Abstract
One-third of schwannomas occur in head and neck region, mostly in the parapharyngeal region. Cystic change is seen in only 4% of schwannomas. The diagnosis of such tumors remains a challenge due to the rarity of these lesions and limited utility of fine needle aspiration cytology. When cystic, branchial cleft cyst is an important differential diagnosis more so due to radiological resemblance. We present a case of 42-year-old male with left sided neck mass masquerading as branchial cleft cyst clinically and radiologically. Multiple sections examined from the cystic areas of the mass revealed lymphoid aggregates beneath the cyst wall in addition to the schwannomatous areas.
Key words: cystic schwannoma, branchial cyst, lymphoid aggregates
Introduction
One-third of schwannomas occur in head and neck region, the most common site being the parapharyngeal space.1 Cystic change is uncommon in these tumors being reported in 4% of schwannomas.2 The diagnosis of such tumors remains a challenge due to the rarity of these lesions and limited utility of fine needle aspiration cytology (FNAC) which is otherwise helpful in the diagnosis of other common lesions at this site. When cystic, branchial cleft cyst is an important differential diagnosis.3 The diagnosis in such cases can be made on histopathology. However there may be areas in a typical schwannoma which may mimic branchial cyst leading to further diagnostic confusion. It is important to differentiate between these two entities. Branchial cyst is known to recur and needs long term follow-up whereas schwannoma rarely recurs if completely excised. We hereby present a case of 42-year-old male with left sided neck mass diagnosed as schwannoma with areas of cystic degeneration and focal branchial cyst like morphology on histopathology.
Case Report
A 42-year-old male presented with complaints of a painless swelling in the left side of neck since 3 years gradually increasing in size. There was no history of trauma. Family and past history of tuberculosis was also negative. On examination, a 5×4 cm swelling was noted on the lateral side of neck in the middle third of anterior border of sternocleidomastoid. It was soft, mobile, non tender and not moving with deglutition. There were no neurological deficits. The skin over the swelling was freely mobile and not associated with discoloration. A clinical diagnosis of branchial cyst was made and the patient was sent for FNAC. The aspirate yielded only blood and was inconclusive.
Doppler neck was done which revealed a large cystic lesion in the posterior triangle adjacent to left external carotid artery and jugular vein with no communication or internal vascular supply. This cystic lesion also showed internal echoes and septation within the tumor. Computed tomography (CT) neck and thorax showed a well defined cystic lesion in the posterior triangle in relation to middle one-third of sternocleidomastoid having thin wall and internal septations (Figure 1a). The lesion was found to be displacing the sternocleidomastoid muscle anteriorly with maintained fat planes. No evidence of any significant lymphadenopathy was noted. The patient also started complaining of pain which was recent in onset and associated with burning sensation. Magnetic resonance imaging (MRI) soft tissue neck suggested the possibility of a third branchial cleft cyst (Figure 1b).
Figure 1.
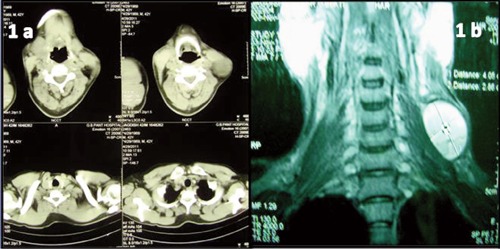
a) Computed tomography neck and thorax showed a well defined cystic lesion in the posterior triangle in relation to middle one-third of sternocleidomastoid having thin wall and internal septations. b) Magnetic resonance imaging of soft tissue neck was suggestive of third branchial cleft cyst.
The patient underwent FNAC three more times which were again inconclusive. The mass was finally excised. Per-operatively it was found to be well encapsulated and not adherent to the adjacent soft tissue. We received a cystic 4×4×3 cm mass. On cut section the cyst was divided by thin septae of 1 mm to 2 mm with a solid area of 2×2×1 cm at one pole. The cyst was filled with grey brown hemorrhagic material (Figure 2). Multiple sections were taken from both the solid and cystic areas. On morphological examination, the lesion revealed typical features of schwannoma with both hypercellular and hypocellular areas (Figure 3a). The Antoni A areas were cellular composed of spindle cells arranged in a palisading fashion. In Antoni B areas tumor cells were separated by abundant edematous fluid forming cystic spaces and giving a myxoid appearance. Mitosis was infrequent. Section from the cyst wall showed wall lined by hemosiderin laden macrophages distinctly positive on Prussian blue stain. However, typical schwannomatous areas were seen beneath the hemosiderin laden macrophages (Figure 3b). There was presence of lymphoid aggregates beneath the cyst wall resembling branchial cleft cyst like areas (Figure 3c). On immunohistochemistry, the tumor cells were strongly positive for S100 and focally for NSE (Figure 3d).
