Abstract
The term ‘‘Stammzelle’’ (stem cells) originally appeared in 1868 in the works of Ernst Haeckel who used it to describe the ancestor unicellular organism from which he presumed all multicellular organisms evolved. Since then stem cells have been studied in a wide spectrum of normal and pathological conditions; it is remarkable to note that ectopic arterial calcification was considered a passive deposit of calcium since its original discovering in 1877; in the last decades, resident and circulating stem cells were imaged to drive arterial calcification through chondro-osteogenic differentiation thus opening the idea that an active mechanism could be at the basis of the process that clinically shows a Janus effect: calcifications either lead to the stabilization or rupture of the atherosclerotic plaques. A review of the literature underlines that 130 years after stem cell discovery, antigenic markers of stem cells are still debated and the identification of the osteoprogenitor phenotype is even more elusive due to tissue degradation occurring at processing and manipulation. It is necessary to find a consensus to perform comparable studies that implies phenotypic recognition of stem cells antigens. A hypothesis is based on the singular morphology and amitotic mechanism of division of osteoclasts: it constitutes the opening to a new approach on osteoprogenitors markers and recognition. Our aim was to highlight all the present evidences of the active calcification process, summarize the different cellular types involved, and discuss a novel approach to discover osteoprogenitor phenotypes in arterial wall.
Keywords: Osteocalcin, Osteoprogenitor, Stem cells markers, Arterial calcification, Resident and circulating
Core tip: We review state of art on active arterial calcification, introduce new insight in arterial osteoprogenitors (OPs) phenotypes and the concept of amitosis. Analysis of literature of all markers used to define mesenchymal stem cells and OPs revealed the evident incongruity between the actual studies: each research has its own panel of antigen markers. Still, osteocalcin resulted the most promising marker of resident and circulating OPs. A new technique allows maintaining DNA/RNA integrity in highly calcified or ectopic bone formation: new studies should consider this technique and the particular division of OPs to identify them.
INTRODUCTION
Physiological and pathological mechanisms of vascular calcification
Previously considered passive and degenerative, vascular calcification is now recognized as a pathobiological process sharing many features with embryonic bone formation[1]. Vascular cell differentiation responds to microenvironmental and mechanical cues, since substrates of great stiffness, such as fibronectin, promote osteochondrogenic differentiation, whereas distensible substrates, such as laminin, promote smooth muscle or adipogenic differentiation[2]. The biomineralization process begins from the so-called crystallization nucleators, which trigger the formation of a primary crystal nucleus, together with the removal of the mineralization inhibitors [ankylosis protein, nucleotide pyrophosphatase, matrix glutamyl protein (MGP)]. The extracellular matrix vesicles contain deposits of calcium and alkaline phosphatase (ALP), pyrophosphatase, etc., which increase the inorganic phosphates in the vesicles[3]. They also stimulate the production of osteopontin, another nucleation inhibitor[4]. During the vessel calcification there are active processes similar to those in the bone biomineralization. In depositions in both tunica interna and media of the vessel wall, matrix vesicles have been identified[5]. Post-mortem studies have shown that vessel wall may contain a typical bone, cartilage or adipose tissue, with bone as the predominating type of metaplasia (10%-15% of samples), appearing in various morphological forms, from amorphous calcium deposits to mature bone tissue[6].
The increasing interest in vascular calcifications derives from the fact that in the atheromatous disease they were considered a form of plaque regression, while more recently the extent of calcification was associated with a worse prognosis, albeit the real impact of calcification within a specific lesion is unclear[7]. Moreover, vascular calcification is commonly seen during other systemic disease, such as diabetes, end-stage renal disease and calciphylaxis, and it is generally considered as a bad outcome predictor[8]. In the coronary arteries the extent and dimensions of the calcification seem to play a key role, since small depositions increase the probability of atherosclerotic plaque rupture, especially on their edges, while with individual, large calcification foci such risk is even likely to decrease[8,9]. In a study on 10 stables and 10 ruptured coronary artery post-mortem specimens, calcifications did not significantly affect the stability of the atheroma, in contrast with the significant reduction in stability associated with the lipid content. Removing the calcification led to a statistically insignificant change in stress[7]. Anyway, vascular calcification is considered a worsening factor, probably due to the association with the general risk factors: a study by Iribarren et al[10] found that aortic arch calcification was associated with coronary heart disease risk both in men and women. Thus aortic arch calcification may reflect the general burden of disease or be a marker of a more aggressive disease.
Histological patterns of vascular calcification
Histologically, arterial calcifications can be classified in calcifications of the tunica intima, principally related to atherosclerosis and, calcifications of the tunica media, unrelated to atherosclerosis (Monckeberg’s type)[6,11].
The intimal atherosclerotic calcifications are the most common form of arterial calcification. Calcium accumulation is initiated by an increase in the plaque of modified lipids, pro-inflammatory cytokines, phosphate and lipoprotein complexes, as well as foci of necrosis[1,6]. In vitro studies have shown that pro-inflammatory cytokines, oxidized low-density lipoprotein (LDL) or other macrophage release products promote the osteogenesis and the calcium accumulation[12-14], while some studies correlated the vascular calcification with the duration of the hypercholesterolemia[15] and with inflammation in vivo[16]. The so-called punctate deposits start in the deeper intimal regions, adjacent to the media, but very large deposits, involving the whole intima, can be seen[11]. In this tissue, hematopoietic, osteoblast-like and osteoclast-like cells were described[17,18]. A finest and more diffuse pattern of calcification, involving the whole intima was recently described due to processing techniques that do not require decalcification[19].
The medial calcification was firstly described by Monckeberg more than a century ago[20]. Since then, the “railroad track” medial calcification was observed in patients with diabetes and chronic renal disease[21,22], as well as in young patients without substitue patent with evident patent metabolic disorders[23,24]. In aging, medial calcification may develop by unknown etiology, or result from associated conditions such as chronic renal failure, diabetes, neuropathies and denervations[1,11,25]. In any case, these calcifications are likely to occur in not-atherosclerotic arterial segments[26].
Premises for a stem cell origin of vascular calcification
Classically, the heterotopic calcifications that can be found in the atheromatous plaques, in not-atheromatous arteries, as well as in many tissue, have been subdivided in active and passive[11]. The active calcifications follow different (and still unclear) mechanisms that can lead to a true ossification of the vessel wall[27,28].
While very rare in veins[29], ectopic calcification in arteries has been noted for many decades. In 1877, Howse found bone-formation in the wall of a ruptured axillary artery[30]. Until recently, however, this phenomenon was simply viewed as a passive consequence of aging[31]. However, as already observed in the 1900s, this condition was reported in the aorta of a girl eight years of age, aorta of adults between the ages of sixteen and twenty-four years, in an infant of fifteen months old and in an ossified aorta in a child of three years[30].
Regardless to the arterial layer, calcifications are found in different vessels as coronaries, distal arteries and aorta. As stated above, clinical outcome depends mainly on the degree and the location of calcification, additionally to the underlying disorder[32]. Several models postulating mechanisms for the formation and inhibition of calcification have now been proposed[33]. These are the active model; the passive physicochemical model; and the arterial osteoclast-like cells model. One model doesn’t exclude the other.
