Abstract
A new series of salicyl glycoconjugates containing hydrazide and hydrazone moieties were designed and synthesized. The bioassay indicated that the novel compounds had no in vitro fungicidal activity but showed significant in vivo antifungal activity against the tested fungal pathogens. Some compounds even had superior activity than the commercial fungicides in greenhouse trial. The results of RT-PCR analysis showed that the designed salicyl glycoconjugates could induce the expression of LOX1 and Cs-AOS2, which are the specific marker genes of jasmonate signaling pathway, to trigger the plant defense resistance.
Introduction
In the past two decades, the goal of sustainable and green agriculture had been inspiring researchers to explore the feasibility of restricting toxic agrochemical usage to reduce their impact on environment and food chains. One of the alternatives, which had been studied intensively in recent years, was to make use of plant defense potentials. Induction of plant defense resistance in crops by chemical or biological elicitors had drawn increasing attentions and was considered as a prospective strategy for disease control [1], [2].
During the long process of co-evolution, plants had evolved lots of defense mechanisms to deal with pests and pathogens. Following plant-pathogen interaction, a number of plant defense responses could be induced (e.g., callus deposition, PR-protein accumulation, et al.) at the site of infection, and also in uninfected tissues, activated by signal molecules associated with defense responses, which resulted in increased resistance to subsequent infections. The systemic acquired resistance is a “whole-plant” defense response that occurred following an earlier localized exposure to a pathogen. Activation of systemic acquired resistance required the accumulation of endogenous salicylic acid [3]–[5]. Besides the salicylic acid dependent defense signaling pathway, the others had also been reported. For example, endogenous jasmonic acid and methyl jasmonate were also the potent signaling molecules which could induce a large set of defense responses [6]. Systemic acquired resistance possessed low specificity, was not easily overcome by new pathogens which emerged frequently.
Chemical elicitors are agrochemicals which do not show a direct effect on pathogens and lacked fungicidal activity themselves but induce defense mechanisms, which clearly distinguish them from conventional pesticides [7]. Some of these agrochemicals are known to have signaling functions in planta, such as benzothiadiazole [8]–[13], which is a functional analog of salicylic acid, while others may mimic the attack of a pathogen, such as harpin [14] or flagellin [15].
Saccharides are known as potent elicitors [16]. The fragments of chitin and chitosan, which act as elicitors in many plants, could induce the production of nitric oxide and hydrogen peroxide in some plant epidermal cells [17]–[20]. Even neutral saccharides, such as β-glucans derived from cellulose or laminarin [21], [22], are capable of enhancing plant resistance. The accumulation of phytoalexins could be induced by branched hexa (β-D-glucopyranosyl)-D-glucitols in soybean [23], [24]. Oligoglucans with polymerization between 8 and 17 could induce the chitinase activity in tobacco BY-2 suspension cells [25], [26]. The phenolic pathway could be rapidly induced by the mannose and glucose disaccharides in Rubus cells [27]. It is evident that saccharides have the ability to trigger defense responses in plants, enhance resistance toward infection, and even support plant growth [28], [29].
In our previous work, some 1,3,4-oxadiazole [30], benzoylureas [31]–[33], acylhydrazones [34], [35], diacylhydrazines [36]–[40], semicarbazide [41], pyrazole and 1,2,4-triazole [42] derivatives containing 5-phenyl-2-furan were designed and synthesized. All the compounds had considerable and diverse bioactivities such as insecticidal, fungicidal, and antitumor activities. Thus, 5-phenyl-2-furan was regarded as an active scaffold in drug design. In this study, we focused on the molecular design and synthesis of novel salicyl glycoconjugates as elicitors against plant diseases. We present here the preparation and characterization of the new elicitors based on salicylic acid and 5-phenyl-2-furan moiety (Figure 1), and show that these compounds could induce the systemic acquired resistance against pathogenic infections in cucumber.
Figure 1. General synthetic procedure for salicylic glycoconjugates.
Materials and Methods
Instruments
All the melting points were determined with a Cole-Parmer melting point apparatus (Cole-Parmer, Vernon Hills, Illinois, USA) while the thermometer was uncorrected. Optical rotation data were recorded on a KRUSS P8000 instrument (KRUSS, Karlsruhe, Germany). IR spectra were recorded on a Nicolet NEXUS-470 FTIR spectrometer (International Equipment Trading Ltd., Vernon Hills, Illinois, USA) with KBr pellets. 1H NMR spectra were recorded with Bruker DPX300 (Bruker, Fallanden, Switzerland) and JEOL JNM-ECS400 (JEOL Ltd., Tokyo, Japan), while tetramethylsilane was used as an internal standard. Analytical thin-layer chromatography was carried out on silica gel 60 F254 plates, and spots were visualized with ultraviolet light. Elemental analysis was carried out with a Flash EA 1112 elemental analyzer (Thermo Finnigan, Bremen, Germany). Mass spectra were measured on a Bruker APEX IV spectrometer (Bruker, Fallanden, Switzerland).
Synthetic procedures
General synthetic procedure for hydrazides 2a and 2b
Preparation of hydrazides 2a and 2b: Esters 1a and 1b (30 mmol) was suspended in 100 mL methanol and reacted with 98% hydrazine monohydrate (60 mmol, 2.9 mL) under reflux for 12 h. The solid was filtered, washed with methanol and dried to afford hydrazides 2a and 2b.
