Abstract
Background
Peritoneal carcinomatosis (PC) is a difficult clinical challenge in colorectal cancer (CRC) because conventional treatment modalities could not produce significant survival benefit, which highlights the acute need for new treatment strategies. Our previous case-control study demonstrated the potential survival advantage of cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) over CRS alone. This phase II study was to further investigate the efficacy and adverse events of CRS+HIPEC for Chinese patients with CRC PC.
Methods
A total of 60 consecutive CRC PC patients underwent 63 procedures consisting of CRS+HIPEC and postoperative chemotherapy, all by a designated team focusing on this combined treatment modality. All the clinico-pathological information was systematically integrated into a prospective database. The primary end point was disease-specific overall survival (OS), and the secondary end points were perioperative safety profiles.
Results
By the most recent database update, the median follow-up was 29.9 (range 3.5–108.9) months. The peritoneal cancer index (PCI) ≤20 was in 47.0% of patients, complete cytoreductive surgery (CC0-1) was performed in 53.0% of patients. The median OS was 16.0 (95% confidence interval [CI] 12.2–19.8) months, and the 1-, 2-, 3-, and 5-year survival rates were 70.5%, 34.2%, 22.0% and 22.0%, respectively. Mortality and grades 3 to 5 morbidity rates in postoperative 30 days were 0.0% and 30.2%, respectively. Univariate analysis identified 3 parameters with significant effects on OS: PCI ≤20, CC0-1 and adjuvant chemotherapy over 6 cycles. On multivariate analysis, however, only CC0-1 and adjuvant chemotherapy ≥6 cycles were found to be independent factors for OS benefit.
Discussion
CRS+HIPEC at a specialized treatment center could improve OS for selected CRC PC patients from China, with acceptable perioperative safety.
Introduction
Peritoneal carcinomatosis (PC) from colorectal cancer (CRC) is characterized by the implantation of tumor nodules throughout the peritoneal cavity and production of intractable ascites. PC is found in about 8–15% CRC patients at first treatment [1], with a significant negative impacts on both the survival and the quality of life because of refractory ascites, progressive intestinal obstruction and uncontrollable abdominal pain, with approximately 30% of the CRC patients died from this problem [2]. Up to now, the oncology community usually considers CRC PC as a virtually untreatable öterminal condition, for which only palliative measures such ?as systemic chemotherapy, with or without limited surgery and best support care, with limited median overall survival (OS) about 6 months [3]–[5].
Increasing studies on this problem has gradually resulted in revolutions in both the basic pathological sciences and clinical approaches to CRC PC. Different from CRC liver metastases, CRC PC is now regarded as regional tumor progression, suitable for radical therapeutic strategies with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotheropy (HIPEC), which are likely to achieve prominent clinical benefit in selected patients [6]–[8]. Although only one phase III prospective randomized controlled clinical trial [6] demonstrated the superiority of this new strategy, it has been considered to justify this comprehensive treatment, much like the evolutionary history of liver resection as a standard procedure for selected patients with CRC liver metastasis. Nevertheless, overwhelming majority of relevant researches came from the Western countries, and there have been no systematic clinical studies from China, where the problem is particularly acute due to the large number of such patients. To address the clinical problem, we have performed a series of preclinical and clinical studies on the feasibility, efficacy and safety of this multidisciplinary therapeutic approach in animal models [9] and in clinical setting [10]–[12], and established a designated CRS+HIPEC program at our institution, which has been in steady operation for over 10 years. As our phase I study [10] and case-control study [12] (the OS was 8.5 in Control group versus 13.7 months in Study group, P = 0.02) suggested potential therapeutic benefit of CRS+HIPEC for CRC PC patients, we proceeded to this prospective phase II study with enlarged sample size to further investigate the efficacy and safety of CRS+HIPEC and postoperative chemotherapy for the treatment of CRC PC in Chinese patients.
Patients and Methods
Ethics statement
All patients had signed the informed consent form, and the study protocol was approved by the institutional review board of Zhongnan Hospital of Wuhan University. This study was registered in the clinical trial registry by US National Cancer Institute. The registry name is “Surgery plus Intraoperative Peritoneal Hyperthermic Chemotherapy (IPHC) to Treat Peritoneal Carcinomatosis” and ClinicalTrials.gov Identifier is “NCT00454519”. Detailed study information is available at Clinical-Trials.gov (http://www.clinicaltrials.gov/ct2/show/NCT00454519).
Patients Selection
This prospective phase II study included 60 consecutive Chinese patients of CRC PC treated by 63 CRS+HIPEC procedures from February 2005 to October 2013 at the Department of Oncology, Zhongnan Hospital of Wuhan University. The preoperative evaluations, major inclusion and exclusion criteria were reported previously [11]. In addition, those patients were excluded who received neoadjuvant chemotherapy or any adjuvant chemotherapy within preoperative 6 months. One patient with advanced age and another one with extensive CRS but could not tolerate HIPEC were excluded. The flowchart of this study was shown in Figure 1.
Figure 1. The flowchart of this phase II clinical study.
Initially 62 patients were enrolled into this study, and 2 were excluded. A total of 60 patients underwent 63 CRS+HIPEC procedures. CRS = cytoreductive surgery, HIPEC = hyperthermic intraperitoneal chemotherapy, SC = systemic chemotherapy, PIC = perioperative intraperitoneal chemotherapy.
CRS plus HIPEC Procedure
CRS+HIPEC were conducted by a designated team focusing on PC treatment, with detailed description reported previously [10]–[12]. In brief, maximal CRS was performed to remove the primary tumor with acceptable margins, any involved adjacent tissues and organs, regional lymph nodes, and peritonectomy [13]. Unresectable tumors were cauterized with ball-tipped electrosurgical device at the maximal electric power, especially on the edge of tumor nodules. The completeness of cytoreduction (CC score) [13] was evaluated before HIPEC, which was implemented by the open Colliseum technique, with 120 mg of cisplatin and 30 mg of mitomycin C each dissolved 6 L of heated saline (drug concentration cisplatin 20 µg/mL, mitomycin C 5 µg/mL), as these concentration has been confirmed to be safe and effective for HIPEC by Fujimoto et al [14]. The heated perfusion solution was infused into the peritoneal cavity at a rate of 500 mL/min through the inflow tube introduced from an automatic hyperthermia chemotherapy perfusion device. The temperature of the perfusion solution in peritoneal space was kept at 43.0±0.5 °C and monitored with a thermometer on real time. The total HIPEC time was 90 min, after which the perfusion solution in the abdominal cavity was removed through the suction tube. After operation, the patient was delivered to the intensive care unit for recovery.
Postoperative Chemotherapy
Adjuvant chemotherapy included systemic chemotherapy mainly with FOLFOX (oxaliplatin, leucovorin and 5-FU) or FOLFIRI (irinotecan, leucovorin and 5-FU) regimens, and perioperative intraperitoneal chemotherapy (PIC) through the intraperitoneal chemotherapy port mainly using docetaxel (75 mg/m2, on day 1, every 3 weeks) and carboplatin (at Calvert formula: area under the curve, AUC 5; on day 1, every 3 weeks), all dosed on the base of body surface area calculation [7], [15], [16]. PIC were launched after the patients postoperative physical conditions recovered, and systemic chemotherapy were delivered with PIC synchronously or alternately.