In some cases arteries can evolve into mature bone tissue histomorphologically indistinguishable from skeletal bone[11]. In our practice, this evolution occurs in at least 5% of diseased arteries. In Figure 1, a Haematoxylin-Eosin staining of a section of carotid atherosclerotic plaques revealed the presence of osteocyte cells within bone lacunae-like mature structure in development; lamellar bone is also visible.
Figure 1.
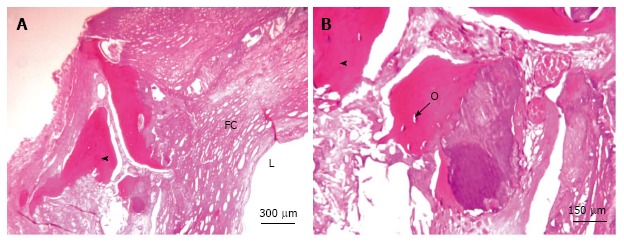
Carotid artery calcifications, hematoxylin and eosin staining. A: Sheet-like calcifications; B: Osteocytes are visible within the bone lacunae-like mature structure in development with lamellar bone. L: Lumen; FC: Fibrous cap; Arrowhead: Ossification; O: Osteocytes.
One of the most recent mechanism proposed in order to shine a light on active vascular calcification is the possible role of stem/progenitor cells, either resident in the vessel wall or circulating cells deriving from the bone marrow. In addition, chondrocyte-like cells, typically not expressed in normal arteries, osteoblast-like cells and multinucleated osteoclast-like cells (OLCs) are found in calcified arteries[17,33]. These cells are recognizable thanks to their peculiar morphology and positivity to specific histological markers; osteoprotegerin, osteopontin (OPN), osteocalcin (OCN), MGP and bone matrix protein (BMP)[34].
The present review focuses on the current and most recent knowledge on the mechanisms of active vascular calcification ascribable to resident and circulating cells that acquire the plasticity of the stem/progenitor cells and that trigger or participate to the vascular calcification processes.
CIRCULATING STEM CELLS
The passive model of vascular calcification has been progressively abandoned, since evidence of a genetic and active process has been observed.
Bone marrow (BM)-derived mesenchymal stem cells (MSC) have the ability to differentiate into many stromal cell types, as myocytes, fibroblasts, astrocytes, adipocytes, chondrocytes and osteocytes; the last two are referred as osteo-progenitors[34].
Progenitors are proliferative cells with a limited capacity for self-renewal and are often unipotent. Accumulating evidence indicates that the mobilization and recruitment of circulating or tissue-resident progenitor cells that give rise to endothelial cells (ECs) and smooth muscle cells (SMCs) can participate in atherosclerosis, neointima hyperplasia after arterial injury, and transplant arteriosclerosis[35]. Specifically progenitor cells can contribute to calcification: BM contains both osteoblast and osteoclast precursors termed as osteoprogenitors (OPs) associated with bone remodeling[36]. This novel mechanism was named “circulating cell theory”: the bone marrow derived cell population may seed the arteries and contribute to disease or repair[37]. The mobilization is the process under the regulation of cytokines in which immature cells from the BM are recruited to the blood[38].
Another common mechanism that can explain the recruitment of circulating OPs in arteries is homing[39]; in response to stress signal, injury, inflammation, repair or abnormal cytokine signalling, circulating cells cross the endothelium and invade the target tissue[40]. The endothelial phenotype selectively modulates bone marrow-derived stem cells homing: indeed different endothelial phenotypes hold functional differences. As an example, coronary artery endothelium enables the fastest bone marrow stromal cells integration. Transmigration requires the interaction of vascular cell adhesion molecule-1, very late antigen-4, β1 integrins, metalloproteinases (MMP) secretion and cytokines[40].
Recently, a primitive CD14-positive cell population was defined and named monocyte-derived multipotent cells (MOMCs). These cells show a fibroblast-like morphology and the expression of several stem cell markers such as CD14, CD45, CD105, CD34 and type I collagen, but lack expression of CD117 (c-kit) or CD133. These characteristics are quite peculiar[41]. Due to this hybrid phenotype, a subpopulation of these cells is likely to overlap the endothelial progenitor cells (EPC) originally described by Asahara et al[42], characterized by the co-expression of CD14 and CD34. Conversely, the so-called monocyte-derived endothelial progenitor cells are described as a MOMC subpopulation positive for CD14 but with low expression for CD34. These cells have the ability to differentiate also into osteoblasts, adipocytes, or neuronal cells[43].
Another subset of these cells showed bone resorption capacity on dentine slices and expression of genes for cathepsin K and calcitonin receptor, characteristic of functional osteoclasts[41]. MOMCs express receptor activator of nuclear factor-kB ligand (RANKL), which is required for osteoclast formation from mononuclear precursors. These results indicate that human MOMCs can express RANKL and differentiate into functional osteoclasts without RANKL-expressing accessory cells.
Under specific stimulations (PDGF: Platelet-Derived Growth Factor, interleukin IL-4, IL-13) CD14+ monocyte precursors can also differentiate into fibrocytes[44]. Discovered in 1994, fibrocytes are bone marrow-derived mesenchymal progenitors that co-express hematopoietic stem cell genes, markers of the monocyte lineage, and fibroblast products. Fibrocytes constitute another source of circulating cells able to differentiate in fibroblasts, myofibroblasts and adipocytes[45].
In valve and arteries, myofibroblasts contribute to cardiovascular ossification; Vattikuti observed that adventitial activated myofibroblasts cells are diverted to the osteoblasts lineage: the hypothesis is that myofibroblasts, responding to vascular smooth muscle cell osteopontin production contributes to calcification in diabetes. Moreover pericytic myofibroblasts expressed BMP-2, a powerful bone morphogen[46].
RESIDENT STEM CELLS
Mesenchymal stem cells
Bone marrow-derived MSC which reside in the vessel wall can differentiate in several cell types, including osteoblasts, chondrocytes and endothelial cells[47-51].
Previous results from our group showed that it is possible to isolate and culture spindle-shaped resident cells with the characteristics of MSC directly from the vessel wall of thoracic aortas harvested from multiorgan and tissue donors. These vessel-wall MSC (vw-MSC) are CD45- and show low expression for CD34, but most co-express CD44, CD90 and CD105, like the bone marrow-derived MSC[52]. Moreover, at reverse transcription polymerase chain reaction these cells express transcripts of embryonic stem cell (OCT4, IL6 and BCRP-1) and hematopoietic stem cell (c-Kit, BMI-1)[52]. Years after we confirmed that vw-MSC expressed the stemness markers Stro-1, Notch-1 and OCT4, and that they were able to differentiate into adipogenic, chondrogenic and leiomyogenic lineages, when cultured in induction media[53]. Recently, Klein et al[54] described a CD44+ population of “vascular wall-resident multipotent stem cells”, expressing also CD90 and CD73, and negative for CD34 and CD45. Moreover, vw-MSC were also isolated and cultured from arterial specimens frozen up to 5 years, and showed positivity for HLA-G, Stro-1, Oct-4 and Notch-1, in addition to the above mentioned[55].
