Abstract
Herpes zoster is a clinical syndrome which usually presents with a localized, vesicular rash in a dermatomal distribution. Cutaneous dissemination rarely occurs in immunocompetent patients, therefore little is known about the baseline demographic, clinical characteristics, management and outcome of these patients. Herein, we report a case of disseminated cutaneous herpes zoster in an immunocompetent patient along with a review and analysis of 28 cases previously reported in the literature.
Key words: herpes zoster, disseminated, cutaneous, immunocompetent
Introduction
Varicella zoster virus (VZV) is an exclusively human double-stranded DNA virus of the Herpesviridae family. The primary VZV infection, called varicella or chickenpox, presents clinically as a disseminated vesicular rash, typically during childhood. After the initial infection, this virus enters a lifelong latent state within the sensory ganglia. Under unknown conditions, the VZV reactivates and travels along the nerve axon to the skin to cause a localized vesicular rash in a dermatomal distribution, called herpes zoster (HZ) or shingles.1
Varicella zoster virus-specific cell-mediated immunity (CMI) is required to halt the virus reactivation. During young adulthood, VZV-specific CMI is robust which explains the infrequent occurrence of HZ in this age group.2 With aging, the VZV-specific CMI declines, especially after age of 60.3-5 This decline in CMI correlates with increased incidence of HZ in the elderly population.2 Humoral immunity does not appear to protect against reactivation of VZV as antibodies levels are preserved throughout all age groups. Also, patients with humoral immunity deficiency (i.e. B-cell deficiency) are not more prone to develop severe varicella infections.2 VZV antibodies are believed to be important in preventing the acquisition of a new infecting virus.6
Among immunocompetent patients, HZ is considered a self-limited, localized infection commonly complicated by post-herpetic neuralgia. In contrast, patients with T-cell deficiency, such as HIV patients and bone marrow transplant recipients, can present with severe cutaneous and visceral disseminated disease.1 Cutaneous dissemination of HZ among immunocompetent hosts has been previously reported in the literature mainly as a single case reports or small case series. However, little is known about the baseline demographic, clinical characteristics, management and outcome of these patients. Therefore, we present here another case of disseminated cutaneous HZ (DCHZ) in an immunocompetent patient and summarize the data on the existed published cases.
Case Report
A 95-year-old Caucasian woman developed a cluster of vesicular lesions over the left lower lip, which spread to involve the left malar region and earlobe with associated burning-like pain over the affected area. She was also noted to have vesicular lesions over the lower left labial and gingival mucosa. Initially, the patient was empirically started on amoxicillin 500 mg orally three times daily and acyclovir 800 mg orally five times daily for possible dental infection and HZ, respectively. A dentist ruled out odontogenic infection and amoxicillin was discontinued. However, while on day two of antiviral therapy, new vesicular lesions appeared throughout the trunk, upper and lower extremities. She was admitted to the hospital for further evaluation and treatment. Patient had a history of coronary artery disease (CAD) and chronic obstructive pulmonary disease (COPD) with no recent used of steroids in the past eight months. On examination, cluster of vesicular lesions were noted over the V1, V2 and V3 branches of the left V cranial nerve. There were also scattered vesicular lesions involving the oral mucosa, as described above, and the trunk, upper and lower extremities (Figure 1). Ophthalmologic evaluation did not reveal any corneal abnormities. Vesicle fluid from face and trunk skin lesion were positive for VZV and negative for herpes simplex by polymerase chain reaction. The patient was also found to have right lower lobe pneumonia based on chest x-ray infiltrate. Her initial white blood cell (WBC) count was 35,000 cell/L and elevated procalcitonin to 0.75 ng/mL. Piperacillin-tazobactam 3.375 g intravenously every 6 hours and acyclovir 5 mg/kg intravenously every 8 hours were started. Her pro-calcitonin and WBC count normalized while on antibiotic treatment. Intravenous acyclovir was continued for 7 days and subsequently changed to oral formulation to complete 14 days of treatment. Chest, abdomen and pelvis computer tomography was negative for malignancies. Immunoglobulin levels and serum protein electrophoresis were within normal limits. After six months of follow-up, no malignancy has been identified.
Figure 1.