Study Endpoints and Their Definition
The primary endpoint was the disease specific overall survival (OS), defined as the time interval from the first treatment to death due to the disease for synchronous PC, and from CRS+HIPEC to death due to the disease for metachronous PC. The secondary endpoints were the perioperative adverse events, defined as complications directly attributable to the treatment within 30 days of CRS+HIPEC, based on NCI Common Terminology Criteria (CTC) for Adverse Events version 4.0 [17].
Follow-up
All patients received regular follow-up once every 3 months for the first 2 years, and once every 6 months thereafter. The follow-up package included physical examination, serum tumor markers (CEA/CA125/CA19-9), abdominopelvic CT scans and/or gastrointestinal iodine contrast media. The last follow-up was on March 11, 2014, and the overall follow-up rate was 100%.
Statistical Analysis
All data analyses were performed using the SPSS statistical software for windows, version 20.0. The numerical data were directly recorded, and the category data were recorded into different categories. OS comparisons were analyzed with Kaplan-Meier cumulative survival curve and log rank test, and multivariate Cox regression analysis was performed to delineate the independent predictors. The OS comparisons were further stratified by PCI scores, with PCI ≤20 defined as low PCI (LPCI), and >20 as high PCI (HPCI) [13]. A 2-sided P<0.05 value was considered as statistically significant.
Results
Major Clinicopathological Characteristics and Perioperative Treatment
A total of 60 CRC PC patients received 63 CRS+HIPEC procedures, including 3 patients each receiving 2 CRS+HIPEC procedures (2 patients due to tumor recurrence and 1 patient due to the second primary cancer). Major clinico-pathologic characteristics of the patients were listed in Table 1.
Table 1. Major clinico-pathologic characteristics of the 60 patientsa.
| Items | Value, n (%) | |
| Gender | ||
| Male | 26 (43) | |
| Female | 34 (57) | |
| Age (years) | ||
| <60 | 46 (77) | |
| ≥60 | 14 (23) | |
| Median KPS score (range) | 80 (50–100) | |
| Primary tumor | ||
| Carcinoma of colon | 35 (58) | |
| Carcinoma of rectum | 25 (42) | |
| Histopathology | ||
| Adenocarcinoma, well/intermediately differentiated | 26 (43) | |
| Adenocarcinoma, poorly/mucinous/signet-ring cell carcinoma | 34 (57) | |
| PC timing b | ||
| Synchronous | 24 (40) | |
| Metachronous | 36 (60) | |
| PCI scores b | ||
| ≤20 | 28 (47) | |
| >20 | 32 (53) | |
| Median PCI scores (range) | 21 (1–39) | |
| Ascites at surgery a | ||
| ≤1,000 mL | 45 (71) | |
| >1,000 mL | 18 (29) | |
| Surgical procedures-organ resection | ||
| Resection of jejunum | 3 (5) | |
| Resection of ileum | 16 (29) | |
| Resection of ileocecus | 16 (29) | |
| Ascending colectomy | 21 (38) | |
| Transverse colectomy | 26 (47) | |
| Descending colectomy | 10 (18) | |
| Sigmoidectomy | 14 (25) | |
| Rectectomy | 14 (25) | |
| Splenectomy | 4 (7) | |
| Resection ovarian/fallopian tube | 20 (36) | |
| Hysterectomy | 14 (25) | |
| Partial hepatectomy | 2 (4) | |
| Cholecystectomy | 6 (11) | |
| Number of organ resected b , c | ||
| 1–3 resections | 33 (59) | |
| 4–7 resections | 23 (41) | |
| Peritonectomy b | ||
| Greater/Lesser/Omentum | 60 (100) | |
| Left diaphragmatic copula | 15 (25) | |
| Right diaphragmatic copula | 21 (35) | |
| Right colon gutter | 20 (33) | |
| Left colon gutter | 17 (28) | |
| Liver round ligament/sickle ligament | 19 (32) | |
| Douglas pouch | 5 (8) | |
| Anterior wall peritoneum | 20 (33) | |
| Pelvic peritoneum | 32 (53) | |
| Mesenteric fulguration | 35 (58) | |
| Peritoneal resection area b | ||
| 1–3 resections | 30 (50) | |
| 4–6 resections | 15 (25) | |
| 7–12 resections | 15 (25) | |
| CC scores b | ||
| 0 | 17 (28) | |
| 1 | 15 (25) | |
| 2–3 | 28 (47) | |
| Number of anastomosis b | ||
| None or Ostomy only | 26 (43) | |
| = 1 | 27 (45) | |
| >1 | 7 (12) | |
| Postoperative chemotherapy cycles | ||
| <6 | 15 (15) | |
| ≥6 | 45 (75) | |
| Median postoperative chemotherapy cycles (range) | 8 (2–18) | |
| Postoperative intraperitoneal chemotherapy cycles d | 4 (1–10) | |
| Median duration of hospitalization (days) (range) | 21 (11–58) | |
| Median follow-up (months)(range) | 29.9 (4.5–109.9) | |
3 patients each underwent 2 operations;
According to the first surgery;
4 patients without organ resection;
Except for 4 patients who developed intestinal fistula after operation.
Surgical procedures and major intraoperative parameters including blood loss, duration of operation, and fluid balance parameters were listed in Table S1.
After operation, all the 60 patients received systemic chemotherapy and 56 patients received PIC procedures except four patients who had intestinal leakage. The PIC was delivered on day 8 post-operation (range, 3–14 days), except one patient who received PIC on postoperative day 20 due to severe gastroplegia, and with median 4 cycles of PIC for each patient. The median cycles of adjuvant chemotherapy (SC and/or PIC) was 8 (range, 2–18). None of the patients received any molecular targeting agents.
Survival Analysis
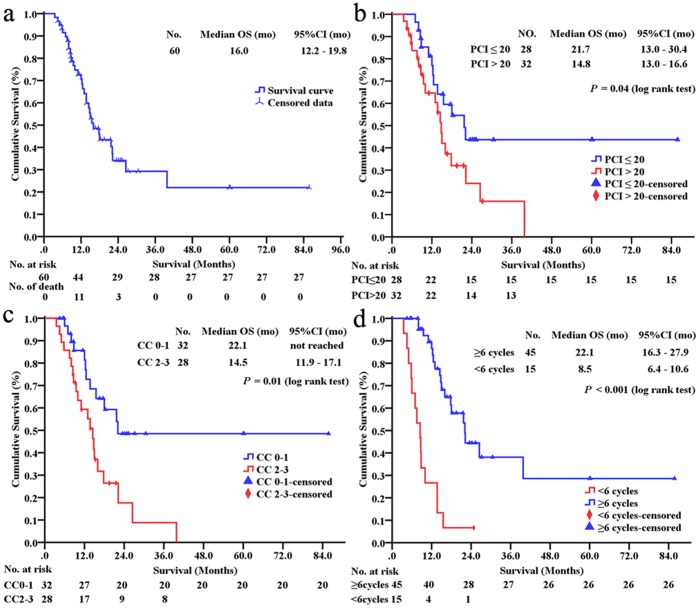
At the last follow-up, the median OS was 16.0 (95% CI 12.2–19.8) months (Figure. 2a). The 1-, 2-, 3- and 5-year survival rates were 70.5%, 34.2%, 22.0% and 22.0%, respectively. The OS comparisons were stratified based on major clinico-pathological factors (Table S2.). Male patients, colon cancer PC, well/intermediately differentiated adenocarcinoma, synchronous PC and the volume of ascites at surgery ≤1,000 mL had a tendency for improved OS; and PCI ≤20 (P = 0.04, Figure. 2b) and CC0-1 (P = 0.01, Figure. 2c) and postoperative chemotherapy cycles ≥6 (P<0.001, Figure. 2d) could obtain statistically better OS benefit.