Recently it was hypothesized that MSC might play a role in the pathogenesis of atherosclerosis, and it was demonstrated that, under particular conditions, MSC in culture acquire an osteoblastic phenotype via the activation of the Wnt pathway[56]. In hyperlipidemic rats treated with angioplasty to have a vascular damage, MSC started the vessel wall remodelling and triggered calcification, mediated by paracrine BMP-2[57], which is considered one of the main mediators in the differentiation of MSC (and others) along the osteoblastic lineage[58,59]. Interestingly, MSC cultured in a uremic serum for one month (mimicking partly the renal failure stimuli) hyperexpressed alkaline phosphatase, osteopontin, Runx2 and showed an up-regulation of BMP-2[60].
SMC
SMC of the human arterial wall show a great phenotypic plasticity, since it was demonstrated that in culture they can differentiate in almost all mesenchymal lineages (except adipocytic), and in particular conditions they can calcify[61,62]. These cells were originally described as calcifying vascular cells (CVC), i.e., SMC that under cAMP stimulus undergo osteoblast differentiation (with expression of alkaline phosphatase, type I collagen and matrix glutamyl protein), aggregate and form mineralized nodules[12]. The matrix carboxyglutamic acid protein (MGP)-deficient mice are a well-known animal model characterized by a progressive calcification of not-atherosclerotic arteries: in these mice vascular SMC were replaced by mineralizing chondrocyte-like cells[63]. The possibility of a phenotypic transition by the cells of the arterial wall opened new possibilities in the theories of the active calcification model.
Steitz et al[64] demonstrated the phenotypic transition of cultured bovine aortic smooth muscle cells into mineralizing cells: after 10 d from the administration of β-glycerophosphate, the smooth muscle cells lost their contractile properties (and the smooth muscle α-actin expression) and acquired an osteocalcin- and osteopontin-positive phenotype. Years later, researchers from the same group demonstrated that vascular SMC from MGP-knock-out mice expressed Runx2/Cbfa1 and gave rise to osteogenic precursors[65]. In SMC from human arteries, an increased expression of osteo- and chondrogenic transcription factors (Cbfa1, Msx2, Sox9) was observed concomitantly with a decreased expression of muscle markers[66]. SMC cultured in 2D scaffolds and treated 2 wk with lyso-phosphatidylcholine (LPC) underwent transdifferentiation to CVCs by up-regulation of the Runx-2 gene[67], while more recently the same authors demonstrated that using 3D cultures (a more reliable model of in vivo conditions) the growth and mineralization of cultured SMC is even more efficient, and adjustable by external factors such as LPC (enhancer) and Schnurri-3 (inhibitor)[68].
Neoangiogenesis and endothelial cells
According to several observations, neoangiogenesis and vascular calcification are closely correlated: first of all, neovessels can simply be considered as means of transportation for progenitor cells in the tissue, but endothelial cells are able to produce cytokines that can stimulate osteoprogenitor cells, in vitro and in vivo. Moreover, many growth factor (such as FGF-2 and VEGF) can stimulate both neoangiogenesis and the activation of osteoblasts and osteoclasts[8]. Endothelial cells cultured under pro-atherogenic stimuli produce pro-osteogenic factor, such as BMP-2[69]. This is particularly interesting, considering that most of plaque neoangiogenesis derive from adventitial vasa vasorum, and can drive many progenitor cells, pericytes, and inflammatory stimuli, including cytokines, in the media and intima layers[70,71].
The potentiality of endothelial cells to become directly a source of stem cells was demonstrated in a diabetic mouse model by Yao et al[72], who found that the stimulation with BMP-4 induced endothelial-to-mesenchymal transition and expression of osteogenic markers in aortic endothelial cells. In cultured human aortic endothelial cells, high glucose concentrations cause the acquisition of a “chondrocyte-like” phenotype, with the expression of STRO-1, CD44 and SOX9[73]. Previous in vivo data from our group have demonstrated that quiescent vasa vasorum in normal arteries from healthy subjects express markers of progenitor cells, namely Nestin and WT1, thus showing proliferative potential[74]. The same phenotype is expressed by intraplaque neoangiogenesis, and particularly Nestin is correlated with complicated plaques[75].
Osteoclast-like giant cells
Like in the normal bone tissue, the calcification of the vessel wall and/or atheromatous plaque is likely to depend on a balance between pro-osteogenic and anti-osteogenic stimuli. In this setting the so-called osteoclast-like giant cells (Figure 2) play a role in calcium reabsorption, as it was demonstrated decades ago by the findings that apoE-knockout mice lacking also the gene for macrophage colony stimulating (M-CSF, a cytokine involved in osteoclast survival) developed massive arterial calcifications[76]. The origin of the OLC in the vessel wall are not clear yet, and whether they derive from resident stem cells, from circulating hematopoietic precursors, from a differentiation of mononuclear cells or from other cells not yet established is still to be clarified. The mononuclear cells commonly found in atheromatous plaques share many phenotypic and genetic features with osteoclasts and they have a hematopoietic origin, while many circulating precursor cells express receptors for RANK and M-CSF, both essential for the osteoclast activity[11].
Figure 2.
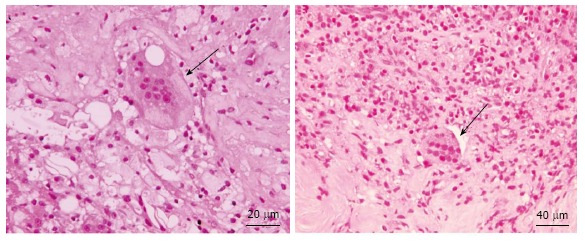
Osteoclasts-like giant cells admixed with inflammatory infiltrate. Arrows point osteoblast cells.
Pericytes and macrophage progenitor cells
Pericytes share several phenotypic markers with CVCs, including α-actin, β-actin, and the 3G5 epitope of monoclonal antibody-defined ganglioside antigen[8]. The putative role of pericytes as a “reservoir” of progenitor cells, and their potential to differentiate into several cell types, including osteoblasts, is well known[66,77,78]. In the last three decades, using different models, a lot of evidence have been adduced that pericytes can undergo chondro- and osteogenic differentiation[50,79,80]. After 8 wk of culture, pericytes have been shown to proliferate and form multicellular clumps with a mineralized matrix containing type I collagen, Gla protein, osteocalcin and osteopontin[81,82]. Furthermore, culturing these cells in a chondrogenic media (TGF-β3: Transforming growth factor β3) pericytes undergo chondrogenic differentiation[50]. Other authors hypothesize that adventitial pericytes (expressing activating Msx2 and other osteoblastic transcription factors) might also be able to stimulate the production of alkaline phosphate, the Wnt pathway activation and the β-catenin nuclear activation in medial cells (SMC)[83]. This represents an interesting example of indirect stimulus towards calcification mediated by the synergic cross-talk between different cells of the vessel wall. Indeed, arterial adventitia contain different progenitor cells, as it was demonstrated in murine aorta, where a population of Sca-1+/CD45+ macrophage progenitor cells has been recently described, which represents a reservoir of non-circulating precursors cells[84].
