Abstract
The aim of this paper is to investigate p53 gene expression in the central and peripheral zones of glioblastoma multiforme using a real-time reverse transcription polymerase chain reaction (RT-PCR) technique in patients who use cell phones ≥3 hours a day and determine its relationship to clinicopathological findings and overall survival. Sixty-three patients (38 males and 25 females), diagnosed with glioblastoma multiforme (GBM), underwent tumor resection between 2008 and 2011. Patient ages ranged from 25 to 88 years, with a mean age of 55. The levels of expression of p53 in the central and peripheral zone of the GBM were quantified by RT-PCR. Data on p53 gene expression from the central and peripheral zone, the related malignancy and the clinicopatholagical findings (age, gender, tumor location and size), as well as overall survival, were analyzed. Forty-one out of 63 patients (65%) with the highest level of cell phone use (≥3 hours/day) had higher mutant type p53 expression in the peripheral zone of the glioblastoma; the difference was statistically significant (P=0.034). Results from the present study on the use of mobile phones for ≥3 hours a day show a consistent pattern of increased risk for the mutant type of p53 gene expression in the peripheral zone of the glioblastoma, and that this increase was significantly correlated with shorter overall survival time. The risk was not higher for ipsilateral exposure. We found that the mutant type of p53 gene expression in the peripheral zone of the glioblastoma was increased in 65% of patients using cell phones ≥3 hours a day.
Key words: glioblastoma multiforme, p53 gene expression, reverse transcriptase polymerase chain reaction
Introduction
Glioblastoma multiforme (GBM) is the most malignant glial tumor. It develops from astrocytes and is the most aggressive primary cancer in humans. Invading cells grow rapidly and form their own blood vessels, making them difficult to remove surgically or to treat.1,2 GBM remains one of the most lethal forms of cancer, with a median survival of 10 to 12 months. It is a primary brain tumor comprising of 12-15% of all intracranial neoplasms and 50-60% of astrocytic tumors.3 GBMs rarely metastasize. Instead, it causes death through invasion into normal brain tissues and growth into cerebral hemispheres,4,5 as a space occupying lesion of the white matter with infiltration to its surrounding parenchyma.5
Histological gliomas are graded based on the presence of specific histological markers, including necrosis, nuclear pleomorphism, mitotic activity and vascular proliferation.3
Two genetic pathways have been described in GBM development: primary and secondary.6 Primary (de novo) GBM grows rapidly without evidence of a pre-existing low-grade tumor and represents the most frequent presentation. Patients tend to be older (mean age 62 years) and have a short clinical history.7 On the other hand, secondary GBM develops more slowly, by progression from a low-grade (WHO grade II), or anaplastic astrocytoma,8 and usually has a longer clinical course in younger patients (mean age 45 years).7
Many factors have proved to influence the process of tumor-genesis.1 Alterations in the p53 gene are one of the distinct features seen in primary, as well as in secondary, GBM.7 It has been concluded that p53 mutations are involved in disease progression, especially from low-grade astrocytomas or anaplasic astrocytomas.9 The p53 gene encodes a 393-amino acid protein that possesses five conserved regions. The p53 protein is a transcription factor that plays a vital role in regulating cell growth, DNA repair and apoptosis, in response to stressful conditions.10 p53 is a short-lived protein under complex regulation, including reversible cycles of post-translational modification, such as phosphorylation, acetylation, and ubiquitination.11
Abnormalities of p53 have been reported in de novo GBM; 28% of these tumors were shown to possess a p53 mutation, while 65% of secondary GBMs show p53 mutations.7 The incidence of p53 protein accumulation is more frequently seen than are p53 mutations.12,13
There are different morphological zones of the GBM, as already known and described in previous studies.8,14 The central regions of the glioblastoma multiforme are almost entirely necrotic, with only scattered islands of viable neoplastic tissue, mostly around blood vessels. Immediately adjacent to the central necrosis is densely cellular tumor tissue consisting of highly anaplastic astrocytoma cells. Magnetic resonance imaging (MRI) with and without contrast is the imaging method of choice. These lesions typically have an enhancing ring observed on T1-weighted images and a broad surrounding zone of edema apparent on T2-weighted images. Several pathological studies have clearly shown that the area of enhancement does not represent the outer tumor border, because infiltrating glioma cells can be identified easily within, and occasionally beyond, a 2-cm margin.15 The main objectives of this study are to evaluate the discrete expression of p53 in the central and peripheral zone (within 2-cm margin) of the GBMs in patients who use cell phones ≥3 hours a day, and further, to investigate its role in overall survival. Cell phones transmit and receive electromagnetic waves, mainly at frequencies of 800-1900 MHz.
