Abstract
Visible and invisible displacement tasks have been used widely for comparative studies of animals’ understanding of object permanence, with evidence accumulating that some species can solve invisible displacement tasks and thus reach Piagetian stage 6 of object permanence. In contrast, dogs appear to rely on associative cues, such as the location of the displacement device, during invisible displacement tasks. It remains unclear, however, whether dogs, and other species that failed in invisible displacement tasks, do so due to their inability to form a mental representation of the target object, or simply due to the involvement of a more salient but potentially misleading associative cue, the displacement device. Here we show that the use of a displacement device impairs the performance of dogs also in visible displacement tasks: their search accuracy was significantly lower when a visible displacement was performed with a displacement device, and only two of initially 42 dogs passed the sham-baiting control conditions. The negative influence of the displacement device in visible displacement tasks may be explained by strong associative cues overriding explicit information about the target object’s location, reminiscent of an overshadowing effect, and/or object individuation errors as the target object is placed within the displacement device and moves along a spatiotemporally identical trajectory. Our data suggest that a comprehensive appraisal of a species’ performance in object permanence tasks should include visible displacement tasks with the same displacement device used in invisible displacements, which typically has not been done in the past.
Keywords: object permanence, visible displacements, invisible displacements, associative cues, Canis familiaris
The ability to locate objects that have disappeared from view has high ecological relevance for vertebrate species, for the purpose of both finding food and avoiding predators. It requires an understanding that an object continues to exist when it is out of sight (object permanence). Jean Piaget first introduced a six-stage developmental schema for infants’ mastering of object permanence tasks (Piaget, 1954), which was later extended by Uzgiris and Hunt (1975) to a 15-point scale. The series of tasks consist of visible displacements, in which a target object or reward is visibly moved behind an occluder in variations of increasing complexity, as well as invisible displacements, in which the target object is first placed into an opaque displacement device and then, still hidden inside the device, moved behind an occluder and left there (see e.g. Pepperberg, Willner, & Gravitz, 1997 or Zucca, Milos, & Vallortigara, 2007, for detailed descriptions). This framework has been adopted widely in comparative psychology and animal cognition research and has proven useful for valid comparisons of cognitive performance between diverse taxa (Pepperberg, 2002).
Comparative work has uncovered considerable variation in performance in object permanence tasks across animal taxa. Some species solve invisible displacements, reaching Piagetian stage 6 of object permanence, e.g. great apes (Call, 2001; Collier-Baker, Davis, Nielsen, & Suddendorf, 2006; Natale, Antinucci, Spinozzi, & Poti, 1986), tamarins (Neiworth et al., 2003), marmosets (Mendes & Huber, 2004), some psittacids (Pepperberg & Funk, 1990; Pepperberg et al., 1997), and some corvids (Hoffmann, Rüttler, & Nieder, 2011; Ujfalussy, Miklósi, & Bugnyar, 2013; Zucca et al., 2007). Others have consistently failed in invisible or even visible displacement tasks, e.g. cats (Doré, 1986, 1990) as well as some monkey (de Blois, Novak, & Bond, 1998; de Blois & Novak, 1994; Natale et al., 1986) and bird species (Dumas & Wilkie, 1995; Plowright, Reid, & Kilian, 1998). Some individuals of the species that failed in invisible displacement tasks used the displacement device as a cue to decide where to start searching for the target object and either searched at the device’s final location or at the first or the last hiding place visited by the device (e.g. cats: Doré 1986, 1990, and on occasion rhesus monkeys: de Blois & Novak, 1994). On other occasions location preferences became apparent (de Blois et al., 1998; de Blois & Novak, 1994).
For domestic dogs (Canis familiaris), reports over the last twenty years were highly contradictory. While early work suggested that they can solve invisible displacement tasks as adults (Gagnon & Doré, 1992, 1994), this conclusion was later revised by Doré et al. (1996), who found that dogs failed to track objects in transposition tasks (see also Fiset & Plourde 2013; Miller et al. 2009; Rooijakkers et al. 2009), and Collier-Baker et al. (2004), who found that dogs used the location of the displacement device as a cue to make their choices in invisible displacement tasks (see also Fiset & Leblanc 2007). In particular, the dogs did not perform above chance if the displacement device was removed at the end of the hiding procedure, or if it was placed next to one of the non-baited target boxes (Collier-Baker et al., 2004; Fiset & Leblanc, 2007). Also, both in rotation tasks and in transposition tasks, dogs exhibit particular problems when a container is present at the location where they saw the target object disappear, that is in 180° rotation tasks (Miller et al., 2009) and in switch and substitution transposition tasks (Doré et al., 1996; Fiset & Plourde, 2013). These results also indicate that dogs tend to follow competing location or object cues, at least when they are uncertain about the target object’s location.
The animals’ use of the displacement device as a cue during invisible displacement tasks raises the question whether their failure is indeed purely due to the inability to track invisible movements of the target, or whether the use of a displacement device itself introduces a level of difficulty that contributes to the subjects’ failure in these tasks. This question cannot be answered by the currently available literature since existing studies typically used a displacement device only for invisible but not for visible displacements. We are aware of only two exceptions: Call (2001) used opaque and transparent displacement devices in his study of chimpanzee object permanence. However, the latter study cannot be used to decide whether poor performance in invisible displacement tasks may be due to the use of the displacement device, because the chimpanzees passed both the visible and the invisible displacement task in this study. Goulet and colleagues also used a transparent displacement device in one of their experiments investigating object permanence in cats (Goulet, Doré, & Rousseau, 1994). Their results suggest that the use of a displacement device can lead to reduced performance in visible displacement trials in this species, even if the device does not affect visibility of the target during the hiding procedure. It remains unclear, however, whether this effect occurs widely across species. Also, it remains uncertain whether animals perceive objects contained within a transparent Plexiglas container in the same way as objects contained within an opaque container with one side open (see also Horner & Whiten, 2007; Seed, Call, Emery, & Clayton, 2009).
In the present study, we therefore specifically address the question whether the poor performance of dogs in invisible displacement tasks is at least partly due to the use of a displacement device. If dogs fail in invisible displacement tasks purely due to failure to track invisible movements of objects, we predict that the use of the same displacement device has no negative effect on performance in visible displacement tasks. In this scenario, the displacement device would only be used as a cue by the dogs if no other information about the target’s location is available to them. If, on the other hand, the displacement device provides a distracting or confusing cue that can override even explicit information about the location of the target, we predict that the use of the displacement device has a negative effect on performance in visible displacement tasks. Previous studies that demonstrated a confounding effect of the displacement device in dogs (e.g. Collier-Baker et al., 2004; Fiset & Leblanc, 2007) cannot answer this question, since they demonstrated the confounding effect only for invisible displacements. The study of Gagnon & Dore (1993) gives a hint however, that the performance of dogs is likely to be affected by the use of a displacement device. While they did not perform visible displacements with the target object displaced visibly within the device, they performed side-by-side displacements of the target object and the displacement device and found a reduced performance in this condition compared to standard visible displacements without displacement device (Gagnon & Doré, 1993).
In humans, a wealth of data shows that women outperform men, or vice versa, in various cognitive tasks (Halpern, 2000; Kimura, 1999). Also in some non-human animals, sex differences in cognitive performance in particular tasks, such as in spatial cognition or in conditioning tasks, are well established (Dalla & Shors, 2009; Healy, Bacon, Haggis, Harris, & Kelley, 2009). These differences may occur as a consequence of differential selection on particular cognitive skills in males and females (Halpern, 2000; Healy et al., 2009; Kimura, 1999), or as a side effect of sex-specific brain differentiation (Halpern, 2000; Kimura, 1999). In a recent study, we found that female dogs outperformed males in a physical cognition task related to object permanence (Müller, Mayer, Dörrenberg, Huber, & Range, 2011). A secondary aim of this study was therefore to evaluate the hypothesis that male and female dogs differ in their performance in object permanence tasks.