The role of the adventitial cells in the regulation of the functions of the vessel wall, both physiologically and in pathological conditions including calcifications, surely deserves future in-depth analyses.
DEFINITIONAL CRITERIA OF OSTEOGENIC LINEAGES
Osteoblastic “profile” and mechanisms
As shown above, several in vitro and animal models have demonstrated that a main mechanism of vascular calcification is represented by BMP-2 and 4. BMP-2 upregulates Runx2, which induces the production of type I collagen and alkaline phosphatase[85,86]. As demonstrated in murine models, MGP is the principal inhibitor of BMP-2, and a loss of MGP leads to tissue calcification[63]. One of the master genes essential for driving differentiation of mesenchymal cells into terminally differentiated osteoblasts is Osterix[11], that can be also found expressed in endothelial cells of the diseased arterial wall (Figure 3).
Figure 3.
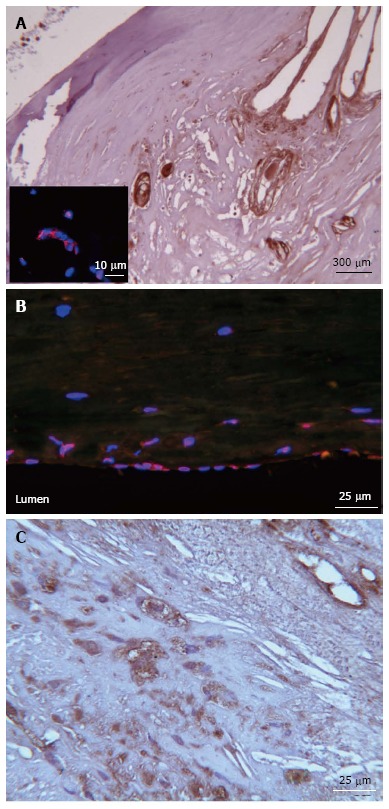
Osterix and osteocalcin expression in carotid plaques. A: Osterix immunohistochemistry (IHC) positivity in vessels single-label immunofluorescence micrographs representing Osterix (red) detectable in the nucleus of endothelial cells of a single vessel; B: Osterix (red) is also seen in the carotid endothelium layer, nuclei (blue) were counterstained with DAPI (4',6-diamidino-2-phenylindole); C: Cytoplasmic and extracellular matrix osteocalcin IHC pattern.
Recently, the receptor activator of NF-kB ligand (RANKL) was identified as another key molecule in the differentiation of osteoblasts and osteoclasts: in apoE-/- mice, the immunostaining for RANKL was diffusely positive in activated chondrocytes involved in the vascular ossification process[87], and its serum level seems to increase with ageing proportionally to the risk of cardiovascular events[88].
Osteopontin is a normal component of the bone and plays a role in the regulation of the mineralization. In calcified human plaques, OPN is expressed in SMC, endothelial cells and macrophages[89,90].
Osteocalcin is one of the most studied markers of osteoblast lineage. OCN is synthesized by osteoblasts and is the major component of the bone matrix (1%-2%). OCN is capable of binding hydroxyapatite (HA) thanks to his glutamyl (GLA) residues. Five Ca2+ ions are bound by 3 GLA residues carboxylated by vitamin K1[91], thus the OCN can dock on the HA and add calcium and growth crystal leading to the grow of bone. Transcription of OCN is regulated by Vitamin D3. In addition to binding to hydroxyapatite, OCN functions in cell signaling and the recruitment of osteoclasts and osteoblasts[92].
In patients with peripheral artery disease, the percentage of circulating bone marrow-derived OPs, positive to OCN, increased with the severity of aortic calcification[93]. Wang et al[94] demonstrated that in injured arteries the release of TGFβ mobilize MSC from the blood stream to the neointima. In a mouse model LDLR -/-, Nestin+/Sca+ cells were all recruited in the calcified arteries were OCN+ osteoblastic cells were seen: they observed that MSC generated OCN+ osteoblastic cells in the calcified lesions and that the migration of MSC to the lesions depends on TGFβ production from the lesions. Finally, when TGFβ receptor 1 was inhibited in mice there was a decrease of the number of MSC in the blood concomitant to their recruitment to the arterial lesions at the calcified lesions.
Different studies correlate the amount of circulating OCN-positive cells to the presence of coronary disease. Flammer et al[95] counted with flow cytometry the blood circulating population of cells positive to both immature EPC markers CD133+, CD34-, KDR+ and OCN. They observed that this fraction of cells, OCN+ EPC, increased in patients with cardiovascular risk factors compared to patients with a stable coronary artery disease history. Of note that the blood circulating cells expressing OCN have been shown to be able to calcify in vitro and in vivo[96]. In a similar study, Gössl et al[97] compared the fraction of EPC circulating cells CD34+/KDR+/OCN+ in 3 groups; the control group (normal coronary arteries/no endothelial dysfunction) versus two groups with coronary atherosclerosis: early coronary atherosclerosis (ECA: Normal coronary arteries but with endothelial dysfunction) and late coronary atherosclerosis (LCA: Severe, multi-vessel coronary artery disease). The number of CD34+/KDR+/OCN+ cells were increased by -2-fold in the ECA patients, with smaller increases in the LCA patients.
The prevalence and extent of calcification seems to have a genetic component that appears to be partially independent of those involved in atherogenesis. Specific genes that have been linked to arterial calcification in humans are also involved in atherosclerosis and include angiotensin I-converting enzyme, apo E, E-selectin, MMP-3, MGP, CC chemokine receptor 2, and estrogen receptor α[11].
New processing techniques of calcified tissue
Due to the tissue composition, morphological analysis of calcified or bone-like tissue is often incomplete: the decalcification procedure degrades the 3D structure of cells and hydrolyses the nucleic acid molecule[98]. Decalcification procedure with ethylenediaminetetraacetic acid or chloride acid put significant limitations to the study of ectopic tissue calcification. Based on this consideration, we recently decided to apply a new technique to preserve nuclear morphology and nucleic acid content, whilst preserving the 3D cellular structure. This protocol was patented at the Massachusetts Institute of Technology of Cambridge (Patent number WO2006009860 A3)[99,100]. Thanks to this method, a new set of cells missed for almost 100 years[101] were discovered: the shape of the nucleus was difficult to spot because of the standard 2.5 µm cut. Metakaryotic cells, also called bell-shaped cells, were identified first in developing fetus, then in adult cancerous tissue and finally in vascular tissue and represent the first possible evidence of stem cells lineages[53,100,102].
Briefly, the spreading protocol[99,100] is based on the digestion of Carnoy fixed tissue with of a collagenase type II enzyme that slowly disaggregate calcified tissue, after maceration in acetic acid, tissue are spread on a slide in a single monolayer of cells. At this point, standard immunohistochemical and molecular analyses could be performed: the result is that morphology of single cells from mineralized tissues is visible (Fittipaldi et al unpublished data).
CONCLUSION
As it is evident from Table 1, the literature of the last 20 years concerning stem cells is characterized by a general incongruity about which marker panel defines the progenitor cells and the different progenitor lineages. This is reflected by the Babel-like terminology used to define the progenitor cells by different groups. This issue is even foggier when it comes to the OPs identification, which became crucial in the last years, with the acceptance of the active model of vascular calcification[11].