Figure 2. Kaplan-Meier curves.
The disease-specific overall survival in patients with CRC PC treated by CRS+HIPEC and adjuvant chemotherapy regimen (a). The statistical significance in overall survival (OS) comparisons of those patients including PCI (b), CC (c) and postoperative adjuvant chemotherapy cycles (d). mo = months, CRC = colorectal cancer, PC = peritoneal carcinomatosis, CRS = cytoreductive surgery, HIPEC = perioperative intraperitoneal chemotherapy, PCI = peritoneal cancer index, CC = completeness of cytoreduction.
Special Analysis on Long-term Survivors
There were 15 patients surviving over 20 months (Table 2), including 7 patients who had disease free survival (DFS) with LPCI and CC-0 resection, 3 patients survived with tumor (SWT), and 5 patients died with HPCI and non-CC-0 resection. It worth noting that 4 patients with HPCI and CC-2/3 resection also achieved long-term OS of 21.5, 22.2, 26.5 and 39.8 months, respectively.
Table 2. Major treatment and follow-up features of long-term survivors (OS >20 months).
| No. | Gender/age(yr) | PC origin/PC timing | PCI | Histopathology | CRS | CC | Survival(months) | Comments |
| 1 | M/36 | Colon ca,Synchronous PC | 6 | Adenocarcinoma, well differentiated | Left hemicolectomy, greater omentum resection, left peritoneum and musculus trasversus abdominis, mesenteric fulguration | 0 | 85.8, DFS | |
| 2 | M/36 | Colon ca,Synchronous PC | First:15Second: 0 | Adenocarcinoma, intermediatelydifferentiated | The first surgery: transverse colectomy, resection of part jejunum, greater omentum resection;The second surgery: rectectomy, colostomy of sigmoid colon (second primary cancer: rectal cancer) without HIPEC | 0 | First:55.0DFSTotal: 60.2, DFS | SAE (the first surgery): abdominal hemorrhage 4 h post operation, reoperation to stop bleeding; Pelvic and abdominal adhesions were found in second surgery |
| 3 | M/47 | Colon ca,Synchronous PC | 15 | Adenocarcinoma, intermediatelydifferentiated | Ascending colectomy, resection of ileocecus | 0 | 60.0, DFS | |
| 4 | F/57 | Colon ca,Synchronous PC | 24 | Adenocarcinoma, intermediatelydifferentiated | Right hemicolectomy, hysterectomy and resection ovarian/fallopian tube, greater omentum, left and right diaphragmatic copula peritoneum, left and colon gutter peritoneum, pelvic peritoneum, liver round ligament, mesenteric fulguration | 0 | 27.2, SWT | |
| 5 | F/37 | Colon ca,Metachronous PC | 1 | Adenocarcinoma, mucinous | Greater omentum | 0 | 25.3, DFS | |
| 6 | F/65 | Colon ca,Metachronous PC | 5 | Adenocarcinoma, intermediatelydifferentiated | Resection ovarian/fallopian tube, greater omentum, liver round ligament | 0 | 24.8, DFS | |
| 7 | F/68 | Colon ca,Synchronous PC | 6 | Adenocarcinoma, intermediatelydifferentiated | Right hemicolectomy, resection ovarian/fallopian tube, greater/lesser omentum | 0 | 24.5, DFS | |
| 8 | M/60 | Colon ca,Metachronous PC | First:15Second: 18 | Adenocarcinoma, mucinous | The first surgery: right hemicolectomy, greater omentum, pelvic peritoneum resection, mesenteric fulgurationThe second surgery: resection of ileocecum, rectectomy; received HIPEC as before | First:1Second: 2 | 30.5, SWT | Not found vast pelvic and abdominal conglutination in second surgery |
| 9 | F/55 | Colon ca,Metachronous PC | 16 | Adenocarcinoma, intermediatelydifferentiated | Left hemicolectomy, rectectomy, hysterectomy and resection ovarian/fallopian tube, greater omentum | 0 | 24.2, DFS | |
| 10 | M/41 | Rectal ca,Metachronous PC | 20 | Adenocarcinoma, poorlydifferentiated | Rectectomy, greater omentum, left/right diaphragmatic copula resection, colon sigmoideum colostomy | 1 | 22.1, D | |
| 11 | F/54 | Colon ca,Metachronous PC | 7 | Adenocarcinoma, mucinous | Sigmoidectomy, rectectomy, greater omentum, pelvic peritoneum resection | 1 | 21.7, D | |
| 12 | M/30 | Colon ca,Synchronous PC | First: 32Second: 39 | Adenocarcinoma, mucinous | The first surgery: right hemicolectomy, resection of part jejunum, greater omentum, left diaphragmatic copula, left/right colon gutter, liver round ligament/sickle ligament resection, mesenteric fulgurationThe second surgery: lump-like tumor on hepatic portal, subcutaneous plate-like tumor; without HIPEC | First:2Second: 3 | 39.8, D | |
| 13 | F/37 | Colon ca,Metachronous PC | 26 | Adenocarcinoma, mucinous | Descending colectomy, resection of part jejunum, left/right diaphragmatic copula, left/right colon gutter, anterior wall peritoneum, pelvic Peritoneum resection, mesenteric fulguration | 2 | 26.5, D | |
| 14 | M/26 | Colon ca,Synchronous PC | 28 | Adenocarcinoma, mucinous | Greater/lesser omentum, liver round ligament/sickle ligament, anterior wall peritoneum resection, mesenteric fulguration | 2 | 22.2, D | |
| 15 | M/43 | Colon ca,Synchronous PC | 39 | Adenocarcinoma, mucinous | Pancolectomy, greater omentum | 3 | 21.5, SWT |
M = male, F = female, ca = carcinomatosis, D = died, DFS = disease free survival, SWT = survival with tumor, SAE = serious adverse event.
Serious Adverse Events (SAE)
SAE (grade 3–5) occurred in 19 (30.2%) of 63 procedures, including postoperative intestinal obstruction (n = 10), intestinal leakage (n = 4), hemorrhage (n = 1), diarrhea (n = 1), septicaemia (n = 1), severe hypoalbuminemia (n = 1), and delirium (n = 1). Detailed accounts of the 19 SAEs were listed in Table 3; No SAE-related death occurred in perioperative period.
