Abstract
Purpose
To compare the relative merits among robotic surgery, laparoscopy, and laparotomy for patients with endometrial cancer by conducting a meta-analysis.
Methods
The MEDLINE, Embase, PubMed, Web of Science, and Cochrane Library databases were searched. Studies clearly documenting a comparison between robotic surgery and laparoscopy or between robotic surgery and laparotomy for endometrial cancer were selected. The outcome measures included operating time (OT), number of complications, length of hospital stay (LOHS), estimated blood loss (EBL), number of transfusions, total lymph nodes harvested (TLNH), and number of conversions. Pooled odds ratios and weighted mean differences with 95% confidence intervals were calculated using either a fixed-effects or random-effects model.
Results
Twenty-two studies were included in the meta-analysis. These studies involved a total of 4420 patients, 3403 of whom underwent both robotic surgery and laparoscopy and 1017 of whom underwent both robotic surgery and laparotomy. The EBL (p = 0.01) and number of conversions (p = 0.0008) were significantly lower and the number of complications (p<0.0001) was significantly higher in robotic surgery than in laparoscopy. The OT, LOHS, number of transfusions, and TLNH showed no significant differences between robotic surgery and laparoscopy. The number of complications (p<0.00001), LOHS (p<0.00001), EBL (p<0.00001), and number of transfusions (p = 0.03) were significantly lower and the OT (p<0.00001) was significantly longer in robotic surgery than in laparotomy. The TLNH showed no significant difference between robotic surgery and laparotomy.
Conclusions
Robotic surgery is generally safer and more reliable than laparoscopy and laparotomy for patients with endometrial cancer. Robotic surgery is associated with significantly lower EBL than both laparoscopy and laparotomy; fewer conversions but more complications than laparoscopy; and shorter LOHS, fewer complications, and fewer transfusions but a longer OT than laparoscopy. Further studies are required.
Introduction
Endometrial carcinoma is the most common female genital tract malignancy in Western countries [1]. It is also the most common gynecologic cancer overall; 1 of every 40 women worldwide will develop endometrial cancer. Surgery is a major component of the diagnosis and treatment of endometrial cancer. Endometrial cancer is increasingly being treated with more minimally invasive approaches, including laparoscopy [2]. However, these minimally invasive approaches to the treatment of endometrial cancer have been limited due to long operation times (OTs), safety considerations, and other factors [3]–[5]. Thus, robotic surgery, the most novel minimally invasive technique, was developed to help overcome the technical limitations of laparoscopy and laparotomy. This technique enables surgeons to more easily perform complex procedures through improved visualization, more accurate instrument control, and improved ease of use of instruments [3]. Reza et al. [4] found that robot-assisted hysterectomy was associated with a longer OT but shorter length of hospital stay (LOHS), lower estimated blood loss (EBL), and fewer transfusions and complications than was open surgery. O'Neill et al. [5] showed that robot-assisted hysterectomy offers benefits with respect to a shorter LOHS and fewer blood transfusions than seen with open surgery.
However, the laparoscopic approach is reportedly a feasible alternative to conventional surgical treatment in patients with endometrial carcinoma [6]. Whether robotic surgery is superior to laparoscopy or laparotomy remains unclear. Therefore, the present meta-analysis compared the outcomes of the three currently used surgical approaches in patients with endometrial cancer: robotic surgery, laparoscopy, and laparotomy.
Materials and Methods
Literature search
The electronic databases of PubMed, MEDLINE, EMBASE, Web of Science, and the Cochrane Library were searched to identify eligible English-language publications (from January 1990 to September 2013). The following text and key words were used in the search: “laparoscopy and laparotomy,” “robotic-assisted with laparotomy,” “robotic versus open,” “robotic versus laparoscopic,” “robotics versus laparoscopy,” “robotics or laparoscopy,” “robotics and laparotomy,” “robotic versus laparotomy,” and “robotic-assisted laparoscopy, laparoscopy, and laparotomy” in combination with “endometrial cancer.” Logical combinations of these and related terms were used to maximize sensitivity. Finally, additional relevant articles were identified by searching the references of eligible articles.
Selection criteria
The inclusion criteria for this meta-analysis were analysis of either a retrospective or prospective cohort; comparison of robotic surgery with laparoscopy or laparotomy for treatment of endometrial cancer; evaluation of the following six outcomes: OT, number of complications, LOHS, EBL, number of transfusions, total lymph nodes harvested (TLNH), and number of conversions; and clear documentation of the surgical techniques being compared (either “robotic” and “laparoscopy” or “robotic” and “laparotomy”). When the same institution reported more than one study, either the higher-quality or most recent publication was included in the analysis to avoid including the same patients.