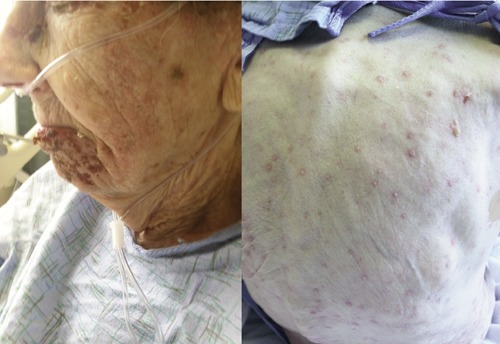
Clinical photograph of a patient with disseminated cutaneous herpes zoster. On the left, cluster of vesicular lesions involving the mandibular branch of the trigeminal nerve. On the right, diffuse vesicular rash with erythematous base involving the back.
Discussion and Conclusions
An electronic search on PubMed and Google using the terms zoster, cutaneous, dissemination and immunocompetent in different combinations was conducted. Only cases of immunocompetent adult patients (age 16 or older) with DCHZ were included. We considered the following conditions immunosuppressive: use of antineoplastic and immunosuppressive medications, cancer, HIV infection, immunologic disorders, and organ transplants.7 After performing additional literature review, milliary tuberculosis (TB) was also added to the immunosuppressive conditions, as it has been associated with T cell deficiency.8 DCHZ was defined as >20 skin lesions beyond the primary or adjacent dermatomes.9 Sixteen related publications were identified. After review, the subjects in one of the articles were excluded from the tabulation due to the limited individual information available for analysis.10 A total of 33 cases were reviewed of which six were excluded due to use of prednisolone (2 cases), history of rheumatoid arthritis (1 case), history of idiopathic thrombocytopenic purpura (1 case) and milliary TB (2 cases). A total of 28 cases were included for tabulation (Table 1).11-24
Table 1.
Characteristics of patient with disseminated cutaneous herpes zoster.
| Case (ref.) | Age (yrs) | Sex | Comorbidities | Dermatome | Non-cutaneous manifestations | Interval to dissemination (days) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 (11) | 22 | F | ACTH injections | C2-C4 | Uvula lesions | 2 | NR | Recovered |
| 2 (11) | 63 | F | ACTH injections | CN V1 | Ophthalmic zoster | 5 | NR | Recovered |
| 3 (11) | 37 | F | None | CN V1 and V2 | Palate lesions | 5 | NR | Recovered |
| 4 (11) | 58 | M | None | CN V1 | Lip and palate lesions, ophthalmic zoster | 6 | NR | Recovered |
| 5 (11) | 72 | F | None | C1-C3 | 4 | NR | Recovered | |
| 6 (11) | 54 | F | None | CN V1 | 3 | NR | Recovered | |
| 7 (12) | 75 | F | Rheumatic heart disease s/p mitral and tricuspid valve replacement | Left T10 | 8 | IV acyclovir | recovered | |
| 8 (13) | 43 | M | None | Right T8 | 2 | NR | NR | |
| 9 (13) | 50 | M | None | Right thigh | 1 | NR | NR | |
| 10 (14) | 70 | F | CVA, depression, malnutrition | Left CN V2 | NR | Oral acyclovir | Recovered | |
| 11 (14) | 79 | M | None | Left L3 | NR | Oral acyclovir | Recovered | |
| 12 (15) | 24 | M | None | Left T5 | Aseptic meningitis | 3 | NR | Recovered |
| 13 (16) | 37 | F | None | T2 | Aseptic meningitis | NR | IV acyclovir | Recovered |
| 14 (17) | 72 | F | DM | Right peroneal | Left facial and right peroneal nerve paresis | 12 | IV acyclovir then IV vidaravine | Right foot palsy at 6 months |
| 15 (3) | 39 | M | None | Right T6 | 9 | Oral valacyclovir hen IV acyclovir | Recovered | |
| 16 (18) | 75 | M | DM, angina | Left CN V and C2 | Ramsay-Hunt | 11 | IV acyclovir | Recovered |
| 17 (19) | 97 | F | HTN, CHF | Right CN V3 | 4 | Oral valacyclovir | NR | |
| 18 (20) | 82 | F | DM, HTN | Right L1-L2 | Right femoral-peroneal paresis | NR | Oral and IV acyclovir | Leg motor weakness at 6 months |
| 19 (21) | 76 | M | HTH, angina, prostate CA s/p resection* | Right S3-S4 and bladder atonies | Intestinal NR | IV acyclovir | Recovered | |
| 20 (22) | 29 | M | None | CN VII | Ramsay-Hunt | 6 | None | Recovered |
| 21 (22) | 51 | M | TB LAD | NR | 7 | None | PHN | |
| 22 (22) | 25 | M | None | CN V | 7 | None | Recovered | |
| 23 (22) | 40 | F | None | NR | 5 | None | Recovered | |
| 24 (23) | 79 | M | None | Left T7-T8 | NR | IV acyclovir | PHN | |