2-mercaptobenzohydrazide ( 2a ). Light yellow solid: yield 90%. m.p. 114–115°C. IR (KBr): νmax 3342, 3123, 1664, 1574, 1505, 1454, 1323, 1223, 1053 cm−1. 1H NMR (300 MHz, DMSO-d6): 4.65 (s, 2H, NH2), 5.16 (s, 1H, SH), 7.29–7.31 (m, 1H, PhH), 7.42–7.45 (m, 1H, PhH), 7.65–7.69 (m, 2H, PhH), 9.89 (s, 1H, CONH). ESI-MS: m/e 169.1 [M+H]+. Anal. Calcd. (%) for C7H8N2OS: C, 49.98; H, 4.79; N, 16.65. Found: C, 50.16; H, 4.91; N, 16.45.
2-hydroxybenzohydrazide ( 2b ). White solid: yield 92%. m.p. 147–148°C. IR (KBr): νmax 3623, 3468, 1667, 1549, 1531, 1464, 1245, 1062 cm−1. 1H NMR (300 MHz, DMSO-d6): δ 4.53 (s, 2H, NH2), 5.23 (s, 1H, OH), 6.72–6.75 (m, 1H, PhH), 7.30–7.32 (m, 1H, PhH), 7.76–7.79 (m, 1H, PhH), 7.85–7.88 (m, 1H, PhH), 9.79 (s, 1H, CONH). ESI-MS: m/e 153.1 [M+H]+. Anal. Calcd. (%) for C7H8N2O2: C, 55.26; H, 5.30; N, 18.41. Found: C, 55.52; H, 5.14; N, 18.59.
General synthetic procedure for hydrazides 5a–d and hydrazones 7a–g
The key intermediates hydrazides 5a–d were obtained almost quantitatively by the hydrazinolysis of compounds 4a∼d in alcohol. Compounds 5a∼d were condensed with 5-substituted phenyl-2-furfural to form the glycosyl hydrazones 7a–g. All the chemical characterization was given in reference [35].
Bioassays
In vitro fungicidal activity
In vitro fungicidal activity of the salicylic glycoconjugates against Colletotrichum orbiculare, Fusarium oxysporum, Rhizoctonia solanii, and Phytophthora capsici were evaluated using mycelium growth rate test [43]–[45]. The tested compounds were dissolved in DMSO (dimethyl sulfoxide) and mixed with sterile molten potato dextrose agar to a final concentration of 50 µg/mL. In vitro fungicidal activity of the salicylic glycoconjugates against Sphaerotheca fuliginea was evaluated using colonized detached leaves method [43]–[45]. The conidial suspensions were prepared by seeding about 2×105 spores mL−1 conidia in a 0.05% Tween 80 solution, and the DMSO solution of compounds (5000 µg/mL) was diluted with conidial suspension to a final concentration of 50 µg/mL. The solution was sprayed with a hand sprayer on the surface of the detached leaves which were inoculated with S. fuliginea.
P. capsici was maintained on oat medium at 17°C. C. orbiculare, F. oxysporum, and R. solanii were maintained on potato dextrose agar medium at 4°C. Five commercial fungicides: thiophanate-methyl, benomyl, chlorothalonil, validamycin, and dimethomorph were used as controls against the above mentioned fungal pathogens under the same conditions. Three replicates were performed. The relative inhibition rate of the synthetic compounds compared to blank control was calculated via the following equation:
In which, I stands for the rate of inhibition (%), C is the diameter of mycelia in the blank control test (in mm), and T is the diameter of mycelia in the presence of tested compounds (in mm).
In vivo Antifungal Activity
Using the pot culture test [42], [46], the in vivo antifungal activities of the salicylic glycoconjugates against C. orbiculare, F. oxysporum, S. fuliginea, R. solanii, and P. capsici were evaluated in greenhouse along with five commercial fungicides, 70% thiophanate-methyl WP, 70% benomyl WP, 50% chlorothalonil WP, 3% validamycin AS, and 50% dimethomorph WP as controls.
The culture plates were cultivated at 24±1°C. Germination was conducted by soaking cucumber seeds in water for 2 h at 50°C and then keeping the seeds moist for 24 h at 28°C in an incubator. When the radicles were 0.5 cm, the seeds were grown in plastic pots containing a 1∶1 (v/v) mixture of vermiculite and peat. Cucumber plants used for inoculations were at the stage of two seed leaves. Ten plants were used for each treatment.
Tested compounds were confected to 2.5% EC (emulsifiable cocentration) formulations, in which pesticide emulsifier 500 (0.375%) and pesticide emulsifier 600 (2.125%) were the additives, DMSO (0.1%) was the solvent, and xylene was the co-solvent. The formulation was diluted to a concentration of 500 µg/mL with water. The solution was sprayed with a hand sprayer on the surface of seed leaves which were then inoculated with C. orbiculare, S. fuliginea, and R. solanii, respectively. Tested compounds and commercial fungicides were applied by irrigation at seedling stage, which were then inoculated with F. oxysporum and P. capsici, respectively. Three replicates for each treatment were applied.
Inoculations of C. orbiculare and S. fuliginea were carried out by spraying conidial suspension, and inoculation of R. solanii was carried out by spraying a mycelial suspension. F. oxysporum assay was carried out by embryo root inoculation, and P. capsici assay was carried out by irrigation inoculation.
Three replicates for each treatment were applied. After inoculation, the plants were maintained at 24±1°C and above 80% relative humidity.