The exclusion criteria for this meta-analysis were as follows: the above-mentioned outcomes of interest were not reported for the two techniques or it was impossible to calculate these outcomes from the published results, it was impossible to extract the appropriate data from the published results, neither the surgical outcomes nor patient parameters were clearly reported, and patients with endometrial cancer were not evaluated.
Data extraction and quality assessment
Data extraction was carried out by two reviewers (L.R. and J.J.), and quality assessment was performed by another two reviewers (Y.X. and F.S.). Any disagreements were resolved by discussion between the reviewers. For continuous variables, the sample size, mean, and standard deviation (SD) were calculated. For dichotomous variables, the total number of patients in each group and the number of patients with each outcome of interest were calculated. Some studies reported median rather than mean values and range or interquartile range rather than SD; in such cases, the mean and SD were estimated [7]. Studies that gave no information on the SD or range were excluded from the meta-analysis. The study quality was assessed using the criteria developed by the Newcastle–Ottawa Scale (NOS) for quality assessment [8].
Statistical analysis
The meta-analysis was performed using Review Manager (version 5.1.4; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008) and Stata (version 11.2; StataCorp LP; College Station, TX, USA) software. All test results were considered to be statistically significant at p<0.05. We analyzed dichotomous variables by estimating odds ratios with their 95% confidence interval (95% CI) and continuous variables using the weighted mean difference (WMD) with the 95% CI. The pooled effect was calculated using either a random-effects or fixed-effects model. Heterogeneity was evaluated with χ2 and I2 values. We considered significant heterogeneity to be present when χ2 was within the 10% level of significance (p<0.10) and the I2 statistic was>50%. If the I2 statistic was>50%, indicating significant heterogeneity, the random-effects model was used. Otherwise, the fixed-effects model was used. Possible publication bias was assessed by Begg's funnel plots and Egger's regression test.
Results
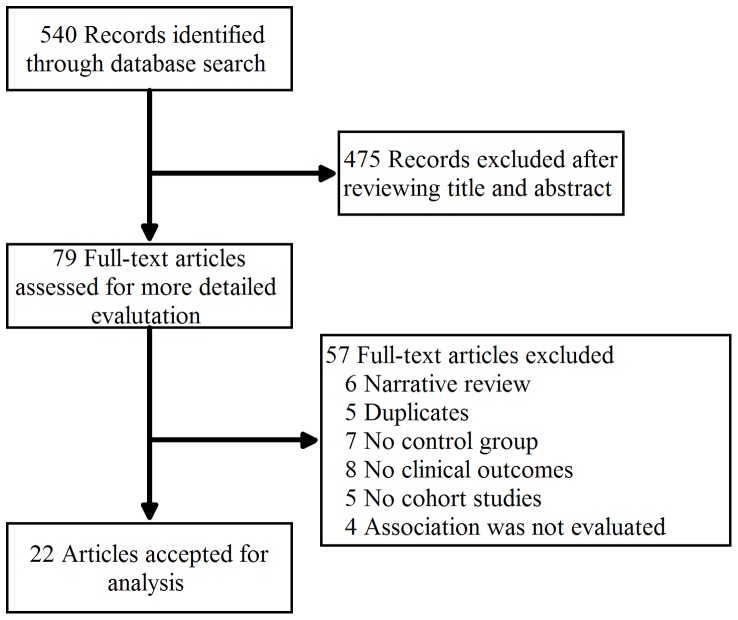
In total, 540 studies were identified and screened for retrieval using the above-described strategy. After screening the title or abstract, 475 studies were excluded and 79 were retrieved and evaluated in detail. Fifty-seven of these studies met the exclusion criteria, and 22 satisfied the selection criteria and were included in this meta-analysis (Fig. 1).
Figure 1. Flow diagram of identification of relevant studies in the present meta-analysis.
The patients' demographic and clinical characteristics are depicted in Table 1. The majority of studies (16/22, 72%) were carried out in the United States; there was one study each from Switzerland, Korea, Turkey, Spain, France, and Italy. Of all 22 studies, 8 compared robotic surgery and laparoscopy [9]–[16], 6 compared robotic surgery and laparotomy [17]–[22], and 8 compared robotic surgery, laparoscopy, and laparotomy [2], [19], [21], [23]–[27]. The 8 studies that compared robotic surgery and laparoscopy included 3403 participants (1822 who underwent robotic surgery and 1581 who underwent laparoscopy). The 6 studies that compared robotic surgery and laparotomy included 1017 participants (445 who underwent robotic surgery and 572 who underwent laparotomy).