| 25 (23) | 80 | M | DM, HTN | Right C3-C4 | NR | IV acyclovir | Recovered | |
| 26 (23) | 71 | M | CAD, HTN, Dementia | Left T8 | NR | IV acyclovir | Recovered | |
| 27 (24) | 69 | M | Right CN V1 | 3 | IV acyclovir | Recovered | ||
| 28 (our case) | 95 | F | CAD, COPD | Left CN V1, V2, V3 | Gingiva lesions | 4 | Oral and IV acyclovir | Recovered |
M, male; F, female; CN, cranial nerve; s/p, status post; ME, meningoencephalitis; CVA, cerebrovascular accident; HTN, hypertension; DM, diabetes mellitus; CHF, congestive heart failure; CA, carcinoma; PHN, post-herpetic neuralgia; COPD, chronic obstructive pulmonary disease; TB, tuberculosis; LAD, lymphadenopathy; NR, not reported.
*Patient did not have any evidence of cancer recurrence
The average age of the patients was 59 years (range 22-97) with 54% men vs 46% women. Forty-six percent of these patients had at least one comorbidity, of which hypertension and diabetes were the most common (Table 1). Initial presentation of the rash was localized to one or several dermatomes that most frequently involved cranial nerves (42%) followed by thoracic (27%), lumbosacral (19%) and cervical (15%) dermatomes. On average, cutaneous dissemination occurred five days (range 1-12 days) after the initial dermatomal skin rash. Extracutaneous manifestations were reported in twelve cases (43%), four of which had mucosal lesion (including one herpes ophthamicus), two had central nervous system manifestations, two had cranial or peripheral nerve paresis, two had Ramsay-Hunt syndrome, one had herpes ophthalmicus and one had visceral involvement. In all cases with reported antiviral treatment (15 cases) intravenous antiviral acyclovir was prescribed except for three patients that were treated with oral acyclovir (2 cases) and oral valacyclovir (1 case). All patients with reported outcomes recovered without sequelae, except for four patients who developed segmental paresis (2 cases) and post-herpetic neuralgia (2 cases).
Although disseminated HZ occurs more often in immunocompromised patients, VZV viremia seems to occur in all patients with HZ irrespective of T-cell immune status or clinical evidence of dissemination.25,26 Kronenberg et al. were able to amplify VZV DNA from serum samples of all tested immunocompetent patients (n=10) with localized HZ.27 Based on this, the potential for developing disseminated disease exists in all patients with HZ, and those with more profound deficiency of the CMI seem to be at increased risk of developing disseminated clinical disease. In a study by Koc et al., cutaneous dissemination among bone marrow transplant patients was observed in 17% of HZ cases.25 Overall, cutaneous dissemination have been reported in 10 to 40% of all immunocompromised patients with HZ.1 The prevalence of DCHZ in immunocompetent patients has not been established. Furthermore, the exact mechanism by which some apparently immunocompetent patients will develop disseminated zoster is not clearly understood.
In almost all reported cases (89%) reviewed here, patients initially presented with a localized HZ involving one or several dermatomes followed by a secondary diffuse papulovesicular rash which helps distinguish disseminated HZ from primary varicella infection of adults. Initial cranial nerve involvement was most common, which may suggest that these patients are most prone to cutaneous dissemination. This is in contrast with study by Mittal et al. (not tabulated) where the majority of patients had initial thoracic segments involvement. Age-related decline of VZV CMI seems to be one of the most important risk factor for VZV reactivation and subsequent HZ.1 This correlates with the average age of the patients (65 years) reviewed here. However, other factors might be involved in the cutaneous dissemination of HZ, as 25% of the reported cases were younger than 40 years.