Figure 2.
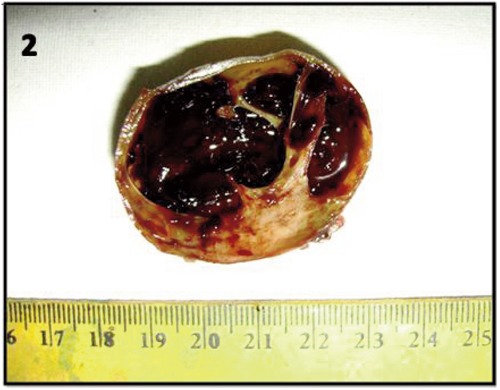
On cut section the cyst was filled with grey brown hemorrhagic material divided by thin septae with a solid area at one pole.
Figure 3.
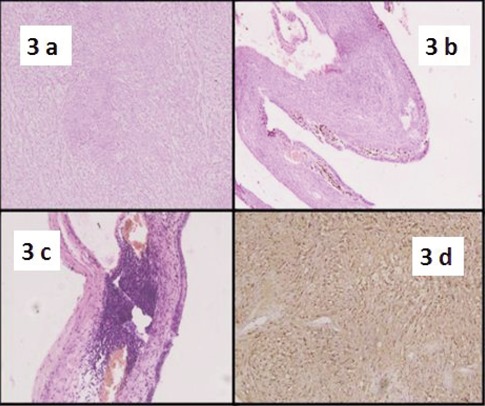
a) Schwannoma with both hypercellular and hypocellular areas (Hematoxylin and Eosin, 10×). b) Typical schwannomatous areas seen beneath the hemosiderin laden macrophages (Hematoxylin and Eosin, 10×). c) Presence of lymphoid aggregates beneath the cyst wall resembling branchial cleft cyst like areas (Hematoxylin and Eosin, 10×). d) Tumor cells strongly positive for S100 (DAB, 10×).
Discussion
Schwannomas are neurogenic tumors, which arises from the cells forming the neural sheath.4 About one-third occur in the head and neck region.1 They may arise from any cranial, peripheral or autonomic nerve.3 Schwannoma can arise from all cranial nerves in the head and neck region, the most common being the eighth cranial nerve.5 However in approximately 10-40% of cases, origin of nerve remains unidentified.6,7
The most common location of schwannomas of the head and neck region is the parapharyngeal space. Other locations include submandibular space, paranasal sinuses, cheek and oral cavity which are quite rare.5
When arising from the parapharyngeal space, they present as asymptomatic, slow growing neck masses along the medial border of sternocleidomastoid.2 Pre-operative diagnosis may be difficult because most do not have neurological deficits at presentation and varied much commoner differential diagnoses may have to be considered. These include branchial cleft cyst, paraganglioma, malignant lymphoma and metastatic cervical lymphadenopathy.8 Hoarseness is the most common symptom, if present. A paroxysmal cough may be elicited on palpating the mass, a clinical sign seen in vagal schwannoma.1 The diagnostic difficulty may be further compounded by presence of marked cystic change which is noted in 4% of schwannomas.2 Cystic schwannomas expand more rapidly than non cystic schwannomas and are often comparatively large due to cystic expansion. The cystic change has been attributed to necrosis, mucinous degeneration, hemorrhage and micro-cysts formation. Antoni B areas displaying loosely arranged spindle cells within a myxoid matrix are particularly prone to degeneration and cyst formation.2 In such cases, FNAC often yield hypocellular fluid, instead of material from the adjoining cellular areas. Hemorrhage within the cystic space may yield only blood thus resulting in consideration of a vascular lesion. Therefore, the pre-operative diagnosis of cystic schwannoma represents a diagnostic challenge not only due to its rarity but also due to the limited accuracy of FNAC techniques.2 Ultrasound-guided FNAC can contribute substantially as it may aid in aspiration from the hypercellular area.
