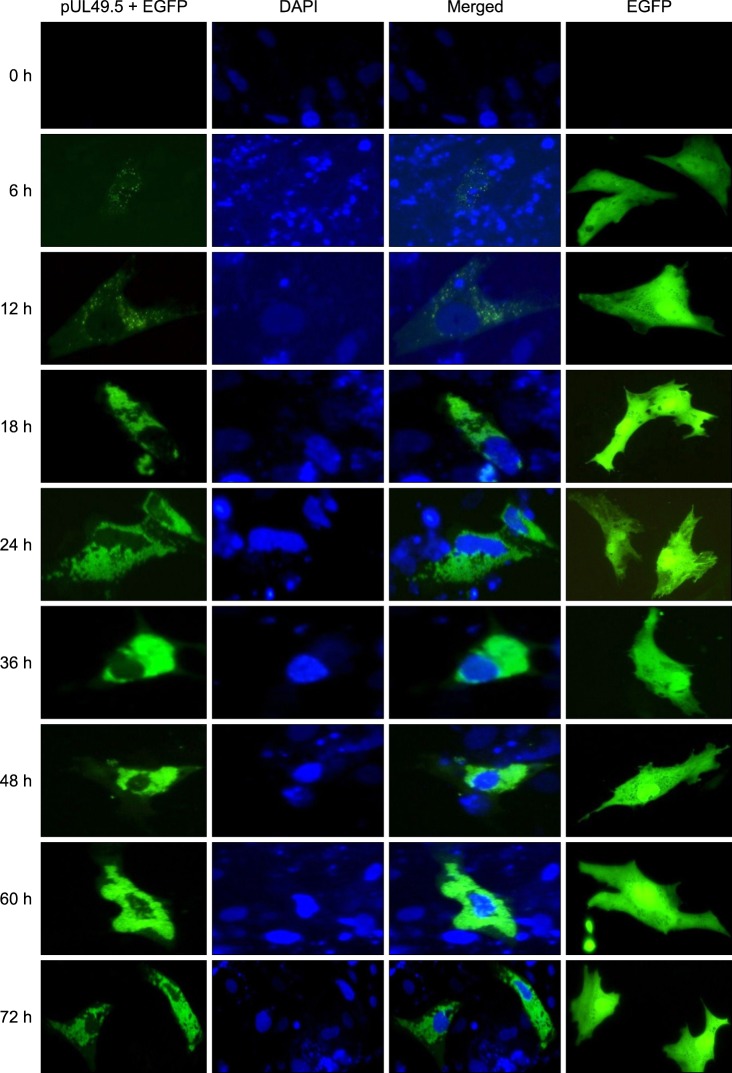
The DEFs were transfected with pEGFP-C1/pUL49.5 to confirm the intracellular localization of DEV pUL49.5. At 0 h after transfection, no EGFP was detected. Green fluorescence (almost invisible) first appeared 6 h after transfection, and was then clearly observed 12 h following transfection. After that, the fluorescence intensity gradually increased before reaching a peak 60 h after transfection, and finally decreased slightly 72 h following transfection. The green fluorescence was widely distributed in the cytoplasm and was particularly intense in the area around the nucleus that was stained with DAPI. Meanwhile, DEFs were transfected with the pEGFP-C1 plasmid as a negative control. The resulting fluorescence showed that EGFP was distributed throughout the entire cell at all time points (Fig. 5). These results showed that the DEV pUL49.5 protein localized in the cytoplasm.
Fig. 5.
Intracellular localization and distribution of DEV pUL49.5 in DEFs. In the first column, green indicates expression of the pUL49.5+EGFP fusion protein. In the second column, blue represents the cell nuclei counter-stained with DAPI. Merged images are shown in the third column. In the fourth column, green represents EGFP expression as a negative control.