Materials and Methods
Sixty-three patients (38 male and 25 female) with diagnosed GBM (WHO grade IV astrocytoma) underwent tumor resection at Mashhad University of Medical Sciences (Mashhad, Iran) between 2008 and 2011. Patient ages ranged from 25 to 88 years with a mean age of 55. The patients and/or their legal guardians gave written informed consent for use of their specimens. The cell phones used in our study transmitted and received electromagnetic waves, mainly at frequencies of 1100-1900 MHz.
Tumor specimens were obtained by surgical resection, quickly frozen, and kept at −80°C until use. Histopathologic examination was carried out on the formalin-fixed, paraffin-embedded specimens. Each specimen was classified according to established World Health Organization Criteria. The presence or absence of high cellularity, nuclear atypia, mitoses, microvascular proliferation and necrosis were recorded. All of the sixty-three tumors were classified as de novo (primary) GBM. For each neoplasm, three zones were identified: i) a central region of necrosis; ii) a densely cellular ring that has been shown in MRI to be congruent with the area of contrast enhancement, and a peripheral zone of lesser cellular density or tumor infiltration (within 2-cm margin of the area of enhancement in MRI). Forty-nine patients underwent total surgical resection with postoperative radiotherapy and chemotherapy (Table 1). Intraoperative neuronavigation was used in all patients. To determine the extent of surgical resection, we performed postoperative MRI. Resection was judged to be total when there were no residual lesion mass, subtotal when less than 10% of the preoperative mass remained, and partial when more than 10% of the mass remained. All patients were re-evaluated after receiving initial adjuvant therapy (Table 1); MRI was performed at periodic follow up visits. Clinical details, including the Karnofsky performance status at the time of diagnosis, the extent of surgery, date of recurrence (or regrowth) on MRI, and date of death were recorded. Occupational exposure to other electromagnetic fields was excluded in all patients.
Table 1.
Summary of clinical data and p53 gene expression related to clinicopathological findings (N=63).
| Parameters | Patients |
|---|---|
| Extent of surgery | |
| Biopsy only | 5 (8%) |
| Partial resection | 9 (14%) |
| Radical resection | 49 (78%) |
| Radiotherapy | |
| Radical (>54Gy) | 38 (60%) |
| High dose palliative (40-53Gy) | 16 (25%) |
| Palliative (<40Gy) | 9 (15%) |
| Temozolomide | |
| Yes | 42 (67%) |
| No | 21 (33%) |
| Temozolomide and radical radiotherapy | |
| Yes | 26 (41%) |
| No | 37 (59%) |
| Alive | |
| Yes | 2 (3%) |
| No | 61 (97%) |
| Male (n=38;P=0.114) | |
| Central | 0.1605±0.1458* |
| Peripheral | 0.0772±0.1181* |
| Female (n=25; P=0.999) | |
| Central | 0.0101±0.0084* |
| Peripheral | 0.0227±0.0330* |
| Average age at glioblastoma operation (yr) | 55 |
| Sex ratio (M/F) | 38/25 (1.5) |
| P53 labeling index | 5.3±4.1% |
| Cell phone use/p53 gene | |
| ≥3 hours/day (P=0.034), central | 0.1609±0.1407* |
| ≥3 hours/day (P=0.034), peripheral | 0.0740±0.1330* |
| ≥3 hours/day (P=0.161), central | 0.0850±0.1401* |
| ≥3 hours/day (P=0. 161), peripheral | 0.0523±0.0821* |
| Mutant 59 (93.6%) | 0.0643± 0.0236* |
| Wild type 4 (6.34%) | 0.2348±0.1276* |
| Handedness | |
| Right-handed | 58 (92%) |
| Left-handed | 5 (8%) |
| Tumor location | |
| Right, frontal | 5 (8%) |
| Right, temporal | 20 (32%) |
| Right, parietal | 8 (13%) |
| Right, occipital | 3 (5%) |
| Left, frontal | 4 (6%) |
| Left, temporal | 7 (11%) |
| Left, parietal | 10 (16%) |
| Left, occipital | 6 (10%) |
| Cumulative use | |
| Never or rarely | 3 (5%) |
| <13hr | 8 (13%) |
| 13 to 100 hr | 36 (57%) |
| >100hr | 10 (16%) |
| >500 hr | 6 (10%) |
| Specific absorption rate | |
| Range | 0.66-1.53 w/kg |
| Average | 1.42 w/kg |
*Mean ± standard deviation.