Table 1.
Phenotypic markers for circulating and resident progenitors cells
| Name of the progenitors | Antigens | Notes (origin) | Ref. |
| MSC, EPC and CEC in human | |||
| MSC | CD105+, CD73+, CD90+, CD14-, CD34-, CD45-, CD79-, and CD19- | Resident from various tissues | Dominici et al[103] |
| vw-MPSC | CD44+, CD90+, CD73+, CD34-, CD45- | Resident from arterial adventitia | Klein et al[54] |
| vw-MSC | CD44+, CD90+, CD73+, CD105+, CD29+, CD166+, Stro-1, Notch-1 and Oct-4 | Resident from aortic arches, thoracic and femoral arteries | Pasquinelli et al[52,53] |
| Circulating MSC | CD105+, CD166+, CD54+, CD55+, CD13+, CD44+; CD34-, CD45-, CD14-, CD31-, CD133- | Circulating and resident from bone marrow; cartilage; synovial membrane; peripheral blood; umbilical cord blood; teeth | Qian et al[104] |
| Circulating EPC | CD34+, CD133+, VEGFR2+ (KDR) | Circulating and resident from bone marrow; peripheral blood; umbilical cord blood; hematopoietic stem cells; hemangioblast; fat tissues | |
| Circulating total PC | CD133+; CD34+; CD133+/CD34+ | Circulating | Baker et al[105] |
| Circulating EC | CD146+/CD31+ | Circulating | |
| Circulating EPC | CD34+ VEGFR+ CD133+ | Circulating | |
| Circulating EPC | (CD34+/CD133–/KDR+/CD45–) expressing VDR+ or OCN+, and VDR+ and OCN+ | Circulating (in chronic kidney disease) | Cianciolo et al[106] |
| Circulating EPC | CD133+, CD34+, KDR+ | Circulating (in rheumatoid arthritis with coronary Calcification) | Yiu et al[107] |
| OPs in human | |||
| OP | CD44+, CD63+, CD146+, Stro-1+ | Resident bone marrow | Gronthos et al[108] |
| CVC | 3G5+ | Resident from arteries (Pericytes markers) | Bostrom et al[109] |
| Circulating osteocalcin-positive mononuclear cells | OCN+ | Circulating bone marrow derived | Pal et al[93] |
| Circulating progenitor cells (Pro-calcific differentiation) | CD34+/OCN+; CD34+/BAP+; CD34+/OCN+/BAP+; OCN+/KDR+ ratio; BAP+/KDR+ ratio; OCN+/ BAP+/KDR+ ratio | Circulating (in diabete mellitus) | Fadini et al[110] |
| Circulating EPC expressing osteocalcin | OCN+/CD133+/CD34-/KDR+ | Circulating (in cardiovascular disease) | Flammer et al[95] |
| Circulating EPC expressing osteocalcin | OCN+/CD133+/CD34-/ KDR+ | Circulating (in coronary atherosclerosis, plaques instability) | Gössl et al[97] |
| Circulating T cells | CD28- CD8 +T cells | Circulating (in calcific aortic stenosis) | Winchester et al[111] |
| OPs in mouse models | |||
| Mesenchymal OPs | CD45-/TER119-/ Sca-1+/PDGFRa+ | Resident in the mouse bone marrow | Morikawa et al[112] |
| Bone marrow-derived calcifying cells | Sca-1+/PDGFRa- and Sca-1+/PDGFRa+ | Resident from mice aorta | Cho et al[113] |
| Circulating osteogenic cells | Sca-1+, PDGFRa+, CD45-, CD44+, CXCR4+ | Circulating in ectopic bone formation in a mouse model | Otsuru et al[114] |
MSC: Mesenchymal stromal cells; EPC: Endothelial progenitors cells; CEC: Circulating endothelial cells; vw-MPSC: Vascular wall-resident multipotent stem cells; vw-MSC: Vascular wall-resident multipotent mesenchymal stem cells; PC: Progenitors cells; CVC: Calcifying vascular cells; OPs: Osteoprogenitors; CD: Cluster of differentiation; Oct-4: Octamer-binding transcription factor 4; VEGFR: Vascular endothelial growth factor receptor; OCN: Osteocalcin; VEGFR2 (KDR): Kinase insert domain receptor; VDR: Vitamin D receptor; BAP: Bone alkaline phosphatase; Sca-1: Stem cells antigen-1; PDGFR: Platelet-derived growth factor receptor; CXCR-4: C-X-C chemokine receptor type 4.
It becomes necessary to uniform the phenotypic definition of osteoprogenitor cells. Paradoxically, the typical morphology of resident osteoblasts and osteoclasts could be of help, since these cells are easily recognizable at optical microscopy. Therefore, the morphology could constitute the basis of the future identification of those resident cells which deserve more attention for the identification of their phenotype. These observations will push towards the study of alternative methods of morphological analysis, including the spreading analysis on calcified tissue, which opens the novel possibility to have more information on DNA and proteins composing bone-like tissue. Twenty years ago, in a study on ectopic bone formation, Solari et al[115] found that osteoclasts undergo amitotic division, and that a budding process is responsible of their division (Figure 4). Recently, these results were fuelled by the finding that some cells with the characteristics of stem cells divide by an amitotic mechanism, using a RNA-DNA intermediate[116]. We recently found cells with the same characteristics in adult pathological arteries (Fittipaldi et al unpublished data).
Figure 4.
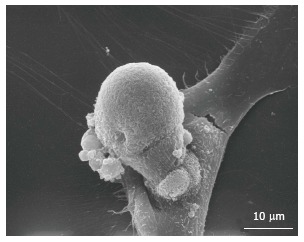
Scanning electronic microscopy. Budding process in vascular wall-resident multipotent stem cells in vitro.
Most of the definitions (and incongruity) of the stem cells derive from osteo-chondrogenic differentiation studies on cultured cells. In these in vitro models, cells are induced to differentiate by definite exogenous stimuli, which do not correspond to the vessel wall microenvironment during the in vivo calcification process. In our opinion, another way to overcome these incongruities in the future, apart from morphology, is the molecular approach, i.e., the identification of one or more markers to locate in situ the progenitor cells and the osteogenic precursors in the vessel wall, as well as the definition of the resident amitotic cells. A promising approach to definitely decipher all the markers characterizing the osteoprogenitor cells could be a combined mRNA profiling and gene set analysis, as already performed on the early and late EPC[117], in order to be able to apply more doable techniques such as immunohistochemistry, immunofluorescence or in situ hybridization.