The fungicidal activity was evaluated when the untreated cucumber plant (blank control) fully developed symptoms. The area of inoculated leaves covered by disease symptoms was assessed and compared to that of untreated ones to determine the average disease index. The relative control efficacy of compounds compared to the blank assay was calculated via the following equation:
where I is relative control efficacy, CK is the average disease index during the blank assay and PT is the average disease index after treatment during testing.
RT-PCR for detection of pathogenesis-related gene expression
Tested compounds (500 µg/mL) were sprayed with a hand sprayer on the surface of the cucumber (Cucumis sativus) seed leaves, which were collected after 24 h, 48 h, and 72 h. The leaves were treated by liquid nitrogen. RNA isolation was performed with the RNAiso Plus Kit (Takara Bio). First-strand cDNA was synthesized from 100 µg/mL total RNA, which was quantified with QuantiT RNA Assay Kit (Invitrogen), by reverse transcription using the QuantiTect Reverse Transcription Kit (QIEGEN). Gene-specific primers (Table S1 in File S1) were designed and actin was used as the housekeeping gene [47], [48]. Each reaction mixture (30 µL) contained 1 µL of the cDNA template, 100 pmol of each primer, 10 µL of Premix Ex Taq HS (Takara Bio), and 20 µL reaction buffer. The thermal cycling conditions were as follows: initial denaturation (94°C, 5 min), followed by 40 cycles of denaturation (94°C, 30 s), annealing (30 s) and extension (72°C, 30 s), and one final cycle of extension (72°C, 5 min). Finally, RT-PCR products were separated by electrophoresis and visualized in 1% agarose gel.
Ethics statement
No specific permits were required for the described field studies. No specific permissions were required for these locations. We confirm that the location is not privately-owned or protected in any way. We confirm that the field studies did not involve endangered or protected species.