Reverse transcription polymerase chain reaction for p53 gene amplification
To investigate the expression level of p53 in human glioblastoma, we employed a reverse transcriptase real-time reverse transcription polymerase chain reaction (RT-PCR) assay using GAPDH primers. Total RNA was extracted from the tumor tissues of each glioblastoma patient. After the reverse transcription, GAPDH primers were used for cDNA amplification. RNA integrity was confirmed with parallel RT-PCR amplification using GAPDH primers. PCR products were electrophoresed on agarose gels containing ethidium bromide and visualized by UV photography. The detailed procedure was as follows. Total RNA was extracted from frozen tissues using the LiCl/Urea method.16 For cDNA synthesis, 3 g of total RNA was annealed to oligo (dT) 15 in 20 L total volume containing 4 L of 5 XM-MLV RT buffer (50 mM Tris HCl, pH 8.3, 7.5 mM KCl, 3 mM MgCl2, and 10 mM DTT), 0.5 mM dNTP, 20 U RNase and 200 U M-MLV RT (Promega, Germany) and was incubated at 37°C for 1.5 hr. The PCR mixture contained 1X PCR buffer (50 mM KCl,10 mM Tris-HCl, pH 9.0, and 0.1% Triton X-100), 1 mM MgCl2, 0.2 M of each primer, 0.2 mM dNTP, 2.5 U of Taq DNA polymerase (Promega, Germany) and 1.5 g of cDNA for a final volume of 50 L. The amplification was performed in the DNA Thermal Cycler. Twenty-five PCR cycles were performed, consisting of 1 min at each temperature (94°C, 53°C, 72°C), except for the initial cycle (5 min at 94°C) and the final extension step (5 min at 72°C). Primer sequences for p53 and GAPDH (reference gene) were as follows.
Sense primer: 5’-CCT GTC ATC TTC TGT CCC TTC -3’ Antisense primer: 5’-GCT GTG ACT GCT TGT AGA TGG -3’ for p53 resulting in a 50-bp PCR product, Sense primer: 5’-GGC CAA GAT CAT CCA TGA CAA CT-3’ Antisense primer: 5’-ACC AGG ACA TGA GCT TGA CAA AGT-3’ for GAPDH resulting in a 110-bp PCR product. (Figure 1)
Figure 1.
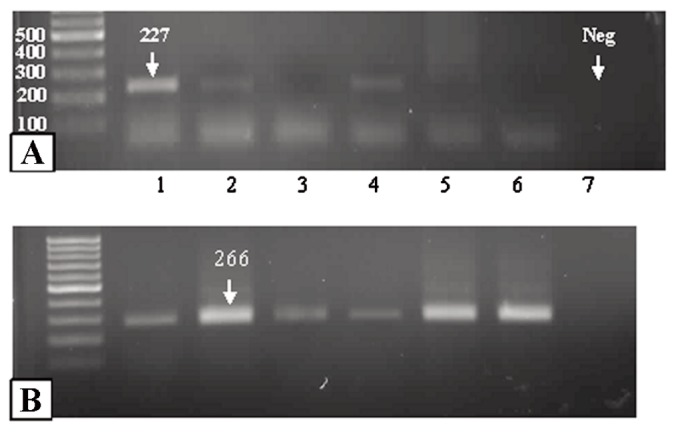
P53 amplification detected by reverse transcriptase polymerase chain reaction in a control brain (B); glioblastomas (A, lane 1), and negative control (lane 7). Note regular 100bp bands of GAPDH mRNA as a positive control (B, lane 2).