Footnotes
P- Reviewer: Latif N, Paraskevas KI S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
References
- 1.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 3.Anderson HC, Sipe JB, Hessle L, Dhanyamraju R, Atti E, Camacho NP, Millán JL. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–1055. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanimura A, McGregor DH, Anderson HC. Matrix vesicles in atherosclerotic calcification. Proc Soc Exp Biol Med. 1983;172:173–177. doi: 10.3181/00379727-172-41542. [DOI] [PubMed] [Google Scholar]
- 6.Karwowski W, Naumnik B, Szczepański M, Myśliwiec M. The mechanism of vascular calcification - a systematic review. Med Sci Monit. 2012;18:RA1–R11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 8.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 9.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci USA. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–2815. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 11.Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 12.Tintut Y, Parhami F, Boström K, Jackson SM, Demer LL. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–7553. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- 13.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105:650–655. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 14.Proudfoot D, Davies JD, Skepper JN, Weissberg PL, Shanahan CM. Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation. 2002;106:3044–3050. doi: 10.1161/01.cir.0000041429.83465.41. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt HH, Hill S, Makariou EV, Feuerstein IM, Dugi KA, Hoeg JM. Relation of cholesterol-year score to severity of calcific atherosclerosis and tissue deposition in homozygous familial hypercholesterolemia. Am J Cardiol. 1996;77:575–580. doi: 10.1016/s0002-9149(97)89309-5. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 17.Doherty TM, Uzui H, Fitzpatrick LA, Tripathi PV, Dunstan CR, Asotra K, Rajavashisth TB. Rationale for the role of osteoclast-like cells in arterial calcification. FASEB J. 2002;16:577–582. doi: 10.1096/fj.01-0898hyp. [DOI] [PubMed] [Google Scholar]
- 18.Doherty TM, Shah PK, Rajavashisth TB. Cellular origins of atherosclerosis: towards ontogenetic endgame? FASEB J. 2003;17:592–597. doi: 10.1096/fj.02-0913hyp. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick LA, Severson A, Edwards WD, Ingram RT. Diffuse calcification in human coronary arteries. Association of osteopontin with atherosclerosis. J Clin Invest. 1994;94:1597–1604. doi: 10.1172/JCI117501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mönckeberg JG. Über die reine Mediaverkalkung der Extremitätenarterien und ihr Verhalten zur Arteriosklerose. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medicin. 1903;171:141–167. [Google Scholar]
- 21.Reaven PD, Sacks J. Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia. 2005;48:379–385. doi: 10.1007/s00125-004-1640-z. [DOI] [PubMed] [Google Scholar]
- 22.Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, Maekawa K, Yamakawa T, Imanishi Y, Inaba M, et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49:417–425. doi: 10.1053/j.ajkd.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Mori H, Yamaguchi K, Fukushima H, Oribe Y, Kato N, Wakamatsu T, Uzawa H. Extensive arterial calcification of unknown etiology in a 29-year-old male. Heart Vessels. 1992;7:211–214. doi: 10.1007/BF01744607. [DOI] [PubMed] [Google Scholar]
- 24.Top C, Cankir Z, Silit E, Yildirim S, Danaci M. Mönckeberg’s sclerosis: an unusual presentation--a case report. Angiology. 2002;53:483–486. doi: 10.1177/000331970205300418. [DOI] [PubMed] [Google Scholar]
- 25.Goebel FD, Füessl HS. Mönckeberg’s sclerosis after sympathetic denervation in diabetic and non-diabetic subjects. Diabetologia. 1983;24:347–350. doi: 10.1007/BF00251822. [DOI] [PubMed] [Google Scholar]
- 26.Proudfoot D, Shanahan CM. Biology of calcification in vascular cells: intima versus media. Herz. 2001;26:245–251. doi: 10.1007/pl00002027. [DOI] [PubMed] [Google Scholar]
- 27.Hunt JL, Fairman R, Mitchell ME, Carpenter JP, Golden M, Khalapyan T, Wolfe M, Neschis D, Milner R, Scoll B, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33:1214–1219. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 28.Jeziorska M, McCollum C, Wooley DE. Observations on bone formation and remodelling in advanced atherosclerotic lesions of human carotid arteries. Virchows Arch. 1998;433:559–565. doi: 10.1007/s004280050289. [DOI] [PubMed] [Google Scholar]
- 29.Verma V, Cronin DC, Dachman AH. Portal and mesenteric venous calcification in patients with advanced cirrhosis. AJR Am J Roentgenol. 2001;176:489–492. doi: 10.2214/ajr.176.2.1760489. [DOI] [PubMed] [Google Scholar]
- 30.Harvey WH. Experimental bone-formation in Arteries. J Med Res. 1907;17:25–34. [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 32.Leroux-Berger M, Queguiner I, Maciel TT, Ho A, Relaix F, Kempf H. Pathologic calcification of adult vascular smooth muscle cells differs on their crest or mesodermal embryonic origin. J Bone Miner Res. 2011;26:1543–1553. doi: 10.1002/jbmr.382. [DOI] [PubMed] [Google Scholar]
- 33.Doherty TM, Asotra K, Fitzpatrick LA, Qiao JH, Wilkin DJ, Detrano RC, Dunstan CR, Shah PK, Rajavashisth TB. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci USA. 2003;100:11201–11206. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal SN, Golledge J. Osteo-progenitors in vascular calcification: a circulating cell theory. J Atheroscler Thromb. 2011;18:551–559. doi: 10.5551/jat.8656. [DOI] [PubMed] [Google Scholar]
- 35.Campagnolo P, Wong MM, Xu Q. Progenitor cells in arteriosclerosis: good or bad guys? Antioxid Redox Signal. 2011;15:1013–1027. doi: 10.1089/ars.2010.3506. [DOI] [PubMed] [Google Scholar]
- 36.Kassem M, Mosekilde L, Rungby J, Mosekilde L, Melsen F, Eriksen EF. Formation of osteoclasts and osteoblast-like cells in long-term human bone marrow cultures. APMIS. 1991;99:262–268. doi: 10.1111/j.1699-0463.1991.tb05148.x. [DOI] [PubMed] [Google Scholar]
- 37.Sata M, Tanaka K, Nagai R. Circulating osteoblast-lineage cells. N Engl J Med. 2005;353:737–738; author reply 737-738. doi: 10.1056/NEJM200508183530719. [DOI] [PubMed] [Google Scholar]
- 38.Cottler-Fox MH, Lapidot T, Petit I, Kollet O, DiPersio JF, Link D, Devine S. Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 2003:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- 39.Pignolo RJ, Kassem M. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 2011;26:1685–1693. doi: 10.1002/jbmr.370. [DOI] [PubMed] [Google Scholar]
- 40.Pourrajab F, Forouzannia SK, Hekmatimoghadam SH, Kord MT. Molecular Strategies Contributing to Efficient Homing of Bone Marrow Stem Cells. Int J Cardiovasc Res. 2012;1:3. [Google Scholar]