Table 3. Detail data of the patients with SAE # (n = 19, 30.2%).
| Events | Gender/Age | Primary tumor | PC time | PCI | Resection | No.Anastomosis | CC | Surgery time (min) | POD | SAEs | Intervention a | OS b(month) | |
| Organ | Peritoneal | ||||||||||||
| Intestinal obstruction (n = 10, 15.9%) | |||||||||||||
| M/27 | Colon | Syn | 24 | 5 | 2 | 1 | 3 | 485 | 4 | CIO/AI | CT | 8.0, D | |
| M/47c | Colon | Met | 27 | 1 | 1 | 1 | 2 | 225 | 7 | IIO/AI | CT | 4.5, D | |
| M/52 | Colon | Met | 10 | 4 | 3 | 1 | 0 | 610 | 13 | CIO/AI | CT | 8.0, D | |
| M/47 | Colon | Syn | 26 | 5 | 2 | 1 | 1 | 670 | 13 | CIO/AI | CT | 15.0, D | |
| M/25 | Colon | Syn | 28 | 1 | 6 | 1 | 2 | 650 | 12 | CIO/AI | CT | 22.2, D | |
| F/62 | Colon | Met | 18 | 1 | 5 | 1 | 3 | 510 | 8 | IIO/AI | CT | 15.3, S | |
| F/72 | Rectum | Met | 5 | 3 | 2 | 1 | 0 | 405 | 6 | CIO/AI | CT | 8.8, S | |
| M/61 | Colon | Syn | 29 | 1 | 9 | 1 | 3 | 585 | 7 | IIO/AI | CT | 11.1, S | |
| F/45 | Colon | Met | 24 | 2 | 5 | 2 | 1 | 390 | 5 | IIO/AI | CT | 5.8, S | |
| F/31 | Colon | Met | 24 | 5 | 6 | 2 | 1 | 740 | 5 | CIO/AI and combined gastroplegia | CT | 5.5, S | |
| Intestinal leakage (n = 4, 12.1%) | |||||||||||||
| M/33 | Colon | Met | 26 | 4 | 6 | 1 | 2 | 540 | 16 | limited peritonitis syndrome | CT | 5.0, D | |
| M/47c | Colon | Met | 27 | 1 | 1 | 1 | 2 | 610 | 7 | sepsis, generalized peritonitis, abdominal abscess | CT | NA | |
| F/55 | Colon | Syn | 16 | 7 | 1 | 1 | 0 | 525 | 6 | limited peritonitis syndrome | reoperation | 24.2, S | |
| F/71 | Rectum | Met | 9 | 1 | 1 | 1 | 0 | 300 | 4 | limited peritonitis syndrome | CT | 7.0, S | |
| Hemorrhage (n = 1, 1.6%) | |||||||||||||
| F/36 | Rectum | Syn | 15 | 0 | 1 | 2 | 0 | 210 | 4h | Hemorrhage shock | reoperation | 60.2, S | |
| Diarrhea (n = 1, 1.6%) | |||||||||||||
| M/60c | Colon | Met | 26 | 1 | 4 | 1 | 2 | 465 | 8 | over 8 stools per day and abdominal pain | CT | 13.7, D | |
| Septicemia (n = 1, 1.6%) | |||||||||||||
| M/60c | Colon | Met | 26 | 1 | 4 | 1 | 2 | 465 | 8 | Staphylococcus aureus septicemia | CT | NA | |
| Hypoalbuminemia (n = 1, 1.6%) | |||||||||||||
| M/47 | Colon | Met | 15 | 3 | 2 | 1 | 1 | 420 | 2 | 16.1 g/L | CT | 11.5, S | |
| Delirium (n = 1, 1.6%) | |||||||||||||
| M/60c | Colon | Met | 26 | 1 | 4 | 1 | 2 | 465 | 8 | grades 3 | drug intervene | NA | |
| Median | 47 | 24 | 2 | 3 | 1 | 2 | 485 | 7 | |||||
all patients recovered after intervention;
none of the patients died of SAE;
different SAEs developed in the same patient;
Common Terminology Criteria for Adverse Events version 4.0;
POD = on postoperative days, M = male, F = female, Syn = synchronous, Met = metachronous, CIO = complete intestinal obstruction, IIO = incomplete intestinal.
obstruction, AI = adynamic ileus CT = conservative treatment D = death, S = survival, NA = not applicable.
Ten patients developed gastrointestinal obstruction within 2 weeks after operation, of whom 9 patients gradually recovered by active conservative therapy, and 1 patient with severe gastroplegia restored to normal gastrointestinal function 13 days after operation; there were no obvious electrolyte disturbance, serious infection or sepsis during the conservative therapy. Four patients developed intestinal leakage, including 2 patients with mild anastomosis leakage and limited peritonitis syndrome on postoperative days 4 and 16, respectively, and these 2 patients recovered after 3 months and 7 days, respectively. The third patient developed colonic stump fistula and vaginal-stump fistula on postoperative days 6, received a reoperation to reconstruct the anastomosis and repair vaginal-stump on postoperative days 33, and the patient is surviving and well with DFS of 24.2 months. The fourth patient developed severe anastomosis leakage, generalized peritonitis, abdominal abscess formation and septicemia due to Escherichia coli. With abdominal drainage, intensified antibiotics, and total parenteral alimentation nutrition support, this patient survived 3 months after the procedure. One patient with abdominal hemorrhage 4 h post operation had reoperation to stop the bleeding. This patient recovered well and he had DFS of 55.0 months, until November 2013 when he developed a second primary rectal cancer, which was successfully resected, and he is living well with no evidence for any tumor recurrence, with a total DFS of 60.2 months. Another SAE case was a 60-year-old male patient who developed septicemia along with grade 3 inflammatory diarrhea, abdominal pain and delirium on day 8 post-operation, due to infection by Staphylococcus aureus as confirmed by blood culture. The septicemia was controlled in 5 days after antibiotics therapy, and the patient fully recovered in about 10 days. The patient survived for 13 months. The last SAE case developed sustained grade 3 hypoalbuminemia (16.1 g/L) from postoperative days 2 to 9, and treated with high-volume plasma and albumin transfusion, and the patient gradually recovered without serious consequences.
A binary logistic regression analysis was conducted to study the correlation of SAEs with major treatment parameters. No significant correlations between SAEs and gender, age, KPS scores, primary tumor, PC time, histopathology, organ and peritoneal resection area, number of anastomosis, operation duration, PCI scores, and CC scores.
Other adverse events (grades 1–2) occurred in 54 (85.7%) of the 63 procedures, including mild hypoalbuminemia (n = 36), liver and kidney dysfunctions (n = 12), delayed incision healing (n = 4), atelectasis (n = 1), and deep vein thrombosis (n = 1).
Univariate and Multivariate Analysis on Predictors of Survival
Ten parameters were included for univariate analysis respectively (Table 4), and 3 covariates indicative of improved survival including PCI ≤20, CC0-1, and postoperative chemotherapy cycles ≥6.