Table 1. Patient demographic and clinical characteristics.
| First Author, Year(Ref.#) | Study type | Country | No.of patients | Mean age(year) | Outcomes meased | ||||
| RS | LS | OS | RS | LS | OS | ||||
| Veljovich et al., 2008 | Prospective cohort | USA | 25 | 4 | 131 | 59.5 | 54 | 63 | BMI,OT,EBL,LOHS,complications, uterine weight |
| Boggess et al., 2008 | Prospective cohort | USA | 108 | 81 | 138 | 61.9 | 62.0 | 64.0 | BMI,OT,LOHS,conversion,EBL,total nodes,stage,complication,transfusion |
| Bell et al.,2008 | Retrospective cohort | USA | 40 | 30 | 40 | 63.0 | 68.4 | 72.3 | BMI,OT,EBL,TLNH,Uterine weight,average cost,LOHS |
| DeNardis et al., 2008 | Retrospective cohort | USA | 56 | - | 106 | 58.9 | - | 6.5 | BMI,FIGO stage,Grade,OT,EBL,LOHS,Transfusion rate,TLNH |
| Magrina et al., 2008 | Prospective cohort | USA | 27 | 31 | 35 | 50 | 31 | 35 | BMI,OT,EBL, LOHS,FIGO stage,transfusion,readmission, TLNH |
| Seamon[1] et al.,2009 | Prospective cohort | USA | 32 | 17 | 14 | 55.0 | 52.8 | 42.0 | BMI,FIGO stage,OT,EBL,TLNH,Surgical margins,Depth of invasion,complications,transfusions,LOHS,Follow-up,Survival status |
| Seamon[2] et al.,2009 | Prospective cohort | USA | 92 | - | 162 | 58 | - | 62 | BMI,TLNH,conversion,transfusion,complications,OT,LOHS |
| Hoekstra1 et al.,2009 | Prospective cohort | USA | 32 | 7 | 26 | 62 | 59 | 56 | BMI,Grade,Stage,OT,EBL,TLNH,LOHS,Conversion,Complications |
| Cardenas-Goicoechea et al.,2010 | Retrospective cohort | USA | 102 | 173 | - | 62 | 59.6 | - | BMI,Tumor type,TLNH,Uterine weight,FIGO stage,Surgical time,Conversion,Blood transfusion,Estimated blood loss,LOHS |
| Sarlos et al., 2010 | Prospective cohort | Switzerland | 40 | 40 | - | 47 | 43.6 | - | BMI,OT,EBL,Uterus weight,LOHS,Wound infection,Personnel costs |
| Jung et al.,2010 | Prospective cohort | Korea | 28 | 25 | 56 | 52.89 | 49.88 | 50.20 | BMI,OT,Uterine weight,TLNH,Conversion, Overall complications,Transfusion,FIGO stage,LOHS |
| Göçmen et al., 2010 | Prospective cohort | Turkey | 10 | - | 12 | 55.7 | - | 56.4 | BMI,EBL,Histology,FIGO stage,OT,conversions,LOHS,transfusion, complication,TLNH |
| Lim et al.,2010 | Prospective cohort | USA | 56 | 56 | - | 62.5 | 61.4 | - | BMI,OT,EBL,TLNH,LOHS |
| Subramaniam et al., 2011 | Retrospective cohort | USA | 73 | - | 104 | 57 | - | 61.3 | BMI,Grade,Uterine weight,TLNH,OT,EBL,LOHS,Wound complications,30 Day mortality |
| Goel et al.,2011 | Retrospective clinical data | USA | 59 | - | 38 | 66.5 | - | 59.5 | BMI,OT,TLNH,EBL,Weight of uterus,FIGO stage,LOHS,Grade |
| Martino et al., 2011 | Retrospective cohort | USA | 101 | 114 | - | 61.8 | 63.6 | - | BMI,Stage,TLNH,Total drug interventions |
| Coronado et al.,2012 | Retrospective cohort | Spain | 71 | 84 | 192 | 67.3 | 65.9 | 64.7 | BMI,Grade,Stage,TLNH,OT,EBL,transfusions,conversion, complications,LOHS,Intra-operative |
| ElSahwi et al., 2012 | Retrospective cohort | USA | 155 | - | 150 | 62.4 | - | 65 | BMI,LOHS,Stage,Grade,Uterine weight,TLNH,EBL,OT, Conversion |
| Venkat et al., 2012 | Retrospective cohort | USA | 27 | 27 | - | 58.2 | 60.2 | - | BMI,Height,Weight,Hypertension,Smoker,TLNH,Uterine weight,LOSH,EBL,Stage,Grade,histology |
| Wright et al., 2012 | Perspective | USA | 1437 | 1027 | - | - | - | - | Complication,Transfusion,LOHS,hospital cost |
| Seror et al.,2013 | Retrospective cohort | France | 40 | 106 | - | 66.27 | 66.91 | - | BMI,Height,Weight,Hypertension,Hereditary history,OT,LOHS,Transfusion,FIGO stage,Complications |
| Fagotti et al., 2013 | Retrospective case-controlled | Italy | 19 | 38 | - | 62.0 | 61.9 | - | BMI,OT,EBL,LOHS,complications |
BMI(kg/m2):body mass index;OT(min):operating time; EBL(ml):estimated blood loss; FIGO: International Federation of Gynecology and Obstetrics;LOHS(h):length of hospital stay;TLNH(%): total lymph nodes harvested;RS:robotic surgery;LS: laparoscopy surgery;OS: open surgery;-:not available;
Quality assessment
The present review evaluated no randomized controlled trials; all included studies were retrospective or prospective studies. The study characteristics and participant features are given in Table 1. The main characteristics of the 22 studies were assessed using the NOS. All studies scored moderately well on the NOS. A score of 7 was attained by eight studies [11], [13], [18], [19], [25]–[27], a score of 8 was attained by seven studies [10], [12], [14]–[16], [20], [22], and a score of 9 was attained by seven studies [2], [9], [17], [21], [23], [24], [28].
