Abstract
According to the minimal criteria of the International Society of Cellular Therapy, mesenchymal stem cells (MSCs) are a population of undifferentiated cells defined by their ability to adhere to plastic surfaces when cultured under standard conditions, express a certain panel of phenotypic markers and can differentiate into osteogenic, chondrogenic and adipogenic lineages when cultured in specific inducing media. In parallel with their major role as undifferentiated cell reserves, MSCs have immunomodulatory functions which are exerted by direct cell-to-cell contacts, secretion of cytokines and/or by a combination of both mechanisms. There are no convincing data about a principal difference in the profile of cytokines secreted by MSCs isolated from different tissue sources, although some papers report some quantitative but not qualitative differences in cytokine secretion. The present review focuses on the basic cytokines secreted by MSCs as described in the literature by which the MSCs exert immunodulatory effects. It should be pointed out that MSCs themselves are objects of cytokine regulation. Hypothetical mechanisms by which the MSCs exert their immunoregulatory effects are also discussed in this review. These mechanisms may either influence the target immune cells directly or indirectly by affecting the activities of predominantly dendritic cells. Chemokines are also discussed as participants in this process by recruiting cells of the immune systems and thus making them targets of immunosuppression. This review aims to present and discuss the published data and the personal experience of the authors regarding cytokines secreted by MSCs and their effects on the cells of the immune system.
Keywords: Mesenchymal stem cells, Immunomodulation, Cytokines, Chemokines, Dendritic cells
Core tip: Autoimmune diseases affect approximately 5% of the human population, leading to serious disability and effective methods to treat these diseases are still not perfect. Mesenchymal stem cells (MSCs) are assumed to be promising agents, both for regenerative medicine and cell therapy for autoimmune disorders. Under the influence of some factors, mesenchymal stem cells secrete cytokines which induce suppression of the immune response. Studies on the secreted cytokines and the precise mechanisms involved in these suppressive mechanisms would create possibilities for efficient application of MSCs as a therapeutic means for treatment of autoimmune diseases.
INTRODUCTION
Maintenance of immunological self-tolerance and immune homeostasis in the organism is under the control of a complex and sophisticated process of immunoregulation and its dysfunction could be a critical factor in the development of autoreactive and potentially life-threatening conditions. Profound understanding of the precise mechanisms underlying this immunoregulatory process could lay the ground to develop a more suitable and efficient therapy for autoimmune diseases. Regulation of the immune response by mesenchymal stem cells (MSCs) is mediated by a number of cell subtypes and secreted factors and recently new cell-based therapeutic approaches have emerged as successful strategies for treatment of various inflammatory and autoimmune conditions. In the last decades, mesenchymal stem cells, one type of adult stem cells, have gained considerable interest as extremely promising cell therapeutic agents[1,2] due to their unique combination of immunomodulatory properties and self-renewal and multilineage differentiation capacity[3,4]. MSCs have been shown to exert profound anti-inflammatory and immunomodulatory effects on almost all the cells of the innate and adaptive immune systems via a variety of mechanisms, notably cytokine and chemokine secretion[5].
Mesenchymal stem cells are a population of undifferentiated multipotent adult stem cells that naturally reside within the human body and are generally defined as plastic-adherent, fibroblast-like cells possessing extensive self-renewal properties and potential to differentiate in vitro and in vivo into a variety of mesenchymal lineage cells[4,6]. MSCs were initially described in the bone marrow by Friedenstein et al[7,8] as a small subpopulation of colony-forming unit fibroblasts which could be distinguished from the rest of the bone marrow cells on the basis of their plastic adherence, spindle-shaped appearance and rapid expansion[7].
After their initial discovery in bone marrow (BM-MSCs), MSCs were isolated and characterized from a wide variety of other adult and fetal tissues, including adipose tissue (AT-MSCs), umbilical cord, dental pulp, skin, tendon, skeleton, muscle, spleen, brain, liver, periosteum, placenta, synovial and amniotic fluids[9,10]. MSCs from different sources may display some differences in the expression of surface markers. However, in general, the phenotypes of these cells are very similar and in the absence of an individual specific marker, MSCs are commonly defined by a panel of cell surface markers that include CD73, CD90 (Thy-1), CD105 (endoglin) and MHC class I, as well as the adhesion molecules CD44, CD29, CD54 (ICAM-1; intercellular adhesion molecule 1), CD106 (VCAM-1; vascular cell adhesion molecule) and CD166[11]. MSCs do not express hematopoietic markers such as CD34, CD45, CD14 and CD11 or co-stimulatory molecules like CD80, CD86 and CD40[11].
According to the minimal criteria of the International Society of Cellular Therapy (ISCT, 2006), the required functional and phenotypic features for defining MSCs include: (1) plastic adherence of the isolated cells under standard culture conditions; (2) positive expression of CD105, CD90 and CD73 markers in at least 95% of a cell population and lack of expression of CD34, CD45, CD11b, CD14, CD19 or CD79a and HLA-DR markers in greater than 95% of the culture, as measured by flow cytometry; and (3) trilineage differentiation potential into osteoblasts, adipocytes and chondroblasts in in vitro culture with specific stimuli[12].
Besides this, trilineage multipotency experimental data have demonstrated that MSCs can also differentiate into other mesodermal lineages, such as skeletal myocytes[13,14], cardiomyocytes[15], tenocytes[16,17] and endothelial cells[18,19]. Moreover, it has been reported that under appropriate conditions, MSCs have the capacity to differentiate into types of cells of endodermal and ectodermal lineages, including hepatocytes[20,21], neuronal cells with neuron-like functions[22-24], insulin-producing cells[25,26], photoreceptor cells[27], renal tubular epithelial cells[28], epidermal and sebaceous duct cells[29]. In addition to their comprehensive differentiation potential, MSCs have the ability to migrate and engraft at sites of inflammation and injury in response to cytokines, chemokines and growth factors[30,31]. At a wound site, they can exert local reparative effects through transdifferentiation into tissue-specific cell types or via the paracrine secretion of soluble factors with anti-inflammatory and wound healing activities[32-34].
Another aspect that makes MSCs of particular clinical interest is the finding that they exert a wide range of immunomodulatory activities affecting both cell-mediated and humoral immune response. A search in the PubMed data base reveals 149 papers, while the ScienceDirect data base contains 495 papers in peer-reviewed journals describing animal models developed to study various aspects of the immunomodulatory effects of MSCs in the period of 2001-2014. The promising results obtained prompt clinical trials in humans using MSCs as a biological agent for immunomodulation. According to the web site of Clinical Trials.gov (a service of the United States National Institutes of Health), more than 418 clinical trials are currently under way to assess the clinical effects of mesenchymal stem cells isolated from various sources, with the greater part of the trials studying the immunomodulatory effect of autologous or allogeneic MSCs in autoimmune diseases such as ulcerative colitis, multiple sclerosis, primary Sjogren’s syndrom, systemic scleroderma, Crohn’s disease etc. Similarly, numerous trials are devoted to the effect of MSCs on modulating the reactions after allogeneic transplantation, such as chronic graft-versus-host disease (GVHD), poor graft function, etc.
In general, the data from these studies have shown that MSCs exert immunomodulatory effects by both cell-to-cell contacts and by secreting biologically active substances, growth factors, cytokines and chemokines.
MSCs have been shown to inhibit T-cell activation and proliferation triggered by mitogenic or antigenic stimulation with allogeneic cells (mixed lymphocyte cultures) or nominal antigens[35,36]. MSCs can also influence T-cell responses indirectly through suppression of CD34+ progenitor cell and monocyte-derived dendritic cell differentiation, as well as through inhibition of their antigen-presenting functions[37-40]. A number of studies have demonstrated that MSCs have the capacity to inhibit B-cell proliferation, differentiation and immunoglobulin production in vitro[41,42] as well as to down-regulate the proliferation, cytokine production and cytotoxicity of NK cells[43,44]. Their ability to promote the generation and to maintain the activity of different subtypes of regulatory T cells (Тr1, CD4+FoxP3+, CD8+FoxP3+) is well documented, especially CD4+FoxP3+, also known as Tregs[45-48]. In addition, MSCs are considered as not being inherently immunogenic as they express low-intermediate levels of HLA class I antigens and either do not express or express negligibly low levels of HLA class II antigens and co-stimulatory molecules, such as CD80, CD86 and CD40[49,50]. Therefore, they should be able to escape not only from the recognition by alloreactive T cells[49,51], but also the cell-specific lysis by cytotoxic T lymphocytes (CTLs)[52] and freshly isolated alloreactive NK cells[53]. Some of these in vitro properties have already been successfully clinically exploited for the treatment of disorders such as acute graft-versus-host disease[54,55], multiple sclerosis[56] and systemic lupus erythematosus[57].
Although the precise mechanisms underlying MSCs immunomodulation are still not completely understood, a number of soluble factors involved in the process have already been identified.
The present review discusses some MSC secreted cytokines which are involved in regulation of the immune response. For the purposes of this review, the term “immunoregulation” is used in a very strict sense as an influence on immunocompetent cells. It should be pointed out that the immunomodulatory effects of MSCs are jointly executed by both secretory factors and direct cell-to-cell contacts. In that case, cytokines most commonly do not directly affect the target cells but interact with other biologically active factors to achieve the effect of immunosuppression. There are some papers describing fine differences in MSC secreted cytokine profiles with immunoregulatory effects but in this review the generally accepted cytokines most often cited in the literature are discussed. The mechanisms of immunomodulation by direct cellular contacts will not be discussed in this review.
MSCs isolated from different tissues are different in some fine specifics as mentioned above. However, no data have been published describing significant differences in the profiles of secreted cytokines by different types of MSCs. Most authors report either a lack of differences or find some quantitative differences in the levels of cytokines secreted by AT-MSCs or BM-MSCs[58-60]. Our experimental data also show some quantitative differences in the cytokine secretion[37]. Similar findings are reported when embryonic, fetal and adult MSCs have been compared[61].
MSCs secrete cytokines either “spontaneously” or after induction by other cytokines, the most important being IFNγ, TNFα and IL-1β[62-64], and it should be underlined that MSCs are not always immunosuppressive. It is assumed that their effects are determined by the local conditions of the microenvironment and sometimes the pro-inflammatory IFNγ, TNFα and IL-1β cytokines may induce secretion of anti-inflammatory immunosuppressive factors. Engagement of certain Toll-like receptors (TLR) expressed by MSCs can determine their pro or anti-inflammatory effects[65-67]. The definition of cytokines as pro or anti-inflammatory is quite far from their real effects because it seems that there is not a single cytokine which is not engaged in both types of reactions. Nevertheless, that definition is quite convenient and will be used further in the present review. The most important immunoregulatory cytokines described in the literature are presented in Table 1.
Table 1.