- 41.Seta N, Kuwana M. Derivation of multipotent progenitors from human circulating CD14+ monocytes. Exp Hematol. 2010;38:557–563. doi: 10.1016/j.exphem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 43.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, et al. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–322. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 44.Yeager ME, Frid MG, Stenmark KR. Progenitor cells in pulmonary vascular remodeling. Pulm Circ. 2011;1:3–16. doi: 10.4103/2045-8932.78095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strieter RM, Keeley EC, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells, fibrocytes, in promoting pulmonary fibrosis. Trans Am Clin Climatol Assoc. 2009;120:49–59. [PMC free article] [PubMed] [Google Scholar]
- 46.Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–E696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- 47.Boström KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109:564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50:304–311. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Hirschi KK, Goodell MA. Hematopoietic, vascular and cardiac fates of bone marrow-derived stem cells. Gene Ther. 2002;9:648–652. doi: 10.1038/sj.gt.3301722. [DOI] [PubMed] [Google Scholar]
- 50.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 51.Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, Markham AF, Jack A, Emery P, McGonagle D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 52.Pasquinelli G, Tazzari PL, Vaselli C, Foroni L, Buzzi M, Storci G, Alviano F, Ricci F, Bonafè M, Orrico C, et al. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–1634. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- 53.Pasquinelli G, Pacilli A, Alviano F, Foroni L, Ricci F, Valente S, Orrico C, Lanzoni G, Buzzi M, Luigi Tazzari P, et al. Multidistrict human mesenchymal vascular cells: pluripotency and stemness characteristics. Cytotherapy. 2010;12:275–287. doi: 10.3109/14653241003596679. [DOI] [PubMed] [Google Scholar]
- 54.Klein D, Benchellal M, Kleff V, Jakob HG, Ergün S. Hox genes are involved in vascular wall-resident multipotent stem cell differentiation into smooth muscle cells. Sci Rep. 2013;3:2178. doi: 10.1038/srep02178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valente S, Alviano F, Ciavarella C, Buzzi M, Ricci F, Tazzari PL, Pagliaro P, Pasquinelli G. Human cadaver multipotent stromal/stem cells isolated from arteries stored in liquid nitrogen for 5 years. Stem Cell Res Ther. 2014;5:8. doi: 10.1186/scrt397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xin H, Xin F, Zhou S, Guan S. The Wnt5a/Ror2 pathway is associated with determination of the differentiation fate of bone marrow mesenchymal stem cells in vascular calcification. Int J Mol Med. 2013;31:583–588. doi: 10.3892/ijmm.2013.1242. [DOI] [PubMed] [Google Scholar]
- 57.Liao J, Chen X, Li Y, Ge Z, Duan H, Zou Y, Ge J. Transfer of bone-marrow-derived mesenchymal stem cells influences vascular remodeling and calcification after balloon injury in hyperlipidemic rats. J Biomed Biotechnol. 2012;2012:165296. doi: 10.1155/2012/165296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 59.Hruska KA, Mathew S, Lund RJ, Memon I, Saab G. The pathogenesis of vascular calcification in the chronic kidney disease mineral bone disorder: the links between bone and the vasculature. Semin Nephrol. 2009;29:156–165. doi: 10.1016/j.semnephrol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kramann R, Couson SK, Neuss S, Kunter U, Bovi M, Bornemann J, Knüchel R, Jahnen-Dechent W, Floege J, Schneider RK. Exposure to uremic serum induces a procalcific phenotype in human mesenchymal stem cells. Arterioscler Thromb Vasc Biol. 2011;31:e45–e54. doi: 10.1161/ATVBAHA.111.228601. [DOI] [PubMed] [Google Scholar]
- 61.Balica M, Boström K, Shin V, Tillisch K, Demer LL. Calcifying subpopulation of bovine aortic smooth muscle cells is responsive to 17 beta-estradiol. Circulation. 1997;95:1954–1960. doi: 10.1161/01.cir.95.7.1954. [DOI] [PubMed] [Google Scholar]
- 62.Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Boström K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 63.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 64.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 65.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 67.Vickers KC. Integrated Investigation of Low Density Lipoprotein Modifications, Lipoprotein-associated Phospholipase A2, and Vascular Smooth Muscle Cell Osteogenic Trans-differentiation in Human Atherosclerosis and Vascular Calcification. PhD Thesis. Houston, TX: Baylor College of Medicine, Department of Medicine; 2008. pp. 1–453. [Google Scholar]
- 68.Castro-Chavez F, Vickers KC, Lee JS, Tung CH, Morrisett JD. Effect of lyso-phosphatidylcholine and Schnurri-3 on osteogenic transdifferentiation of vascular smooth muscle cells to calcifying vascular cells in 3D culture. Biochim Biophys Acta. 2013;1830:3828–3834. doi: 10.1016/j.bbagen.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cola C, Almeida M, Li D, Romeo F, Mehta JL. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun. 2004;320:424–427. doi: 10.1016/j.bbrc.2004.05.181. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol. 1993;143:164–172. [PMC free article] [PubMed] [Google Scholar]
- 71.Guzman RJ. Clinical, cellular, and molecular aspects of arterial calcification. J Vasc Surg. 2007;45 Suppl A:A57–A63. doi: 10.1016/j.jvs.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yao Y, Jumabay M, Ly A, Radparvar M, Cubberly MR, Boström KI. A role for the endothelium in vascular calcification. Circ Res. 2013;113:495–504. doi: 10.1161/CIRCRESAHA.113.301792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang R, Gao M, Wu M, Liu H, Zhang X, Liu B. High glucose mediates endothelial-to-chondrocyte transition in human aortic endothelial cells. Cardiovasc Diabetol. 2012;11:113. doi: 10.1186/1475-2840-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vasuri F, Fittipaldi S, Buzzi M, Degiovanni A, Stella A, D’Errico-Grigioni A, Pasquinelli G. Nestin and WT1 expression in small-sized vasa vasorum from human normal arteries. Histol Histopathol. 2012;27:1195–1202. doi: 10.14670/HH-27.1195. [DOI] [PubMed] [Google Scholar]
- 75.Fittipaldi S, Vasuri F, Degiovanni A, Pini R, Mauro R, Faggioli G, D’Errico-Grigioni A, Stella A, Pasquinelli G. Nestin and WT1 expression in atheromathous plaque neovessels: Association with vulnerability. Histol Histopathol. 2014:May 26; Epub ahead of print. doi: 10.14670/HH-29.1565. [DOI] [PubMed] [Google Scholar]
- 76.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P, et al. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol. 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 77.Doherty MJ, Canfield AE. Gene expression during vascular pericyte differentiation. Crit Rev Eukaryot Gene Expr. 1999;9:1–17. [PubMed] [Google Scholar]
- 78.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 79.Sato K, Urist MR. Induced regeneration of calvaria by bone morphogenetic protein (BMP) in dogs. Clin Orthop Relat Res. 1985;(197):301–311. [PubMed] [Google Scholar]
- 80.Diaz-Flores L, Gutierrez R, Lopez-Alonso A, Gonzalez R, Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992;(275):280–286. [PubMed] [Google Scholar]
- 81.Schor AM, Allen TD, Canfield AE, Sloan P, Schor SL. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci. 1990;97(Pt 3):449–461. doi: 10.1242/jcs.97.3.449. [DOI] [PubMed] [Google Scholar]