Publication bias
Funnel plot analysis was performed for those studies that compared the numbers of overall complications, conversions, and transfusions between robotic surgery and laparoscopy. None of the studies lay outside the limits of the 95% CIs, and there was no evidence of publication bias among the studies (Fig. 2). The funnel plot for robotic surgery versus laparotomy showed no publication bias because there were no studies with a smaller mean difference (0–50) or higher variability (SE 16–20).
Figure 2. Funnel plot for main operative outcomes (complications, conversions, and transfusions) among all studies that compared robotic surgery and laparoscopy.
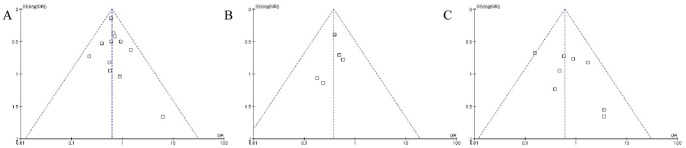
(A) Publication bias regarding complications (Begg's test: Z = 0.48, p = 0.63; Egger's test: t = 1.03, p = 0.032). (B) Publication bias regarding conversions (Begg's test: Z = 1.22, p = 0.022; Egger's test: t = −1.64, p = 0.20). (C) Publication bias regarding transfusions (Begg's test: Z = 0.62, p = 0.54; Egger's test: t = 1.55, p = 0.17).
Operative outcomes of robotic surgery versus laparoscopy
OT
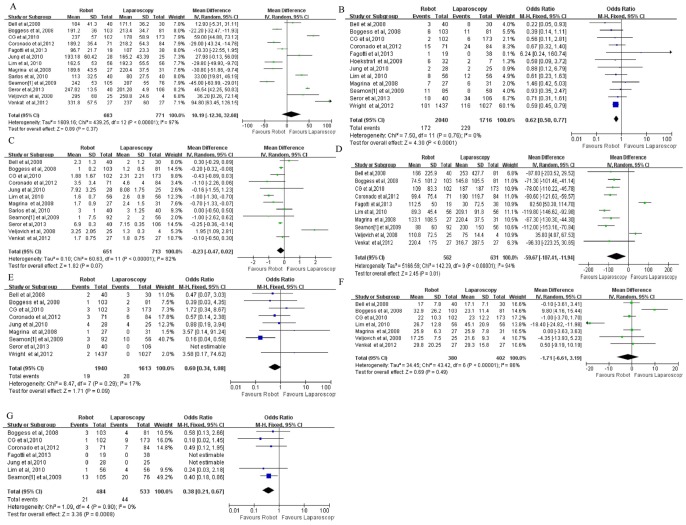
Thirteen studies showed no statistically significant differences in OT between robotic surgery and laparoscopy. Analysis of the pooled results also showed that the two types of surgery did not significantly differ in this regard (WMD, 10.19; 95% CI, −12.30–32.68; p = 0.37) (Fig. 3A).
Figure 3. Comparison of robotic surgery and laparoscopy with respect to (A) operating time, (B) complications, (C) length of hospital stay, (D) estimated blood loss, (E) transfusions, (F) total number of lymph nodes harvested, and (G) conversions.
OR: odds ratio; WMD: weighted mean difference.