Cytokines secreted by mesenchymal stem cells and the corresponding target cells
| Cytokines secreted by MSCs | Target cells |
| IL-10 | Mph, Neu, DCs, Th1, Tregs, Tr1, tumor cells |
| IL-6 | Neu, Mo, DCs, B, Th2, Tregs, Th17, CD8+FoxP3+ |
| TGFβ | Mph, NK, DCs, B, T, Tregs |
| Chemokines | Neu, Mo, NK, Eo, Baso, DCs, Ly |
| CCL-2/MCP-1 | Mph, EC, PL, Th2, Th17 |
| CCL-5/RANTES | Neu, Mo, DCs, Th1, Tregs, CD8+FoxP3+ |
| IDO | Mo, DCs, B, T, Tregs |
| VEGF | DCs, EC, Th1, Th17, Tregs |
| ICAM | T, MSCs |
| PGE2 | Mph, Mo, NK, DCs, T, Tr1 |
MSCs: Mesenchymal stem cells; TGFβ: Transforming growth factor β; CCL: CC chemokine ligand; MCP-1: Monocyte chemotactic protein 1; RANTES: Regulated on activation, normal T cell expressed and secreted; IDO: Indoleamine-2,3-dioxygenase; VEGF: Vascular endothelial growth factor; ICAM: Intercellular adhesion molecule; PGE2: Prostaglandin E2; Mph: Macrophages; Neu: Neutrophils; DCs: Dendritic cells; Th: T helpers; Tregs: T regulatory cells; Tr1: T regulatory 1; Mo: Monocytes; B: B cells; NK: Natural killers; T: T cells; Eo: Eosinophils; Baso: Basophils; Ly: Lymphocytes; EC: Endothelial cells; PL: Plasma cells.
INTERLEUKIN 10
Interleukin 10 (IL-10) is pleiotropic cytokine identified in the 1980s and characterized by its anti-inflammatory effect related to the induction of immune tolerance[68-71]. It has been established that IL-10 suppresses the functions of macrophages and neutrophils[70,72], inhibits the Th1 immune response[70,73-76], influences NF-kB synthesis[77] and causes expression of anti-inflammatory molecules, such as protease inhibitors[78] and IL-1 and TNFα antagonists[79].
The major function of IL-10 in induction of immune tolerance is its effect on the antigen presenting cells and particularly on the dendritic cells (DCs). IL-10 suppresses the secretion of pro-inflammatory cytokines (TNFα, IL-1, IL-6, IL-8, IL-12) by DCs and the expression of MHC II molecules, as well as co-stimulatory complex B7 on their surface[75-77]. In parallel to that, IL-10 is capable of inducing anergy of T lymphocytes by directly inhibiting the phosphorylation of CD28. In that way, one of the basic immunosuppressive mechanisms is executed by IL-10 by inducing a tolerogenic type of dendritic cells with reduced HLA-II and B7 expression and by suppression of CD28 (the partner of B7) expression on the surface of the T lymphocytes. This “two sided” suppression of the second signal which is unconditionally needed for activation of the T lymphocytes induces a deep anergy in this cell population[37,69-71,76].
Further on, IL-10 is directly engaged in the induction of immune tolerance by two types of T regulatory lymphocytes: Tregs and Tr1[76]. IL-10 is one of the cytokines related to the generation of Tregs[73] which secretes IL-10 by itself and this process has been described both for “natural” FoxP3+ Tregs and for FoxP3+ Tregs generated after response to a specific antigen[70,73,80].
A specific feature of IL-10 and some other cytokines is that the producing cells are both the source and target of the cytokine effect and this predominantly affects the dendritic and T regulatory cells. A good example is that tolerogenic DCs secrete IL-10 and thus induce the generation of regulatory T helpers (FoxP3 and Tr1) which secrete IL-10 inducing tolerogenic phenotype of DCs[70,71,76]. Likewise, many other cytokines IL-10 can also act in an autocrine loop.
The effect of IL-10 is mediated via its binding to its specific receptor (IL-10R) and subsequent interaction between JAK1 and STAT-3[73,77], a mechanism which is common for many other cytokines. Besides the antigen-presenting cells and particularly tolerogenic DCs, Tregs and Tr1, other immune cells secrete IL-10 and these include T and B lymphocytes, NK cells, neutrophils and macrophages[76,80]. The role of IL-10 secreted by Th2 helpers is well known[76,80] but some recently published data show that this cytokine in a somewhat paradoxical manner is secreted by both Th1 and Th17 cells. Quite often these “double secreting” cells (IL-10 simultaneously with IFNγ or IL-17) use IL-10 to suppress their own pro-inflammatory effect, both directly and/or with the help of tolerogenic antigen-presenting cells[71,74].
IL-10 is considered to be a classical cytokine inducing immune tolerance but there are data which show that, similarly to most cytokines, IL-10 acts in more than one way. Its pro-immune effect has been described in tumorigenesis[70,72] and IL-10 is detected in a tumor environment and shown to have an anti-tumor effect. It is assumed that this effect is due to inhibition of the tumor angiogenesis and enhancement of the nitric oxide secretion. IL-10 is also connected to the inhibition of the expression of MHC by the tumor cells which makes them an easier target for the NK cells. Some pro-inflammatory effects of IL-10 have been demonstrated which seem to lead to enhanced apoptosis of Tregs and stimulation of the antigen up-take by the antigen-presenting cells[70].
IL-10 is the cytokine most commonly discussed in relation to the immunoregulatory effects of MSCs. Nevertheless, the published data demonstrating the secretion of IL-10 by MSCs are quite contradictory. Almost half of the papers discussed in the present review report positive secretion of IL-10 by MSCs[62,66,81-84], while the other half and our own experimental results reject such a possibility[60,64,69,78,85-88]. It is quite logical to support the concept proposed by some authors claiming that MSCs secrete IL-10 under specific conditions with the inflammatory environment and presence of cytokines (IFNγ, IL-1b and TNFα) which activate certain Toll-like receptors on MSCs[63,65,67]. Although there is no definite opinion about the conditions under which MSCs secrete IL-10, their role is indisputable as a factor which causes indirect stimulation of IL-10 secretion by other cells. It has been shown that MSCs secrete factors which up-regulate the secretion of IL-10 by peripheral blood mononuclear cells (PBMCs)[59], as well as by tolerogenic macrophages[89] and tolerogenic DCs[37,69,90]. It is also assumed although not undoubtedly proven that MSCs induce generation of Tregs[59,62,90] and our results show that when cultured in MSC conditioned medium, the fraction of CD4+FoxP3+ lymphocytes is increased and this effect is directly induced by MSCs without any involvement of DCs[69].
IL-6
IL-6 was identified in 1986 as a factor stimulating B lymphocytes[91]. It is now known that it is a pleiotropic cytokine with a key role in a multitude of processes such as regulation of the immune response, hematopoiesis, inflammation, cell survival, apoptosis, cell proliferation and oncogenesis[91,92]. The action of IL-6 is mediated by its binding with a membrane IL-6 receptor (mIL-6R) and gp130 as gp130 interacts with the JAK-STAT system[91]. A small fraction of cells show expression of mIL-6R but almost all cell types express gp130. Cells expressing only gp130 can bind the complex IL-6/soluble IL-6R (sIL-6R), a process known as trans-signaling, which makes a lot of cell populations susceptible to the effects of IL-6[93,94]. Some authors believe that the effect of IL-6 mediated by trans-signaling (IL-6/sIL-6R) is related to a pro-inflammatory effect, while the “classical” pathway (IL-6/mIL-6R) of activation is connected to the anti-inflammatory action of the cytokine[94]. Such an assumption sounds quite logical, keeping in mind the dual nature of IL-6 because of its pro-inflammatory and/or anti-inflammatory effects[75,82]. IL-6 is routinely described as a classical pro-inflammatory cytokine based on well proven effects of this cytokine. In concert with IL-1 and TNFα, this cytokine induces secretion of acute phase proteins, causes neutrophil recruitment, expression of cell adhesive molecules and a switch from neutrophil to macrophage induced inflammation[72,75,94]. IL-6 stimulates T cell proliferation[64,72] and together with IL-4 participates in the generation of the Th2 immune response[94]. IL-6 has a significant role in the triggering of humoral immune response by stimulating the B cell differentiation and secretion of antibodies[95]. Some recent data demonstrate that IL-6 together with TGFβ is engaged in regulation of the balance between the pro-inflammatory Th17 and immunosuppressive response mediated by Tregs as both cytokines acting together induce expression of RORγt which is the major transcription factor defining the Th17 cells[94,95].
Recently, simultaneously to the proven pro-inflammatory function of IL-6, quite convincing data have piled up about its function as an anti-inflammatory cytokine. It has been established that IL-6 suppresses the secretion of many pro-inflammatory cytokines, such as IL-1, TNFα, GM-CSF, IFNγ, but on the other hand, it induces the synthesis of glucocorticoids, IL-10, IL-1 receptor antagonist and soluble receptor for ТNFα[72,75,79,96]. It has been demonstrated that IL-6 exerts its anti-inflammatory effect both locally and systemically because mice deficient to IL-6 gene have increased production of pro-inflammatory TNFα, GM-CSF and MIP-2[72]. Moreover, some new results show that IL-6 is a key factor in the formation and functions of the CD8+FoxP3+ cell population, which is related to suppression of the Th17 immune response[97]. Altogether, these data show that IL-6 has a “two sided” engagement in the modulation of the immune response by regulatory and Th17 cells. On one hand, IL-6 together with TGFβ induces formation of Th17 cells and on the other hand, IL-6 directly inhibits this response by its effect on CD8+FoxP3+ (Figure 1).
Figure 1.
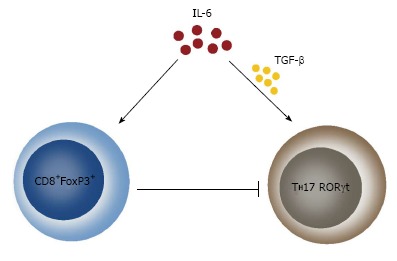
Effect of Interleukin 6 on Th17 formation. IL-6 exerts a dual effect on the generation of Th17 cells. On one side, IL-6 in concert with TGFβ facilitates the generation of this cell population and on the other side, IL-6 inhibits the Th17 cells by inducing the generation of CD8+FoxP3+ T lymphocytes. TGFβ: Transforming growth factor β.