Method
Subjects
We tested a cohort of Border Collies in three testing periods: between 6 and 8 months, between 12 and 14 months, and between 18 and 21 months of age. Sample sizes varied between conditions and testing periods (see Table 1), but the full sample consisted of 42 subjects (15 males, 27 females). All subjects lived as pet dogs with their owners, who volunteered to participate in this study. We tested dogs of a single breed with the aim of reducing variability induced by breed differences and we chose Border Collies due to their high availability and motivation to work with humans. Also, we have no reason to assume that Border Collies were selected for better performance in object permanence tasks and performance in such tasks did not differ between breed groups in an earlier study (Gagnon & Doré, 1992).
Table 1. Overview of test conditions.
| Condition | Number of trials per session/criterion | Number of dogs testeda |
|---|---|---|
| pretrials | 3 trials/3 correct choices | 42 |
| standard visible displacement | 10 trials/at least 7 correct choicesb | 42 |
| control | 10 trials/no criterion | 36 |
| A-not-B error | 9 trials/at least 7 correct choices and no more than 1 A-not-B errorc. | 36 |
| original displacement device condition | 10 trials/at least 7 correct choicesb | 21 |
| simplified displacement device condition | 10 trials/at least 7 correct choicesb | 29 |
| visible displacement with final touch | 10 trials/at least 7 correct choicesb | 13 |
| visible displacement with initial sham-baiting | 10 trials/at least 7 correct choicesb | 11 |
| invisible displacement | 10 trials/at least 7 correct choicesb | 2 |
| invisible displacement with final touch | 10 trials/at least 7 correct choicesb | 2 |
Note. For detailed descriptions of conditions see text, for example trials see supplementary videos.
Sample sizes decrease across conditions for two reasons: 1) some dogs did not return for testing in later sessions due limited owner availability (N=19); and 2) some dogs failed to reach criterion in one condition and were not tested in subsequent conditions (N=21).
one-tailed binomial test with chance probability at 0.33: p < .02
an A-not-B error was counted when a dog made an incorrect choice in the first trial after the baited location had been changed, and chose the box that had been baited in the immediately preceding trials.
Experimental Setup
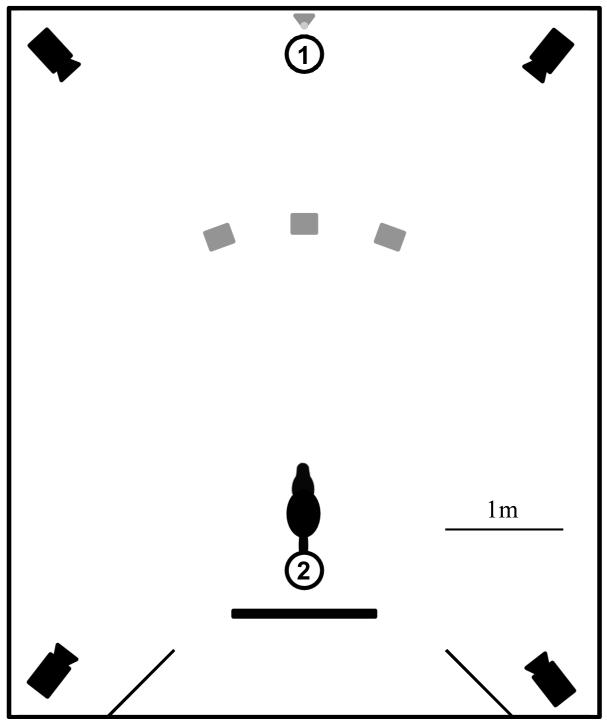
The setup was closely matched to the one used by Collier-Baker et al. (2004). Testing took place in a 5 by 6 m sized room. Three wooden target boxes (30 cm high, 20 cm wide and 13 cm deep) were arranged 40 cm from each other, 2 m from of the dog’s position (Figure 1) with their openings facing away from the subject. Each box was mounted on a 45 by 45 cm wooden board to ensure that dogs could not knock them over and had a piece of paper with notes for the experimenter fixed to the top. Experimenter 1 (E1), who performed the ball hiding, took position at the wall in front of the subject; experimenter 2 (E2), who handled the dog, took a seat behind the dog during trials (Figure 1). The displacement device was modelled after the one used in previous studies (Collier-Baker et al., 2004; Gagnon & Doré, 1994); it consisted of a triangular wooden box (20 cm high, 16 cm wide and 14 cm deep) attached to a 1m stick, with the inside lined with white paper to ensure good contrast to the target object. When not in use, the device was placed against the wall behind E1’s back. A 1.2 m wide temporary wall was set up behind E2’s chair for use in control trials (see below). Tennis ball imitations attached to a 1m transparent nylon string were used as a target objects. The balls were cleaned thoroughly with water between tests with different dogs to remove excessive saliva and were replaced frequently when getting worn. All experiments were recorded with an array of four video cameras placed in the four corners of the testing room. The owner was not present in the room but could observe testing via video link outside the testing room, with three exceptions where the owner was present in the room (behind the temporary wall) due to separation issues of the dog.
Figure 1. Experimental setup.
The circles represent start positions for experimenters 1 and 2, the grey squares mark locations of the three target boxes, and the grey triangle marks the start and end location of the displacement device. The partition behind experimenter 2 is used during control trials (cf. Procedure section of Methods). The setup (with the exception of the cameras) is drawn to scale.
Procedure
Before testing, we trained the dogs to touch the target ball with the nose or the paw, first when placed on the floor in various locations in the room, later when placed into one of the three target boxes. That is, the dogs learned that they would receive a food reward for touching the ball. This procedure aimed to bring ball-motivated and food-motivated dogs onto roughly equal levels of motivation during the test trials. It lasted between about 1 minute for intrinsically ball-motivated dogs and 30 minutes (with breaks) for dogs that were not ball motivated.
Each test trial started with E2 bringing the dog to its start position, 2 m from the array of target boxes (cf. Figure 1) for all conditions except for trials of the control condition (see below). During trials of all conditions, E1 did not touch the ball but operated it by means of the attached string. Once E2 had put on a blind fold, E1 called the dog’s name, tossed out the ball towards the dog and performed the hiding procedure according to the condition tested (see below). At the end of the hiding procedure, E1 returned to the start position at the wall (Figure 1), stood with the hands flat to the side and the gaze directed at the toes for three seconds before tapping the floor with the feet to signal E2 to release the dog. During the hiding procedure, E1 could observe from the corner of his eyes whether the subject remained oriented towards the target array. If the dog turned its head away from the target array briefly during the hiding process, the hiding process was delayed until the dog paid attention again. In cases where the dog turned its head away for more than 2 seconds, the trial was aborted and restarted after a few seconds (4% of all trials).
If the dog approached the correct box first and touched or retrieved the ball, it was rewarded either with a treat or with the experimenter throwing the ball for the dog to retrieve (depending on the dog’s preference); if the dog approached an incorrect box first, it was not rewarded. If the dog did not find the ball within 2 s after inspecting the first box, E1 stepped forward, showed the location of the ball to the dog and picked it up without allowing the dog to touch it. At the end of each trial, E1 retrieved the ball from the dog if applicable and E2 returned the dog to its start position.