In a recent study, the following chronic conditions were associated with an increase risk of HZ: allergic rhinitis, COPD, CAD, cerebrovascular accident, depression, diabetes, hyperlipidemia, hypothyroidism and osteoarthritis.7 In our review, nine patients (32%) had at least one of these chronic conditions. Of them, only diabetes has been directly linked to VZV-CMI deficiency. Okamoto et al. demonstrated that VZV-CMI was significantly lower than healthy volunteers.28 These findings corroborate other clinical studies, showing an increased risk of HZ in patients with diabetes.29,30 We identified four patients with diabetes (Table 1) plus three other patients (not tabulated) with DCHZ.10 In these cases cutaneous dissemination might be due to an underlying VZV-CMI deficiency. Except for diabetes, the other above mentioned conditions have not been directly linked to CMI deficiency and their role as risk factor for HZ need to be further investigated. In patients with COPD, treatment of acute exacerbations entails systemic steroid which could attenuate the CMI, however it is not clear if previous remote steroid treatment may affect VZV-specific immunity. Our patient had a history of COPD but there was no history of steroid use for at least eight months, and therefore no immunosuppressive condition was identified.
As the cutaneous dissemination of HZ is thought to be via viremia, patients are often treated with intravenous antivirals to prevent cutaneous and visceral dissemination. In our review, three patients presented with central nervous system or visceral disease complications which reaffirm the need of aggressive treatment at diagnosis. Intravenous acyclovir was the most common antimicrobial therapy instituted and just a few cases received oral antiviral therapy only.
There are several limitations in the cases reviewed here. Several of these reports were published several decades ago when diagnostics tests were limited or certain immunosuppressive conditions were not yet fully recognized such as human immunodeficiency virus infection (specially in the young patients). Moreover, follow-up information was lacking in many of these cases which makes it difficult to exclude underlying malignancy or immunocompromized states that can initially present with cutaneous dissemination of herpes zoster but their own diagnosis can be delayed. Two patients were also receiving adrenocorticotropic hormone injections for unknown reasons, leaving the possibility of an unidentified abnormality of the immune system.
Overall, cutaneous dissemination of HZ in the patients reviewed was associated with low morbidity and mortality. This could be a result of appropriate antiviral therapy or to a sufficient VZV-CMI to overcome the infection.
In conclusion, DCHZ can occur in any immunocompetent host, although it is more predominant in older patients. Despite cutaneous dissemination, overall mortality and morbidity is low. Chronic comorbidities may play additional role and further research is needed to identify the exact mechanisms by which they affect VZV CMI.
References
- 1.Arvin AM.Varicella-zoster virus. Clin Microbiol Rev 1996;9:361-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvin A.Aging, immunity, and the varicella-zoster virus. N Engl J Med 2005;352: 2266-7 [DOI] [PubMed] [Google Scholar]
- 3.Burdett C, Mendoza N, Arora A, et al. A rare case of disseminated shingles in an immunocompetent patient following a 7-day treatment with oral valacyclovir. J Clin Virol 2008;43:233-5 [DOI] [PubMed] [Google Scholar]
- 4.Miller AE.Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology 1980;30:582-7 [DOI] [PubMed] [Google Scholar]
- 5.Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis 2003;188: 1336-44 [DOI] [PubMed] [Google Scholar]
- 6.Arvin AM.Humoral and cellular immunity to varicella-zoster virus: an overview. J Infect Dis 2008;197:S58-60 [DOI] [PubMed] [Google Scholar]
- 7.Joesoef RM, Harpaz R, Leung J, Bialek SR.Chronic medical conditions as risk factors for herpes zoster. Mayo Clin Proc 2012; 87:961-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davoudi S, Rasoolinegad M, Younesian M, et al. CD4+ cell counts in patients with different clinical manifestations of tuberculosis. Braz J Infect Dis 2008;12:483-6 [DOI] [PubMed] [Google Scholar]