Contrary to IL-10, there is no doubt that IL-6 is secreted by MSCs and almost all authors agree with this statement[69,78,82,84,86,87]. Its secretion by MSCs has been demonstrated both in mice and in humans[62,66] and is detected either after induction with TNFα, IL-1b and IFNγ or spontaneously[37,61-63,72,95]. When MSCs were tested for 120 cytokines at mRNA and protein levels, it was established that IL-6 has the highest expression and the conclusion was made that IL-6 was the basic cytokine responsible for the immunoregulatory effects of MSCs[60]. After the secretion of IL-6 by MSCs, a suppression of the apoptosis of neutrophils is observed[64,68,81] and this effect could be very important for the connection between defects of apoptosis and triggering of autoimmune reactions. However, it is still not truly clarified whether IL-6 causes generation of classical Treg cells (CD4+FoxP3+), although there is no doubt about its direct effect on the generation of CD8+FoxP3+ cells. The fact that MSCs secrete active factors, increasing the numbers of Tregs, has been proven in a number of experiments[59,62,69] but there are no sure data that this effect is mediated via IL-6. It should be stated that such a mechanism is quite probable, keeping in mind the effect of IL-6 on the generation of CD8+FoxP3+.
MSCs can be both a source and a target of the effects of IL-6. It has been established that under the influence of IL-6, MSCs can transform malignant cells and have tumorogenic properties and this effect is mediated through the mechanism of trans-signaling[98]. These facts raise questions about the interactions between MSCs and the tumor microenvironment which is most commonly very rich in IL-6.
INTERACTIONS BETWEEN IL-6 AND IL-10
IL-6 stimulates the secretion of IL-10 by different types of cells and this effect has been proved without any doubt but the reverse interaction has not been demonstrated so far[96]. The effect of IL-6 on monocytes and dendritic cells is of particular importance for the complex process of immunoregulation. Some publications describe a pathway in which MSCs secrete IL-6 which directly or via induction of autocrine secretion of IL-10 influences the monocyte activity inhibiting their differentiation as dendritic cells[37,81]. Both IL-6 and the autocrine reacting IL-10 also suppress the capacity of DCs to present antigens and thus a population of immature tolerogenic dendritic cells is formed which secrete IL-10[37,38,59,64,69,99,100]. Its effect stimulates the generation of T regulatory cells secreting IL-10 by themselves and potentiating further formation of tolerogenic DCs[62,85]. However, it should be noted that IL-6 and IL-10 are not the only cytokines involved in these complex interactions, for example, prostaglandin E2 (PGE2) which is another immunosuppressive factor secreted by MSCs interacting with IL-6 in suppression of the DCs differentiation[68].
TRANSFORMING GROWTH FACTOR BETA
One of the most prominent immunomodulatory cytokines produced and constitutively secreted by MSCs is transforming growth factor beta (TGFβ). As a pleiotropic cytokine, TGFβ regulates multiple fundamental cellular functions, including proliferation, differentiation, migration, adhesion and apoptosis, that affect numerous biological processes such as development, wound healing, carcinogenesis, angiogenesis and immune responses[101]. TGFβ is a member of a superfamily of dimeric polypeptide growth factors that consists of about 40 members in vertebrates, also including bone morphogenetic proteins (BMPs), activins, inhibins, growth differentiation factors (GDFs) and glial cell line-derived neurotrophic factor (GDNF)[102]. In mammals, three homologous TGFβ isoforms have been identified (TGFβ1, TGFβ2 and TGFβ3) that are controlled by specific genes[103]. Each isoform may exert a distinct role which depends on the target cell type, its state of differentiation and growth conditions[103].
TGFβ is now established as a principal mediator of immune regulation which plays an essential role in orchestrating the initiation and resolution of inflammatory responses, as well as in induction and maintenance of immune tolerance by influencing leukocyte proliferation, differentiation, activation and survival[104,105]. The diversity of modulatory activities that TGFβ exerts on the immune cell functions is quite extensive and includes effects such as inhibition of effector T-cell proliferation and function, generation of regulatory T cells from naïve T lymphocytes, attenuation of cytokine production and cytolytic activity of NK cells, suppression of B cells, dendritic cells and macrophages[105].
As TGFβ is constitutively produced by MSCs and most of its effects on immune cells mentioned above have also been demonstrated to be intrinsic features to MSCs, it is reasonable to assume the putative involvement of TGFβ as a mediator of their broad immunoregulatory properties.
It has been reported that MSCs isolated from human bone marrow were able to suppress CD4+ and CD8+ T-cell proliferation induced by cellular or nonspecific mitogenic stimuli and that this effect could be reversed by the addition of monoclonal anti-TGFβ1 neutralizing antibodies[35]. Later, it was shown that human bone marrow-derived MSCs, activated by blood CD14+ monocytes, secreted TGFβ1 which is responsible for inhibition of T-lymphocyte responses[106]. It has also been observed that TGFβ1 was involved in a cell contact-dependent inhibition of T-cell proliferation by MSCs[107]. Furthermore, MSCs obtained from dental pulp were found to produce TGFβ and to suppress the proliferation of PBMCs, which could be neutralized with anti-TGFβ antibodies[108]. In contrast, the addition of TLR-3 agonist augmented the suppressive potential of dental pulp-derived MSCs and potentiated TGFβ secretions by these cells[108].
Numerous mechanisms have been suggested to be involved in TGFβ-mediated inhibition of T-cell proliferation, differentiation and effector functions. One pathway by which TGFβ exerts its anti-proliferative effect on T lymphocytes is through blockade of the production of the T-cell mitogenic cytokine IL-2[109]. Functional analysis revealed that this is most likely due to impaired IL-2 gene transcription as a result of inhibition of IL-2 promoter/enhancer activity[109]. In another study[110], the transcription factor Smad3 was also shown to be critical for TGFβ1-mediated inhibition of IL-2 expression. Moreover, it has been demonstrated that the addition of exogenous IL-2 partially but not completely reversed the antiproliferative effects of TGFβ, indicating the suppressive activity of TGFβ on both production and intracellular signaling of IL-2[111].
TGFβ also inhibits cell proliferation through controlling the expression of cell cycle regulators, including up-regulation of cyclin-dependent kinase inhibitors (CKIs) p15, p21 and p27 and down-regulation of cell cycle-promoting factors, such as c-myc, cyclin D2 and cyclin E[112-115]. However, it has been reported that TGFβ is able to suppress the proliferation of T cells from mice deficient for all three CKIs mentioned above, demonstrating their dispensable role in this process[116]. In addition, a Smad3-dependent down-regulation of CDK4 has been described, suggesting a potential mechanism underlying resistance of Smad3-/- T cells to the induction of growth arrest by TGFβ[116].
TGFβ is a strong suppressor of T-cell differentiation and effector functions. In the presence of TGFβ, CD8+ T cells fail to acquire CTL function and CD4+ T lymphocytes do not become Th1 or Th2 cells[117]. The inhibition of T-cell differentiation occurs even in the presence of added IL-2, while at the same time T-cell proliferation remains unaffected[118].
One of the possible mechanisms of inhibition of T-cell differentiation by TGFβ is associated with decreased expression of IL-12 receptor β2-chain (IL-12Rβ2) and therefore with possible blockade of IL-12 signaling, which is required for Th1-cell development[119]. However, a more recent study has demonstrated that inhibition of T-bet (T-box expressed in T cells), a transcriptional activator of Th1 development, was critical for TGFβ-induced suppression of Th1-cell differentiation and that down-regulation of IL-12Rβ2 expression appeared not to be important for the TGFβ-mediated effect but rather was an event secondary to T-bet inhibition[120]. It has also been shown that restoration of T-bet expression through retroviral transduction of T-bet into developing Th1 cells abrogated the inhibitory effect of TGFβ[120] which indicated that T-bet was the most critical and primary target for the inhibition of Th1 differentiation by TGFβ. In addition, TGFβ can also function indirectly to suppress Th1-cell differentiation by inhibiting IFNγ production by NK cells[121]. In this regard, it has been found that bone marrow-derived MSCs were able to suppress NK cell proliferation and IFNγ production through the secretion of TGFβ1 and prostaglandin E2[43].
TGFβ has also been found to potently down-regulate Th2-cell differentiation. A few studies[122,123] have shown that TGFβ-mediated prevention of Th2-cell development is due to suppressed expression of the transcription factor GATA-3, a key transcriptional activator of Th2-cell differentiation[124]. Moreover, TGFβ is able to induce the transcription factor Sox-4 and therefore negatively regulate GATA-3 function indirectly by two distinct mechanisms[125]. First, Sox-4 binds directly to GATA-3, preventing its transcriptional activity, and second, Sox-4 binds to the promoter of IL-5, a Th2 cytokine, and prevents GATA-3-mediated induction of gene expression[125].
In addition to suppressing proliferation, TGFβ has also been demonstrated to inhibit CD8+ T-cell effector functions through down-regulation of the expression of several essential CTL effector molecules such as perforin[126], Fas ligand (FasL)[127] and IFNγ[128,129]. Furthermore, the release of cytolytic granules by CTLs can be selectively suppressed by Tregs in a TGFβ-dependent manner[130].
Another important immunosuppressive activity of TGFβ could be its implication in the development of regulatory T cells. TGFβ promotes the conversion of naive CD4+T cells to Treg cells by induction of transcription factor FoxP3[131-133]. Several reports have indicated an essential role for both Smad2 and Smad3 transcription factors in TGFβ-mediated induction and maintenance of Foxp3 expression[134-137]. For instance, it was demonstrated that Smad2 and Smad3 double deficiency lead to complete ablation of FoxP3 upregulation by TGFβ, suggesting a functional redundancy between these two transcription factors in the induction of Tregs[137].
A recent paper has shown that both TGFβ1 and prostaglandin E2 derived from MSCs contributed to allogeneic MSCs induction of CD4+CD25+ FoxP3+ regulatory T cells that possess the ability to suppress alloantigen-driven proliferative responses in a mixed lymphocyte reaction[46]. Later, MSC-derived TGFβ1 was reported to be largely responsible for the increase in Treg frequency based on knockdown studies, thereby protecting breast cancer cells from immune clearance[138].
Recently, a mouse model of ragweed-induced asthma was described in which iv injected MSCs were capable of suppressing Th2-driven allergic responses via secretion of TGFβ[139]. The results suggested that IL-4 and/or IL-13 were able to activate the STAT6 pathway in MSCs which resulted in an increase of their TGFβ production. It seemed that TGFβ secreted by MSCs could mediate its beneficial effects (i.e., inhibition of eosinophil infiltration and excess mucus production in the lung, decreased levels of Th2 cytokines (IL-4, IL-5 and IL-13) in bronchial lavage and lowered serum levels of Th2 immunoglobulins (IgG1 and IgE), either alone or together with recruited Treg cells[139].
CHEMOKINES
Chemokines are a family of structurally related peptides with comparatively small molecules (7,5-12,5 kDa) with chemoattractive properties[140]. Their physiological role is participation in processes like regulation of inflammation, cell differentiation and migration of immune cells, as well as angiogenesis[141]. Chemokines are produced and secreted by various cell types as a response to pro-inflammatory stimuli with the aim to attract and activate neutrophils, monocytes, lymphocytes and other effector cells to sites of infection[140].