The dogs were tested in a series of conditions of increasing difficulty, starting from standard visible displacements without a displacement device to invisible displacements with final touch (see Table 1 for an overview). Dogs that reached a pre-defined criterion in a particular condition (cf. Table 1) moved on to the next condition. Dogs that repeatedly failed in one condition were not tested in the subsequent, more difficult conditions, assuming that they would have very little chance of solving them. This approach was chosen for logistical reasons, in our study with a comparably large number of subjects and conditions, but it comes with the limitation that for the more difficult conditions sample size decreases and samples are biased towards good performers.
Conditions were presented in sessions consisting of 10 trials, with the exception of pretrials (3 trials) and the A-not-B condition (12 trials per session; cf. Table 1). A maximum of three sessions were completed per testing day. Dog and both experimenters left the room for breaks of at least 5 minutes between sessions. Additionally, breaks were introduced within sessions if the dog did not pay attention to the hiding process for several attempted trials.
Pretrials
For pretrials, a single target box was placed alternately in one of the three possible locations (Figure 1) and the ball was hidden in the box by means of the attached string. This condition corresponds to task 4 of the Uzgiris and Hunt scale (Piaget stage 4).
Standard visible displacement condition
E1 started trials of the standard visible displacement condition by tossing out the ball towards the dog (randomly between two target boxes). E1 then pulled the ball slowly on the string attached to it to place it into one of the three target boxes (cf. supplementary video 2). The baited box was chosen pseudorandomly so that each box was baited three or four times per session and the same box was never baited more than twice in a row. This condition corresponds to task 7 in the Uzgiris and Hunt scale (Piaget stage 5a). Based on consistent previous results (Collier-Baker et al., 2004; Gagnon & Doré, 1992, 1994; Triana & Pasnak, 1981), we predicted that the majority of dogs would solve this task in their first session. Dogs that solved the standard visible displacement condition were subsequently tested in two conditions, a control condition to rule out that the dogs found the ball by means of olfactory cues and the A-not-B condition.
Control condition
The procedure for control trials was identical to standard visible displacement trials except that the dog did not observe the hiding process. That is, the start position of dog and E2 was behind the temporary wall (Figure 1) and they returned to their usual start position, 2 m in front of the array, after the hiding process had been completed, upon which the release signal was given by E1 (cf. supplementary video 3). This condition controls for the possibility that the dogs may locate the baited box by smell or by means of the transparent nylon string attached to the ball, which extended out of the target box in all conditions (see also Fiset & Leblanc, 2007). The control condition was always presented as the last one on the corresponding testing day, so that any saliva deposited on the ball throughout testing, which offers numerous associative cues for finding the object, would be maximal in this condition.
A-not-B condition
For the A-not-B condition, the ball was hidden in plain sight as in the standard visible displacement condition, but the target boxes were chosen in the pre-specified order of box A for three trials followed by box B for three trials and so forth (trial order: AAABBBCCCAAA). Box A was chosen randomly among the three target boxes (left, middle, right). In cases where a dog did not achieve two successive correct choices at the end of a trial triplet, trials with the same target box were repeated until this was the case, before shifting to the next target box (e.g. AAABBBBCCCAA in a case where the dog chose incorrectly in the second B trial). The first three A trials were disregarded for the criterion in this condition (cf. Table 1). The A-not-B condition is a variation of task 5 of the Uzgiris and Hunt scale, with three instead of two target boxes, and tests for occurrence of the A-not-B error, a perseverative search error (Piaget, 1954). Based on previous results (Gagnon & Doré, 1992, 1994), we predicted that few dogs would commit A-not-B errors. Dogs that passed the criterion for the A-not-B condition were tested in visible displacement conditions with displacement device involving the same displacement device later used for invisible displacements.
Visible displacements with displacement device
In these conditions, the ball was transferred into one of the target boxes using the displacement device, with the opening of the device facing towards the dog during the whole hiding process. For these and all subsequent conditions, the baited box was chosen pseudorandomly. Initially, the dogs were presented with a visible displacement condition for which the ball was first transferred into one of the target boxes by means of the string (as for the standard visible displacements) and then transferred to a different box with the displacement device (henceforth: original displacement device condition, cf. supplementary video 4). As this condition turned out to be unsolvable for the dogs (cf. results) it was subsequently replaced by a simplified version (henceforth: simplified displacement device condition). For this condition, the displacement device was first (randomly) placed at the left or right extension of the array of target boxes with the opening facing towards the dog. The ball was then tossed out towards the dog next to the displacement device, placed into the displacement device by means of the attached string and transferred into one of the target boxes using the device (cf. supplementary video 5). For both the original and the simplified displacement device condition, the displacement device was shown to be empty and then returned to its position at the wall behind E1 (cf. Figure 1) at the end of the hiding procedure. That is, the displacement device was removed from the searchable stimulus array in order to avoid the possibility that the displacement device itself, being a salient cue, could be considered as a search option by the dogs (cf. Collier-Baker et al., 2004). The simplified version was presented to all dogs that had previously failed in the original version (N=19) as well as to some dogs that proceeded directly from the A-not-B condition (N=10). Performance in the simplified version did not differ between these two groups (GLM: t28=0.83, p=0.41). Dogs that did not reach the criterion for the simplified displacement device condition in their first session were tested in up to two further sessions of the same condition. Dogs that passed the criterion (cf. Table 1) were subsequently tested with one of the two sham-baiting conditions (half of the subjects in each).
Sham-baiting conditions
For the final touch condition, the hiding procedure was the same as for the simplified displacement device condition, but was followed by sham-baiting another target box with the empty displacement device (cf. supplementary video 6). For the initial sham-baiting condition, the ball was transferred from the displacement device into one target box but immediately retrieved back into the device and visibly transferred to another target box (cf. supplementary video 7). For both conditions, the sham-baited box was chosen pseudorandomly among the two non-baited boxes so that each of the three boxes was sham-baited three or four times within a session of ten trials. Dogs that reached the criterion for the final touch condition were subsequently tested in the initial sham-baiting condition and vice versa. These conditions served to determine whether the dogs had solved the simplified displacement device condition by using a simple rule such as “go to where the displacement device was last” or “go to where the displacement device went first” (cf. Collier-Baker et al., 2004; Gagnon & Doré, 1992). Dogs that reached criterion for both the final touch condition and the initial sham-baiting condition were subsequently tested with two invisible displacement conditions.
Invisible displacement conditions
The single invisible displacement condition matched the simplified displacement device condition, but the displacement device was turned by 180° on its vertical axis after the ball had been placed into it, so that the opening faced away from the dog (cf. supplementary video 8). After transferring the ball into one of the target boxes, the displacement device was turned back to show the dog that it was empty, then it was returned to its position at the wall behind E1 (Figure 1). This condition corresponds to task 13 of the Uzgiris and Hunt scale (Piaget stage 6). For the invisible displacement condition with final touch, the procedure was the same as for single invisible displacements, but E1 sham-baited a different target box with the displacement device after it was shown to be empty, and then returned it to its position at the wall (cf. supplementary video 9). The sham-baited box was again chosen pseudorandomly. Following earlier studies, we predicted that no dog would solve both of the invisible displacement conditions. However, the dogs in our study may have benefitted from the substantial experience with (visible) displacements and may thus be able to solve the invisible displacement tasks as well. If the dogs pass the single invisible displacement condition but fail in the invisible displacement condition with final touch, this will replicate the finding of Collier-Baker at al. (2004), namely that dogs “solve” invisible displacement tasks by choosing the hiding location last visited by the displacement device.
A repetition of the control condition was intended for dogs that passed all other conditions to ensure that they had not learned over time to use olfactory cues, or to follow the transparent nylon string, to solve the tasks. However, since none of the subjects solved all other conditions, this repetition was ultimately not carried out.