Table 4. Analysis of independent factors influencing survival.
| Covariate | Univariate analysis | Multivariate analysis | ||||||
| χ2 | P | HR | 95% CI | χ2 | P | HR | 95% CI | |
| PCC (≥6 vs.<6) | 22.20 | <0.001 | 5.8 | 2.8–12.0 | 24.91 | <0.001 | 7.2 | 3.3–15.5 |
| CC score (CC0-1 vs. CC2-3) | 7.58 | 0.01 | 2.8 | 1.3–5.6 | 10.26 | <0.001 | 3.4 | 1.6–7.2 |
| PCI score (≤20 vs. >20) | 4.03 | 0.045 | 2.1 | 1.0–4.2 | ||||
| Primary tumor (colon vs. rectum) | 2.78 | 0.10 | 1.9 | 0.9–3.9 | ||||
| PC timing (Syn vs. Met) | 2.71 | 0.10 | 1.8 | 0.9–3.8 | ||||
| Histopathology (well vs. poorly differentiated) | 2.36 | 0.12 | 1.8 | 0.8–3.9 | ||||
| Age (≥60 vs. <60) | 1.81 | 0.18 | 2.1 | 0.7–5.9 | ||||
| Gender (male vs. female) | 1.43 | 0.23 | 1.5 | 0.3–3.1 | ||||
| Ascites (≤1,000 mL vs. >1,000 mL) | 1.25 | 0.26 | 1.5 | 0.7–3.2 | ||||
| SAE (no vs. yes) | 0.00 | 0.99 | 1.0 | 0.4–2.3 | ||||
PCC = postoperative chemotherapy cycles, Syn = synchronous, Met = metachronous.
Multivariate Cox regression analysis identified 2 variables including CC scores and postoperative adjuvant chemotherapy cycles as independent predictors for better survival (Table 4). Compared with CC2-3 and postoperative chemotherapy cycles <6, CC0-1 and postoperative chemotherapy cycles ≥6 were about 3 times (Hazard Ratio = 3.4, 95% CI 1.6–7.2, P<0.001) and 7 times (Hazard Ratio = 7.2, 95% CI 3.3–15.5, P<0.001) more likely to improve survival, respectively.
Analysis of Predictors of Survival for HPCI Patients: Univariate and Multivariate Analysis
As our data contained 32 (53%) cases with HPCI (PCI >20), we conducted a subgroup analysis on such patients. Survival analysis of the patients with HPCI by Kaplan-Meier (Table S3.) found the median OS was 15.0 vs. 9.5 months in synchronous (n = 15) vs. metachronous (n = 17) subgroups (P = 0.12); 17.8 vs. 6.0 months in postoperative chemotherapy cycles ≥6 (n = 23) vs. <6 (n = 9) subgroups (P<0.001); 14.8 vs. 13.7 months in no-SAE (n = 23) vs. SAE (n = 13.7) subgroups (P = 0.21), respectively.
Four parameters were included for multivariate analysis, and only postoperative adjuvant chemotherapy cycle was identified as independent predictor for better survival. The chemotherapy over 6 cycles was about 15 times more likely to improve survival in contrast to less 6 cycles (Chi-square = 15.0, 95% CI 3.7–61.0, P<0.001).
Discussion
Over the past 10 years, we have established a designated PC program, with both preclinical [9], [18] and clinical studies [10], [11] to demonstrated the efficacy of CRS+HIPEC for PC treatment. This phase II clinical study is a part of our comprehensive treatment strategy for PC, producing several lines of new evidence. First, for CRC patients with both synchronous and metachronous PC, the median disease specific OS could reach 16.0 months (95% CI 12.2–19.8 months) by such combined treatment approach, and the 3-yr survival rate could reach 22.0%, even though none of them received any molecular targeting therapy. Second, the median disease specific OS could reach 22.0 months for patients with synchronous PC, but 14.5 months for those with metachronous PC. Although such differences did not reach statistical significance, possibly due to follow-up durations and sample size, they do suggest that CRC patients with synchronous PC could obtain better survival benefit from such treatment. Third, completeness of CRS has an important independent impact on survival. Therefore, every effort should be made during surgery to reduce the tumor burden. Fourth, adjuvant systemic chemotherapy after CRS+HIPEC could bring about significant survival advantages for such patients. All the 15 long-terms survivors had over 6 cycles of post-operative systemic chemotherapy. These major results, once again, provide data to support the rational for CRS+HIPEC, that is to remove the bulky tumor by surgical resection, to eliminate the micro-metastasis and seeding nodules by the heated chemotherapeutic perfusion, and to boost the treatment efficacy by postoperative systemic and intraperitoneal chemotherapies [6], [19].
To our knowledge, there have been about 15 clinical trials (Table S4.) on the treatment of CRC PC, including 5 phase I studies [20]–[24], 9 phase II studies [25]–[33], and 1 phase III study [6]. These studies covered the surgical procedures, drug treatment selection, pharmacological evaluation and adverse events. In 5 phase I studies on a total number of 109 patients, it was found that HIPEC at temperature 40–46°C for 30–90 min, with oxliplatin, MMC, pegylated liposomal doxorubicin, and irrinotecan, were feasible with acceptable efficacy and side effects. In 9 phase II studies on 562 CRC PC patients, the median overall survivals ranged from 19.8 months to 41.0 months, and 3-yr survival rate reaching 63.7% at the most. In the only phase III study, the median OS was 22.2 months in CRS+HIPEC vs. 12.6 months in control group (Surgery+Systemic Chemotherapy). Moreover, all these studies also indicated that while the treated patients obtained better efficacy the SAEs were also acceptable. Therefore, the CRS+HIPEC have become increasingly accepted as the treatment of choice at designated centers for selected patients with CRC PC. Moreover, a nationwide application of standardized CRS+HIPEC protocol in the Netherlands has produced more encouraging long-term survival of about 33.0 months [34]. In addition, 3 large sample-size multicenter retrospective studies in France and Italy also showed a mean OS of 23.4 months [7], [16], [35]. Compared with these international studies, our results on Chinese patients treated with standardized CRS+HIPEC protocols have added, for the first time, the new evidence from China on this increasing international database.
Several factors have been recognized to have important impacts on the clinical outcomes of this combined treatment, including PCI, CC, SAEs, lymph nodes status, synchronous vs. metachronous PC, and systemic chemotherapy. For PCI, the Sugarbaker PCI system has been adopted as the current standard recording system [13], and out of the total 39 cores, PCI ≤20 is usually regarded as LPCI and >20 is regarded as HPCI [36], [37]. In this study, of the 32 patients (53% of the total) with HPCI, the median OS was 14.8 months (95% CI 13.0–16.6 months). This is comparable to most other studies [35], [38], [39] showing a median OS of about 12 months for patients with PCI >20. Sugarbaker et al [38]. also reported the 5-year survival rates of 50%, 20%, and 0%, respectively for patients with PCI ≤10, 11–20, and >20. Our results suggest that HPCI Chinese patients still could benefit from HIPEC procedure rather than the traditional treatment, at the specialized treatment center. However, it was indeed the one cause to shorten OS in this study in contrast to LPCI patients. Therefore, for HPCI patients especially for patients with synchronous PC, intensified chemotherapy to reduce the tumor burden first and then followed by CRS+HIPEC could be a sensible strategy in future work.