It has been established that in vitro cultured MSCs constitutively secrete a multitude of different members of the chemokine family, such as CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CCL7 (MCP-3), CCL20 (MIP-3α), CCL26 (eotaxin-3), CXCL1 (GROα), CXCL2 (GROβ), CXCL5 (ENA-78), CXCL8 (IL-8), CXCL10 (IP-10), CXCL11 (i-TAC), CXCL12 (SDF-1) and CX3CL1 (fractalkine)[142]. Our own data have shown that MSCs isolated from human bone marrow or adipose tissue secrete IL-8, GROα, MCP-1, RANTES and SDF-1 and these chemokines can be demonstrated to be present in MSC conditioned medium[59,69].
It is quite reasonable to assume that the types and the combinations of chemokines expressed by MSCs could vary depending on the specific microenvironment and contacts with surrounding cells, especially as the latter are immune cells. The target cells attracted by the cited group of chemokines are neutrophils, monocytes, eosinophils, basophils, T and B lymphocytes, DCs, NK cells, hematopoietic and endothelial progenitors[142].
These data might suggest that the MSC secreted chemokines just have a chemoattractive effect which does not seem to be related to immunoregulation. Nevertheless, chemokines could be considered a crucial element in exerting the immunomodulatory activity of MSCs in vivo because it is assumed that the chemokines mediate the interactions between MSCs and other types of immunocompetent cells. By attracting immune cells in close proximity with MSCs, the secreted chemokines provide direct cell-to-cell contact as well as a possible paracrine immunoregulatory effect of other effector molecules also secreted by the MSCs. Thus, Ren et al[143] established that the chemokines CXCL9, CXCL10 and CXCL11 stimulate the migration of T cells in the proximity of MSCs and that these cells are targets of the local suppressive effect of nitrogen oxide secreted by the stem cells. Nevertheless, MSC secreted chemokines predominantly exert chemotactic activity and many data point to their direct role in the process of immunomodulation.
Monocyte chemoattractant protein-1 (CCL2/MCP-1)
CCL2 is a key chemokine regulating the recruitment and migration of cells of the monocyte-macrophage system. It is secreted from monocytes and other types of cells, including endothelial cells, microglial cells, NK cells etc[144]. CCL2 is related to multiple disorders associated with accumulation of activated monocytes, including atherosclerosis, bronchial asthma, inflammatory processes of the intestines etc[144]. CCL2 plays a role of direct mediator for angiogenesis and its effect is manifested by formation of new blood vessels, as proven in animal models[145]. Much data shows that CCL2 modulates the T cell immune response, causing a switch from Th0 to Th2 with predominant secretion of IL-4[146,147]. The role of CCL2 in immune regulation has been proven by the fact that it induces secretion of MCPIP1 (MCP-1 induced protein-1) which acts as RNAse and stimulates mRNA degradation for some cytokines such as IL-6 and IL-1[148]. MCPIP1 acts as a negative regulator of CCL2 and inhibits macrophage activation[149]. It has also been established that CCL2, CCL5 and some other chemokines induce proliferation and activation of specific CD56+ cytolytic cells designated as CHAK (CC chemokine-activated killer) which act similarly to the IL-2 activated cells (LAK)[150].
Some recent studies report that CCL2 is one of the factors associated with the immune modulation caused by MSCs. Secretion of this chemokine by the MSCs causes enhanced FasL dependent apoptosis of T lymphocytes. The apoptotic T cells stimulate secretion of higher levels of TGFβ by macrophages and the latter cytokine is associated with generation of CD4+FoxP3+ Tregs[151]. Other authors comment about the anti-apoptotic effect of CCL2 and describe inhibition of caspase 3 in the cell line of embryonic cardiomyoblasts cultured in the presence of MSC conditioned medium[152]. So it seems that there are data suggesting a dual function of CCL2, either pro-apoptotic or anti-apoptotic depending on the microenvironment and the general cytokine profile. It has been shown that CCL2 mediated in an autocrine manner the migration of MSCs towards the site of inflammation, ischemic damage, trauma or a developing malignant process and there the MSCs exert their immunomodulating effect[152].
Some data have been reported demonstrating that the inhibiting effect of MSCs on the immunoglobulin production by plasma cells is the result of the effector effect of CCL2 and CCL7 chemokines secreted by the MSCs[153]. It has been established that this effect is due to inhibition of the phosphorylation of STAT3 which causes activation of the transcription factor PAX5 and suppression of the immunoglobulin synthesis[153]. This assumption is substantiated by the fact that neutralizing the CCL2 neutralizes the suppressive effect of MSCs on plasma cells[153]. A possible participation of CCL2 in the inhibition of the pro-inflammatory CD4+ Th17 cells caused by MSCs has been hypothesized as an alleviation of clinical symptoms observed in EAE (experimental autoimmune encephalomyelitis)[154]. Furthermore, it has been established that MSC conditioned medium exerts an inhibitory effect on the activation of CD4 T cells obtained from EAE mice. This effect is mediated via CCL2-dependent suppression of STAT3 phosphorylation[154]. In addition, the key role of CCL2 produced by MSCs has been supported by the fact that MSCs isolated from CCL2 knock-out mice and injected in EAE mice do not demonstrate any therapeutic effect[154].
Regulated on activation, normal T-cell expressed and secreted (RANTES/ССL5)
RANTES/ССL5 was initially identified as a product secreted by activated T lymphocytes[155] which mediates the chemotactic activity of some cell types, including monocytes, lymphocytes and dendritic cells. It is engaged in regulation of leucocyte migration, angiogenesis[156,157] and some processes of wound healing[158]. CCL5 is a mighty activator of leucocytes and neutrophils, the effect of which is similar to that of mitogenic stimuli[159]. Besides its functions as a chemokine, CCL5 participates in the anti-viral immune response by blocking HIV replication in vitro and the disease progress[160,161]. CCL5 inhibits the T cell response and maybe functions as a blocking factor (suppressor of alloantigen specific T cells) by inducing cell apoptosis by modulating Bcl-2 levels and by a caspase independent mechanism[162]. There are data that CCL5 is involved in blocking the development of monocytes and memory Th1 cells[163]. CCL5 secreted by NKT cells leads to formation of CD8+ FoxP3+ cells which is the probable mechanism to induce tolerance in alloreactive T cells[164]. CCL5, similarly to CCL2, stimulates the migration of MSCs to sites of tissue damage in an autocrine manner and there are data that some tumors stimulate de novo secretion of CCL5 by MSCs with the aim to support metastases, the invasiveness and the mobility of tumor cells[165,166].
Data reported by different authors show that the effects of the chemokines should not be interpreted in one way. Most probably, chemokines secreted by MSCs do not only recruit various types of immune cells in order to exert immunomodulation but on the other hand, they act in an autocrine manner leading to migration of stem cells to the sites of tissue damage and at a later stage, support the immunomodulatory properties of the MSCs.
INDOLEAMINE-2,3-DIOXYGENASE
Indoleamine-2,3-dioxygenase (IDO) is the tryptophan-catabolizing enzyme that possesses immunosuppressive and antimicrobial effects. IDO is one of the key immunoregulators secreted by MSCs, tumors and during pregnancy. IDO is expressed by a wide range of MSCs, like decidual MSCs[167], amnionic fluid MSC[168], multipotent adult progenitor cells (MAPCs)[169], umbilical cord MSCs[170], AT-MSCs[171] etc. IDO expression is species specific. Murine MSCs possess very little IDO[172], while human MSCs do just the opposite - express an abundant amount of IDO. MSCs from monkey, pig and humans utilize IDO, whereas mouse, rat, rabbit, hamster[173] and equine[174] MSCs do not produce IDO. This variation should be considered when mouse MSCs are used as a model for studying immunoregulation properties since differences in expression of molecules involved in the process by murine and human MSCs are unquestionable.
Not activated MSCs normally express low levels of IDO, but on stimulation with inflammatory cytokines, mainly IFNγ, the IDO mRNA levels are found to be elevated[175]. IDO is not an exclusive mechanism for MSCs immunomodulation in basal states but is essential for MSC suppression in the presence of IFNγ[176]. Glucocorticoids, budesonide or dexamethasone treatment of MSCs also lead to enhanced IDO expression and is able to regenerate IDO synthesis in over-passaged MSCs[177]. Damage associated molecular patterns (DAMPs) are also involved in the IDO expression regulated by MSCs[178]. IDO is expressed after stimuli generated by the crosstalk of MSCs and cells co-cultured with them[168,179].
The cross-talk of MSCs and PBMCs causes increased IL-10 and IDO expression from MSCs that seems to be the mechanism responsible for the immunosuppressive action of the human amnionic fluid stem cells[168]. After IFNγ priming of MSCs, the IDO expression leads to B-cell growth arrest and apoptosis[180], in contrast to not activated MSCs that are IDO negative and support B-cell proliferation and survival. The addition of 1-methyl-DL-tryptophan (1-MT), an IDO inhibitor, also restored the proliferative capacity of both naive and pre-activated T cells[181]. The feedback regulation between MSCs and activated T cells may limit the immunosuppressive effects of MSCs only to sites containing ongoing inflammatory responses where the activated T cells induce the up-regulation of IDO from MSCs[179].
Direct and indirect pathways are engaged in MSC meditated immunosuppression. The catabolic activity of IDO, secreted by MSCs, can directly suppress T-cell proliferation as a result of rapid tryptophan degradation.
In addition to the direct mechanism, an indirect pathway is described and is provided through the MSC mediated differentiation of monocytes into IL-10 secreting, CD206+ immunosuppressive M2 macrophages which contribute to T-cell suppression[182].
Induction of regulatory T cells is another indirect mechanism for immunoregulation explored by MSCs. IDO expression is responsible for induction of IL-10+IFNγ+CD4+ regulatory T type 1 [T(R)1]-like cells by MSCs[183]. Neutralization of IDO is also a reason for Treg reduction[184]. Feedback regulation between Tregs and MSCs exist since Tregs do not alter the secretion of IFNγ by immune cells and hence contribute to MSC activation. MSCs by themselves secrete IDO and are able to induce the production of IL-10 from Tregs[185].
As described above, MSCs can suppress dendritic cell maturation and function, mediated by soluble factors which also include IDO. It was demonstrated that MSCs inhibit the maturation of DCs through the stimulation of IL-10 secretion and by activating the JAK1 and STAT3 signaling pathway[186].
VASCULAR ENDOTHELIAL GROWTH FACTOR
The VEGF family are the key mediators of angiogenesis and it is largely known that this process plays a critical role in tumor progression as well as in acute and chronic inflammation. The main mechanism of action of VEGF is as endothelial cell mitogen that stimulates angiogenesis by promoting endothelial cell survival, proliferation, migration and differentiation.