- 9.Whitley RJ, Gnann JW, Jr., Hinthorn D, et al. Disseminated herpes zoster in the immunocompromised host: a comparative trial of acyclovir and vidarabine. The NIAID Collaborative Antiviral Study Group. J Infect Dis 1992;165:450-5 [DOI] [PubMed] [Google Scholar]
- 10.Mittal RR, Maninder Disseminated herpes zoster. Indian J Dermatol Venereol Leprol 1995;61:148-9 [PubMed] [Google Scholar]
- 11.Merselis JG, Jr., Kaye D, Hook EW.Disseminated herpes zoster. A Report of 17 Cases. Arch Intern Med 1964;113:679-86 [DOI] [PubMed] [Google Scholar]
- 12.Dirbas FM, Swain JA.Disseminated cutaneous herpes zoster following cardiac surgery. J Cardiovasc Surg (Torino) 1990;31:531-2 [PubMed] [Google Scholar]
- 13.Sun ZH, Guo YY, Li M, Yao ZR.Disseminated herpes zoster in immunocompetent patients not due to varicella-zoster virus gene mutation. Chin Med J (Engl) 2013;126:3193. [PubMed] [Google Scholar]
- 14.O’Toole EA, Mooney EE, Walsh JB, et al. Disseminated herpes zoster in the elderly. Ir J Med Sci 1997;166:141-2 [DOI] [PubMed] [Google Scholar]
- 15.Karp SJ.Meningitis and disseminated cutaneous zoster complicating herpes zoster infection. J Neurol Neurosurg Psychiatry 1983;46:582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriuchi H, Moriuchi M, Sun CC, Trucksis M.Disseminated cutaneous zoster and aseptic meningitis in a previously healthy patient. J Infect 1997;35:183-5 [DOI] [PubMed] [Google Scholar]
- 17.Takahama H, Tsukahara N, Hirayama M, et al. Isolated double herpes zoster paresis involving the left facial nerve and the right peroneal nerve following disseminated herpes zoster. J Dermatol 2007;34:349-52 [DOI] [PubMed] [Google Scholar]
- 18.Yoon KJ, Kim SH, Lee EH, Choi JH.Disseminated herpes zoster in an immunocompetent elderly patient. Korean J Pain 2013;26:195-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capron J, Steichen O.Disseminated zoster in an elderly patient. Infection 2009;37:179-80 [DOI] [PubMed] [Google Scholar]
- 20.Kim JB, Jung HJ, Lee JM, et al. Disseminated herpes zoster with a zoster paresis-induced femoral fracture. Geriatr Gerontol Int 2012;12:168-71 [DOI] [PubMed] [Google Scholar]
- 21.Revenga F, Ruiz R, Vanaclocha F. [Transient intestinal and bladder atonies in a geriatric patient with disseminated herpes zoster]. An Med Interna 1992;9:624-5 [Article in Spanish] [PubMed] [Google Scholar]
- 22.Nigam P, Dayal SG, Dubey AL.Disseminated zoster. J Dermatol 1980;7: 443-7 [DOI] [PubMed] [Google Scholar]
- 23.Chyen LH, Wee CM.Disseminated cutaneous zoster can occur in healthy individuals: a case series. Singapore Fam Physician 2011;37:52-4 [Google Scholar]
- 24.Gupta S, Jain A, Gardiner C, Tyring SK.A rare case of disseminated cutaneous zoster in an immunocompetent patient. BMC Fam Pract 2005;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koc Y, Miller KB, Schenkein DP, et al. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant 2000;6:44-9 [DOI] [PubMed] [Google Scholar]
- 26.Bezold G, Lange M, Pillekamp H, Peter RU.Varicella zoster viraemia during herpes zoster is not associated with neoplasia. J Eur Acad Dermatol Venereol 2002;16:357-60 [DOI] [PubMed] [Google Scholar]
- 27.Kronenberg A, Bossart W, Wuthrich RP, et al. Retrospective analysis of varicella zoster virus (VZV) copy DNA numbers in plasma of immunocompetent patients with herpes zoster, of immunocompromised patients with disseminated VZV disease, and of asymptomatic solid organ transplant recipients. Transpl Infect Dis 2005;7:116-21 [DOI] [PubMed] [Google Scholar]
- 28.Okamoto S, Hata A, Sadaoka K, et al. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis 2009;200:1606-10 [DOI] [PubMed] [Google Scholar]
- 29.Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect 2013;67:463-9 [DOI] [PubMed] [Google Scholar]
- 30.Aldaz P, Diaz JA, Loayssa JR, et al. [Herpes zoster incidence in diabetic patients]. An Sist Sanit Navar 2013;36:57-62 [Article in Spanish] [DOI] [PubMed] [Google Scholar]