Colocalization of DEV pUL49.5 and gM in DEFs
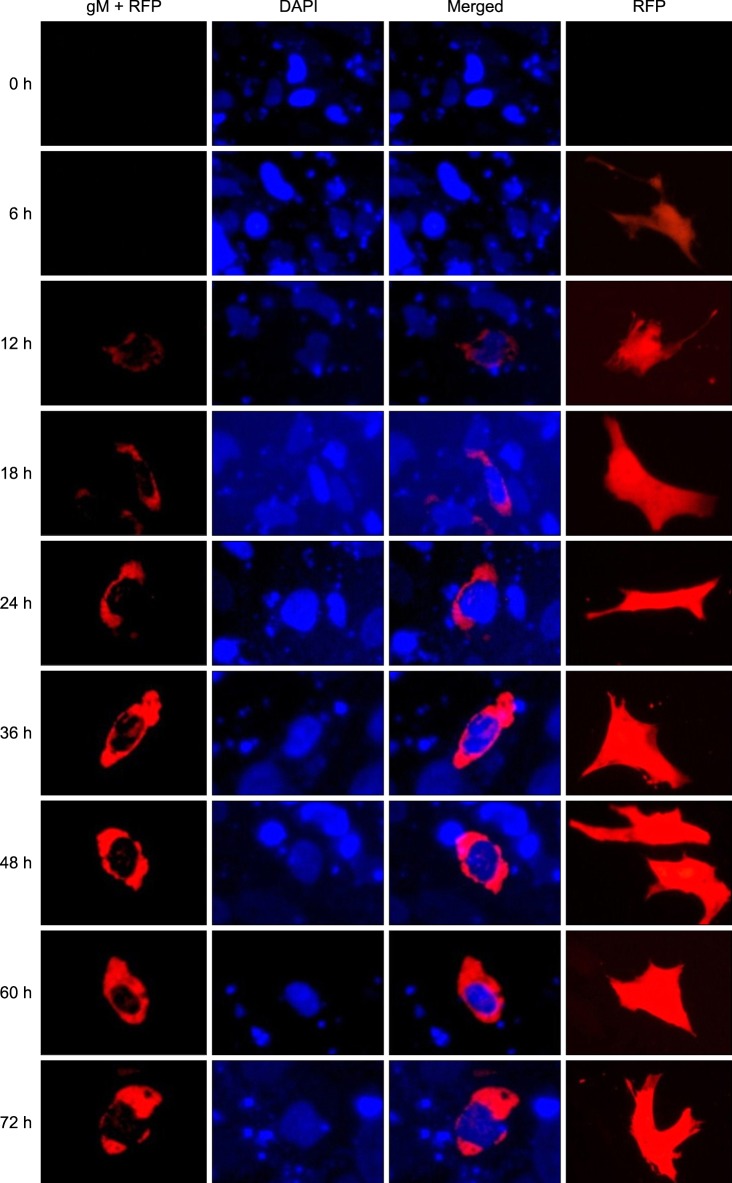
DEFs were also transfected with pDsRed1-N1/gM to confirm the intracellular localization of DEV gM. As shown in Fig. 6, red fluorescence appeared in the cytoplasm of the transfected cells, particularly in the area around the nucleus. Fluorescence was first detected 12 h after transfection. The intensity increased rapidly thereafter, achieved a maximum 60 h following transfection, and then slightly decreased 72 h after transfection. In the control cells, red fluorescence appeared in all regions.
Fig. 6.
Intracellular localization and distribution of DEV gM in DEFs. In the first column, red represents expression of the gM+RFP fusion protein. In the second column, blue represents nuclei counter-stained with DAPI. The third column contains merged images. In the fourth column, red represents the expression of RFP as a negative control.