Six proteins of the vascular endothelial growth factor (VEGF) family are described (VEGF-A,-B,-C,-D,-E and PlGF). VEGF-A interacts with two receptors, VEGF-R1 and -R2, which are expressed on endothelial cells and on some immune cells. In addition to its best known function in angiogenesis, VEGF has a role in immunity and inflammation. VEFG is responsible for recruitment of inflammatory cells and expression of co-stimulatory molecules on recruited and resident mononuclear cells. As a result, pro-inflammatory Th1 and Th17 cytokines are up-regulated[187]. Vascular endothelial growth factor is a key mediator in the development of T cell priming and in the polarization to type 1 and type 17 T helper cells in the airways. Affecting functions of memory T cells in pro-inflammatory responses has also been described after VEGF stimulation[188]. VEGF also have an indirect immunosuppressive function on lymphocyte activation and proliferation by increasing IDO secretion from dendritic cells[189].
VEGF-A secreted by tumor cells is involved in immunosuppression via down regulation of the transcription factor NF-κB and as a result, there is an inhibition of dendritic cell maturation, trafficking and antigen presentation[190-192]. An increased VEGF plasma level in cancer patients correlated to the presence of immature DCs and immature myeloid cells in the peripheral blood[193,194]. These findings are substantiated by results from mouse model studies showing that treatment with anti-VEGF antibody increases the numbers and enhances the functions of DCs[195-197]. VEGF-A administration decreases splenic T cells and suppresses their function[198]. Placental growth factor (PlGF), a VEGF-R1 ligand, also impedes DC differentiation[190]. In vitro experiments have demonstrated that PlGF could block the capacity of human myeloid-derived DCs to stimulate a Th1 response[199].
MSCs are a potent source of VEGF. It has been shown that high expression levels of VEGF were maintained during prolonged culture periods and that in vivo hMSCs engrafted into immunodeficient mice could survive and secreted human VEGF[200]. MSCs from decidua were also found to secrete VEGF[167]. Measurement of secreted VEGF-A by ELISA in serum-free medium from cultured MSCs showed a reproducible concentration of 4.1 ± 0.9 ng[201]. Wang et al[202] hypothesized that hypoxia or TNFα activates MSCs which are able to release VEGF by STAT3 and p38 MAPK dependent mechanisms. Human MSCs that released VEGF in response to TLR-2 and NOD-1 ligands were also described[203].
INTERCELLULAR ADHESION MOLECULE
Intercellular adhesion molecule-1 (ICAM-1) is a membrane glycoprotein belonging to the immunoglobulin superfamily. Expressed on endothelial cells, leukocytes (lymphocytes and monocytes) and MSCs[204,205], ICAM-1 (CD54) is a ligand that binds primarily the heterodimeric, leukocyte-restricted β2-integrin receptors-αLβ2 (LFA-1), αMβ2 (MAC-1). ICAM-1 plays important functions in leukocyte transmigration through vessels, cell to cell adhesion impacting immune responsiveness during infections and disease pathogenesis. The level of membrane expression of ICAM on endothelia and MSCs is up-regulated by pro-inflammatory cytokines (IL-1, IL-6, TNFα) and IFNγ from activated T cells[204,206,207] and does not depend on intercellular adhesion. Generally, MSCs are renowned for their immune-suppressive function which is crucially dependent on membrane expression of ICAM-1, as demonstrated in a mouse experimental model. It was unambiguously shown that blocking antibodies against ICAM-1 receptors or ICAM-1 deficiency of MSCs abrogated the suppressive effect of MSCs on activated T cells. Strengthening the adhesion of MSCs to T cells via ICAM-1 proportionally potentiates the function of MSCs represented by lagging of T cells proliferation[204]. Besides the direct role of ICAM-1 in MSCs interaction with immune cells, the importance of membrane expression of ICAM-1 spans MSCs migration[208], proliferation and differentiation capacity[209]. Seemingly indirectly related, the essence of the processes like migration, proliferation and differentiation of MSCs is also regulated by the inflammatory environment[66]. Therefore, specifically attracted to the sites of inflammation, like tissue damage, carcinogenesis and infection, MSCs participate in immune modulation, tissue repair and cell differentiation processes.
Apart from the membrane form of ICAM, a soluble ICAM (sICAM) also exists which is formed after shedding by proteolytic cleavage from the cell membrane[210,211] or by coding of specific mRNA transcripts in cells[212]. Elevated amounts of a biologically active form of sICAM is detected in serum, cerebrospinal fluid, synovial fluid, urine and sputum in pathologies with an underlying inflammatory status, like autoimmune and degenerative diseases[213-215] and tumor pathogenesis[216,217]. Different reports point to various cell sources of sICAM in health and pathologies, including endothelial cells[218], peripheral blood mononuclear cells, keratinocytes, epidermoid carcinoma cell lines, melanoma cells[219] and tumors[216,217,220]. sICAM can be secreted spontaneously or after specific inductions[220]. Limited data demonstrate that some but not all MSCs are a source of sICAM. Profiles of cytokine arrays revealed high expression of sICAM from human MSCs derived from umbilical cord and deciduas[221,222] and null expression from bone marrow-derived MSCs[221]. The exact physiological role of sICAM in health and pathology is still not completely revealed but reports demonstrate its potential to stimulate endothelial cell differentiation in conditions with angiogenic growth in tumorigenesis[223]. In relation to this finding, a speculation imposes that the process of massive angiogenesis which takes place during placentation might be related to the secretion of sICAM from umbilical cord-derived and decidua-derived MSCs. Hypothetically, the lack of such a requirement for bone marrow-derived MSCs suggests acquisition of varying functions of MSCs according to the tissue localization. Furthermore, the importance of sICAM secreted from human umbilical cord-derived MSCs for microglia functioning and neuronal survival is depicted in a model of Alzheimer’s disease[222].
Insufficiently explored, the paracrine function of sICAM seems to counteract the classical biological function of membrane ICAM by preventing leukocyte interactions. sICAM affects trafficking of immune cells via hampering attachment to endothelial cells[224] and blocks immune response development due to deteriorated immune cell contacts. In addition, the increased sICAM during inflammation probably affects MSC migration, proliferation and differentiation and detailed exploration of their biology can help understand and modulate the regulatory properties of MSCs in different pathologies.
PROSTAGLANDIN E2
Prostaglandins (PGs) are products of cyclooxygenases (COX) synthesis from arachidonic acid. COX1 is constitutively expressed from virtually all tissues, while COX2 is induced under inflammatory conditions (by LPS, IL-1, TNFα for example)[225]. COX2 is shown to preferentially metabolize prostaglandin E2 (PGE2)[226] that acts as a messenger molecule through a paracrine and autocrine manner on surrounding cells.
Together with IDO, PGE2 is another major effector molecule responsible for immunoregulatory competence of MSCs[183]. MSCs constitutively produce detectable levels of PGE2[44,227,228]. Under inflammatory conditions of the environment, PGE2 is induced, substantially increasing secreted amounts from MSCs. LPS as well as cytokines like IFNγ, TNFα, IL-1β are mediators directly regulating PGE2 production from MSCs[227,229,230]. Multiple studies show that direct contact of PBMCs, monocytes and NK cells with MSCs induces PGE2 augmentation via the mentioned cytokines[44,227-229]. Activated by environmental signals, PGE2 from MSCs exert regulatory influence on the activation status, proliferation, differentiation and function of immune cells from adaptive and innate immunity. Acting by a contact or paracrine manner[229,231], PGE2 has a systemic anti-inflammatory effect of reducing TNFα, IL-6 and vascular permeability in an experimental model of sepsis[230]. Particularly, the cellular targets of PGE2 are PBMCs, NK cells, monocytes, macrophages and the transitional processes of differentiation of monocytes into immature DCs[228,230,232]. PGE2 indirectly affects polyclonally or allogenically activated PBMCs by substantial suppression of proliferation and IFNγ secretion[227,229,231]. Simultaneously, the effect on T cells is accompanied by a prevailing bias towards IL-4 production[227] and induction of regulatory IL-10 secreting T cells[183,233]. The influence of MSCs on T cells that represent the effector arm of adaptive immunity is shown to be mediated via the antigen presenting cells (APCs). They are subjected to the direct effect of PGE2, resulting in reduced effectiveness of reaching the stage of immature DCs from monocytes showing an affected phenotype as a low number of CD1a cells and decreased expression of co-stimulatory CD80, CD86[228] and antigen-presenting molecules MHC II[231]. Furthermore, when co-cultured with MSCs, the production of IL-12 from APCs (especially DCs) is low[228,231,232], while IL-10 (from DCs and macrophages) is increased[227,230]. In total, when differentiating in the presence of MSCs, DCs stay immature in a tolerogenic state and unable to elicit a Th1 immune response. On the other hand, MSCs do not affect the differentiation of immature into mature DCs. The latter demonstrates normal expression of CD80, CD86, CD83 receptors, normal capacity for T cell activation and even increased IL-12 secretion[228]. Retained in an undifferentiated state, DCs when in co-culture with MSCs largely deteriorate/aggravate the cytotoxicity properties of NK cells as well. Investigations show that due to changed chemokine profile and reduced IL-12 secretion from DCs, NK cells do not properly recruit to DCs[232]. Under these circumstances, NK cells have low activation, diminished IFNγ secretion and cytotoxicity against their targets[232], including the reactivity against MSCs[234]. Summarizing the influence of PGE2 on immune cells with regulatory function endows MSCs a central place in controlling of inflammatory responses. Extremely sensitive to activation signals from the environment, MSCs seem to link the crossroads between innate and adaptive immunity. By suppressing inflammatory mediators, they participate in the activation of feed-back processes, counteracting non-self[55,183] and autoimmune reactivity[233,235,236], leading the immune system to a steady homeostatic state.
CONCLUSION
A general conclusion can be drawn that MSCs can realize their immunoregulatory functions even when they are an object of different stimuli. One of the mechanisms to exert these functions is secretion of cytokines which can directly influence the effector immune cells. In addition to that, when secreting cytokines MSCs are involved in complex multi-directional interactions, including predominantly dendritic cells and different subtypes of T regulatory cells (Figure 2). Detailed elucidation of these interactions might be of key importance for the effective application of mesenchymal stem cells in therapy for autoimmune diseases.
Figure 2.
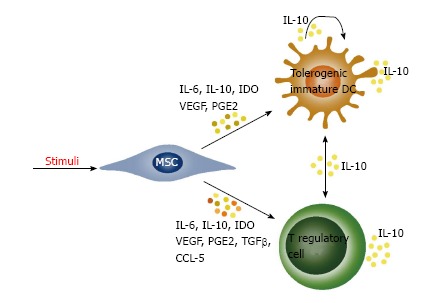
Mesenchymal stem cells provide an immunoregulatory effect by interactions with dendritic cells and T regulatory cells. Under the influence of cytokines secreted by MSCs and autocrine secreted interleukin-10 (IL-10), the dendritic cells acquire an immature tolerogenic phenotype characterized by a low expression of MHC II and B7 molecules, as well as a higher secretion of IL-10. The secretion of IL-10 induces generation of different subtypes of regulatory T cells which further secrete IL-10 and induce tolerogenic phenotype in dendritic cells. Cytokines secreted from MSCs also lead directly to formation of regulatory T cells. VEGF: Vascular endothelial growth factor; PGE2: Prostaglandin E2; MSCs: Mesenchymal stem cells; IDO: Indoleamine-2, 3-dioxygenase; TGFβ: Transforming growth factor β; DCs: Dendritic cells; IL: Interleukin; CCL: CC chemokine ligand.