Complications
Twelve studies showed a significantly higher number of complications in robotic surgery than in laparoscopy. Analysis of the pooled results also showed that the number of complications was significantly lower in robotic surgery than in laparoscopy (OR, 0.62; 95% CI, 0.50–0.77; p<0.0001) (Fig. 3B).
LOHS
Twelve studies showed no significant difference in LOHS between robotic surgery and laparoscopy. Analysis of the pooled results also showed that the two types of surgery did not significantly differ in this regard (WMD, −0.23; 95% CI, −0.47–0.02; p = 0.07) (Fig. 3C).
EBL
Ten studies showed significantly lower EBL in robotic surgery than in laparoscopy. Analysis of the pooled results also showed significantly lower EBL in robotic surgery than in laparoscopy (WMD, −59.67; 95% CI, −107.41–11.94; p = 0.01) (Fig. 3D).
Transfusion
Nine studies showed no significant difference in the number of transfusions between robotic surgery and laparoscopy. Analysis of the pooled results also showed that the two types of surgery did not significantly differ in this regard (OR, 0.60; 95% CI, 0.34–1.08; p = 0.09) (Fig. 3E).
TLNH
Seven studies showed no significant difference in the TLNH between robotic surgery and laparoscopy. Analysis of the pooled results also showed that the two types of surgery did not significantly differ in this regard (WMD, −1.71; 95% CI, −6.61–3.19; p = 0.49) (Fig. 3F).
Conversions
Seven studies showed significantly fewer conversions in robotic surgery than in laparoscopy. Analysis of the pooled results also showed that the number of conversions was significantly lower in robotic surgery than in laparoscopy (OR, 0.38; 95% CI, 0.21–0.67; p = 0.0008) (Fig. 3G).
Operative outcomes of robotic surgery versus laparotomy
OT
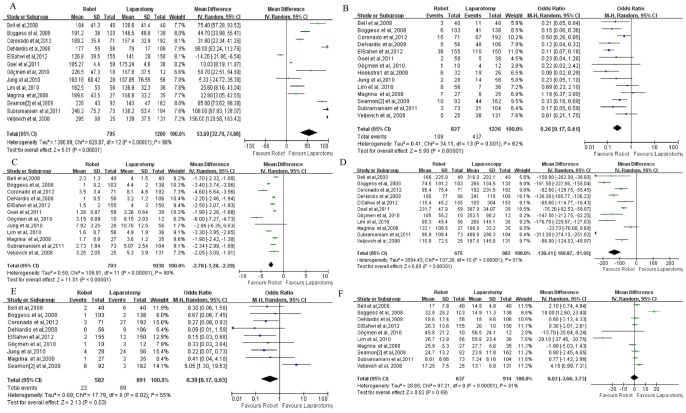
Thirteen studies showed that the OT was significantly longer in robotic surgery than in laparotomy. Analysis of the pooled results also showed that the OT was significantly longer in robotic surgery than in laparotomy (WMD, 53.69; 95% CI, 32.7–74.68; p<0.00001) (Fig. 4A).
Figure 4. Comparison of robotic surgery and laparotomy with respect to (A) operating time, (B) complications, (C) length of hospital stay, (D) estimated blood loss, (E) transfusions, and (F) total number of lymph nodes harvested.
OR: odds ratio; WMD: weighted mean difference.
Complications
Fourteen studies showed significantly fewer complications in robotic surgery than in laparotomy. Analysis of the pooled results also showed significantly fewer complications in robotic surgery than in laparotomy (WMD, 0.26; 95% CI, 0.17–0.41; p<0.00001) (Fig. 4B).
LOHS
Twelve studies showed that the LOHS was significantly shorter in robotic surgery than in laparotomy. Analysis of the pooled results also showed that the LOHS was significantly shorter in robotic surgery than in laparotomy (WMD, −2.78; 95% CI, −3.28 to −2.29; p<0.00001) (Fig. 4C).
EBL
Eleven studies showed significantly lower EBL in robotic surgery than in laparotomy. Analysis of the pooled results also showed significantly lower EBL in robotic surgery than in laparotomy (WMD, −130.41; 95% CI, −168.87 to −91.95; p<0.00001) (Fig. 4D).
Transfusion
Nine studies showed significantly fewer transfusions in robotic surgery than in laparotomy. Analysis of the pooled results also showed significantly fewer transfusions in robotic surgery than in laparotomy (WMD, 0.39; 95% CI, 0.17–0.93; p = 0.03) (Fig. 4E).
TLNH
Ten studies showed no significant difference in TLNH between robotic surgery and laparotomy. Analysis of the pooled results also showed that the two types of surgery did not differ in this regard (WMD, 0.03; 95% CI, −3.66–3.73; p = 0.99) (Fig. 4F).