Results and Discussion
Synthesis
The synthetic routes of 2-mercaptobenzohydrazide 2a, 2-hydroxybenzohydrazide 2b and glycosyl hydrazides 5a–d were shown in Figure 1. The hydrazides 5a–d were obtained almost quantitatively by hydrazinolysis of the esters 4a–d in alcohol. Finally, the hydrazides 5a–d were reacted with aldehyde 6 by condensation to form the glycosyl hydrazones 7a–g.
Fungicidal activity
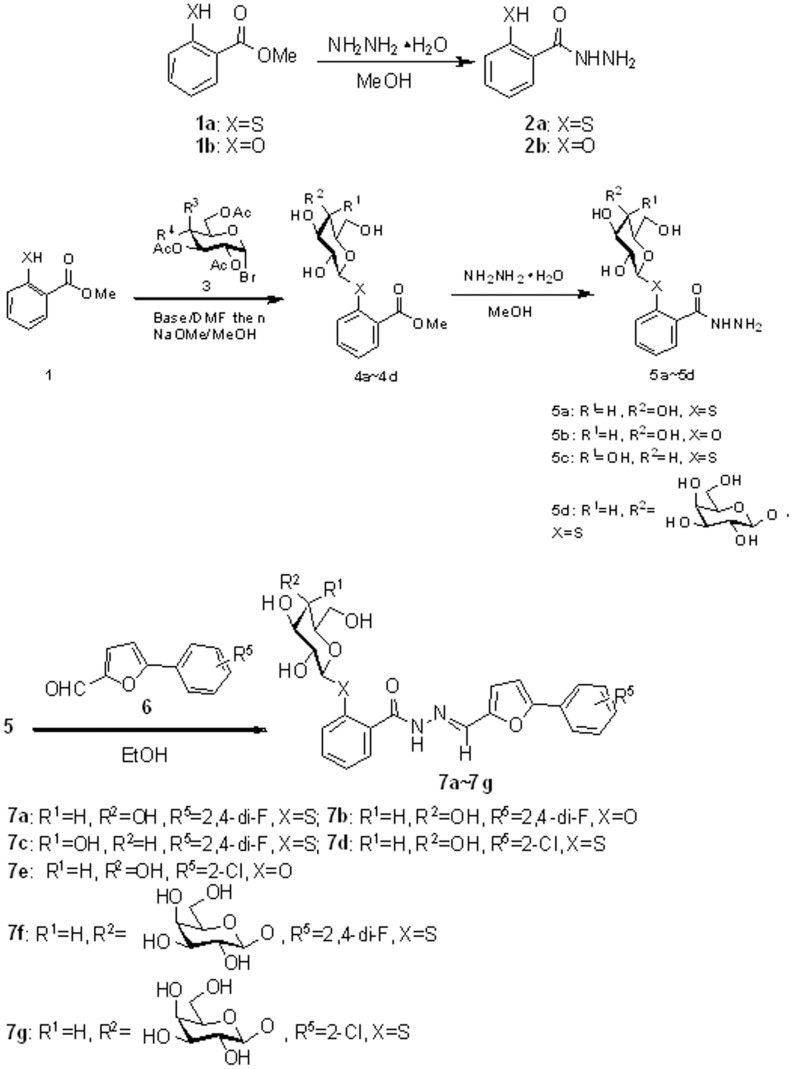
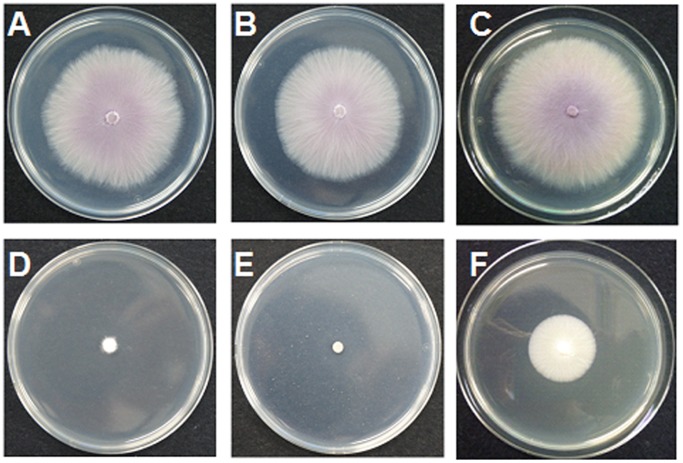
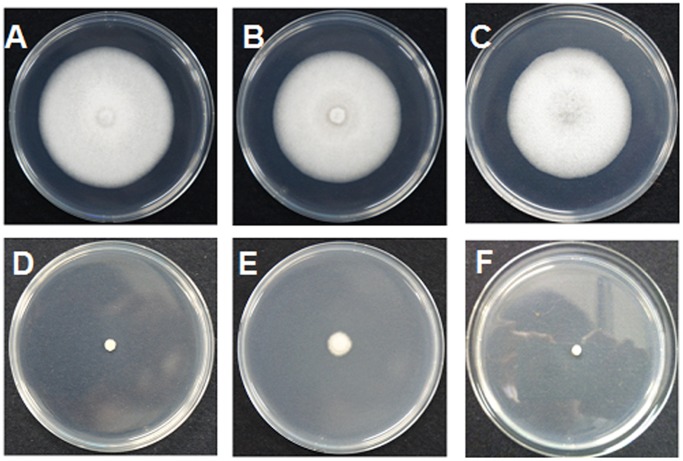
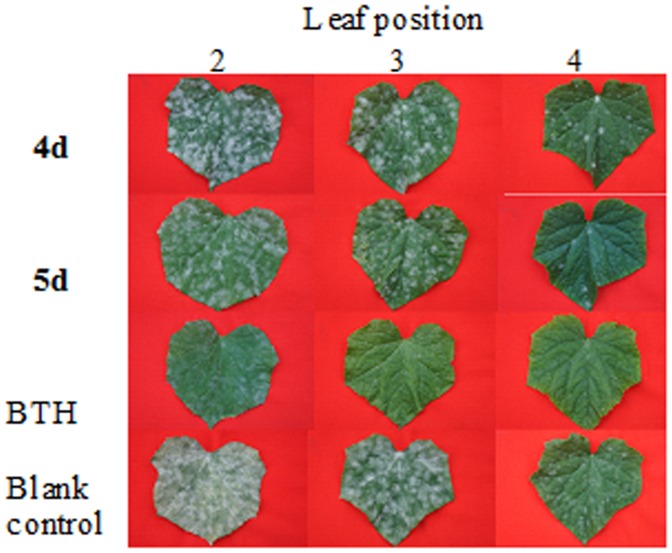
The in vitro fungicidal results were shown in Table 1. The hydrazides 2a and 2b showed excellent activity against the tested fungi (Figures 2 and 3). For example, the inhibitory rates of the hydrazides 2a and 2b against C. orbiculare were 97.3% and 95.4%, which were better than thiophanate-methyl (91.0%). After modification of sugars, the in vitro activity of all the derivatives was decreased and they exhibited poor inhibitory rates. Although the in vitro activity of these glycosides was not encouraging, the in vivo tests gave promising results (Table 2), with all the carbohydrate derivatives showing considerable activity, especially against F. oxysporum (Table 2), C. orbiculare (Figure 4), and S. fuliginea (Figure 5). Among them, hydrazide 5d and hydrazone 7f had activity of 71.0% and 74.9% on F. oxysporum, respectively, which is similar to the control benomyl (74.5%) against the same pathogen. 5d also showed good activity of 68.6% and 73.9% against C. orbiculare and S. fuliginea, respectively. Some hydrazones 7 exhibited promising activity against P. capsici. For examples, 7c showed an inhibitory rate of 83.5%, and the inhibitory rates of 7a, 7d and 7e were more than 75%.
Table 1. In vitro fungicidal activity against five fungus species at 50 µg/mL.