For CC score, CC0 patients had much better survival benefit than CC1-3 patients, with reported median OS 33.0 months vs. 10.0 months for CC0 vs. CC1-3 [6], . In this study, 17 patients with CC0 cytoreduction reached mean survival of 50.1 months, again supporting the effort to reduce tumor burden as much as possible. Among these 17 patients with a CC0 resection, 15 were in LPCI category and 2 in HPCI category, again confirming the importance of selecting LPCI patients for treatment. To achieve complete cytoreduction, however, a wide resection is usually necessary involving several organs and abdominal regions. In our study, usually 2 to 3 organ resections and 3 to 4 peritoneal regions are resected in one CRS procedure, which is long, complex and technically difficult. This will inevitably increase the blood and fluid losses, hemodynamic disturbances, and increase risks for SAEs [40], with perioperative morbidity rate ranging from 14.8% to 57.0% and mortality rate from 0.0% to 12.0% [8]. In 2 multicenter studies by Glehen et al [7] and Elias et al [16], the perioperative mortality rate was 4% and 3%, respectively. In the present study, the 30-day perioperative SAE rate of 30.2% and mortality rate of 0% also fell into the reported ranges. The overall morbidity and mortality are comparable with conventional gastrointestinal surgery and acceptable, if patients are treated at specialized PC centers, as demonstrated in a meta-analysis by Chua et al [41]. It was reported in a recent Japanese study that HPCI and complete resection rates were closely associated with high morbidity rate [42]. In our study, however, our binary logistic regression did not reveal any significant correlation between SAE and HIPEC with CC0-1. Although SAE was not identified as an independent prognostic factor, possibly due to relatively small sample size, it has significant negative impacts on the quality of life, and increase the treatment cost. Therefore, intensified perioperative care should be delivered so as to minimize the risks for SAEs.
Our study found that postoperative adjuvant chemotherapy also played an important role in the comprehensive therapeutic strategy. All the 60 patients received systematic chemotherapy and 56 cases had PIC within postoperative 4 weeks. Because of peritoneal-plasma partition [43], only a minimal chemotherapy drugs could penetrate into the abdominal cavity, resulting in reduced drug concentration in PC nodules. Appling PIC in addition to systematic chemotherapy could have double effects on PC nodules from a bi-directional approach, which has been proved to play a key role for better survival in recent reports [16], [25], [28], [29], and in our multivariate analysis of predictors of survival.
Of particular attention is the 15 patients with prolonged survival over 20 months in this series, including 5 patients survived over 30 months and 1 over 80 months. Five major factors for such favorable outcome are synchronous PC, well/intermediately differentiated adenocarcinoma, mucinous adenocarcinoma, LPCI and CC0-1 resection. Seven of the 15 patients with LPCI and CC0 showed the median OS from 24.2 months to 85.8 months in this study, again confirming the crucial importance of complete cytoreduction for better survival, as reported previously [6], [29], [34]–[36], [44]–[48]. However, what deserve special attention are four patients with HPCI and CC2-3 resection still obtained long-term survival (OS ranging from 21.5–39.8 months). These patients shared the following features in common: less than 45 years old, synchronous PC, mucinous adenocarcinoma, adjuvant chemotherapy (SC and/or PIC) over 6 cycles and without SAE. These features could help better selecting patients from this category for such combined treatment in the future.
The differences in OS definition between our study and many other studies made it not sensible for direct comparison. In our study, the OS was defined as the time interval from the first surgery to death due to the disease for synchronous PC, and from CRS+HIPEC to death due to the disease for metachronous PC. Therefore, the objective of this definition was to evaluate the impact of CRS+HIPEC on survival. In our definition, the OS is the entire survival time for patients with synchronous PC. For patients with metachronous PC, the time interval from first surgery to the subsequent development of PC was not included in our OS consideration. In this study, the median OS was 22.2 months in synchronous subgroup and 14.5 months in metachronous subgroup, respectively (P = 0.09). Of course, the actual survival time in metachronous PC group could have been much longer if it had been estimated from the first treatment of colorectal cancer. In other studies, however, the definition was different. They defined OS as the time interval from first diagnosis to cancer-related death. For example, in a recent study by Kerscher et al [49] focusing on a comprehensive large CRC database (n = 2,406) analysis to decipher the characteristics of PC, the “OS was estimated from the diagnosis of the primary tumour”. Therefore, this study revealed the median OS was 8.0 months for patients with synchronous PC (n = 115), and 30.0 months for patients with metachronous PC (n = 141). Another example is by Jayne et al [50] studying 3,019 CRC patients, including 214 cases with synchronous PC and 135 cases with metachronous PC. Again, this study also defined OS as the time interval from “initial diagnosis of colorectal cancer”. The median OS was 7.0 months for synchronous PC and 28.0 months for metachronous PC (P<0.001). Therefore, because the definition of OS is different in our study, we just to emphasize that CRS+HIPEC could have better effects on selected patients with synchronous PC than those with metachronous PC. However, our multivariate analysis demonstrated that synchronous/metachronous PC was not an independent prognostic factor.
The OS differences between our study and those in the West could also be accounted for by the tumor biological differences between the Chinese and Western colorectal cancer patients. It has already been documented that Chinese colorectal cancer patients are approximately 10 years younger than those in the West [51], and tumor biology in younger patients are often more aggressive and lethal. Another fact is significantly higher percentages of rectal cancer origin in Chinese colorectal cancer patients. Table S5 summarized 9 representative studies to illustrate such differences. Among the 7 studies from China, including 6 from mainland China [52]–[57] and 1 from Hong Kong [58] on Chinese residents, over 40% of colorectal cancer patients were rectal origin. In comparison, among the 2 Western studies including one from UK [59] and another from France [16], less than 30% of patients were rectal primaries. Therefore, these differences could have considerable impacts on tumor biology, treatment strategy and clinical outcomes.
Conclusion
In summary, this phase II study has provided additional evidence from China that CRS+HIPEC could bring survival benefits for selected CRC PC patients at specialized treatment centers.
Supporting Information
Intraoperative parameters.
(DOC)
OS comparisons stratified by major clinico-pathological factors.
(DOC)
Analysis of independent factors influencing survival for patients with HPCI.
(DOC)
Major studies on CRS+HIPEC, either single-arm or controlled studies.
(DOC)
Nine large clinical-demographic studies on Chinese and Western colorectal cancer patients.
(DOC)
RAW data for manuscript PONE-D-14-23603.