Footnotes
P- Reviewer: Kan L, Yang FC S- Editor: Gong XM L- Editor: Roemmele A E- Editor: Lu YJ
References
- 1.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinders ME, Leuning DG, de Fijter JW, Hoogduijn MJ, Rabelink TJ. Mesenchymal stromal cell therapy for cardio renal disorders. Curr Pharm Des. 2014;20:2412–2429. doi: 10.2174/13816128113199990477. [DOI] [PubMed] [Google Scholar]
- 3.Le Blanc K, Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Siegel G, Schäfer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87:S45–S49. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- 6.Tropel P, Noël D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 9.Lotfinegad P, Shamsasenjan K, Movassaghpour A, Majidi J, Baradaran B. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: a hope in cell therapy. Adv Pharm Bull. 2014;4:5–13. doi: 10.5681/apb.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plock JA, Schnider JT, Solari MG, Zheng XX, Gorantla VS. Perspectives on the use of mesenchymal stem cells in vascularized composite allotransplantation. Front Immunol. 2013;4:175. doi: 10.3389/fimmu.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law S, Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. Am J Stem Cells. 2013;2:22–38. [PMC free article] [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.De Bari C, Dell’Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 15.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940–952. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 18.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 19.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 20.Pournasr B, Mohamadnejad M, Bagheri M, Aghdami N, Shahsavani M, Malekzadeh R, Baharvand H. In vitro differentiation of human bone marrow mesenchymal stem cells into hepatocyte-like cells. Arch Iran Med. 2011;14:244–249. [PubMed] [Google Scholar]
- 21.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, Rice HE. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294:371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 24.Tropel P, Platet N, Platel JC, Noël D, Albrieux M, Benabid AL, Berger F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–2876. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- 25.Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. doi: 10.1371/journal.pone.0001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23:7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 29.Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325–335. doi: 10.1111/j.1743-6109.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 30.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 31.Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007;69:1–9. doi: 10.1111/j.1399-0039.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 35.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 36.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 37.Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I, Kyurkchiev DS. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37–42. doi: 10.1016/j.imlet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 39.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34 -derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Sun B, Wang D, Ji Y, Kong Q, Wang G, Wang J, Zhao W, Jin L, Li H. Murine bone marrow mesenchymal stem cells cause mature dendritic cells to promote T-cell tolerance. Scand J Immunol. 2008;68:607–615. doi: 10.1111/j.1365-3083.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 41.Bochev I, Elmadjian G, Kyurkchiev D, Tzvetanov L, Altankova I, Tivchev P, Kyurkchiev S. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol Int. 2008;32:384–393. doi: 10.1016/j.cellbi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 43.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 44.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 45.Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, Sportoletti P, Falzetti F, Tabilio A. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–318. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 46.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 48.Ye Z, Wang Y, Xie HY, Zheng SS. Immunosuppressive effects of rat mesenchymal stem cells: Involvement of CD4 CD25 regulatory T cells. Hepatobiliary Pancreat Dis Int. 2008;7:608–614. [PubMed] [Google Scholar]
- 49.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 50.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 51.McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 52.Angoulvant D, Clerc A, Benchalal S, Galambrun C, Farre A, Bertrand Y, Eljaafari A. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology. 2004;41:469–476. [PubMed] [Google Scholar]
- 53.Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 54.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 55.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 56.Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008;265:131–135. doi: 10.1016/j.jns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond) 2014;11:1. doi: 10.1186/1476-9255-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kyurkchiev D, Ivanova-Todorova E, Bochev I, Mourdjeva M, Kyurkchiev S. Differences between adipose tissue-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells as regulators of the immune response. In: Hayat MA, editor. Stem cells and cancer stem cells, volume 10. Netherlands: Springer; 2013. pp. 71–84. [Google Scholar]
- 60.Park CW, Kim KS, Bae S, Son HK, Myung PK, Hong HJ, Kim H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells. 2009;2:59–68. doi: 10.15283/ijsc.2009.2.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan CK, Wu KH, Lee YS, Hwang SM, Lee MS, Liao SK, Cheng EH, See LC, Tsai CN, Kuo ML, et al. The comparison of interleukin 6-associated immunosuppressive effects of human ESCs, fetal-type MSCs, and adult-type MSCs. Transplantation. 2012;94:132–138. doi: 10.1097/TP.0b013e31825940a4. [DOI] [PubMed] [Google Scholar]
- 62.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Dazzi F, Krampera M. Mesenchymal stem cells and autoimmune diseases. Best Pract Res Clin Haematol. 2011;24:49–57. doi: 10.1016/j.beha.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 65.DelaRosa O, Dalemans W, Lombardo E. Mesenchymal stem cells as therapeutic agents of inflammatory and autoimmune diseases. Curr Opin Biotechnol. 2012;23:978–983. doi: 10.1016/j.copbio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 68.Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ivanova-Todorova E, Bochev I, Dimitrov R, Belemezova K, Mourdjeva M, Kyurkchiev S, Kinov P, Altankova I, Kyurkchiev D. Conditioned medium from adipose tissue-derived mesenchymal stem cells induces CD4+FOXP3+ cells and increases IL-10 secretion. J Biomed Biotechnol. 2012;2012:295167. doi: 10.1155/2012/295167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 71.Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heo YJ, Joo YB, Oh HJ, Park MK, Heo YM, Cho ML, Kwok SK, Ju JH, Park KS, Cho SG, et al. IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients. Immunol Lett. 2010;127:150–156. doi: 10.1016/j.imlet.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 74.O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 75.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 76.Yang S, Li W, Liu W, Gao C, Zhou B, Li S, Li Y, Kong Y. IL-10 gene modified dendritic cells induced antigen-specific tolerance in experimental autoimmune myocarditis. Clin Immunol. 2006;121:63–73. doi: 10.1016/j.clim.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 77.Sultani M, Stringer AM, Bowen JM, Gibson RJ. Anti-inflammatory cytokines: important immunoregulatory factors contributing to chemotherapy-induced gastrointestinal mucositis. Chemother Res Pract. 2012;2012:490804. doi: 10.1155/2012/490804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 79.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: Induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 80.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller W, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 2011;10:410–415. doi: 10.1016/j.autrev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Blaber SP, Webster RA, Hill CJ, Breen EJ, Kuah D, Vesey G, Herbert BR. Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med. 2012;10:172. doi: 10.1186/1479-5876-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Engela AU, Baan CC, Dor FJ, Weimar W, Hoogduijn MJ. On the interactions between mesenchymal stem cells and regulatory T cells for immunomodulation in transplantation. Front Immunol. 2012;3:126. doi: 10.3389/fimmu.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 85.Djouad F, Bouffi C, Ghannam S, Noël D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5:392–399. doi: 10.1038/nrrheum.2009.104. [DOI] [PubMed] [Google Scholar]
- 86.Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 87.Perrini S, Ficarella R, Picardi E, Cignarelli A, Barbaro M, Nigro P, Peschechera A, Palumbo O, Carella M, De Fazio M, et al. Differences in gene expression and cytokine release profiles highlight the heterogeneity of distinct subsets of adipose tissue-derived stem cells in the subcutaneous and visceral adipose tissue in humans. PLoS One. 2013;8:e57892. doi: 10.1371/journal.pone.0057892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Solovyeva VV, Salafutdinov II, Martynova EV, Khaiboullina SF, Rizvanov AA. Human adipose derived stem cells do not alter cytokine secretion in response to the genetic modification with pEGFP-N2 plasmid DNA. World Appl Sci J. 2013;26:968–972. [Google Scholar]
- 89.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bassi EJ, Aita CA, Câmara NO. Immune regulatory properties of multipotent mesenchymal stromal cells: Where do we stand? World J Stem Cells. 2011;3:1–8. doi: 10.4252/wjsc.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 92.Yun UJ, Park SE, Jo YS, Kim J, Shin DY. DNA damage induces the IL-6/STAT3 signaling pathway, which has anti-senescence and growth-promoting functions in human tumors. Cancer Lett. 2012;323:155–160. doi: 10.1016/j.canlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Garbers C, Hermanns HM, Schaper F, Müller-Newen G, Grötzinger J, Rose-John S, Scheller J. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012;23:85–97. doi: 10.1016/j.cytogfr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 95.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 96.Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 97.Nakagawa T, Tsuruoka M, Ogura H, Okuyama Y, Arima Y, Hirano T, Murakami M. IL-6 positively regulates Foxp3+CD8+ T cells in vivo. Int Immunol. 2010;22:129–139. doi: 10.1093/intimm/dxp119. [DOI] [PubMed] [Google Scholar]
- 98.Cui X, Liu J, Bai L, Tian J, Zhu J. Interleukin-6 induces malignant transformation of rat mesenchymal stem cells in association with enhanced signaling of signal transducer and activator of transcription 3. Cancer Sci. 2014;105:64–71. doi: 10.1111/cas.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noël D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 100.Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1:2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 102.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 103.Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003;98:257–265. doi: 10.1016/s0163-7258(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 104.Wahl SM, Swisher J, McCartney-Francis N, Chen W. TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol. 2004;76:15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- 105.Yoshimura A, Muto G. TGF-β function in immune suppression. Curr Top Microbiol Immunol. 2011;350:127–147. doi: 10.1007/82_2010_87. [DOI] [PubMed] [Google Scholar]