Detection of p53 mutations using a yeast-based p53 functional assay
To detect functionally inactivated p53 mutations, a yeast-based transactivation assay called functional analysis of separated alleles in yeast (FASAY) was performed as described previously.17 When more than 20% of the yeast transformants showed a His2 phenotype, we considered them positive for p53 mutation and carried out further p53 sequencing.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS/PC 8, Chicago, IL, USA). A P value of less than 0.05 was accepted as statistically significant. The Wilcoxon Signed Ranks Test was used to evaluate differences in the clinicopathological findings (age, gender, tumor location and tumor size) of the patients and p53 expression in the central and peripheral zone of the primary GBMs. The chi-square and Fisher’s exact tests were used to analyze differences in sex ratio and p53 expression.
Results
The results of this study are summarized in Table 1. The mean age of the patients with primary GBMs at the time of surgery was 55 years. The incidence of primary GBM was higher in the males than in the females of our study but the difference was not statistically significant. P53 mutations that inactivated normal p53 function were detected in 59 out of 63 (93.6%) of the primary GBMs (P≤0.05). Among the 59 tumors with p53 mutations, 9 showed a 100% yeast His2 phenotype, 40 showed more than 75% His2 phenotype, and 10 showed 20-75% His2 phenotype (data not shown).
Among patients with tumors who reported having used hand-held cellular telephones regularly for at least six months before diagnosis, the laterality of the tumor was not significantly associated with the self-reported laterality of the cellular telephone use (Table 1).
There was a specific relationship between p53 gene expression and the clinicopathological findings. Forty-one out of 63 cases (65%) with the highest level of cell phone use (≥3 hours/day) showed a higher mutant p53 expression in the peripheral zone of the glioblastoma; the difference was statistically significant (P=0.034).
Demographic and clinical characteristics for the whole group (n=63) are listed in (Table 1). Fifty-eight patients (92%) had either partial or total radical resection of their tumor. The mean time from surgery to commencing radiotherapy was 27 days (mean 32.4 days for radical cases and 23.9 for palliative cases). At the time of analysis, 61 (97%) patients had died. The median overall survival time was 6.7 months, but for cases with lower p53 gene expression in the central zone of the GBM, it was 10 months (P=0.024) (Figure 2).
Figure 2.
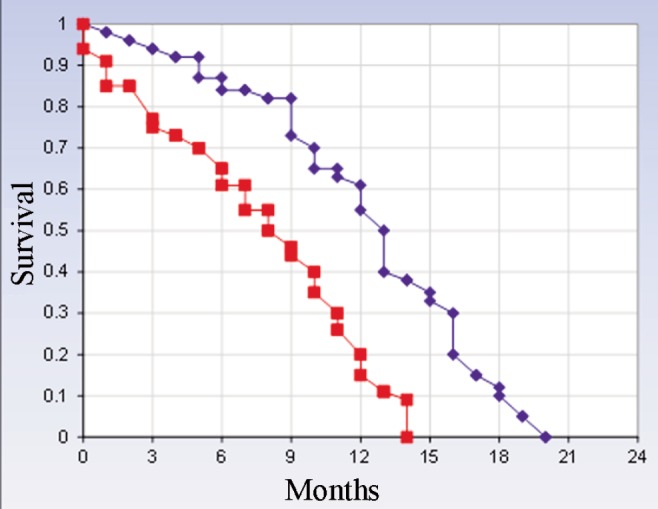
Kaplan-Meier overall survival curves in glioblastoma patients with p53 gene expression in the central and peripheral zones. Patients with higher p53 gene expression in the peripheral zone using cell phones 3 hours a day had shorter overall survival than those with lower p53 gene expression; the difference was statistically significant (P=0.024). The average specific absorption rate by our patients was 1.42 w/kg. Blue line: mutant type p53 gene expression in the central zone; red line: mutant type p53 gene expression in the peripheral zone.