(XLSX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the grants for New Strategies to Treat Peritoneal Carcinomatosis from Hubei Sciences and Technology Bureau (No. 2008BCC011, No. 2060402-542), the Science Fund for Doctorate Mentors by China’s Ministry of Education (No. 20120141110042), and the Fundamental Research Fund for the Central Universities (No. 2012303030212). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Al-Shammaa HA, Li Y, Yonemura Y (2008) Current status and future strategies of cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. World J Gastroenterol 14: 1159–1166 10.3748/wjg.14.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brücher BL, Piso P, Verwaal V, Esquivel J, Derraco M, et al. (2012) Peritoneal carcinomatosis: cytoreductive surgery and HIPEC–overview and basics. Cancer Invest 30: 209–224 10.3109/07357907.2012.654871 [DOI] [PubMed] [Google Scholar]
- 3. Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, et al. (2000) Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88: 358–363 [DOI] [PubMed] [Google Scholar]
- 4. Jayne DG, Fook S, Loi C, Seow-Choen F (2002) Peritoneal carcinomatosis from colorectal cancer. Br J Surg 89: 1545–1550 10.1046/j.13652168.2002.02274.x [DOI] [PubMed] [Google Scholar]
- 5. Glehen O, Osinsky D, Beaujard AC, Gilly FN (2003) Natural history of peritoneal carcinomatosis from nongynecologic malignancies. Surg Oncol Clin N Am 12: 729–739,xiii. 10.1016/S1055-3207(03)00044-9 [DOI] [PubMed] [Google Scholar]
- 6. Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, et al. (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal origin. J Clin Oncol 21: 3737–3743 10.1200/JCO.2003.04.187 [DOI] [PubMed] [Google Scholar]
- 7. Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, et al. (2004) Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 22: 3284–3292 10.1200/JCO.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 8. Cao C, Yan TD, Black D, Morris DL (2009) A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 16: 2152–2165 10.1245/s10434-009-0487-4 [DOI] [PubMed] [Google Scholar]
- 9. Tang L, Mei LJ, Yang XJ, Huang CQ, Zhou YF, et al. (2011) Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of gastric cancer with peritoneal carcinomatosis: evidence from an experimental study. J Transl Med 9: 53 10.1186/1479-5876-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang XJ, Li Y, Hassan AHA, Yang GL, Liu SY, et al. (2009) Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival in selected patients with peritoneal carcinomatosis from abdominal and pelvic malignancies: results of 21 cases. Ann Surg Oncol 16: 345–351 10.1245/s10434-008-0226-2 [DOI] [PubMed] [Google Scholar]
- 11. Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, et al. (2011) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 18: 1575–1581 10.1245/s10434-011-1631-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang CQ, Feng JP, Yang XJ, Li Y (2014) Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: A case-control study from a Chinese center. J Surg Oncol 109: 730–739 10.1002/jso.23545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugarbaker PH (2001) Cytoreduction surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol 27: 239–243 10.1053/ejso.2000.1038 [DOI] [PubMed] [Google Scholar]
- 14. Fujimoto S, Takahashi M, Koyabayashi K, Nagano K, Kure M, et al. (1992) Cytohistologic assessment of antitumor effects of intraperitoneal hyperthermic perfusion with mitomycin C for patients with gastric cancer with peritoneal metastasis. Cancer 70: 2754–2760 [DOI] [PubMed] [Google Scholar]
- 15. Sugarbaker PH (1999) Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 43(Suppl): S15–S25 10.1007/s002800051093 [DOI] [PubMed] [Google Scholar]
- 16. Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, et al. (2010) Carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 28: 63–68 10.1200/JCO.2009.23.9285 [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute (nd) Common Terminology Criteria for Adverse Events version 4.0. Available: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 2014 Jan 11.
- 18. Li PC, Chen LD, Zheng F, Li Y (2008) Intraperitoneal chemotherapy with hydroxycamptothecin reduces peritoneal carcinomatosis: results of an experimental study. J Cancer Res Clin Oncol 134: 37–44 10.1007/s00432-007-0242-9 [DOI] [PubMed] [Google Scholar]
- 19. Glehen O, Mohamed F, Gilly FN (2004) Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol 5: 219–228 10.1016/S1470-2045(04)01425-1 [DOI] [PubMed] [Google Scholar]
- 20. Witkamp AJ, de Bree E, Kaag MM, Boot H, Beijnen JH, et al. (2001) Extensive cytoreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer 37: 979–984 10.1016/S0959-8049(01)00058-2 [DOI] [PubMed] [Google Scholar]
- 21. Stewart JH4th, Shen P, Russell G, Fenstermaker J, McWilliams L, et al. (2008) A phase I trial of oxaliplatin for intraperitoneal hyperthermic chemoperfusion for the treatment of peritoneal surface dissemination from colorectal and appendiceal cancers. Ann Surg Oncol 15: 2137–2145 10.1245/s10434-008-9967-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrison LE, Bryan M, Pliner L, Saunders T (2008) Phase I trial of pegylated liposomal doxorubicin with hyperthermic intraperitoneal chemotherapy in patients undergoing cytoreduction for advanced intra-abdominal malignancy. Ann Surg Oncol 15: 1407–1413 10.1245/s10434-007-9718-8 [DOI] [PubMed] [Google Scholar]
- 23. Ceelen WP, Peeters M, Houtmeyers P, Breusegem C, De Somer F, et al. (2008) Safety and efficacy of hyperthermic intraperitoneal chemoperfusion with high-dose oxaliplatin in patients with peritoneal carcinomatosis. Ann Surg Oncol 5: 535–541 10.1245/s10434-007-9648-5 [DOI] [PubMed] [Google Scholar]
- 24. Cotte E, Passot G, Tod M, Bakrin N, Gilly FN, et al. (2011) Closed abdomen hyperthermic intraperitoneal chemotherapy with irinotecan and mitomycin C: a phase I study. Ann Surg Oncol 18: 2599–2603 10.1245/s10434-011-1651-1 [DOI] [PubMed] [Google Scholar]
- 25. Glehen O, Mithieux F, Osinsky D, Beaujard AC, Freyer G, et al. (2003) Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: A phase II study. J Clin Oncol 21: 799–806 10.1200/JCO.2003.06.139 [DOI] [PubMed] [Google Scholar]
- 26.Rouers A, Laurent S, Detroz B, Meurisse M (2006) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: higher complication rate for oxaliplatin compared to Mitomycin C. Acta Chir Belg 106: 302–306. PMID:16910003. [DOI] [PubMed]
- 27. Elias D, Goere D, Blot F, Billard V, Pocard M, et al. (2007) Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: mortality and morbidity in 106 consecutive patients. Ann Surg Oncol 14: 1818–1824 10.1245/s10434-007-9348-1 [DOI] [PubMed] [Google Scholar]
- 28. van Leeuwen BL, Graf W, Pahlman L, Mahteme H (2008) Swedish experience with peritonectomy and HIPEC. HIPEC in peritoneal carcinomatosis. Ann Surg Oncol 15: 745–753 10.1245/s10434-007-9700-5 [DOI] [PubMed] [Google Scholar]
- 29. Yan TD, Morris DL (2008) Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: experimental therapy or standard of care? Ann Surg 248: 829–835 10.1097/SLA.0b013e31818a15b5 [DOI] [PubMed] [Google Scholar]