Discussion
Surgical management has long been the primary therapy for endometrial cancer. Multiple surgical approaches to endometrial cancer have been available since newly developed methods of minimally invasive surgery were introduced. The choice of the most appropriate surgical method is becoming increasingly more important with growth in the obese and morbidly obese populations. To the best of our knowledge, this is the first comprehensive meta-analysis to compare robotic surgery with both laparoscopy and laparotomy for treatment of endometrial cancer. The results of this study show that robotic surgery is superior to laparotomy in terms of the number of complications, LOHS, EBL, and number of transfusions but is inferior to laparotomy in terms of the OT. Additionally, robotic surgery is superior to laparoscopy in terms of the EBL and number of conversions but is generally equivalent to laparoscopy in terms of the OT, number of complications, LOHS, and number of transfusions.
Robotic surgery may offer benefits over laparotomy in terms of reduced numbers of complications and transfusions, shorter LOHS, and lower EBL. The results of this study are consistent with those of previous studies [4]. In addition, the OT was longer in robotic surgery than in laparotomy. However, there was no significant difference in the TLNH between robotic surgery and laparoscopy. The numbers of conversions to laparotomy and complications are critical in minimally invasive surgical procedures for endometrial cancer. They are the most commonly reported outcomes because patients who have undergone conversion to laparotomy have higher complication rates [29]. The number of complications was significantly lower in robotic surgery than in laparoscopy. This result was due to both the inferior visualization of the laparoscopic videoscope and the superior ability of the three-dimensional robotic surgical platform, which enhances operative visualization [11]. Jung et al. [25] found that the high rate of operative complications among patients who underwent laparotomy was caused by the operative wound associated with the procedure.
In the present study, robotic surgery generally resulted in fewer conversions and lower EBL than did laparoscopy. These results can be explained by the robotic platform, which offers increased precision and dexterity, and are consistent with the results of previous studies [2], [23], [25], [29]. There were no significant differences in the OT or LOHS between robotic surgery and laparoscopy. Boggess et al. [2] and Jung et al. [25], however, found that robotic surgery was associated with a shorter LOHS and OT than was laparoscopy. Seamon et al. [30] noted that the OT is not consistently defined, making it difficult to compare this parameter between robotic surgery and laparoscopy in the face of heterogeneous data involving either a lack of the definition of OT in a given publication or the presence of data collection bias (retrospectively versus prospectively collected data). In another study [15], the OT included the time required to place the laparoscopic ports and uterine manipulator with colpotomy ring as well as the robotic docking time. However, other studies did not include these factors in the definition of OT. One possible explanation for the discrepant conclusions among various studies is the learning curve for robotic surgery in the treatment of endometrial cancer. Differences in the LOHS may be explained by differences in the medical insurance systems and cultures of the various countries in which each study was performed. In the present meta-analysis, the number of complications was significantly higher in laparoscopy than in robotic surgery; this may be associated with the OT and LOHS, neither of which showed a significant difference between robotic surgery and laparoscopy.
Our meta-analysis indicates that robotic surgery is associated with fewer complications than is laparotomy. This is similar to the findings of other investigators. Frigerio et al. [31] compared laparoscopy and laparotomy and found fewer postoperative complications among patients who underwent laparoscopic-assisted vaginal hysterectomy. Gil-Moreno et al. [32] compared laparoscopy and laparotomy and found that the amount of blood loss, number of blood transfusions required, and LOHS were significantly lower in the laparoscopic group; however, the OT was significantly longer. On the other hand, we found significantly more complications in robotic surgery than in laparoscopy. This is not completely different from the results of other investigations [17], [30]. Our data also showed a significantly lower EBL, shorter LOHS, and fewer transfusions in robotic surgery than in laparotomy. These findings are also not different from those of other investigations. Bell et al. [23] showed that the transfusion rate was not statistically different between robotic surgery and laparotomy. Finally, our study demonstrated that robotic surgery was associated with more complications than in laparoscopy but fewer complications than in laparotomy.
More prospective studies are needed to fully compare the effectiveness of robotic surgery with that of laparoscopy and laparotomy. The results of this study should be interpreted while taking its limitations into account. First, the data used for the WMD statistical analysis were median and range rather than mean and SD. The mean and SD must be estimated from the median and range, which may result in error or inaccuracy. Second, the learning curve is very important, especially for inexperienced surgeons, and affects the training curve for robotic surgery. As experience with robotic systems increases, the natural expectation is that the OT, LOHS, and transfusion rate will tend to decrease [24]. Finally, this meta-analysis was characterized by heterogeneity in the OT, LOHS, EBL, TLNH, and conversion rate because it was impossible to match the patient characteristics among all studies. Additionally, the random-effects model took between-study variation into consideration, which may have had a limited influence on the results.