| Compd. | Inhibitory rate (%) | ||||
| C. orbiculare | F. oxysporum | S. fuliginea | R. solanii | P. capsici | |
| 2a | 97.3±2.0 | 73.0±2.2 | 73.1±2.1 | 86.5±2.3 | 56.2±2.2 |
| 2b | 95.4±1.3 | 95.5±2.3 | 77.7±2.3 | 79.2±1.7 | 61.7±1.8 |
| 4a | 11.3±1.0 | 12.2±1.1 | 28.1±2.0 | 27.9±1.6 | 13.2±1.1 |
| 4b | 28.6±1.2 | 28.0±1.5 | 10.3±1.5 | 28.9±2.0 | 28.4±1.3 |
| 4c | 12.9±1.2 | 9.3±0.4 | 6.5±0.6 | 18.1±2.0 | 12.9±1.7 |
| 4d | 8.1±0.4 | 11.6±1.0 | 11.5±2.1 | 23.0±2.0 | 2.0±0.4 |
| 5a | 15.2±0.9 | 7.4±0.2 | 10.4±1.7 | 26.1±1.0 | 21.6±1.0 |
| 5b | 19.7±1.1 | 9.4±0.7 | 25.9±2.6 | 12.9±1.1 | 26.7±1.6 |
| 5c | 2.2±0.3 | 3.2±0.1 | 1.3±0.8 | 16.3±2.4 | 23.8±1.2 |
| 5d | 17.6±1.3 | 13.3±1.2 | 15.2±1.4 | 19.5±1.3 | 28.4±2.3 |
| 7a | 24.4±1.2 | 35.3±1.7 | 28.5±2.0 | 15.4±1.0 | 21.4±1.3 |
| 7b | 15.7±1.8 | 24.7±1.4 | 37.5±2.3 | 47.6±1.5 | 31.6±1.8 |
| 7c | 25.5±1.7 | 33.3±1.6 | 17.5±1.1 | 39.4±2.0 | 38.6±1.2 |
| 7d | 13.3±1.9 | 25.3±1.3 | 36.5±1.6 | 38.4±1.3 | 29.0±1.4 |
| 7e | 25.5±1.0 | 21.5±1.1 | 19.6±1.7 | 19.8±1.0 | 41.5±1.5 |
| 7f | 6.0±1.0 | 12.6±1.1 | 27.6±1.6 | 37.6±2.5 | 24.5±1.2 |
| 7g | 11.3±0.6 | 8.2±0.8 | 12.4±1.6 | 21.5±1.5 | 15.7±1.1 |
| DMSO | 1.0±0.3 | 1.9±0.7 | 1.0±0.1 | 1.4±0.5 | 1.0±0.2 |
| Fungicidesa | 91.0±1.3 a | 98.2±1.2 b | 97.5±2.1 c | 91.0±2.1 d | 91.2±2.5 e |
Control fungicides: a, thiophanate-methyl; b, benomyl; c, chlorothalonil; d, validamycin; e, dimethomorph.
Figure 2. In vitro fungicidal activity against Fusarium oxysporum.
A: blank control, B: 5d, C: DMSO, D: 2b, E: benomyl, F: 2a.
Figure 3. In vitro fungicidal activity against Colletotrichum orbiculare.
A: blank control, B: 5d, C: DMSO, D: 2b, E: thiophanate-methyl, F: 2a.
Table 2. In vivo antifungal activity against five fungus species at 500 µg/mL.
| Compd. | Inhibitory rate (%) | ||||
| C. orbiculare | F. oxysporum | S. fuliginea | R. solanii | P. capsici | |
| 2a | 51.8±2.0 | 55.2±2.2 | 48.1±3.1 | 51.7±2.2 | 33.2±1.3 |
| 2b | 61.7±1.1 | 64.5±2.4 | 43.5±2.2 | 51.8±3.1 | 26.8±1.1 |
| 4a | 45.6±2.6 | 43.6±2.3 | 41.9±1.1 | 49.7±2.3 | 12.9±1.2 |
| 4b | 41.6±1.2 | 28.1±1.0 | 39.4±1.7 | 21.5±1.5 | 11.3±0.6 |
| 4c | 51.6±1.7 | 59.7±2.1 | 49.3±2.3 | 17.4±1.9 | 3.2±0.9 |
| 4d | 47.5±1.8 | 34.5±1.6 | 34.7±1.3 | 43.8±1.6 | 6.6±0.3 |
| 5a | 50.3±1.3 | 54.5±1.2 | 50.8±1.7 | 26.0±1.3 | 40.4±2.2 |
| 5b | 53.3±1.8 | 61.8±2.0 | 53.7±2.5 | 45.0±2.0 | 11.3±1.7 |
| 5c | 34.6±1.6 | 55.6±0.8 | 54.5±1.3 | 34.5±1.4 | 24.2±1.0 |
| 5d | 68.6±1.3 | 71.0±2.3 | 73.9±2.6 | 31.2±1.4 | 12.9±1.2 |
| 7a | 41.3±0.5 | 53.8±1.5 | 52.3±1.1 | 45.5±2.1 | 76.0±2.2 |
| 7b | 48.3±1.3 | 33.6±1.5 | 54.5±1.5 | 36.8±1.9 | 68.6±1.5 |
| 7c | 54.8±1.9 | 62.6±1.6 | 34.5±0.7 | 28.6±1.5 | 83.5±1.3 |
| 7d | 12.6±0.3 | 34.5±1.0 | 37.8±1.3 | 36.6±1.9 | 78.5±1.6 |
| 7e | 54.3±2.5 | 54.6±0.9 | 23.3±1.2 | 33.2±1.8 | 77.5±2.0 |
| 7f | 59.6±1.8 | 74.9±1.3 | 14.7±0.8 | 14.5±1.0 | 25.6±1.1 |
| 7g | 54.8±1.5 | 40.3±1.2 | 42.8±1.3 | 33.9±1.5 | 34.7±1.0 |
| DMSO | 2.2±0.6 | 2.9±0.2 | 2.4±0.4 | 2.2±0.8 | 3.1±0.6 |
| Fungicidesa | 76.8±2.3 a | 74.5±2.3 b | 94.6±1.7 c | 81.0±2.7 d | 91.2±2.4 e |
Control fungicides: a, 70% thiophanate-methyl WP; b, 70% benomyl WP; c, 50% chlorothalonil WP; d, 3% validamycin AS; e, 50% dimethomorph WP.