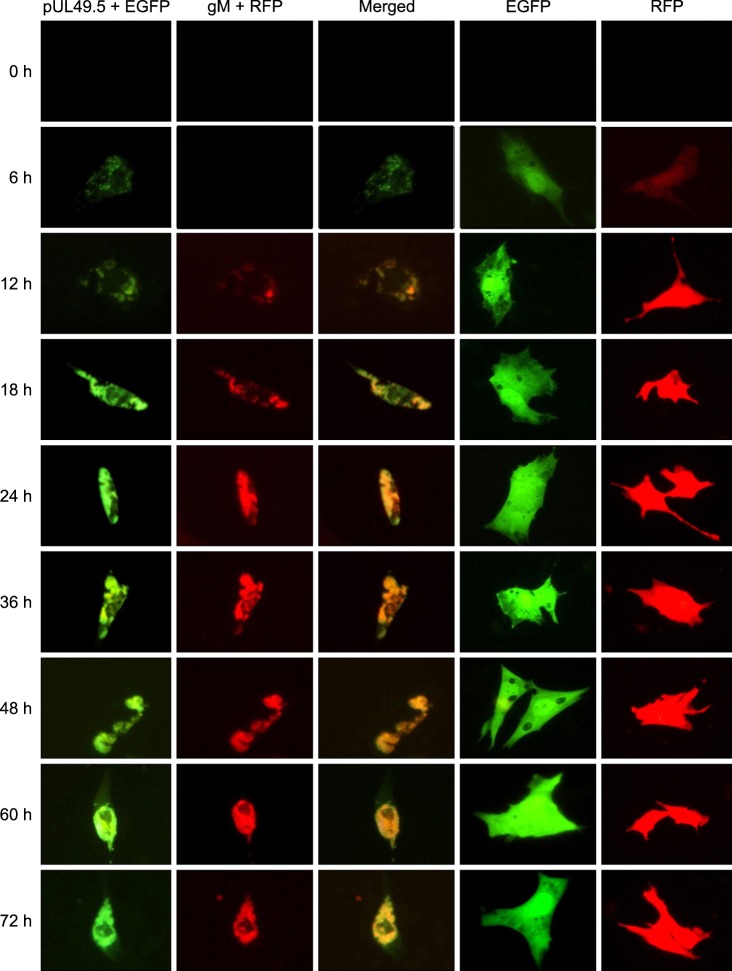
To determine whether DEV pUL49.5 localized in the same region as gM, DEFs were cotransfected with the two recombinant plasmids. Results of the experiment indicated that the two fluorescence signals overlapped. This was first observed 12 h after transfection and continued to persist until the end of the study (Fig. 7).
Fig. 7.
Colocalization of DEV pUL49.5 and gM in DEFs. In the first column, green represents expression of the pUL49.5+EGFP fusion protein. In the second column, red represents expression of the gM+RFP fusion protein. The third column contains merged images. In the fourth column, green represents the expression of EGFP as a negative control. In the fifth lane, red represents the expression of RFP as a negative control.
Discussion
Presently, bioinformatic software is needed for analyzing genes and predicting protein structure. In the current study, the phylogenetic tree showed that DEV pUL49.5 belongs to the herpesvirus UL49.5 superfamily and is most closely related to MeHV-1 gN. This suggested that the structure and function of DEV pUL49.5 is similar to that of MeHV-1 gN. Similar to pUL49.5 of other herpesviruses, DEV pUL49.5 is most likely a membrane protein with a predicted and cleavable signal sequence (first hydrophobic region) as well as a membrane anchor region in the C-terminus [18]. However, analysis of the signal peptide and transmembrane domains indicated that DEV pUL49.5 is extremely likely to be a membrane protein with a predicted and cleavable signal sequence along with two transmembrane domains. In addition, the two hydrophobic regions with a predicted result were located in regions consistent with those of the transmembrane domains in the DEV pUL49.5 sequence. It could be thus concluded that DEV pUL49.5 is different from pUL49.5 proteins of other herpesviruses.