In summary, this is the first meta-analysis to compare three conventional surgical approaches to endometrial cancer (robotic surgery, laparoscopy, and laparotomy) with respect to OT, complications, LOHS, EBL, transfusions, TLNH, and conversions. Overall, the present study has shown that robotic surgery is a feasible and promising method for the treatment of endometrial cancer compared with both laparoscopy and laparotomy. Some articles [13], [18], [19], [26] have reported that the costs associated with robotic surgery are higher than those associated with laparoscopy; however, we believe that robotic surgery can be a feasible alternative technique when robotic costs are reduced. No randomized controlled trials were available for inclusion in this study, which may have biased the interpretation of the results. Randomized controlled trials must be included in future studies to more fully assess the long-term results of this new technology in the field of endometrial cancer.
Supporting Information
PRISMA Checklist.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81101994 and 81072125). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ahmedin Jemal DVM, Siegel R, Xu J (2010) Cancer statistics, 2010. CA Cancer J Clin 5: 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, et al. (2008) A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol 199: 360 e361–369. [DOI] [PubMed] [Google Scholar]
- 3. Herron DM, Marohn M, SAGES-MIRA Robotic Surgery Consensus Group (2008) A consensus document on robotic surgery. Surg Endosc 2: 313–325. [DOI] [PubMed] [Google Scholar]
- 4. Reza M, Maeso S, Blasco JA, Andradas E (2010) Meta-analysis of observational studies on the safety and effectiveness of robotic gynaecological surgery. Br J Surg 97: 1772–1783. [DOI] [PubMed] [Google Scholar]
- 5. O'Neill M, Moran PS, Teljeur C, O'Sullivan OE, O'Reilly BA, et al. (2013) Robot-assisted hysterectomy compared to open and laparoscopic approaches: systematic review and meta-analysis. Arch Gynecol Obstet 287: 907–918. [DOI] [PubMed] [Google Scholar]
- 6. Peiretti M, Zanagnolo V, Bocciolone L, Landoni F, Colombo N, et al. (2009) Robotic surgery: changing the surgical approach for endometrial cancer in a referral cancer center. J Minim Invasive Gynecol 16: 427–431. [DOI] [PubMed] [Google Scholar]
- 7. Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wells GA, Shea B, Connell DO, Peterson J, Welch V, et al. (2007) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Proceedings of the Third Symposium on Systematic Reviews Beyond the Basics: Improving Quality and Impact 7: 3–5. [Google Scholar]
- 9. Cardenas-Goicoechea J, Adams S, Bhat SB, Randall TC (2010) Surgical outcomes of robotic-assisted surgical staging for endometrial cancer are equivalent to traditional laparoscopic staging at a minimally invasive surgical center. Gynecol Oncol 117: 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fagotti A, Corrado G, Fanfani F, Mancini M, Paglia A, et al. (2013) Robotic single-site hysterectomy (RSS-H) vs. laparoendoscopic single-site hysterectomy (LESS-H) in early endometrial cancer: a double-institution case-control study. Gynecol Oncol 130: 219–223. [DOI] [PubMed] [Google Scholar]
- 11. Lim PC, Kang E, Park do H (2010) Learning curve and surgical outcome for robotic-assisted hysterectomy with lymphadenectomy: case-matched controlled comparison with laparoscopy and laparotomy for treatment of endometrial cancer. J Minim Invasive Gynecol 17: 739–748. [DOI] [PubMed] [Google Scholar]
- 12. Martino MA, Shubella J, Thomas MB, Morcrette RM, Schindler J, et al. (2011) A cost analysis of postoperative management in endometrial cancer patients treated by robotics versus laparoscopic approach. Gynecol Oncol 123: 528–531. [DOI] [PubMed] [Google Scholar]
- 13. Sarlos D, Kots L, Stevanovic N, Schaer G (2010) Robotic hysterectomy versus conventional laparoscopic hysterectomy: outcome and cost analyses of a matched case-control study. Eur J Obstet Gynecol Reprod Biol 150: 92–96. [DOI] [PubMed] [Google Scholar]
- 14. Seror J, Bats AS, Huchon C, Bensaid C, Douay-Hauser N, et al. (2013) Laparoscopy vs Robotics in Surgical Management of Endometrial Cancer: Comparison of Intraoperative and Postoperative Complications. J Minim Invasive Gynecol. [DOI] [PubMed] [Google Scholar]