Figure 4. In vivo antifungal activity against Colletotrichum orbiculare.
Figure 5. In vivo antifungal activity against Sphaerotheca fuliginea.
The bioassay results showed that the tested compounds had in vivo antifungal activity against pathogenic fungi of Ascomycota (C. orbiculare, F. oxysporum and S. fuliginea), Basidiomycota (R. solanii), and Oomycete (P. capsici). The observed in vivo antifungal activity also had some association with the issue of pathogen biology. The tested compounds exhibited activity not only against the obligatory parasite pathogen (S. fuliginea), but also against the facultative parasite pathogens (C. orbiculare, F. oxysporum, R. solanii and P. capsici). The tested compounds also showed good activity against the soil-borne fungal disease (F. oxysporum, R. solanii and P. capsici). Also, we confirmed that all of these test compounds were safe for the host plants.
Defense activity of designed compound in plant
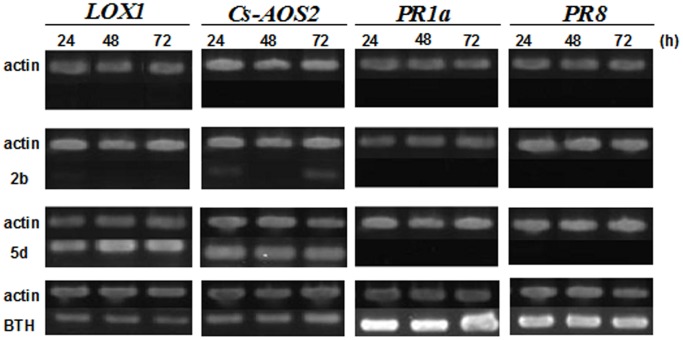
There are two important defense signaling pathways in plant system. One is mediated by salicylic acid and the other is mediated by jasmonic acid. In each defense pathway, there are specific marker genes which expression could be influenced by corresponding signaling molecules. In order to unveil the mode of action of our designed compounds, RT-PCR was performed to check the expression patterns of pathogenesis-related genes (PR1a, PR8, LOX1, Cs-AOS2) (Figure 6). Among them, PR1a and PR8 were the specific marker genes mediated by salicylic acid, whereas LOX1 and Cs-AOS2 were the specific marker genes mediated by jasmonic acid. Our results showed that expressions of the LOX1 and Cs-AOS2 genes were significantly induced by hydrazide 5d, and the expression level was comparable with that mediated by BTH (S-methyl benzo [1], [2], [3]thiadiazole-7-carbothioate). However, hydrazide 5d had no obvious effect on the expressions of PR1a and PR8.
Figure 6. Effect of designed compounds on inducing the expression of pathogenesis-related genes in Cucumis sativus.
Conclusions
A new series of glycosyl hydrazines and hydrozone derivatives were designed and synthesized. Their antifungal tests indicated that most of the salicylic glycoconjugates had no in vitro fungicidal activity but showed considerable in vivo antifungal activity. The plant defense activity showed that expressions of the LOX1 and Cs-AOS2 genes were significantly induced by hydrazide 5d, but the compound had no effect on the expressions of PR1a and PR8. Intriguingly, although the designed compounds were the derivatives of salicylic acid, they did not mimic the mode of action of salicylic acid, but seem to follow the jasmonic acid mediated pathway to induce the plant defense resistance.
Supporting Information
Primers Used in This Study.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial support was provided by the National Key Project for Basic Research (2015CB150605), the National Natural Science Foundation of China (21102173), the State Key Laboratory for Biology of Plant Diseases and Insect Pests (SKLOF201411), the Specialty and Innovation Projects of Guangdong Province University, the President Science Foundation of South China Agricultural University (4200-K13014), the Japan Society for the Promotion of Science (JSPS, ID No. P10100), Advanced Multi-Career Training Program for Postdoctoral Scholars from JST, and the VBL project funding from Chiba University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Franco G (2003) Systemic acquired resistance in crop protection: from nature to a chemical approach. J Agric Food Chem 51: 4487–4503. [DOI] [PubMed] [Google Scholar]
- 2. Terry LA, Joyce DC (2004) Elicitors of induced resistance in postharvest horticultural crops: a brief review. Postharvest Biol Technol 32: 1–13. [Google Scholar]
- 3. Malamy J, Carr JP, Klessig DF, Raskin I (1990) Salicylic acid a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250: 1002–1004. [DOI] [PubMed] [Google Scholar]
- 4. Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, et al. (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261: 754–756. [DOI] [PubMed] [Google Scholar]
- 5. Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206. [DOI] [PubMed] [Google Scholar]
- 6. Beckers G, Spoel S (2006) Fine-tuning plant defence signalling: Salicylate versus jasmonate. Plant Biol 8: 1–10. [DOI] [PubMed] [Google Scholar]
- 7. Tamm L, Thürig B, Fleissbach A, Goltlieb AE, Karavani S, et al. (2011) Elicitors and soil management to induce resistance against fungal plant diseases. NJAS−Wageningen J Life Sci 58: 131–137. [Google Scholar]