DEV pUL49.5 was predicted to be entirely in the cytoplasm with a 66.7% probability that it could appear in the endoplasmic reticulum. The intracellular localization of a protein is important for understanding its function because proteins cooperate towards a common biological function, it means that they should localized in the same subcellular compartment first [22]. The most common methods to assess intracellular localization are direct or indirect immunofluorescence assays [27], immune colloidal gold staining [21], and the expression of recombinant plasmids with fluorescent reporter genes. Gene transfection with constructs expressing fluorescent proteins was used for the present study due to the ease, low error rate, relatively low cost, and ability to monitor dynamic changes associated with this technique [9]. Lipofectamine 2000 [15] was used to transfect DEFs with the recombinant plasmids that contained the cloned genes. Nucleic acid-Lipofectamine 2000 complexes can be added directly to cells in culture medium and produce high transfection rates in the presence or absence of serum [28]. Fluorescent protein has a wide range of applications in molecular biology as a reporter [10,19]. Two fluorescent proteins, EGFP and RFP, derived from jellyfish Aequorea victoria and coral Discosoma spp., respectively, were used in this study [2].
The cytoplasm and endoplasmic reticulum were predicted to be enriched with DEV pUL49.5. In order to prove this hypothesis, the intracellular localization of DEV pUL49.5 was examined using EGFP-tagged DEV pUL49.5. The fluorescence observed within 72 h after transfection revealed that the prediction was correct. Additionally, was found that DEV pUL49.5 expression underwent subtle changes in the cytoplasm. At 6 and 12 h after transfection, DEV pUL49.5 was distributed in a punctate manner. Over time, DEV pUL49.5 expression extended into the entire cytoplasmic region. Thus, we speculated that DEV pUL49.5 was concentrated in the endoplasmic reticulum as hypothesized. Moreover, DEV pUL49.5 expression reached a maximum 60 h after transfection, implying that the UL49.5 gene was expressed during the late stage of the infection cycle.
According to previous reports of other herpesviruses [13], we speculated that DEV pUL49.5 would form a complex with gM in the cytoplasm. Based on studies of HSV-1, PRV, HCMV, MDV, and other herpesviruses, gM and gN have interaction. In HCMV, gM and gN are covalently linked in a complex via a disulfide bond formed between conserved cysteine residues located at the putative second extracellular loop of gM and at the position immediately adjacent to the amino-terminal boundary of the putative transmembrane domain of gN [23]. In addition, a non-covalent bond also contributes to the interaction, and may represent the core-binding mechanism of the gM/gN complex [13]. Although gM in alphaherpesvirus is not essential for viral replication in cell cultures, disruption of this gene reduces viral growth [24]. In contrast, gM is essential for HCMV and MDV replication [4]. Of particular interest, gN has a special function in varicelloviruses. Even when in a complex formation with gM, gN of BHV-1 and equine herpesvirus 4 (EHV-4) helps avoid T cell recognition by mediating TAP inactivation and the down-regulation of cell surface major histocompatibility complex class I (MHC I) expression [17,25].
gM and gN protein interaction has been identified in several types of herpesviruses. DEV gM has been reported to have a cysteine residue and serve as an envelope glycoprotein [16]. Since gN possesses a cysteine residue, it was highly possible that DEV gM and pUL49.5 could be connected with a disulfide bond. In order to confirm this possibility, we first needed to prove that the functional areas of the two proteins were consistent. Colocalization of gM and pUL49.5 was evaluated after the intracellular localization of gM had been identified. Red fluorescence corresponding to DEV gM was first observed at 12 h after transfection, later than that of pUL49.5, but reached its peak at the same time as green fluorescence indicative of pUL49.5 expression (60 h). This finding indicated that the expression of DEV gM started later than that of pUL49.5, while the expression rate of gM was faster. It is worth noting that the fluorescence intensity of EGFP and RFP was different, so the color cannot be a basis for comparison. Furthermore, nuclei stained by DAPI could not be displayed in the merged images because the inverted microscope had only of only two fluorescence channels. According to the merged images showing the two types of fluorescent signals, DEV pUL49.5 and gM were localized the same region, thereby confirming our prediction. Based on our data, it is highly probable that DEV pUL49.5 interacts with gM in the cytoplasm. However, further experiments are required to determine whether DEV pUL49.5 has the same functions as the pUL49.5 protein of other herpesviruses.
Acknowledgments
This research was supported by the China Agricultural Research System (CARS-43-8), Sichuan Province Research Programs (2013HH0042, 2013TD0015, 11ZA084, 12TD005, 2011ZO0034, and 2011JO0040), the Ministry of Education Program (20125103110013), China.