Discussion
Among tumor suppressor genes, p53 appears to play an important role in the pathogenesis of several common malignancies, including GBM.18 P53 has been shown to exert a tumor suppressor effect by inducing apoptosis, activating the cell cycle, stimulating cell differentiation and being involved in DNA repair pathways.19 Mutations in the p53 gene are detected in about 65% of secondary and 28% of de novo GBMs, thus indicating that p53 abnormalities are common in the progression from a low-grade to a high-grade lesion.20 A univariant analysis indicated the presence of p53 mutations and predicted a longer survival in a population-based study, however a multivariate analysis, when adjusted for age, failed to sustain such an association.8 Alternatively, the status of p53 had little effect on the survival of GBM patients.9 In another study, 41 patients with long-term survival (>3 years) versus 48 patients with short-term survival (<1.5 years) showed that p53 expression was more common in long-term survival, irrespective of the specific types of p53 mutation.21 With regard to the morphological heterogeneity of GBM, another study reported that genetic abnormalities seen in the low-grade areas were conserved in the high-grade areas,22 suggesting that these morphologically different cellular subsets were derived from a common transformed clone. These findings might be helpful in detecting the presence or absence of tumor cell infiltrations in the peripheral margin or edematous area of GBMs. In this study, the gene expression of mutant p53 in the central and peripheral zone of GBM was examined to elucidate the relationship between their expression and overall patient survival. High mutant p53 gene expression was observed in the peripheral zone of 65% primary GBM, whereas the expression was only 35% in the central zone. Several initial studies have indicated that p53 was significantly altered in patients with malignant transformation, but not in those no apparent progression.23,24 For example, Chozick et al.24 have shown that among the six patients with malignant degeneration, the average percentage of p53 immunoreactivity was about 27%, as compared to only about 4% in patients with no apparent progression. It has been demonstrated that p53 mutations can be a feature of the subgroup of GBMs that are at the end stage of the progression towards malignancy.25 It remains to be tested whether GBMs arising in the absence of p53 inactivation also show a similar progression pattern. In addition, it will be of interest to see whether this genetic difference correlates with a specific clinical behavior, such as the fulminant clinical course of de novo GBMs or with long postoperative survival.26
This study reviews the association between the use of mobile phones and p53 gene expression in the different zones of glioblastoma multiforme as related to overall patient survival. P53 mutations that inactivated normal p53 function were detected in 59 out of 63 (93.6%) of the primary GBMs. Among the 59 tumors with p53 mutations, 9 showed a 100% yeast His2 phenotype, 40 showed more than 75% His2 phenotype, and 10 showed 20-75% His2 phenotype (data not shown). Of the 63 patients participating in our study, 41 (65%) reported cell phone use of more than three hours a day. Increased mutant p53 gene expression in the peripheral GBM zone of these 41 patients was significantly correlated to their cell phone use (P=0.034) and shorter overall survival time. It would be interesting to see if such a pattern holds true in a larger patient population. Occupational or other exposure to electromagnetic fields was excluded in all patients.
Due to its invasive nature, it is not generally possible to induce E-fields in living human subjects, and surrogate phantoms must be used. For assessing exposure from transmitters located near the body, the most useful quantity is the specific absorption rate (SAR), the amount of radiofrequency energy absorbed from the phone into the local tissues. The SAR of cell phones varies from 0.12 to 1.6 W/kg body weight, depending upon the model. Cell phones operate at different frequencies in different countries and continents. The SAR by our patients was 1.42 w/kg (Table 1).
The influence of p53 mutations in gliomas has already been discussed. Tumor cells carrying p53 mutations are resistant to apoptosis induced by DNA damage, while an overexpression of wild-type p53 enhances the radiosensitivity of glioma cells.27 However, the effect of p53 mutations on the efficacy of radio- and chemosensitivity in patients with gliomas, especially GBMs, remains controversial.
Some have reported that the p53 status of GBM patients did not affect their survival or sensitivity to radiotherapy.28-30 Others observed that the presence of p53 gene mutations in GBM was associated with longer survival and a better radiation response.31,32 Shiraishi et al.9 report that the time to tumor progression after surgery in patients receiving radio- and chemotherapy was not affected by the presence of p53 mutation. Therefore, the p53 gene mutation alone does not account for the radio- and chemoresistance of GBM. In the peripheral zone of glioblastomas, p53 gene expression was associated with a particularly poor prognosis, while expression in the central zone regions was not, suggesting that different p53 domains may affect patient survival differently. Our findings are hypothesis generating and cannot be used to show causality, but merely a correlation between p53 gene abnormalities and cell phone usage. Further investigations may yield information on whether this is also the case in patients with gliomas other than GBM.