- 30.Sideris L, Mitchell A, Drolet P, Leblanc G, Leclerc YE, et al. (2009) Surgical cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis arising from the appendix. Can J Surg 52: 135–141. PMCID: PMC2663512. [PMC free article] [PubMed]
- 31. Quenet F, Goéré D, Mehta SS, Roca L, Dumont F, et al. (2011) Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg 254: 294–301 10.1097/SLA.0b013e3182263933 [DOI] [PubMed] [Google Scholar]
- 32. Hompes D, D’Hoore A, Van Cutsem E, Fieuws S, Ceelen W, et al. (2012) The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol 19: 2186–2194 10.1245/s10434-012-2264-z [DOI] [PubMed] [Google Scholar]
- 33. Glockzin G, Rochon J, Arnold D, Lang SA, Klebl F, et al. (2013) A prospective multicenter phase II study evaluating multimodality treatment of patients with peritoneal carcinomatosis arising from appendiceal and colorectal cancer: the COMBATAC trial. BMC Cancer 7;13: 67 10.1186/1471-2407-13-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuijpers AM, Mirck B, Aalbers AG, Nienhuijs SW, de Hingh IH, et al. (2013) Cytoreduction and HIPEC in The Netherlands: Nationwide Long-term Outcome Following the Dutch Protocol. Ann Surg Oncol 20: 4224–4230 10.1245/s10434-013-3145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cavaliere F, De Simone M, Virzì S, Deraco M, Rossi CR, et al. (2011) Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol. 37: 148–154 10.1016/j.ejso.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 36. Jacquet P, Sugarbaker PH (1996) Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82: 359–374 10.1007/978-1-4613-1247-523 [DOI] [PubMed] [Google Scholar]
- 37. Maggiori L, Elias D (2010) Curative treatment of colorectal peritoneal carcinomatosis: current status and future trends. Eur J Surg Oncol 36: 599–603 10.1016/j.ejso.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 38. Sugarbaker TA, Chang D, Koslowe P, Sugarbaker PH (1996) Patterns of spread of recurrent intra-abdominal sarcoma. Cancer Treat Res 82: 65–77 10.1007/978-1-4613-1247-55 [DOI] [PubMed] [Google Scholar]
- 39. da Silva RG, Sugarbaker PH (2006) Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg 203: 878–886 10.1016/j.jamcollsurg.2006.08.024 [DOI] [PubMed] [Google Scholar]
- 40. Saxena A, Yan TD, Chua TC, Morris DL (2010) Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 17: 1291–1301 10.1245/s10434-009-0875-9 [DOI] [PubMed] [Google Scholar]
- 41. Chua TC, Yan TD, Saxena A, Morris DL (2009) Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? : A systematic review of morbidity and mortality. Ann Surg 249: 900–907 10.1097/SLA.0b013e3181a45d86 [DOI] [PubMed] [Google Scholar]
- 42. Mizumoto A, Canbay E, Hirano M, Takao N, Matsuda T, et al. (2012) Morbidity and Mortality Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy at a Single Institution in Japan. Gastroenterol Res Pract 2012: 836425 10.1155/2012/836425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dedrick RL, Flessner MF (1997) Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst 89: 480–487 10.1093/jnci/89.7.480 [DOI] [PubMed] [Google Scholar]
- 44. Nissan A, Guillem JG, Paty PB, Wong WD, Cohen AM (2004) Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg 91: 747–754 10.1002/bjs.4473 [DOI] [PubMed] [Google Scholar]
- 45. Glehen O, Cotte E, Schreiber V, Sayag-Beaujard AC, Vignal J, et al. (2007) Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol 14: 509–514 10.1245/s10434-006-9167-9 [DOI] [PubMed] [Google Scholar]
- 46. Elias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, et al. (2009) Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 27: 681–685 10.1200/JCO.2008.19.7160 [DOI] [PubMed] [Google Scholar]
- 47. Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, et al. (2010) Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 116: 3756–3762 10.1002/cncr.25116 [DOI] [PubMed] [Google Scholar]
- 48. Chua TC, Morris DL, Saxena A, Esquivel J, Liauw W, et al. (2011) Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol 18: 1560–1567 10.1245/s10434-010-1522-1 [DOI] [PubMed] [Google Scholar]
- 49. Kerscher AG, Chua TC, Gasser M, Maeder U, Kunzmann V, et al. (2013) Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: a longitudinal experience of 2406 patients over two decades. Br J Cancer 16 108: 1432–1439 10.1038/bjc.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jayne DG, Fook S, Loi C, Seow-Choen F (2002) Peritoneal carcinomatosis from colorectal cancer. Br J Surg 89: 1545–1550 10.1046/j.1365-2168.2002.02274.x [DOI] [PubMed] [Google Scholar]
- 51. Whittemore AS, Wu-Williams AH, Lee M, Zheng S, Gallagher RP, et al. (1990) Diet, physical activity, and colorectal cancer among Chinese in North America and China. J Natl Cancer Inst 82: 915–926 10.1093/jnci/82.11.915 [DOI] [PubMed] [Google Scholar]
- 52. Zheng S, Chen K, Liu X, Ma X, Yu H, et al. (2003) Cluster randomization trial of sequence mass screening for colorectal cancer. Dis Colon Rectum 46: 51–58 10.1007/s10350-004-6496-2 [DOI] [PubMed] [Google Scholar]
- 53. Fu JF, Huang YQ, Yang J, Yi CH, Chen HL, et al. (2013) Clinical characteristics and prognosis of young patients with colorectal cancer in Eastern China. World J Gastroenterol 19: 8078–8084 10.3748/wjg.v19.i44.8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu J, Zhong Y, Weixin N, Xinyu Q, Yanhan L, et al. (2007) Preoperative hepatic and regional arterial chemotherapy in the prevention of liver metastasis after colorectal cancer surgery. Ann Surg 245: 583–590 10.1007/s10350-004-6496-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu AG, Yu ZJ, Jiang B, Wang XY, Zhong XH, et al. (2010) Colorectal cancer in Guangdong Province of China: a demographic and anatomic survey. World J Gastroenterol 16: 960–965 10.3748/wjg.v16.i8.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang S, Cui Y, Weng Z, Gong X, Chen M, et al. (2009) Changes on the disease pattern of primary colorectal cancers in Southern China: a retrospective study of 20 years. Int J Colorectal Dis 24: 943–949 10.1007/s00384-009-0726-y [DOI] [PubMed] [Google Scholar]
- 57. Wang T, Cui Y, Huang WS, Deng YH, Gong W, et al. (2009) The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointest Endosc 69: 609–615 10.1016/j.gie.2008.05.017 [DOI] [PubMed] [Google Scholar]
- 58. Ho JW, Lam TH, Tse CW, Chiu LK, Lam HS, et al. (2004) Smoking, drinking and colorectal cancer in Hong Kong Chinese: a case-control study. Int J Cancer 109(4): 587–597 10.1002/ijc.20018 [DOI] [PubMed] [Google Scholar]
- 59. Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, et al. (2012) Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut. 61: 1439–1446 10.1136/gutjnl-2011-300843 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraoperative parameters.
(DOC)
OS comparisons stratified by major clinico-pathological factors.
(DOC)
Analysis of independent factors influencing survival for patients with HPCI.
(DOC)
Major studies on CRS+HIPEC, either single-arm or controlled studies.
(DOC)
Nine large clinical-demographic studies on Chinese and Western colorectal cancer patients.
(DOC)
RAW data for manuscript PONE-D-14-23603.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.