Footnotes
There is no conflict of interest.
References
- 1.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Bevis BJ, Glick BS. Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed) Nat Biotechnol. 2002;20:83–87. doi: 10.1038/nbt0102-83. [DOI] [PubMed] [Google Scholar]
- 3.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 4.Burkhardt C, Himmelein S, Britt W, Winkler T, Mach M. Glycoprotein N subtypes of human cytomegalovirus induce a strain-specific antibody response during natural infection. J Gen Virol. 2009;90:1951–1961. doi: 10.1099/vir.0.010967-0. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs W, Mettenleiter TC. The nonessential UL49.5 gene of infectious laryngotracheitis virus encodes an O-glycosylated protein which forms a complex with the non-glycosylated UL10 gene product. Virus Res. 2005;112:108–114. doi: 10.1016/j.virusres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 7.Jöns A, Granzow H, Kuchling R, Mettenleiter TC. The UL49.5 gene of pseudorabies virus codes for an o-glycosylated structural protein of the viral envelope. J Virol. 1996;70:1237–1241. doi: 10.1128/jvi.70.2.1237-1241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horton P, Nakai K. Better prediction of protein cellular localization sites with the k nearest neighbors classifier. Proc Int Conf Intell Syst Mol Biol. 1997;5:147–152. [PubMed] [Google Scholar]
- 9.Kirby AJ, Camilleri P, Engberts JBFN, Feiters MC, Nolte RJM, Söderman O, Bergsma M, Bell PC, Fielden ML, García Rodríguez CL, Guédat P, Kremer A, McGregor C, Perrin C, Ronsin G, van Eijk MCP. Gemini surfactants: new synthetic vectors for gene transfection. Angew Chem Int Ed Engl. 2003;42:1448–1457. doi: 10.1002/anie.200201597. [DOI] [PubMed] [Google Scholar]
- 10.Kneen M, Farinas J, Li Y, Verkman AS. Green fluorescent protein as a noninvasive intracellular pH indicator. Biophys J. 1998;74:1591–1599. doi: 10.1016/S0006-3495(98)77870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koppers-Lalic D, Reits EAJ, Ressing ME, Lipinska AD, Abele R, Koch J, Marcondes Rezende M, Admiraal P, van Leeuwen D, Bienkowska-Szewczyk K, Mettenleiter TC, Rijsewijk FAM, Tampé R, Neefjes J, Wiertz EJHJ. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc Natl Acad Sci U S A. 2005;102:5144–5149. doi: 10.1073/pnas.0501463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koppers-Lalic D, Verweij MC, Lipińska AD, Wang Y, Quinten E, Reits EA, Koch J, Loch S, Marcondes Rezende M, Daus F, Bieńkowska-Szewczyk K, Osterrieder N, Mettenleiter TC, Heemskerk MHM, Tampé R, Neefjes JJ, Chowdhury SI, Ressing ME, Rijsewijk FAM, Wiertz EJHJ. Varicellovirus UL49.5 proteins differentially affect the function of the transporter associated with antigen processing, TAP. PLoS Pathog. 2008;4:e1000080. doi: 10.1371/journal.ppat.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koyano S, Mar EC, Stamey FR, Inoue N. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J Gen Virol. 2003;84:1485–1491. doi: 10.1099/vir.0.18941-0. [DOI] [PubMed] [Google Scholar]
- 14.Kropff B, Burkhardt C, Schott J, Nentwich J, Fisch T, Britt W, Mach M. Glycoprotein N of human cytomegalovirus protects the virus from neutralizing antibodies. PLoS Pathog. 2012;8:e1002999. doi: 10.1371/journal.ppat.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kullberg M, Mann K, Owens JL. A two-component drug delivery system using Her-2-targeting thermosensitive liposomes. J Drug Target. 2009;17:98–107. doi: 10.1080/10611860802471562. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Huang B, Ma X, Wu J, Li F, Ai W, Song M, Yang H. Molecular characterization of the genome of duck enteritis virus. Virology. 