Limitations of our study
The present work attempts to address an important and timely public health concern, namely, does long-term cell phone usage cause changes in p53 gene expression in the peripheral zone of glioblastoma? We have statistically analyzed our data to the best of our abilities; however, we also recognize the following limitations of the present study. First is the lack of a sufficiently large number of patients. We interviewed those patients with glioma who were eligible, mainly because rapid death and low level of consciousness prevented us from approaching all of them. As early death is most likely in patients with high grade gliomas, it is not surprising that participation rates were not high enough in our prospective study. Second, the design of the study relies on participants recalling the amount of their cell phone use, obtained through questionnaires and/or telephone interviews, rather than the potentially more accurate data that might have been obtained through the cell phone company records of study participants. Reliance on recall by a participant regarding time spent using a cell phone introduces the potential for recall bias, which can contribute to over- or underestimations of exposure time.
In the present study, our analysis did not control for all confounding variables due to the relatively small number of patients. Some biases may be inherent in this type of analysis, and we acknowledge this is a limitation. The number of cases presented here does not allow for any definitive conclusions. But because any connection between cell phone use and glioblastoma is a topic of interest, our work strives to increase the understanding of the molecular mechanism of glioblastoma formation in those patients using cell phones. Future studies should incorporate a larger number of patients. Because each cell phone model has a different specific absorption rate, differentiating between the effects of various models would also be important.
Conclusions
We found that p53 gene expression in the peripheral zone of glioblastoma was increased in 65% of patients who used cell phones more than 3 hours a day and that this increase was significantly correlated with shorter overall survival time. Our data, therefore, expand the understanding of the functions of p53 and demonstrate its role in the peripheral zone of glioblastoma as related to cell phone use and overall survival.
Due to a growing knowledge of GBM heterogeneity and subclassification, it would be reasonable to address these questions more accurately in well-defined subpopulations of patients.
Acknowledgments
Authors would thank Dr. Y. Kazemi from the Department of Internal Medicine and Dr. M.R. Khakzad from the Department of Immunology, Islamic Azad University, Medical Branch for their expertise in the evaluation and analysis of the patient data.
References
- 1.McLendon RE, Rich JN.Glioblastoma Stem cells: a neuropathologist’s view. J Oncol 2011;2011:397195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utsuki S, Oka H, Suzuki S, et al. Pathological and clinical features of cystic and noncystic glioblastomas. Brain Tumor Pathol 2006;23:29-34 [DOI] [PubMed] [Google Scholar]
- 3.Brat DJ, Prayson RA, Ryken TC, Olson JJ.Diagnosis of malignant glioma: role of neuropathology. J Neurooncol 2008;89: 287-311 [DOI] [PubMed] [Google Scholar]
- 4.Demuth T, Berens ME.Molecular mechanisms of glioma cell migration and invasion. J Neurooncol 2004;70:217-28 [DOI] [PubMed] [Google Scholar]
- 5.Groszer M, Erickson R, Scripture-Adams DD, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci USA 2006;103:111-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev 2001;15:1311-33 [DOI] [PubMed] [Google Scholar]
- 7.Ohgaki H.Genetic pathways to glioblastomas. Neuropathol Off J Jpn Soc Neuropathol 2005;25:1-7 [DOI] [PubMed] [Google Scholar]
- 8.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res 2004;64: 6892-9 [DOI] [PubMed] [Google Scholar]
- 9.Shiraishi S, Tada K, Nakamura H, et al. Influence of p53 mutations on prognosis of patients with glioblastoma. Cancer 2002;95:249-57 [DOI] [PubMed] [Google Scholar]
- 10.Vogelstein B, Lane D, Levine AJ.Surfing the p53 network. Nature 2000;408:307-10 [DOI] [PubMed] [Google Scholar]