- 8. Gorlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, et al. (1996) Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 8: 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fan Z, Shi Z, Zhang H, Liu X, Bao L, et al. (2009) Synthesis and biological activity evaluation of 1,2,3-thiadiazole derivatives as potential elicitors with highly systemic acquired resistance. J Agric Food Chem 57: 4279–4286. [DOI] [PubMed] [Google Scholar]
- 10. Zuo X, Mi N, Fan Z, Zheng Q, Zhang H, et al. (2010) Synthesis of 4-methyl- 1,2,3-thiadiazole derivatives via Ugi reaction and their biological activities. J Agric Food Chem 58: 2755–2762. [DOI] [PubMed] [Google Scholar]
- 11. Fan Z, Yang Z, Zhang H, Mi N, Wang H, et al. (2010) Synthesis, crystal structure, and biological activity of 4-methyl-1,2,3-thiadiazole-containing 1,2,4-triazolo [3,4-b][1,3,4] thiadiazoles. J Agric Food Chem 58: 2630–2636. [DOI] [PubMed] [Google Scholar]
- 12. Xu YF, Zhao ZJ, Qian XH, Qian ZG, Tian WH, et al. (2006) Novel, unnatural benzo-1,2,3-thiadiazole-7-carboxylate elicitors of taxoid biosynthesis. J Agric Food Chem 54: 8793–8798. [DOI] [PubMed] [Google Scholar]
- 13. Du Q, Zhu W, Zhao Z, Qian X, Xu Y (2012) Novel benzo-1,2,3-thiadiazole-7- carboxylate derivatives as plant activators and the development of their agricultural applications. J Agric Food Chem 60: 346–353. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Bi Y, Zhang Z, Zhang H, Ge Y (2011) Reduction of latent infection and enhancement of disease resistance in muskmelon by preharvest application of harpin. J Agric Food Chem 59: 12527–12533. [DOI] [PubMed] [Google Scholar]
- 15. Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276. [DOI] [PubMed] [Google Scholar]
- 16. Yamaguchi T, Ito Y, Shibuya N (2000) Oligosaccharide elicitors and their receptors for plant defense responses. Trends Glycosci Glyc 12: 113–120. [Google Scholar]
- 17. Kombrink A, Sanchez-Valle A, Thomma BPHJ (2011) The role of chitin detection in plant-pathogen interactions. Microbes Infect 13: 1168–1176. [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Heng Y, Zhao X, Du Y, Li F (2009) Oligochitosan induced Brassica napus L. production of NO and H2O2 and their physiological function. Carbohydr Polym 75: 612–617. [Google Scholar]
- 19. Yin H, Zhao X, Du Y (2010) Oligochitosan: a plant diseases vaccine–a review. Carbohydr Polym 82: 1–8. [Google Scholar]
- 20. Bautista-Banos S, Hernandez-Lauzardo AN, Velazquez-del Valle MG, Hernandez-Lopez M, Ait Barka E, et al. (2006) Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot 25: 108–118. [Google Scholar]
- 21. Aziz A, Gauthier A, Bezler A, Poinssot B, Joubert JM, et al. (2007) Elicitor and resistance-inducing activities of β-1,4 cellodextrins in grapevine, comparison with β-1,3 glucans and α-1,4 oligogalacturonides. J Exp Bot 58: 1463–1472. [DOI] [PubMed] [Google Scholar]
- 22. Klarzynski O, Plesse B, Joubert JM, Yvin JC, Kopp M, et al. (2000) Linear β-1,3 glucans are elicitors of defense response in tobacco. Plant Physiol 124: 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharp JK, McNeil M, Albersheim P (1984) Purification and partial characterization of a β-glucan fragment that elicits phytoalexin accumulation in soybean. J Biol Chem 259: 11312–11320. [PubMed] [Google Scholar]
- 24. Sharp JK, McNeil M, Albersheim P (1984) The primary structures of one elicitor-active and seven elicitorinactive hexa(β-D-glucopyranosyl)-D-glucitols isolated from the mycelial walls of Phytophthora megasperma f.sp. glycinea . J Biol Chem 259: 11321–11336. [PubMed] [Google Scholar]
- 25. Jamois F, Ferrieres V, Guegan JP, Yvin JC, Plusquellec D, et al. (2005) Glucan-like synthetic oligosaccharides: iterative synthesis of linear oligo-β-(1,3)-glucans and immunostimulatory effects. Glycobiology 15: 393–407. [DOI] [PubMed] [Google Scholar]
- 26. Shinya T, Ménard R, Kozone I, Matsuoka H, Shibuya N, et al. (2006) Novel β-1,3, β-1,6-oligoglucan elicitor from Alternaria alternata 102 for defense response in tobacco. FEBS J 273: 2421–2431. [DOI] [PubMed] [Google Scholar]
- 27. Nita-Lazar M, Heyraud A, Gey C, Braccini I, Lienart Y (2004) Novel oligosaccharide from Fusarium oxysporum L., rapidly induces PAL activity in Rubus cells. Acta Biochim Pol 51: 625–634. [PubMed] [Google Scholar]
- 28. Liu H, Cheng S, Liu J, Du Y, Bai Z, et al. (2008) Synthesis of pentasaccharise and heptasaccharide derivatives and their effects on plant growth. J Agric Food Chem 56: 5634–5638. [DOI] [PubMed] [Google Scholar]