Test periods
The first test period took place when the subjects were 6 to 8 months old. In this period, after the initial training to touch the ball, all dogs were tested with pretrials, standard visible displacements for up to 2 sessions and, if successful, with the control condition, the A-not-B condition and the original displacement device condition. In the second test period, at an age of 12 to 14 months, we first repeated the training procedure done at the beginning of the first period to ensure that the dogs still recognized the ball as the target object. On this occasion, the procedure lasted typically no more than 1 minute. Thereafter, dogs that had not passed the standard visible displacement condition or the A-not-B condition in the first test period were retested in these conditions. Dogs that had passed the two conditions in the first test period received just three “warm-up” trials of the standard visible displacement condition. Thereafter, all dogs were tested with the simplified displacement device condition, for a maximum of two sessions. Dogs that passed this condition were subsequently tested with the final touch and/or initial sham-baiting condition. In the third test period, at an age of 18-21 months, the training procedure was again repeated and three “warm-up” trials were conducted before all dogs were retested with the simplified displacement device condition and, if applicable, with the two sham-baiting conditions and the two invisible displacement conditions.
In 43% of all cases, testing within a testing period was completed in one testing day. In the other cases, testing was completed on a second testing day, 2 to 19 (mean 8) days after the first one.
Scoring and Analysis
For each trial, we coded which target box the dog looked into first (so that the dog could see the ball if it was there). First choices could be determined reliably from video recordings since the boxes’ openings were facing away from the approaching dog. No choice was coded if the dog did not approach one of the three target boxes in a straight line, e.g. when the dog went to explore other parts of the room before approaching one of the boxes, or when the dog passed the boxes to approach E1 before making a choice. No choice trials were excluded from the analyses (4% of all trials). Inter-observer reliability for first choice (correct, incorrect or no choice) based on 20 randomly chosen sessions (204 trials) was high (Cohen’s kappa = 0.97).
All analyses were performed in R 2.15.1 (R Core Team, 2012). To compare performance between conditions, we used generalized linear mixed models (GLMMs; R package lme4, Bates et al. 2012), with logit link, number of correct choices in the numerator and number of trials in the denominator of the binomial response variable, and dog identity included as a random factor. To determine whether group performance differed from chance level for the first session of each condition, we tested whether the intercept differed from the one corresponding to the chance level of 33% in binomial GLMs with the intercept as the only predictor. For these analyses, we used the function “esticon” in R package “doBy” (Højsgaard, Halekoh, with contributions from Robison-Cox, Wright, & Leidi, 2012). Where sample sizes permitted, the subject’s sex was included as a predictor in the models. GLMs use the t-statistic, GLMMs use the z-statistic.
Results
Visible displacements without displacement device
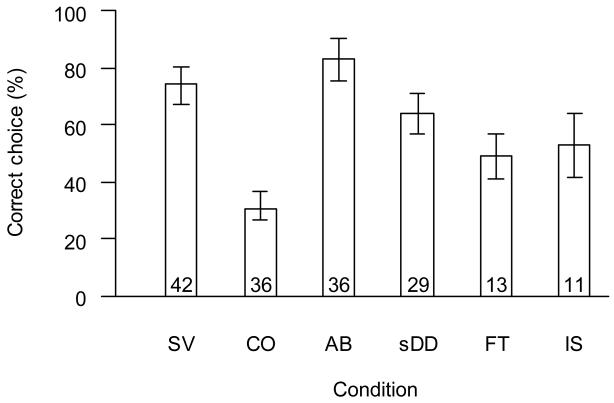
Group performance in the standard visible displacement condition was significantly above chance level in the first session, with 74% correct choices (GLM: t41=6.03, p<0.001; Figure 2). Performance did not differ between males and females (GLM: t40=0.17, p=0.86). Of the 42 subjects tested, 29 reached the individual criterion in their first session, a further 7 subjects reached it in their second session. Group performance was not different from chance level in the control condition, with 31% correct choices (GLM: t35=−0.77, p=0.45; Figure 2) and none of the 36 tested subjects had more than six correct choices. Group performance was also significantly above chance level for the A-not-B condition, with 83% correct choices in the first session (GLM: t35=8.46, p<0.001; Figure 2), and all but 6 of the 36 tested subjects reached the individual criterion. Only two of the latter failed due to A-not-B errors with subsequent perseveration, the other four failed to reach criterion with apparently random errors. Furthermore, errors occurred no more likely in the first trials after a shift to a different target box (A-not-B trials; 13% errors), than in other trials of this condition (19% errors). The nine subjects that had failed either in the standard visible displacement condition or in the A-not-B condition in the first test period, and were re-tested with the same condition in the second test period, performed significantly better in the second period (GLMM, z=5.11, p<0.001, with data of the two conditions pooled due to small sample size). All but one of these subjects reached criterion on the second occasion.
Figure 2. First session performance for visible displacement conditions.
SV: standard visible displacement condition, CO: control condition, AB: A-not-B condition, sDD: simplified displacement device condition, FT: final touch condition, IS: initial sham-baiting condition. Data are shown as mean with 95% confidence intervals. Numbers inside columns give sample sizes.
Visible displacements with displacement device
Only one of the 21 subjects tested in the original displacement device condition in the first test period reached the individual criterion, a stark contrast to the performance in the condition without displacement device (standard visible displacement). Group performance was even marginally below chance with 29% correct choices. Therefore the procedure for the visible displacement condition with displacement device was subsequently simplified for the second test period (cf. Methods).
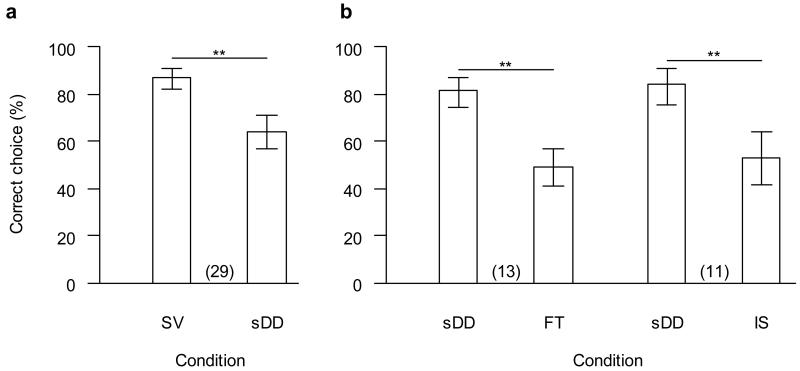
Group performance in the simplified version was significantly above chance in the first session with 63.9% correct choices (GLM: t28=8.02, p<0.001; Figure 2), with no difference between males (N = 8) and females (N = 21; GLM: t27=1.39, p=0.18). However, performance was significantly lower than in the last (and often only) session of the standard visible displacement condition, the same visible displacement but without displacement device (GLMM: z=−6.37, p<0.001; Figure 3a). This effect remained significant when the analysis was restricted to “non-adjacent” trials, where the target object passed behind one of the target boxes before another box was baited, or when the analysis was restricted to “adjacent trials”, where this was not the case (p<0.01 in both cases).
Figure 3. Drop in performance with introduction of displacement device.
a in the first session of the simplified displacement device condition (sDD) compared to the preceding session of the standard visible displacement condition (SV); b in the final touch condition (FT) and the initial sham-baiting condition (IS) compared to the preceding session of the simplified displacement device condition (which is not necessarily the same in both cases). Data are shown as mean with 95% confidence intervals. Numbers in parentheses give sample sizes (paired data). **: p<0.001.