- 15. Venkat P, Chen LM, Young-Lin N, Kiet TK, Young G, et al. (2012) An economic analysis of robotic versus laparoscopic surgery for endometrial cancer: costs, charges and reimbursements to hospitals and professionals. Gynecol Oncol 125: 237–240. [DOI] [PubMed] [Google Scholar]
- 16. Wright JD, Burke WM, Wilde ET, Lewin SN, Charles AS, et al. (2012) Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer. J Clin Oncol 30: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeNardis SA, Holloway RW, Bigsby GEt, Pikaart DP, Ahmad S, et al. (2008) Robotically assisted laparoscopic hysterectomy versus total abdominal hysterectomy and lymphadenectomy for endometrial cancer. Gynecol Oncol 111: 412–417. [DOI] [PubMed] [Google Scholar]
- 18. Gocmen A, Sanlikan F, Ucar MG (2010) Comparison of robotic-assisted surgery outcomes with laparotomy for endometrial cancer staging in Turkey. Arch Gynecol Obstet 282: 539–545. [DOI] [PubMed] [Google Scholar]
- 19. Seamon LG, Cohn DE, Henretta MS, Kim KH, Carlson MJ, et al. (2009) Minimally invasive comprehensive surgical staging for endometrial cancer: Robotics or laparoscopy? Gynecol Oncol 113: 36–41. [DOI] [PubMed] [Google Scholar]
- 20. Subramaniam A, Kim KH, Bryant SA, Zhang B, Sikes C, et al. (2011) A cohort study evaluating robotic versus laparotomy surgical outcomes of obese women with endometrial carcinoma. Gynecol Oncol 122: 604–607. [DOI] [PubMed] [Google Scholar]
- 21. Coronado PJ, Herraiz MA, Magrina JF, Fasero M, Vidart JA (2012) Comparison of perioperative outcomes and cost of robotic-assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Eur J Obstet Gynecol Reprod Biol 165: 289–294. [DOI] [PubMed] [Google Scholar]
- 22. Goel M, Zollinger TW, Moore DH (2011) Surgical staging of endometrial cancer: robotic versus open technique outcomes in a contemporary single surgeon series. J Robotic Surg 5: 109–114. [DOI] [PubMed] [Google Scholar]
- 23. Bell MC, Torgerson J, Seshadri-Kreaden U, Suttle AW, Hunt S (2008) Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol 111: 407–411. [DOI] [PubMed] [Google Scholar]
- 24. Hoekstra AV, Jairam-Thodla A, Rademaker A, Singh DK, Buttin BM, et al. (2009) The impact of robotics on practice management of endometrial cancer: transitioning from traditional surgery. Int J Med Robot 5: 392–397. [DOI] [PubMed] [Google Scholar]
- 25. Jung YW, Lee DW, Kim SW, Nam EJ, Kim JH, et al. (2010) Robot-assisted staging using three robotic arms for endometrial cancer: comparison to laparoscopy and laparotomy at a single institution. J Surg Oncol 101: 116–121. [DOI] [PubMed] [Google Scholar]
- 26. Magrina JF, Kho RM, Weaver AL, Montero RP, Magtibay PM (2008) Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol 109: 86–91. [DOI] [PubMed] [Google Scholar]
- 27. Veljovich DS, Paley PJ, Drescher CW, Everett EN, Shah C, et al. (2008) Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol 198: 679 e671–679; discussion 679 e679–610. [DOI] [PubMed] [Google Scholar]
- 28. ElSahwi KS, Hooper C, De Leon MC, Gallo TN, Ratner E, et al. (2012) Comparison between 155 cases of robotic vs. 150 cases of open surgical staging for endometrial cancer. Gynecol Oncol 124: 260–264. [DOI] [PubMed] [Google Scholar]
- 29. Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, et al. (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365: 1718–1726. [DOI] [PubMed] [Google Scholar]
- 30. Seamon LG, Bryant SA, Rheaume PS, Kimball KJ, Huh WK, et al. (2009) Comprehensive surgical staging for endometrial cancer in obese patients: comparing robotics and laparotomy. Obstet Gynecol 114: 16–21. [DOI] [PubMed] [Google Scholar]
- 31. Frigerio L, Gallo A, Ghezzi F, Trezzi G, Lussana M, et al. (2006) Laparoscopic-assisted vaginal hysterectomy versus abdominal hysterectomy in endometrial cancer. Int J Gynaecol Obstet 93: 209–213. [DOI] [PubMed] [Google Scholar]
- 32. Gil-Moreno A, Diaz-Feijoo B, Morchon S, Xercavins J (2006) Analysis of survival after laparoscopic-assisted vaginal hysterectomy compared with the conventional abdominal approach for early-stage endometrial carcinoma: a review of the literature. J Minim Invasive Gynecol 13: 26–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.