Radiological investigation especially MRI has great role in the pre-operative work-up as it helps in diagnosis and also aids in evaluating the extent of the tumor and its relationship with the jugular vein and the carotid artery.5 The final diagnosis however rests on histopathological examination of the tissue displaying the characteristic Antoni A and Antoni B areas, palisading of bland appearing spindle tumor cells and formation of verocay bodies. Immunohistochemically schwannomas display strong positivity for S100.3
The first documented case of a cystic schwannoma of the neck masquerading as branchial cleft cyst clinically and ultrasonographically was reported by Buchanan et al.9 However histology proved it to be a cystic schwannoma.9 In this patient, besides the secondary degenerative changes of hemorrhage and cystic degeneration, lymphoid aggregates were noted at few foci in the cystic areas of the tumor. Lymphoid follicles and scattered lymphocytes have been reported in gastrointestinal schwannomas.10 Considering the location of the tumor and its cystic nature, branchial cleft cysts form one of the most important differential diagnoses. The presence of lymphoid follicles can further enhance the diagnostic confusion and lead to the misdiagnosis of branchial cyst if FNAC is performed from such an area. Hence, even when lymphoid aggregates appear in a cystic lesion, it is helpful to rule out the possibility of a cystic schwannoma.
It is of paramount importance to differentiate between these two entities both preoperatively and postoperatively as incomplete excision may lead to recurrence especially of branchial cyst. Therefore a case of excised branchial cyst needs long term monitoring.11 Surgical excision is recommended in case of schwannoma and conservative management has also a place in treatment. However, schwannoma rarely recurs if completely excised.12
Conclusions
When dealing with cystic neck masses, the possibility of schwannomas should be considered even when focal branchial cysts like areas are present. A thorough sampling of the tumor would most likely yield typical S100 positive hypocellular and hypercellular areas.
References
- 1.Chang SC, Schi YM.Neurilemmoma of the vagus nerve: a case report and brief literature review. Laryngoscope 1984;94:946-9 [DOI] [PubMed] [Google Scholar]
- 2.Wakoh M, Yonezu H, Otonari T, et al. Two cases of schwannoma with marked cystic changes. Dentomaxillofac Radiol 2005;34:44-50 [DOI] [PubMed] [Google Scholar]
- 3.Lanham PD, Wushensky C.Second brachial cleft cyst mimic: case report. Am J Neuroradiol 2005;26:1862-4 [PMC free article] [PubMed] [Google Scholar]
- 4.Lahoti BK, Kaushal M, Garge S, Aggarwal G.Extra vestibular schwannoma: a two year experience. Indian J Otolaryngol Head Neck Surg 2011;63:305-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma DK, Sohal BS, Parmar TL, Arora H.Schwannomas of head and neck and review of literature. Indian J Otolaryngol Head Neck Surg 2012;64:177-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Ghamdi S, Black MJ, Lafond G.Extracranial head and neck schwannomas. J Otolaryngol 1992;21:186-8 [PubMed] [Google Scholar]
- 7.Sharaki MM, Talaat M, Hamam SM.Schwannoma of the neck. Clin Otolaryngol 1982;7:245-51 [DOI] [PubMed] [Google Scholar]
- 8.Colreavy MP, Lacy PD, Hughes J, et al. Head and neck schwannomas: a 10-year review. J Laryngol Otol 2000;114:119-24 [DOI] [PubMed] [Google Scholar]
- 9.Buchanan MA, Williams SM, Hellquist H, Innes AJ.Cystic schwannoma of the cervical plexus masquerading as a type II second branchial cleft cyst. Eur Arch Otorhinolaryngol 2009;266:459-62 [DOI] [PubMed] [Google Scholar]
- 10.Park KJ, Kim KH, Roh YH, et al. Isolated primary schwannoma arising on the colon: report of two cases and review of the literature. Korean Surg Soc 2011;80:367-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu YJ, Li YD, Qu XZ, et al. [Clinical analysis of branchial cleft cyst (fistula): report of 284 cases]. Shanghai Kou Qiang Yi Xue 2008;17:461-4 [Article in Chinese] [PubMed] [Google Scholar]
- 12.Kang GC, Soo KC, Lim DT.Extracranial non-vestibular head and neck schwannomas: a ten-year experience. Ann Acad Med [PubMed] [Google Scholar]