Of the 29 dogs tested in the simplified displacement device condition, 15 passed the criterion in the second test period, after a maximum of 2 sessions; one further individual passed the criterion in the first session of the third test period (in total its third session). Of the 13 dogs that failed the simplified displacement device condition, 11 showed evidence of a location effect (preference for and/or avoidance of one of the three locations), while two dogs showed evidence for a preference of the side that the displacement device had started from. At group level, errors were more likely to occur in “non-adjacent trials” (44% errors) than in “adjacent trials” (32% errors; GLMM: z=3.28, p=0.001), an effect that had previously been found for chimpanzees as well (Call, 2001). When 22 dogs were retested in the third test period with the simplified displacement device condition, their performance was not improved compared to the performance in the last session of the same condition in the preceding test period, rather on contrary (GLMM: z=−1.87, p=0.06).
Group performance did not differ between the final touch and the initial sham-baiting condition (GLMM: z=0.60, p=0.55). Group performance was significantly above chance for both conditions (GLMs: final touch: 49% correct, t12=3.93, p=0.002; initial sham-baiting: 53% correct, t10=3.49, p=0.006). Note though that group level performance here rather overestimates the performance of dogs in these conditions, since only the best performers in the preceding conditions proceeded to this stage. Performance in the final touch and initial sham-baiting conditions was significantly lower than in the preceding session of the simplified displacement device condition (GLMMs; final touch: z=−4.36, p<0.001; initial sham-baiting: z=−4.92, p<0.001; Figure 3b). For both conditions, the sham-baited box was chosen more likely than the other non-baited box (final touch: z=3.63, p<0.001; initial sham-baiting: z=3.40, p<0.001). Four of the 13 dogs tested reached the criterion for the final touch condition; likewise, 4 of the 11 dogs tested reached the criterion for the initial sham-baiting condition. However, only two dogs, one male and one female, reached the criterion in both conditions and can thus be said to have solved visible displacement with a displacement device.
Invisible displacements with displacement device
The two subjects that had passed the final touch and the initial sham-baiting condition were subsequently tested with two invisible displacement tasks (one session each). Both dogs reached the criterion in the single invisible displacement condition but failed in the invisible displacement condition with final touch.
Discussion
The introduction of a displacement device for visible displacements led to a significant drop in performance, compared to standard visible displacements without displacement device. In addition, sham-baiting of the initial box with the device (equivalent to a double visible displacement), or final touch of a target box with the empty device led to an even further reduction in performance, which stands in contrast to previous studies that performed visible displacements without displacement device (Gagnon & Doré, 1992, 1994; Triana & Pasnak, 1981). These studies provided no evidence for a reduced performance in double compared to single visible displacements in domestic dogs (but see Fiset & Plourde, 2013). A visible displacement with final touch condition was not typically tested in previous studies (but see Mendes & Huber, 2004, who found that a final touch did not affect performance of marmosets). Overall, only two subjects, i.e., 5% of the complete sample, passed all visible displacement conditions, including those involving sham-baiting, even though previous work had found that dogs reliably solve double and even triple visible displacements when performed without displacement device, already from an age of 2 months (Fiset & Plourde, 2013; Gagnon & Doré, 1992, 1994). Taken together, these results indicate that the use of a displacement device, even if it does not reduce visibility of the target during displacements, affects performance in classic object permanence tasks. They also suggest that the failure of dogs in invisible displacement tasks reported previously (e.g. Collier-Baker et al., 2004; Fiset & Leblanc, 2007) may not only be due to a failure to form a mental representation of the outof-view object, as in these studies, like in almost all studies of object permanence, only invisible but not visible displacements were performed with a displacement device. That is, the use of a displacement device in classic Piagetian invisible displacement tasks has likely contributed significantly to the subject’s poor performance in these tasks compared to performance in visible displacements without a displacement device. This finding of course does not indicate in reverse that dogs form mental representations during invisible displacements, evidence against which has accumulated also with tasks that suffer less from possible confounding effects of containers (e.g. rotation and transposition tasks; Doré et al., 1996; Fiset & Plourde, 2013; Miller et al., 2009; Rooijakkers et al., 2009).
Several non-exclusive explanations may account for the effect of the displacement device on performance in visible displacement tasks. Firstly, the movement of the displacement device away from the target box after the end of the hiding procedure may interfere retroactively with the subject’s memory of the target location, as suggested by Gagnon & Dore (1993). This explanation alone cannot account for all of our results, however, since the performance of the subjects in our study dropped significantly from the simplified displacement device condition to the initial sham-baiting condition, even though the movement of the displacement device after the end of the hiding procedure was the same in both conditions.
The second potential explanation is reminiscent of overshadowing effects in classic associative learning theory (Dickinson, 1980; Spetch, 1995): The displacement device may constitute a salient cue that attracts attention at the expense of the smaller target object itself, and thus may lead to incorrect choices as a distractor or as a cue introducing conflicting information. This effect may not only responsible for our results, but may also explain the earlier finding of Gagnon & Dore (1993) that the movement of a second object side-by side with the target object leads to an impaired performance in visible displacement tasks. The interpretation of the displacement device as a distractor or competing cue is supported by a series of studies exploring dogs’ performance in invisible displacement tasks, which showed that dogs fail in particular when a strong associative cue is present at the original hiding location, both for transposition tasks (Fiset & Plourde, 2013; Rooijakkers et al., 2009) and for rotation tasks (Miller et al., 2009). Furthermore, Ashton and De Lillo (2011) have recently shown that dogs may be misled by strong associative cues in search tasks, even if explicit cues indicating the true target location are provided (see also Watson et al., 2001). Also the finding that our subjects were often misled by sham-baiting, in invisible as well as in visible displacement trials, supports the hypothesis that strong associative cues can override explicit information about the target’s location.
Finally, the visible displacement of the target object inside a displacement device may also lead to object individuation errors. Young children (Wilcox & Baillargeon, 1998; Xu & Carey, 1996) as well as non-human primates (e.g. Flombaum, Kundey, Santos, & Scholl, 2004) typically rely on spatio-temporal information to individuate objects and thus may commit individuation errors when spatio-temporal information is consistent with the interpretation that two objects seen at different times represent one and the same object, even though other information would be available that clearly shows otherwise (e.g. different size, shape or colour). For example, object individuation errors are commonly found in children and primates when one object disappears behind an occluder and a different objects appears at the other side at about the time when the first one would be expected to reappear, that is when spatio-temporal information suggest that they may be the same object (the so-called “tunnel effect”; Burke, 1952; Carey & Xu, 2001; Flombaum et al., 2004; but see Mendes, Rakoczy, & Call, 2008 for evidence of object individuation in great apes using a different experimental paradigm). Object individuation has been studied rarely in non-human animals other than primates (but see e.g. Fontanari, Rugani, Regolin, & Vallortigara, 2011). For dogs in particular, Bräuer and Call ( 2011) have recently noted that little is known about how they represent objects. While these authors showed that dogs can individuate objects according to their properties in a violation-of-expectation paradigm, their results to not rule out that conflicting spatio-temporal information would lead to object individuation errors as it does in human children and non-human primates. Given the precedence of spatio-temporal information in object individuation in children and primates, it appears conceivable that the visible displacement of the target object inside the displacement device, along a spatio-temporally identical trajectory, may have led to object individuation errors in our subjects. This phenomenon may in particular be responsible for the drop in performance from the standard visible displacement condition to the simplified displacement device condition, where a displacement device was introduced but did not provide conflicting information (as it did in the sham-baiting conditions).