- 82.Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR, Woodbury RA. The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992;275:287–299. [PubMed] [Google Scholar]
- 83.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Psaltis PJ, Puranik AS, Spoon DB, Chue CD, Hoffman SJ, Witt TA, Delacroix S, Kleppe LS, Mueske CS, Pan S, et al. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res. 2014;115:364–375. doi: 10.1161/CIRCRESAHA.115.303299. [DOI] [PubMed] [Google Scholar]
- 85.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–1425. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- 88.Kiechl S, Werner P, Knoflach M, Furtner M, Willeit J, Schett G. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Expert Rev Cardiovasc Ther. 2006;4:801–811. doi: 10.1586/14779072.4.6.801. [DOI] [PubMed] [Google Scholar]
- 89.Bini A, Mann KG, Kudryk BJ, Schoen FJ. Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1852–1861. doi: 10.1161/01.atv.19.8.1852. [DOI] [PubMed] [Google Scholar]
- 90.O’Brien ER, Garvin MR, Stewart DK, Hinohara T, Simpson JB, Schwartz SM, Giachelli CM. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb. 1994;14:1648–1656. doi: 10.1161/01.atv.14.10.1648. [DOI] [PubMed] [Google Scholar]
- 91.Lee AJ, Hodges S, Eastell R. Measurement of osteocalcin. Ann Clin Biochem. 2000;37(Pt 4):432–446. doi: 10.1177/000456320003700402. [DOI] [PubMed] [Google Scholar]
- 92.Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- 93.Pal SN, Rush C, Parr A, Van Campenhout A, Golledge J. Osteocalcin positive mononuclear cells are associated with the severity of aortic calcification. Atherosclerosis. 2010;210:88–93. doi: 10.1016/j.atherosclerosis.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang W, Li C, Pang L, Shi C, Guo F, Chen A, Cao X, Wan M. Mesenchymal stem cells recruited by active TGFβ contribute to osteogenic vascular calcification. Stem Cells Dev. 2014;23:1392–1404. doi: 10.1089/scd.2013.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flammer AJ, Gössl M, Widmer RJ, Reriani M, Lennon R, Loeffler D, Shonyo S, Simari RD, Lerman LO, Khosla S, et al. Osteocalcin positive CD133+/CD34-/KDR+ progenitor cells as an independent marker for unstable atherosclerosis. Eur Heart J. 2012;33:2963–2969. doi: 10.1093/eurheartj/ehs234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 97.Gössl M, Mödder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314–1325. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wickham CL, Sarsfield P, Joyner MV, Jones DB, Ellard S, Wilkins B. Formic acid decalcification of bone marrow trephines degrades DNA: alternative use of EDTA allows the amplification and sequencing of relatively long PCR products. Mol Pathol. 2000;53:336. doi: 10.1136/mp.53.6.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gostjeva EV, Thilly WG. Stem cell stages and the origins of colon cancer: a multidisciplinary perspective. Stem Cell Rev. 2005;1:243–251. doi: 10.1385/SCR:1:3:243. [DOI] [PubMed] [Google Scholar]
- 100.Gostjeva EV, Zukerberg L, Chung D, Thilly WG. Bell-shaped nuclei dividing by symmetrical and asymmetrical nuclear fission have qualities of stem cells in human colonic embryogenesis and carcinogenesis. Cancer Genet Cytogenet. 2006;164:16–24. doi: 10.1016/j.cancergencyto.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 101.Child CM. Studies on the Relation between Amitosis and Mitosis. IV. Nuclear Division in the Somatic Structures of the Proglottids of Moniezia. V. General Discussion and Conclusions concerning Amitosis and Mitosis in Moniezia. Biological Bulletin. 1907;13:165–184. [Google Scholar]
- 102.Gostjeva EV, Koledova V, Tomita-Mitchell A, Mitchell M, Goetsch MA, Varmuza S, Fomina JN, Darroudi F, Thilly WG. Metakaryotic stem cell lineages in organogenesis of humans and other metazoans. Organogenesis. 2009;5:191–200. doi: 10.4161/org.5.4.9632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 104.Qian H, Yang Y, Li J, Huang J, Dou K, Yang G. The role of vascular stem cells in atherogenesis and post-angioplasty restenosis. Ageing Res Rev. 2007;6:109–127. doi: 10.1016/j.arr.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 105.Baker JF, Zhang L, Imadojemu S, Sharpe A, Patil S, Moore JS, Mohler ER, Von Feldt J. Circulating endothelial progenitor cells are reduced in SLE in the absence of coronary artery calcification. Rheumatol Int. 2012;32:997–1002. doi: 10.1007/s00296-010-1730-9. [DOI] [PubMed] [Google Scholar]
- 106.Cianciolo G, La Manna G, Della Bella E, Cappuccilli ML, Angelini ML, Dormi A, Capelli I, Laterza C, Costa R, Alviano F, et al. Effect of vitamin D receptor activator therapy on vitamin D receptor and osteocalcin expression in circulating endothelial progenitor cells of hemodialysis patients. Blood Purif. 2013;35:187–195. doi: 10.1159/000347102. [DOI] [PubMed] [Google Scholar]
- 107.Yiu KH, Mok MY, Wang S, Ooi GC, Khong PL, Lau CS, Tse HF. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345–350. [PubMed] [Google Scholar]
- 108.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- 109.Boström K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fadini GP, Albiero M, Menegazzo L, Boscaro E, Agostini C, de Kreutzenberg SV, Rattazzi M, Avogaro A. Procalcific phenotypic drift of circulating progenitor cells in type 2 diabetes with coronary artery disease. Exp Diabetes Res. 2012;2012:921685. doi: 10.1155/2012/921685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Winchester R, Wiesendanger M, O’Brien W, Zhang HZ, Maurer MS, Gillam LD, Schwartz A, Marboe C, Stewart AS. Circulating activated and effector memory T cells are associated with calcification and clonal expansions in bicuspid and tricuspid valves of calcific aortic stenosis. J Immunol. 2011;187:1006–1014. doi: 10.4049/jimmunol.1003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cho HJ, Cho HJ, Lee HJ, Song MK, Seo JY, Bae YH, Kim JY, Lee HY, Lee W, Koo BK, et al. Vascular calcifying progenitor cells possess bidirectional differentiation potentials. PLoS Biol. 2013;11:e1001534. doi: 10.1371/journal.pbio.1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun. 2007;354:453–458. doi: 10.1016/j.bbrc.2006.12.226. [DOI] [PubMed] [Google Scholar]
- 115.Solari F, Domenget C, Gire V, Woods C, Lazarides E, Rousset B, Jurdic P. Multinucleated cells can continuously generate mononucleated cells in the absence of mitosis: a study of cells of the avian osteoclast lineage. J Cell Sci. 1995;108(Pt 10):3233–3241. doi: 10.1242/jcs.108.10.3233. [DOI] [PubMed] [Google Scholar]
- 116.Thilly WG, Gostjeva EV, Koledova VV, Zukerberg LR, Chung D, Fomina JN, Darroudi F, Stollar BD. Metakaryotic stem cell nuclei use pangenomic dsRNA/DNA intermediates in genome replication and segregation. Organogenesis. 2014;10:44–52. doi: 10.4161/org.27684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng CC, Chang SJ, Chueh YN, Huang TS, Huang PH, Cheng SM, Tsai TN, Chen JW, Wang HW. Distinct angiogenesis roles and surface markers of early and late endothelial progenitor cells revealed by functional group analyses. BMC Genomics. 2013;14:182. doi: 10.1186/1471-2164-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]