- 29. Kano A, Gomi K, Yamasaki-Kokudo Y, Satoh M, Fukumoto T, et al. (2010) A rare sugar, D-allose, confers resistance to rice bacterial blight with upregulation of defense-related genes in Oryza sativa . Phytopathology 100: 85–90. [DOI] [PubMed] [Google Scholar]
- 30. Cui ZN, Shi YX, Zhang L, Ling Y, Li BJ, et al. (2012) Synthesis and fungicidal activity of novel 2,5-disubstituted-1,3,4-oxadiazole derivatives, J Agric Food Chem. 60: 11649–11656. [DOI] [PubMed] [Google Scholar]
- 31. Yang XL, Wang DQ, Chen FH, Ling Y, Zhang ZN (1998) The synthesis and larvicidal activity of N-aroyl-N′-(5-aryl-2-furoyl) ureas. Pestic Sci 52: 282–286. [Google Scholar]
- 32. Yang XL, Ling Y, Wang DQ, Chen FH (2002) The synthesis and biological activity of N-phenyl-N′-(5-phenyl-2-furoyl) ureas. Chin J Synth Chem 10: 510–512. [Google Scholar]
- 33. Cui ZN, Zhang L, Huang J, Li Y, Ling Y, et al. (2008) 3D-QSAR studies on diacyl urea derivatives containing furan moiety. Acta Chim Sinica 66: 1417–1423. [Google Scholar]
- 34. Cui ZN, Li Y, Huang J, Ling Y, Cui JR, et al. (2010) New class of potent antitumor acylhydrazone derivatives containing furan. Eur J Med Chem 45: 5576–5584. [DOI] [PubMed] [Google Scholar]
- 35. Cui ZN, Yang XL, Shi Y, Uzawa H, Cui J, et al. (2011) Molecular design, synthesis and bioactivity of glycosyl hydrazine and hydrazone derivatives: Notable effects of the sugar moiety. Bioorg Med Chem Lett 21: 7193–7196. [DOI] [PubMed] [Google Scholar]
- 36. Cui ZN, Wang Z, Li Y, Zhou XY, Ling Y, et al. (2007) Synthesis of 5-(chlorophenyl)-2-furancarboxylic acid 2-(benzoyl)hydrazide derivatives and determination of their insecticidal activity. Chin J Org Chem 27: 1300–1304. [Google Scholar]
- 37. Cui ZN, Huang J, Li Y, Ling Y, Yang XL, et al. Synthesis and bioactivity of novel N,N′-diacylhydrazine derivatives containing furan(I). Chin J Chem 26: 916–922. [Google Scholar]
- 38. Li XC, Yang XL, Cui ZN, Li Y, He HW, et al. (2010) Synthesis and bioactivity of novel N,N′-diacylhydrazine derivatives containing furan(II). Chin J Chem 28: 1233–1239. [Google Scholar]
- 39. Cui ZN, Zhang L, Huang J, Yang XL, Ling Y (2010) Synthesis and bioactivity of novel N,N′-diacylhydrazine derivatives containing furan(III). Chin J Chem 28: 1257–1266. [Google Scholar]
- 40. Zhang L, Cui ZN, Yin B, Yang GF, Ling Y, et al. (2010) QSAR and 3D-QSAR studies of the diacyl-hydrazine derivatives containing furan rings based on the density functional theory. Sci China Chem 53: 1322–1331. [Google Scholar]
- 41. Cui ZN, Ling Y, Li BJ, Li YQ, Rui CH, et al. (2010) Synthesis and bioactivity of N-benzoyl-N′-[5-(2′-substituted phenyl)-2-furoyl] semicarbazide derivatives. Molecules 15: 4267–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cui ZN, Shi YX, Cui JR, Ling Y, Li BJ, et al. (2012) Synthesis and bioactivities of novel pyrazole and triazole derivatives containing 5-phenyl-2-furan. Chem Biol Drug Des 79: 121–127. [DOI] [PubMed] [Google Scholar]
- 43. Li XH, Wu DC, Qi ZQ, Li XW, Gu ZM, et al. (2010) Synthesis, fungicidal activity, and structure-activity relationship of 2-oxo and 2-hydroxycycloalkyl- sulfonamides. J Agric Food Chem 58: 11384–11389. [DOI] [PubMed] [Google Scholar]
- 44. Li XH, Pan Q, Cui ZN, Ji MS, Qi ZQ (2013) Synthesis and fungicidal activity of N-(2,4,5-trichlorophenyl)-2-oxo- and 2-hydroxycycloalkyl-sulfonamides. Lett Drug Design Discov 10: 353–359. [Google Scholar]
- 45. Li XH, Cui ZN, Chen XY, Wu DC, Qi ZQ, et al. (2013) Synthesis of 2-acyloxycyclohexylsulfonamides and evaluation on their fungicidal activity. Int J Mol Sci 14: 22544–22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang BL, Shi YX, Ma Y, Liu XH, Li YH, et al. (2010) Synthesis and biological activity of some novel trifluoromethyl-substituted 1,2,4-triazole and bis(1,2,4-triazole) mannich bases containing piperazine rings. J Agric Food Chem 58: 5515–5522. [DOI] [PubMed] [Google Scholar]
- 47. Bovie C, Ongena M, Thonart P, Dommes J (2004) Cloning and expression analysis of cDNAs corresponding to genes activated in cucumber showing systemic acquired resistance after BTH treatment. BMC Plant Biol 4: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferreira RB, Monteiro S, Freitas R, Santos CN, Chen ZJ, et al. (2007) The role of plant defence proteins in fungal pathogenesis. Mol Plant Pathol 8: 677–700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers Used in This Study.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.