Assuming that the displacement device represents a strong associative cue, or leads to object individuation errors, it may appear surprising that the subjects in our study only rarely first approached the displacement device itself when released (less than 2% of all trials involving the displacement device). This finding stands in marked contrast to the results of Collier-Baker et al. (2004), who found that dogs did not choose any of the target boxes in more than 40% of all trials when the displacement device was removed from the searchable stimulus array (similarly to our procedure). Instead they typically approached the displacement device behind the experimenter. We suggest a simple explanation for this discrepancy: Collier-Baker and colleagues had recruited a sample of naïve dogs for the relevant condition, whereas the dogs in our study had been subjected to several sessions with the same general setup, during which they may have learned that the target object will always be found within one of the three target boxes. We therefore suggest that, with this experience, the dogs in our study did not typically consider the displacement device itself as a possible final location of the target object (see also Doré, 1990). Instead, we suggest that the displacement device had a distracting or confusing effect, which resulted in the subjects being unable to reliably choose the baited box among the three available target boxes.
The dogs in our study performed at a somewhat lower level of accuracy in the standard visible displacement condition (74% correct choices in the first session) compared to the performance of dogs in comparable conditions in earlier studies (>85% correct; Collier-Baker et al., 2006; Fiset & Leblanc, 2007; Gagnon & Dore, 1993). In our visible displacement conditions, the target object passed at a small distance behind a target box before being placed into another in some trials, the “non-adjacent” trials (cf. supplementary videos 2 and 5). Assuming that in the previous studies the target object was moved in front of the other boxes on the way to the target box, this methodological difference, together with the lower age of the subjects in our study, could explain this reduced accuracy. However, this methodological difference cannot explain the drop in performance that we suggest was due to the use of the displacement device, since we applied the hiding procedure equally for displacements with and without displacement device.
Only two of the 36 tested subjects committed repeated A-not-B errors in the A-not-B condition, which is in accordance with the finding of Gagnon & Doré (1994) that even dog puppies at two months of age do not commit the A-not-B error. Our result is also unsurprising given the more recent evidence that A-not-B errors in human infants and dogs occur mostly when the hiding procedure involves ostensive cueing which was not the case in our study (Kis et al., 2012; Topál, Gergely, Erdohegyi, Csibra, & Miklósi, 2009; but see Fiset, 2010; Marshall-Pescini, Passalacqua, Valsecchi, & Prato-Previde, 2010 for alternative explanations for the results of Topál et al.).
While our study was not specifically designed to investigate changes in performance with age and experience, as no subjects were tested at 18 months of age without prior experience, our data nevertheless provide some insights into this question. We found some evidence of improved performance in the second test period (at 12 months of age) compared to the first one (at 6 months of age). Eight of the nine subjects that had failed in the standard visible or the A-not-B condition in the first period, and were retested in the second period, passed the respective condition on that occasion. Unfortunately, due to the necessary simplification of methods, no such statement can be made for performance in the visible displacement tasks with displacement device. We found no evidence for further improved performance in the third test period (at 18 months of age), rather on the contrary: the dogs tended to perform more poorly when retested in the simplified displacement device condition in the third period compared to their performance in the same condition in the second period. Overall, these data are in line with the suggestion of Gagnon & Doré (1994) that dog’s skills in displacement tasks are fully developed well before they reach adulthood and do not improve further thereafter. Furthermore, our results do not provide support for the hypothesis that extensive experience with simpler versions of the tasks may lead to improved performance in the more difficult conditions (in particular the invisible displacement tasks as found by Doré, 1986; Gagnon & Doré, 1992 with a different sequence of conditions). Only two subjects (of the 16 tested) passed both sham-baiting conditions of the visible displacements, despite experiencing more than 40 trials of simpler visible displacements in the same experimental setup previously, and neither of them passed the invisible displacement conditions. Our data rather support the notion that prior experience with simpler tasks does not improve performance in more difficult tasks (de Blois & Novak, 1994), or may even lead to worse performance if stronger associations with cues that are misleading in later conditions are formed.
Contrary to our previous results (Müller et al., 2011), we found no evidence for a better performance of females compared to males in the object permanence tasks presented here, despite a reasonably large sample size. Two possible explanations may account for the discrepancy between the present results and those presented by Müller et al. (2011): Firstly, the better performance of females may be restricted to object permanence tasks that require subjects to form a detailed representation of the out-of-sight object (including e.g. its size as in the 2011 study, or its shape, colour etc.), rather than merely its presence, as is the case in classic object permanence tasks like the ones used here. Alternatively, sex differences may appear in violation-of-expectation tests (as in Müller et al., 2011) but not in active choice tasks (as in this study). This suggestion is supported by a series of findings suggesting that performance in active choice tasks and perception tasks may be dissociated (e.g. Bremner, 2000; Daum, Prinz, & Aschersleben, 2009; Houdé, 2000; but see Hespos & Baillargeon, 2006, 2008). Finally, the marked sex difference found previously may represent a spurious result. Only replication of experiments will ultimately allow distinguishing these options.
To conclude, we found that the use of a displacement device negatively affects the performance of dogs in displacement tasks, either because the device constitutes a strong associative cue that can override explicit information about the target’s location, or because it leads to object individuation errors as the target and the device move in a spatio-temporally identical trajectory. Based on our results and similar findings reported previously for cats (Goulet et al., 1994), we suggest that comprehensive appraisals of a species’ performance in object permanence tasks, which have traditionally followed Piaget’s (1954) six-stage developmental schema and/or scale 1 of Uzgiris and Hunt’s (1975) scales of psychological development, should incorporate visible displacement tasks with the displacement device also used in invisible displacements, as these are necessary to determine whether poor performance in invisible displacement tasks is indeed due to failure to form a mental representation of the target object, the classic interpretation (but see Pepperberg & Funk, 1990; Pepperberg, 2002), or at least partly due to distracting or conflicting cues provided by the displacement device itself. In addition, our result support the suggestion that rotation tasks and transpositions tasks may be more reliable indicators for whether animals can follow invisible displacements than the classic invisible displacement tasks following Piaget (1954) and Uzgiris and Hunt (1975), as they do not suffer to from the competing cue provided by a displacement device (see also Barth & Call, 2006; Doré et al., 1996; Miller et al., 2009).
Supplementary Material
Acknowledgments
We thank the dog owners for bringing their dogs to participate in our experiments, Alina Gaugg, Amelie Göschl, Elisabeth Pikhart, Magdalena Weiler and Elena Zanchi for help with the experiments, and Catarina Bacelar for reliability coding. We also thank the anonymous reviewers for their comments on an earlier version of this manuscript. This work was funded by the Austrian Science Fund (FWF grant P21418 to L.H. and F.R.). In addition, L.H was supported by the WWTF project CS11-005, F.R. by the FWF project P21244, and S.R by the DK CogCom Program (FWF Doctoral Programs W1234) and the ESF RNP “CompCog” program (06-RNP-020). The Clever Dog Lab received financial support from Royal Canin and a private sponsor.