2009;391:151–161. doi: 10.1016/j.virol.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Lipińska AD, Koppers-Lalic D, Rychłowski M, Admiraal P, Rijsewijk FAM, Bieńkowska-Szewczyk K, Wiertz EJHJ. Bovine herpesvirus 1 UL49.5 protein inhibits the transporter associated with antigen processing despite complex formation with glycoprotein M. J Virol. 2006;80:5822–5832. doi: 10.1128/JVI.02707-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mach M, Osinski K, Kropff B, Schloetzer-Schrehardt U, Krzyzaniak M, Britt W. The carboxy-terminal domain of glycoprotein N of human cytomegalovirus is required for virion morphogenesis. J Virol. 2007;81:5212–5224. doi: 10.1128/JVI.01463-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.March JC, Rao G, Bentley WE. Biotechnological applications of green fluorescent protein. Appl Microbiol Biotechnol. 2003;62:303–315. doi: 10.1007/s00253-003-1339-y. [DOI] [PubMed] [Google Scholar]
- 20.Masse MJ, Jöns A, Dijkstra JM, Mettenleiter TC, Flamand A. Glycoproteins gM and gN of pseudorabies virus are dispensable for viral penetration and propagation in the nervous systems of adult mice. J Virol. 1999;73:10503–10507. doi: 10.1128/jvi.73.12.10503-10507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayhew TM, Griffiths G, Lucocq JM. Applications of an efficient method for comparing immunogold labelling patterns in the same sets of compartments in different groups of cells. Histochem Cell Biol. 2004;122:171–177. doi: 10.1007/s00418-004-0685-x. [DOI] [PubMed] [Google Scholar]
- 22.Nair R, Rost B. LOC3D: annotate sub-cellular localization for protein structures. Nucleic Acids Res. 2003;31:3337–3340. doi: 10.1093/nar/gkg514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pati SK, Novak Z, Purser M, Arora N, Mach M, Britt WJ, Boppana SB. Strain-specific neutralizing antibody responses against human cytomegalovirus envelope glycoprotein N. Clin Vaccine Immunol. 2012;19:909–913. doi: 10.1128/CVI.00092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y, Bell S, Zenner HL, Lau SYK, Crump CM. Glycoprotein M is important for the efficient incorporation of glycoprotein H-L into herpes simplex virus type 1 particles. J Gen Virol. 2012;93:319–329. doi: 10.1099/vir.0.035444-0. [DOI] [PubMed] [Google Scholar]
- 25.Said A, Azab W, Damiani A, Osterrieder N. Equine herpesvirus type 4 UL56 and UL49.5 proteins downregulate cell surface major histocompatibility complex class I expression independently of each other. J Virol. 2012;86:8059–8071. doi: 10.1128/JVI.00891-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimamura M, Mach M, Britt WJ. Human cytomegalovirus infection elicits a glycoprotein M (gM)/gN-specific virus-neutralizing antibody response. J Virol. 2006;80:4591–4600. doi: 10.1128/JVI.80.9.4591-4600.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verheugt FW, von dem Borne AEG, Décary F, Engelfriet CP. The detection of granulocyte alloantibodies with an indirect immunofluorescence test. Br J Haematol. 1977;36:533–544. doi: 10.1111/j.1365-2141.1977.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Bian Z, Wei D, Zhang JG. miR-29b regulates migration of human breast cancer cells. Mol Cell Biochem. 2011;352:197–207. doi: 10.1007/s11010-011-0755-z. [DOI] [PubMed] [Google Scholar]
- 29.Xing J, Wang S, Li Y, Guo H, Zhao L, Pan W, Lin F, Zhu H, Wang L, Li M, Wang L, Zheng C. Characterization of the subcellular localization of herpes simplex virus type 1 proteins in living cells. Med Microbiol Immunol. 2011;200:61–68. doi: 10.1007/s00430-010-0175-9. [DOI] [PubMed] [Google Scholar]