- 106.Groh ME, Maitra B, Szekely E, Koç ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 107.Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebe L, Zhang Y, Gorin NC, Thierry D, et al. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13:217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tomic S, Djokic J, Vasilijic S, Vucevic D, Todorovic V, Supic G, Colic M. Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev. 2011;20:695–708. doi: 10.1089/scd.2010.0145. [DOI] [PubMed] [Google Scholar]
- 109.Brabletz T, Pfeuffer I, Schorr E, Siebelt F, Wirth T, Serfling E. Transforming growth factor beta and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol Cell Biol. 1993;13:1155–1162. doi: 10.1128/mcb.13.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol. 2004;172:4275–4284. doi: 10.4049/jimmunol.172.7.4275. [DOI] [PubMed] [Google Scholar]
- 111.Ruegemer JJ, Ho SN, Augustine JA, Schlager JW, Bell MP, McKean DJ, Abraham RT. Regulatory effects of transforming growth factor-beta on IL-2- and IL-4-dependent T cell-cycle progression. J Immunol. 1990;144:1767–1776. [PubMed] [Google Scholar]
- 112.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 114.Nelson BH, Martyak TP, Thompson LJ, Moon JJ, Wang T. Uncoupling of promitogenic and antiapoptotic functions of IL-2 by Smad-dependent TGF-beta signaling. J Immunol. 2003;170:5563–5570. doi: 10.4049/jimmunol.170.11.5563. [DOI] [PubMed] [Google Scholar]
- 115.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. P27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 116.Wolfraim LA, Walz TM, James Z, Fernandez T, Letterio JJ. p21Cip1 and p27Kip1 act in synergy to alter the sensitivity of naive T cells to TGF-beta-mediated G1 arrest through modulation of IL-2 responsiveness. J Immunol. 2004;173:3093–3102. doi: 10.4049/jimmunol.173.5.3093. [DOI] [PubMed] [Google Scholar]
- 117.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 118.Sad S, Mosmann TR. Single IL-2-secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J Immunol. 1994;153:3514–3522. [PubMed] [Google Scholar]
- 119.Gorham JD, Guler ML, Fenoglio D, Gubler U, Murphy KM. Low dose TGF-beta attenuates IL-12 responsiveness in murine Th cells. J Immunol. 1998;161:1664–1670. [PubMed] [Google Scholar]
- 120.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 122.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 123.Heath VL, Murphy EE, Crain C, Tomlinson MG, O’Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol. 2000;30:2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 124.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 125.Kuwahara M, Yamashita M, Shinoda K, Tofukuji S, Onodera A, Shinnakasu R, Motohashi S, Hosokawa H, Tumes D, Iwamura C, et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-β and suppresses T(H)2 differentiation. Nat Immunol. 2012;13:778–786. doi: 10.1038/ni.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smyth MJ, Strobl SL, Young HA, Ortaldo JR, Ochoa AC. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8 T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol. 1991;146:3289–3297. [PubMed] [Google Scholar]
- 127.Genestier L, Kasibhatla S, Brunner T, Green DR. Transforming growth factor beta1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med. 1999;189:231–239. doi: 10.1084/jem.189.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahmadzadeh M, Rosenberg SA. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174:5215–5223. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bonig H, Banning U, Hannen M, Kim YM, Verheyen J, Mauz-Korholz C, Korholz D. Transforming growth factor-beta1 suppresses interleukin-15-mediated interferon-gamma production in human T lymphocytes. Scand J Immunol. 1999;50:612–618. doi: 10.1046/j.1365-3083.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- 130.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 131.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3 T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 133.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4 CD25- cells to CD25 Foxp3 regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 134.Jana S, Jailwala P, Haribhai D, Waukau J, Glisic S, Grossman W, Mishra M, Wen R, Wang D, Williams CB, et al. The role of NF-kappaB and Smad3 in TGF-beta-mediated Foxp3 expression. Eur J Immunol. 2009;39:2571–2583. doi: 10.1002/eji.200939201. [DOI] [PubMed] [Google Scholar]
- 135.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, Horwitz DA, Shi W, Zheng SG. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martinez GJ, Zhang Z, Reynolds JM, Tanaka S, Chung Y, Liu T, Robertson E, Lin X, Feng XH, Dong C. Smad2 positively regulates the generation of Th17 cells. J Biol Chem. 2010;285:29039–29043. doi: 10.1074/jbc.C110.155820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Takimoto T, Wakabayashi Y, Sekiya T, Inoue N, Morita R, Ichiyama K, Takahashi R, Asakawa M, Muto G, Mori T, et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol. 2010;185:842–855. doi: 10.4049/jimmunol.0904100. [DOI] [PubMed] [Google Scholar]
- 138.Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 139.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:S460–475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 141.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 142.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 143.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 144.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: Direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 146.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 147.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 148.Xu J, Fu S, Peng W, Rao Z. MCP-1-induced protein-1, an immune regulator. Protein Cell. 2012;3:903–910. doi: 10.1007/s13238-012-2075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liang J, Wang J, Azfer A, Song W, Tromp G, Kolattukudy PE, Fu M. A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J Biol Chem. 2008;283:6337–6346. doi: 10.1074/jbc.M707861200. [DOI] [PubMed] [Google Scholar]
- 150.Maghazachi AA, Al-Aoukaty A, Schall TJ. CC chemokines induce the generation of killer cells from CD56+ cells. Eur J Immunol. 1996;26:315–319. doi: 10.1002/eji.1830260207. [DOI] [PubMed] [Google Scholar]
- 151.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rafei M, Hsieh J, Fortier S, Li M, Yuan S, Birman E, Forner K, Boivin MN, Doody K, Tremblay M, et al. Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood. 2008;112:4991–4998. doi: 10.1182/blood-2008-07-166892. [DOI] [PubMed] [Google Scholar]
- 154.Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, Yuan S, Young YK, Boivin MN, Forner K, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 155.Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, Krensky AM. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 156.Balkwill F. The molecular and cellular biology of the chemokines. J Viral Hepat. 1998;5:1–14. doi: 10.1046/j.1365-2893.1998.00081.x. [DOI] [PubMed] [Google Scholar]
- 157.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 158.Martins-Green M, Petreaca M, Wang L. Chemokines and Their Receptors Are Key Players in the Orchestra That Regulates Wound Healing. Adv Wound Care (New Rochelle) 2013;2:327–347. doi: 10.1089/wound.2012.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schall TJ. Biology of the RANTES/SIS cytokine family. Cytokine. 1991;3:165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- 160.Appay V, Rowland-Jones SL. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 161.Hadida F, Vieillard V, Mollet L, Clark-Lewis I, Baggiolini M, Debre P. Cutting edge: RANTES regulates Fas ligand expression and killing by HIV-specific CD8 cytotoxic T cells. J Immunol. 1999;163:1105–1109. [PubMed] [Google Scholar]
- 162.Ramhorst RE, García VE, Corigliano A, Rabinovich GA, Fainboim L. Identification of RANTES as a novel immunomodulator of the maternal allogeneic response. Clin Immunol. 2004;110:71–80. doi: 10.1016/j.clim.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 163.Weber C, Weber KS, Klier C, Gu S, Wank R, Horuk R, Nelson PJ. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and T(H)1-like/CD45RO( ) T cells. Blood. 2001;97:1144–1146. doi: 10.1182/blood.v97.4.1144. [DOI] [PubMed] [Google Scholar]
- 164.Faunce DE, Stein-Streilein J. NKT cell-derived RANTES recruits APCs and CD8 T cells to the spleen during the generation of regulatory T cells in tolerance. J Immunol. 2002;169:31–38. doi: 10.4049/jimmunol.169.1.31. [DOI] [PubMed] [Google Scholar]
- 165.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 166.Zischek C, Niess H, Ischenko I, Conrad C, Huss R, Jauch KW, Nelson PJ, Bruns C. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann Surg. 2009;250:747–753. doi: 10.1097/SLA.0b013e3181bd62d0. [DOI] [PubMed] [Google Scholar]
- 167.Liu L, Zhao G, Fan H, Zhao X, Li P, Wang Z, Hu Y, Hou Y. Mesenchymal stem cells ameliorate Th1-induced pre-eclampsia-like symptoms in mice via the suppression of TNF-α expression. PLoS One. 2014;9:e88036. doi: 10.1371/journal.pone.0088036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luo C, Jia W, Wang K, Chi F, Gu Y, Yan X, Zou G, Duan T, Zhou Q. Human amniotic fluid stem cells suppress PBMC proliferation through IDO and IL-10-dependent pathways. Curr Stem Cell Res Ther. 2014;9:36–45. doi: 10.2174/1574888x113086660067. [DOI] [PubMed] [Google Scholar]
- 169.Jacobs SA, Pinxteren J, Roobrouck VD, Luyckx A, van’t Hof W, Deans R, Verfaillie CM, Waer M, Billiau AD, Van Gool SW. Human multipotent adult progenitor cells are nonimmunogenic and exert potent immunomodulatory effects on alloreactive T-cell responses. Cell Transplant. 2013;22:1915–1928. doi: 10.3727/096368912X657369. [DOI] [PubMed] [Google Scholar]
- 170.Guo J, Yang J, Cao G, Fan H, Guo C, Ma YE, Qian Y, Chen L, Li X, Chang C. Xenogeneic immunosuppression of human umbilical cord mesenchymal stem cells in a major histocompatibility complex-mismatched allogeneic acute graft-versus-host disease murine model. Eur J Haematol. 2011;87:235–243. doi: 10.1111/j.1600-0609.2011.01635.x. [DOI] [PubMed] [Google Scholar]
- 171.DelaRosa O, Lombardo E, Beraza A, Mancheño-Corvo P, Ramirez C, Menta R, Rico L, Camarillo E, García L, Abad JL, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 172.Ren G, Su J, Zhang L, Zhao X, Ling W, L’huillie A, Zhang J, Lu Y, Roberts AI, Ji W, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 173.Su J, Chen X, Huang Y, Li W, Li J, Cao K, Cao G, Zhang L, Li F, Roberts AI, et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21:388–396. doi: 10.1038/cdd.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carrade DD, Lame MW, Kent MS, Clark KC, Walker NJ, Borjesson DL. Comparative Analysis of the Immunomodulatory Properties of Equine Adult-Derived Mesenchymal Stem Cells. Cell Med. 2012;4:1–11. doi: 10.3727/215517912X647217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liang C, Chen SL, Wang M, Zhai WJ, Zhou Z, Pang AM, Feng SZ, Han MZ. [Synergistic immunomodulatory effects of interferon-gamma and bone marrow mesenchymal stem cells] Zhonghua Xue Ye Xue Za Zhi. 2013;34:213–216. doi: 10.3760/cma.j.issn.0253-2727.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 176.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ankrum JA, Dastidar RG, Ong JF, Levy O, Karp JM. Performance-enhanced mesenchymal stem cells via intracellular delivery of steroids. Sci Rep. 2014;4:4645. doi: 10.1038/srep04645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lotfi R, Eisenbacher J, Solgi G, Fuchs K, Yildiz T, Nienhaus C, Rojewski MT, Schrezenmeier H. Human mesenchymal stem cells respond to native but not oxidized damage associated molecular pattern molecules from necrotic (tumor) material. Eur J Immunol. 2011;41:2021–2028. doi: 10.1002/eji.201041324. [DOI] [PubMed] [Google Scholar]