References
- Ashton RL, De Lillo C. Association, inhibition, and object permanence in dogs’ (Canis familiaris) spatial search. Journal of Comparative Psychology. 2011;125:194–206. doi: 10.1037/a0022584. doi:10.1037/a0022584. [DOI] [PubMed] [Google Scholar]
- Barth J, Call J. Tracking the displacement of objects: a series of tasks with great apes (Pan troglodytes, Pan paniscus, Gorilla gorilla, and Pongo pygmaeus) and young children (Homo sapiens) Journal of Experimental Psychology. Animal Behavior Processes. 2006;32(3):239–252. doi: 10.1037/0097-7403.32.3.239. doi:10.1037/0097-7403.32.3.239. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version. 2012 http://cran.r-project.org/package=lme4. [Google Scholar]
- Bräuer J, Call J. The magic cup: great apes and domestic dogs (Canis familiaris) individuate objects according to their properties. Journal of Comparative Psychology. 2011;125:353–361. doi: 10.1037/a0023009. doi:10.1037/a0023009. [DOI] [PubMed] [Google Scholar]
- Bremner JG. Developmental relationships between perception and action in infancy. Infant Behavior and Development. 2000;23:567–582. doi:10.1016/S0163-6383(01)00058-3. [Google Scholar]
- Burke L. On the tunnel effect. Quarterly Journal of Experimental Psychology. 1952;4:37–41. doi:10.1080/17470215208416611. [Google Scholar]
- Call J. Object permanence in orangutans (Pongo pygmaeus), chimpanzees (Pan troglodytes), and children (Homo sapiens) Journal of Comparative Psychology. 2001;115:159–171. doi: 10.1037/0735-7036.115.2.159. doi:10.1037/0735-7036.115.2.159. [DOI] [PubMed] [Google Scholar]
- Carey S, Xu F. Infants’ knowledge of objects: beyond object files and object tracking. Cognition. 2001;80:179–213. doi: 10.1016/s0010-0277(00)00154-2. doi:10.1016/S0010-0277(00)00154-2. [DOI] [PubMed] [Google Scholar]
- Collier-Baker E, Davis JM, Nielsen M, Suddendorf T. Do chimpanzees (Pan troglodytes) understand single invisible displacement? Animal Cognition. 2006;9:55–61. doi: 10.1007/s10071-005-0004-5. doi:10.1007/s10071-005-0004-5. [DOI] [PubMed] [Google Scholar]
- Collier-Baker E, Davis JM, Suddendorf T. Do dogs (Canis familiaris) understand invisible displacement? Journal of Comparative Psychology. 2004;118:421–433. doi: 10.1037/0735-7036.118.4.421. doi:10.1037/0735-7036.118.4.421. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiology and Behavior. 2009;97:229–238. doi: 10.1016/j.physbeh.2009.02.035. doi:10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum MM, Prinz W, Aschersleben G. Means-end behavior in young infants: the interplay of action perception and action production. Infancy. 2009;14:613–640. doi: 10.1080/15250000903263965. doi:10.1080/I525000090326396. [DOI] [PubMed] [Google Scholar]
- De Blois ST, Novak MA. Object permanence in rhesus monkeys (Macaca mulatta) Journal of Comparative Psychology. 1994;108:318–327. [Google Scholar]
- De Blois ST, Novak MA, Bond M. Object permanence in orangutans (Pongo pygmaeus) and squirrel monkeys (Saimiri sciureus) Journal of Comparative Psychology. 1998;112:137–152. doi: 10.1037/0735-7036.112.2.137. doi:10.1037/0735-7036.112.2.137. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Contemporary animal learning theory. Cambridge University Press; Cambridge, UK: 1980. [Google Scholar]
- Doré FY. Object permanence in adult cats (Felis catus) Journal of Comparative Psychology. 1986;100:340–347. [Google Scholar]
- Doré FY. Search behaviour of cats (Felis catus) in an invisible displacement test: cognition and experience. Canadian Journal of Psychology. 1990;44:359–370. doi: 10.1037/h0084262. doi:10.1037/h0084262. [DOI] [PubMed] [Google Scholar]
- Doré FY, Fiset S, Goulet S, Dumas M-C, Gagnon S. Search behavior in cats and dogs: Interspecific differences in working memory and spatial cognition. Animal Learning and Behavior. 1996;24:142–149. doi:10.3758/BF03198962. [Google Scholar]
- Dumas C, Wilkie DM. Object permanence in ring doves (Streptopelia risoria) Journal of Comparative Psychology. 1995;109:142–150. doi:10.1037//0735-7036.109.2.142. [Google Scholar]
- Fiset S. Comment on “Differential sensitivity to human communication in dogs, wolves, and human infants”. Science. 2010;329:142–b. doi: 10.1126/science.1184045. doi:10.1126/science.1184045. [DOI] [PubMed] [Google Scholar]
- Fiset S, Leblanc V. Invisible displacement understanding in domestic dogs (Canis familiaris): the role of visual cues in search behavior. Animal Cognition. 2007;10:211–224. doi: 10.1007/s10071-006-0060-5. doi:10.1007/s10071-006-0060-5. [DOI] [PubMed] [Google Scholar]
- Fiset S, Plourde V. Object permanence in domestic dogs (Canis lupus familiaris) and gray wolves (Canis lupus) Journal of Comparative Psychology. 2013;127:115–127. doi: 10.1037/a0030595. doi:10.1037/a0030595. [DOI] [PubMed] [Google Scholar]
- Flombaum JI, Kundey SM, Santos LR, Scholl BJ. Dynamic object individuation in rhesus macaques: a study of the tunnel effect. Psychological Science. 2004;15:795–800. doi: 10.1111/j.0956-7976.2004.00758.x. doi:10.1111/j.0956-7976.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- Fontanari L, Rugani R, Regolin L, Vallortigara G. Object individuation in 3-day-old chicks: use of property and spatiotemporal information. Developmental Science. 2011;14:1235–1244. doi: 10.1111/j.1467-7687.2011.01074.x. doi:10.1111/j.1467-7687.2011.01074.x. [DOI] [PubMed] [Google Scholar]
- Gagnon S, Doré FY. Search behavior in various breeds of adult dogs (Canis familiaris): Object permanence and olfactory cues. Journal of Comparative Psychology. 1992;106:58–68. doi: 10.1037/0735-7036.106.1.58. doi:10.1037/0735-7036.106.1.58. [DOI] [PubMed] [Google Scholar]
- Gagnon S, Doré FY. Search behavior of dogs (Canis familiaris) in invisible displacement problems. Animal Learning and Behavior. 1993;21:246–254. doi:10.3758/BF03197989. [Google Scholar]
- Gagnon S, Doré FY. Cross-sectional study of object permanence in domestic puppies (Canis familiaris) Journal of Comparative Psychology. 1994;108:220–232. doi: 10.1037/0735-7036.108.3.220. doi:10.1037/0735-7036.108.3.220. [DOI] [PubMed] [Google Scholar]
- Goulet S, Doré F, Rousseau R. Object permanence and working memory in cats (Felis catus) Journal of Experimental Psychology. Animal Behavior Processes. 1994;20:347–365. doi:10.1037/0097-7403.20.4.347. [PubMed] [Google Scholar]
- Halpern DF. Sex differences in cognitive abilities. 3rd ed Lawrence Erlbaum Associates; London, UK: 2000. [Google Scholar]
- Healy SD, Bacon IE, Haggis O, Harris AP, Kelley LA. Explanations for variation in cognitive ability: Behavioural ecology meets comparative cognition. Behavioural Processes. 2009;80:288–294. doi: 10.1016/j.beproc.2008.10.002. doi:10.1016/j.beproc.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Hespos SJ, Baillargeon R. Décalage in infants’ knowledge about occlusion and containment events: converging evidence from action tasks. Cognition. 2006;99:B31–B41. doi: 10.1016/j.cognition.2005.01.010. doi:10.1016/j.cognition.2005.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespos SJ, Baillargeon R. Young infants’ actions reveal their developing knowledge of support variables: converging evidence for violation-of-expectation findings. Cognition. 2008;107:304–316. doi: 10.1016/j.cognition.2007.07.009. doi:10.1016/j.cognition.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Rüttler V, Nieder A. Ontogeny of object permanence and object tracking in the carrion crow, Corvus corone. Animal Behaviour. 2011;82:359–367. doi:10.1016/j.anbehav.2011.05.012. [Google Scholar]