- 11.Levine AJ.p53, the cellular gatekeeper for growth and division. Cell 1997;88:323-31 [DOI] [PubMed] [Google Scholar]
- 12.Newcomb EW, Cohen H, Lee SR, et al. Survival of patients with glioblastoma multiforme is not influenced by altered expression of p16, p53, EGFR, MDM2 or Bcl-2 genes. Brain Pathol Zurich Switz 1998;8:655-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe K, Sato K, Biernat W, et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin Cancer Res Off J Am Assoc Cancer Res 1997;3:523-30 [PubMed] [Google Scholar]
- 14.Olivier M, Hollstein M, Hainaut P.TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2010;2:a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukundan S, Holder C, Olson JJ.Neuroradiological assessment of newly diagnosed glioblastoma. J Neurooncol 2008;89:259-69 [DOI] [PubMed] [Google Scholar]
- 16.Neubauer A, Neubauer B, He M, et al. Analysis of gene amplification in archival tissue by differential polymerase chain reaction. Oncogene 1992;7:1019-25 [PubMed] [Google Scholar]
- 17.Ishioka C, Frebourg T, Yan YX, et al. Screening patients for heterozygous p53 mutations using a functional assay in yeast. Nat Genet 1993;5:124-9 [DOI] [PubMed] [Google Scholar]
- 18.Prives C, Manfredi JJ.The continuing saga of p53-more sleepless nights ahead. Mol Cell 2005;19:719-21 [DOI] [PubMed] [Google Scholar]
- 19.Akyüz N, Boehden GS, Süsse S, et al. DNA substrate dependence of p53-mediated regulation of double-strand break repair. Mol Cell Biol 2002;22:6306-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkar C, Sinha S, Sharma MC, et al. Supratentorial glioblastoma in adults: identification of subsets and their clinical correlation. Brain Tumor Pathol 2004;21:7-12 [DOI] [PubMed] [Google Scholar]
- 21.Burton EC, Lamborn KR, Forsyth P, et al. Aberrant p53, mdm2, and proliferation differ in glioblastomas from long-term compared with typical survivors. Clin Cancer Res Off J Am Assoc Cancer Res 2002;8:180-7 [PubMed] [Google Scholar]
- 22.Cheng Y, Ng HK, Ding M, et al. Molecular analysis of microdissected de novo glioblastomas and paired astrocytic tumors. J Neuropathol Exp Neurol 1999;58: 120-8 [DOI] [PubMed] [Google Scholar]
- 23.Sarkar C, Ralte AM, Sharma MC, Mehta VS.Recurrent astrocytic tumours - a study of p53 immunoreactivity and malignant progression. Br J Neurosurg 2002;16: 335-42 [DOI] [PubMed] [Google Scholar]
- 24.Chozick BS, Pezzullo JC, Epstein MH, Finch PW.Prognostic implications of p53 overexpression in supratentorial astrocytic tumors. Neurosurgery 1994;35:831-8 [DOI] [PubMed] [Google Scholar]
- 25.Sidransky D, Mikkelsen T, Schwechheimer K, et al. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature 1992;355:846-7 [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi T, Rempel SA, Rutz HP, et al. Turcot’s syndrome of glioma and polyposis occurs in the absence of germ line mutations of exons 5 to 9 of the p53 gene. Cancer Res 1993;53:957-61 [PubMed] [Google Scholar]
- 27.Broaddus WC, Liu Y, Steele LL, et al. Enhanced radiosensitivity of malignant glioma cells after adenoviral p53 transduction. J Neurosurg 1999;91:997-1004 [DOI] [PubMed] [Google Scholar]
- 28.Kraus JA, Glesmann N, Beck M, et al. Molecular analysis of the PTEN, TP53 and CDKN2A tumor suppressor genes in long-term survivors of glioblastoma multiforme. J Neurooncol 2000;48:89-94 [DOI] [PubMed] [Google Scholar]
- 29.Newcomb EW, Cohen H, Lee SR, et al. Survival of patients with glioblastoma multiforme is not influenced by altered expression of p16, p53, EGFR, MDM2 or Bcl-2 genes. Brain Pathol Zurich Switz 1998;8:655-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baxendine-Jones J, Campbell I, Ellison D.p53 status has no prognostic significance in glioblastomas treated with radiotherapy. Clin Neuropathol 1997;16:332-6 [PubMed] [Google Scholar]
- 31.Schiebe M, Ohneseit P, Hoffmann W, et al. Analysis of mdm2 and p53 gene alterations in glioblastomas and its correlation with clinical factors. J Neuroonco 2000;49:197-203 [DOI] [PubMed] [Google Scholar]
- 32.Tada M, Matsumoto R, Iggo RD, et al. Selective sensitivity to radiation of cerebral glioblastomas harboring p53 mutations. Cancer Res 1998;58:1793-7 [PubMed] [Google Scholar]