- 179.Lin W, Oh SK, Choo AB, George AJ. Activated T cells modulate immunosuppression by embryonic-and bone marrow-derived mesenchymal stromal cells through a feedback mechanism. Cytotherapy. 2012;14:274–284. doi: 10.3109/14653249.2011.635853. [DOI] [PubMed] [Google Scholar]
- 180.Maby-El Hajjami H, Amé-Thomas P, Pangault C, Tribut O, DeVos J, Jean R, Bescher N, Monvoisin C, Dulong J, Lamy T, et al. Functional alteration of the lymphoma stromal cell niche by the cytokine context: role of indoleamine-2,3 dioxygenase. Cancer Res. 2009;69:3228–3237. doi: 10.1158/0008-5472.CAN-08-3000. [DOI] [PubMed] [Google Scholar]
- 181.Yang SH, Park MJ, Yoon IH, Kim SY, Hong SH, Shin JY, Nam HY, Kim YH, Kim B, Park CG. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp Mol Med. 2009;41:315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 183.Hsu WT, Lin CH, Chiang BL, Jui HY, Wu KK, Lee CM. Prostaglandin E2 potentiates mesenchymal stem cell-induced IL-10+IFN-γ+CD4+ regulatory T cells to control transplant arteriosclerosis. J Immunol. 2013;190:2372–2380. doi: 10.4049/jimmunol.1202996. [DOI] [PubMed] [Google Scholar]
- 184.Erkers T, Nava S, Yosef J, Ringdén O, Kaipe H. Decidual stromal cells promote regulatory T cells and suppress alloreactivity in a cell contact-dependent manner. Stem Cells Dev. 2013;22:2596–2605. doi: 10.1089/scd.2013.0079. [DOI] [PubMed] [Google Scholar]
- 185.Engela AU, Baan CC, Peeters AM, Weimar W, Hoogduijn MJ. Interaction between adipose tissue-derived mesenchymal stem cells and regulatory T-cells. Cell Transplant. 2013;22:41–54. doi: 10.3727/096368912X636984. [DOI] [PubMed] [Google Scholar]
- 186.Liu WH, Liu JJ, Wu J, Zhang LL, Liu F, Yin L, Zhang MM, Yu B. Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK1/STAT3 signaling pathway. PLoS One. 2013;8:e55487. doi: 10.1371/journal.pone.0055487. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 187.Kim YS, Hong SW, Choi JP, Shin TS, Moon HG, Choi EJ, Jeon SG, Oh SY, Gho YS, Zhu Z, et al. Vascular endothelial growth factor is a key mediator in the development of T cell priming and its polarization to type 1 and type 17 T helper cells in the airways. J Immunol. 2009;183:5113–5120. doi: 10.4049/jimmunol.0901566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Basu A, Hoerning A, Datta D, Edelbauer M, Stack MP, Calzadilla K, Pal S, Briscoe DM. Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol. 2010;184:545–549. doi: 10.4049/jimmunol.0900397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marti LC, Pavon L, Severino P, Sibov T, Guilhen D, Moreira-Filho CA. Vascular endothelial growth factor-A enhances indoleamine 2,3-dioxygenase expression by dendritic cells and subsequently impacts lymphocyte proliferation. Mem Inst Oswaldo Cruz. 2014;109:70–79. doi: 10.1590/0074-0276130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174:215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 191.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 192.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 193.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 194.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- 196.Ishida T, Oyama T, Carbone DP, Gabrilovich DI. Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. J Immunol. 1998;161:4842–4851. [PubMed] [Google Scholar]
- 197.Wada J, Suzuki H, Fuchino R, Yamasaki A, Nagai S, Yanai K, Koga K, Nakamura M, Tanaka M, Morisaki T, et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 2009;29:881–888. [PubMed] [Google Scholar]
- 198.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 199.Lin YL, Liang YC, Chiang BL. Placental growth factor down-regulates type 1 T helper immune response by modulating the function of dendritic cells. J Leukoc Biol. 2007;82:1473–1480. doi: 10.1189/jlb.0307164. [DOI] [PubMed] [Google Scholar]
- 200.Kagiwada H, Yashiki T, Ohshima A, Tadokoro M, Nagaya N, Ohgushi H. Human mesenchymal stem cells as a stable source of VEGF-producing cells. J Tissue Eng Regen Med. 2008;2:184–189. doi: 10.1002/term.79. [DOI] [PubMed] [Google Scholar]
- 201.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 202.Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol. 2007;42:1009–1015. doi: 10.1016/j.yjmcc.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sioud M, Mobergslien A, Boudabous A, Fløisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol. 2010;71:267–274. doi: 10.1111/j.1365-3083.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 204.Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 206.Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): An overview. Eur Cytokine Netw. 2004;15:91–98. [PubMed] [Google Scholar]
- 207.Wong D, Dorovini-Zis K. Upregulation of intercellular adhesion molecule-1 (ICAM-1) expression in primary cultures of human brain microvessel endothelial cells by cytokines and lipopolysaccharide. J Neuroimmunol. 1992;39:11–21. doi: 10.1016/0165-5728(92)90170-p. [DOI] [PubMed] [Google Scholar]
- 208.Fu X, Han B, Cai S, Lei Y, Sun T, Sheng Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-alpha and its possible role in wound healing. Wound Repair Regen. 2009;17:185–191. doi: 10.1111/j.1524-475X.2009.00454.x. [DOI] [PubMed] [Google Scholar]
- 209.Xu FF, Zhu H, Li XM, Yang F, Chen JD, Tang B, Sun HG, Chu YN, Zheng RX, Liu YL, et al. Intercellular Adhesion Molecule-1 Inhibits Osteogenic Differentiation of Mesenchymal Stem Cells and Impairs Bio-Scaffold-Mediated Bone Regeneration In Vivo. Tissue Eng Part A. 2014:Jun 5; Epub ahead of print. doi: 10.1089/ten.tea.2014.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Champagne B, Tremblay P, Cantin A, St Pierre Y. Proteolytic cleavage of ICAM-1 by human neutrophil elastase. J Immunol. 1998;161:6398–6405. [PubMed] [Google Scholar]
- 211.Budnik A, Grewe M, Gyufko K, Krutmann J. Analysis of the production of soluble ICAM-1 molecules by human cells. Exp Hematol. 1996;24:352–359. [PubMed] [Google Scholar]
- 212.Wakatsuki T, Kimura K, Kimura F, Shinomiya N, Ohtsubo M, Ishizawa M, Yamamoto M. A distinct mRNA encoding a soluble form of ICAM-1 molecule expressed in human tissues. Cell Adhes Commun. 1995;3:283–292. doi: 10.3109/15419069509081014. [DOI] [PubMed] [Google Scholar]
- 213.Klimiuk PA, Sierakowski S, Latosiewicz R, Cylwik JP, Cylwik B, Skowronski J, Chwiecko J. Soluble adhesion molecules (ICAM-1, VCAM-1, and E-selectin) and vascular endothelial growth factor (VEGF) in patients with distinct variants of rheumatoid synovitis. Ann Rheum Dis. 2002;61:804–809. doi: 10.1136/ard.61.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tulek N, Aydintug O, Ozoran K, Tutkak H, Duzgun N, Duman M, Tokgoz G. Soluble intercellular adhesion molecule-1 (sICAM-1) in patients with systemic lupus erythematosus. Clin Rheumatol. 1996;15:47–50. doi: 10.1007/BF02231684. [DOI] [PubMed] [Google Scholar]
- 215.Rieckmann P, Michel U, Albrecht M, Brück W, Wöckel L, Felgenhauer K. Cerebral endothelial cells are a major source for soluble intercellular adhesion molecule-1 in the human central nervous system. Neurosci Lett. 1995;186:61–64. doi: 10.1016/0304-3940(95)11282-2. [DOI] [PubMed] [Google Scholar]
- 216.Dymicka-Piekarska V, Guzinska-Ustymowicz K, Kuklinski A, Kemona H. Prognostic significance of adhesion molecules (sICAM-1, sVCAM-1) and VEGF in colorectal cancer patients. Thromb Res. 2012;129:e47–e50. doi: 10.1016/j.thromres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 217.Nakata B, Hori T, Sunami T, Ogawa Y, Yashiro M, Maeda K, Sawada T, Kato Y, Ishikawa T, Hirakawa K. Clinical significance of serum soluble intercellular adhesion molecule 1 in gastric cancer. Clin Cancer Res. 2000;6:1175–1179. [PubMed] [Google Scholar]
- 218.Krenn V, Schedel J, Doring A, Huppertz HI, Gohlke F, Tony HP, Vollmers HP, Muller-Hermelink HK. Endothelial cells are the major source of sICAM-1 in rheumatoid synovial tissue. Rheumatol Int. 1997;17:17–27. doi: 10.1007/pl00006846. [DOI] [PubMed] [Google Scholar]
- 219.Budnik A, Trefzer U, Parlow F, Grewe M, Kapp A, Schopf E, Krutmann J. Human epidermal keratinocytes are a source of soluble ICAM-1 molecules. Exp Dermatol. 1992;1:27–30. doi: 10.1111/j.1600-0625.1992.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 220.Jackson AM, Alexandrov AB, Gribben SC, Esuvarnathan K, James K. Expression and shedding of ICAM-1 in bladder cancer and its immunotherapy. Int J Cancer. 1993;55:921–925. doi: 10.1002/ijc.2910550608. [DOI] [PubMed] [Google Scholar]
- 221.Hwang JH, Shim SS, Seok OS, Lee HY, Woo SK, Kim BH, Song HR, Lee JK, Park YK. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci. 2009;24:547–554. doi: 10.3346/jkms.2009.24.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ, et al. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ. 2012;19:680–691. doi: 10.1038/cdd.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gho YS, Kleinman HK, Sosne G. Angiogenic activity of human soluble intercellular adhesion molecule-1. Cancer Res. 1999;59:5128–5132. [PubMed] [Google Scholar]
- 224.Rieckmann P, Michel U, Albrecht M, Bruck W, Wockel L, Felgenhauer K. Soluble forms of intercellular adhesion molecule-1 (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J Neuroimmunol. 1995;60:9–15. doi: 10.1016/0165-5728(95)00047-6. [DOI] [PubMed] [Google Scholar]
- 225.Crofford LJ. COX-1 and COX-2 tissue expression: Implications and predictions. J Rheumatol Suppl. 1997;49:15–19. [PubMed] [Google Scholar]
- 226.Brock TG, McNish RW, Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J Biol Chem. 1999;274:11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- 227.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 228.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 229.Chen K, Wang D, Du WT, Han ZB, Ren H, Chi Y, Yang SG, Zhu D, Bayard F, Han ZC. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010;135:448–458. doi: 10.1016/j.clim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 230.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 232.Van Elssen CH, Vanderlocht J, Oth T, Senden-Gijsbers BL, Germeraad WT, Bos GM. Inflammation-restraining effects of prostaglandin E2 on natural killer-dendritic cell (NK-DC) interaction are imprinted during DC maturation. Blood. 2011;118:2473–2482. doi: 10.1182/blood-2010-09-307835. [DOI] [PubMed] [Google Scholar]
- 233.Zheng ZH, Li XY, Ding J, Jia JF, Zhu P. Allogeneic mesenchymal stem cell and mesenchymal stem cell-differentiated chondrocyte suppress the responses of type II collagen-reactive T cells in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:22–30. doi: 10.1093/rheumatology/kem284. [DOI] [PubMed] [Google Scholar]
- 234.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 235.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 236.Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]