- Højsgaard S, Halekoh U, Robison-Cox J, Wright K, Leidi AA. doBy - Groupwise summary statistics, general linear contrasts, population means (least-squares-means), and other utilities. R package version. 2012;4:5–5. http://CRAN.R-project.org/package=doBy. [Google Scholar]
- Horner V, Whiten A. Learning from others’ mistakes? Limits on understanding a trap-tube task by young chimpanzees (Pan troglodytes) and children (Homo sapiens) Journal of Comparative Psychology. 2007;121:12–21. doi: 10.1037/0735-7036.121.1.12. doi:10.1037/0735-7036.121.1.12. [DOI] [PubMed] [Google Scholar]
- Houdé O. Inhibition and cognitive development: Object, number, categorization, and reasoning. Cognitive Development. 2000;15:63–73. doi:10.1016/S0885-2014(00)00015-0. [Google Scholar]
- Kimura D. Sex and cognition. MIT Press; Cambridge, MA: 1999. [Google Scholar]
- Kis A, Topál J, Gácsi M, Range F, Huber L, Miklósi A, Virányi Z. Does the A-not-B error in adult pet dogs indicate sensitivity to human communication? Animal Cognition. 2012;15:737–743. doi: 10.1007/s10071-012-0481-2. doi:10.1007/s10071-012-0481-2. [DOI] [PubMed] [Google Scholar]
- Marshall-Pescini S, Passalacqua C, Valsecchi P, Prato-Previde E. Comment on “Differential sensitivity to human communication in dogs, wolves, and human infants”. Science. 2010;329:142–c. doi: 10.1126/science.1187748. doi:10.1126/science.1187748. [DOI] [PubMed] [Google Scholar]
- Mendes N, Huber L. Object permanence in common marmosets (Callithrix jacchus) Journal of Comparative Psychology. 2004;118:103–112. doi: 10.1037/0735-7036.118.1.103. doi:10.1037/0735-7036.118.1.103 103. [DOI] [PubMed] [Google Scholar]
- Mendes N, Rakoczy H, Call J. Ape metaphysics: object individuation without language. Cognition. 2008;106(2):730–749. doi: 10.1016/j.cognition.2007.04.007. doi:10.1016/j.cognition.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Miller HC, Gipson CD, Vaughan A, Rayburn-Reeves R, Zentall TR. Object permanence in dogs: invisible displacement in a rotation task. Psychonomic Bulletin and Review. 2009;16:150–155. doi: 10.3758/PBR.16.1.150. doi:10.3758/PBR.16.1.150. [DOI] [PubMed] [Google Scholar]
- Müller CA, Mayer C, Dörrenberg S, Huber L, Range F. Female but not male dogs respond to a size constancy violation. Biology Letters. 2011;7:689–691. doi: 10.1098/rsbl.2011.0287. doi:10.1098/rsbl.2011.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale F, Antinucci F, Spinozzi G, Poti P. Stage 6 object concept in nonhuman primate cognition: a comparison between gorilla (Gorilla gorilla gorilla) and Japanese macaque (Macaca fuscata) Journal of Comparative Psychology. 1986;100:335–339. [Google Scholar]
- Neiworth JJ, Steinmark E, Basile BM, Wonders R, Steely F, DeHart C. A test of object permanence in a new-world monkey species, cotton top tamarins (Saguinus oedipus) Animal Cognition. 2003;6:27–37. doi: 10.1007/s10071-003-0162-2. doi:10.1007/s10071-003-0162-2. [DOI] [PubMed] [Google Scholar]
- Pepperberg IM. The value of the Piagetian framework for comparative cognitive studies. Animal Cognition. 2002;5:177–182. doi: 10.1007/s10071-002-0148-5. doi:10.1007/s10071-002-0148-5. [DOI] [PubMed] [Google Scholar]
- Pepperberg IM, Funk MS. Object permanence in four species of psittacine birds: An African Grey parrot (Psittacus erithacus), an Illiger mini macaw (Ara maracana), a parakeet (Melopsittacus undulatus), and a cockatiel (Nymphicus hollandicus) Animal Learning and Behavior. 1990;18:97–108. doi:10.3758/BF03205244. [Google Scholar]
- Pepperberg IM, Willner MR, Gravitz LB. Development of Piagetian object permanence in a grey parrot (Psittacus erithacus) Journal of Comparative Psychology. 1997;111:63–75. doi: 10.1037/0735-7036.111.1.63. doi:10.1037/0735-7036.111.1.63. [DOI] [PubMed] [Google Scholar]
- Piaget J. The child’s construction of reality. Basic Books; New York: 1954. [Google Scholar]
- Plowright CMS, Reid S, Kilian T. Finding hidden food: Behavior on visible displacement tasks by mynahs (Gracula religiosa) and pigeons (Columba livia) Journal of Comparative Psychology. 1998;112:13–25. doi:10.1037//0735-7036.112.1.13. [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. http://www.R-project.org/ ISBN 3-900051-07-0. [Google Scholar]
- Rooijakkers EF, Kaminski J, Call J. Comparing dogs and great apes in their ability to visually track object transpositions. Animal Cognition. 2009;12:789–796. doi: 10.1007/s10071-009-0238-8. doi:10.1007/s10071-009-0238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed AM, Call J, Emery NJ, Clayton NS. Chimpanzees solve the trap problem when the confound of tool-use is removed. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:23–34. doi: 10.1037/a0012925. doi:10.1037/a0012925. [DOI] [PubMed] [Google Scholar]
- Spetch ML. Overshadowing in landmark learning: touch-screen studies with pigeons and humans. Journal of Experimental Psychology. Animal Behavior Processes. 1995;21:166–181. doi: 10.1037//0097-7403.21.2.166. doi:10.1037//0097-7403.21.2.166. [DOI] [PubMed] [Google Scholar]
- Topál J, Gergely G, Erdohegyi A, Csibra G, Miklósi A. Differential sensitivity to human communication in dogs, wolves, and human infants. Science. 2009;325:1269–1272. doi: 10.1126/science.1176960. doi:10.1126/science.1176960. [DOI] [PubMed] [Google Scholar]
- Triana E, Pasnak R. Object permanence in cats and dogs. Animal Learning and Behavior. 1981;9:135–139. doi:10.3758/BF03212035. [Google Scholar]
- Ujfalussy DJ, Miklósi A, Bugnyar T. Ontogeny of object permanence in a non-storing corvid species, the jackdaw (Corvus monedula) Animal Cognition. 2013;16:405–416. doi: 10.1007/s10071-012-0581-z. doi:10.1007/s10071-012-0581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgiris IC, Hunt JM. Assessment in infancy: ordinal scales of psychological development. University of Illinois Press; Champaign: 1975. [Google Scholar]
- Watson JS, Gergely G, Csanyi V, Topal J, Gacsi M, Sarkozi Z. Distinguishing logic from association in the solution of an invisible displacement task by children (Homo sapiens) and dogs (Canis familiaris): using negation of disjunction. Journal of Comparative Psychology. 2001;115:219–226. doi: 10.1037/0735-7036.115.3.219. doi:10.1037//0735-7036.115.3.219. [DOI] [PubMed] [Google Scholar]
- Wilcox T, Baillargeon R. Object individuation in infancy: the use of featural information in reasoning about occlusion events. Cognitive Psychology. 1998;37:97–155. doi: 10.1006/cogp.1998.0690. doi:10.1006/cogp.1998.0690. [DOI] [PubMed] [Google Scholar]
- Xu F, Carey S. Infants’ metaphysics: the case of numerical identity. Cognitive Psychology. 1996;30:111–153. doi: 10.1006/cogp.1996.0005. doi:10.1006/cogp.1996.0005. [DOI] [PubMed] [Google Scholar]
- Zucca P, Milos N, Vallortigara G. Piagetian object permanence and its development in Eurasian jays (Garrulus glandarius) Animal Cognition. 2007;10:243–58. doi: 10.1007/s10071-006-0063-2. doi:10.1007/s10071-006-0063-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.