Abstract
G-protein-coupled receptors regulate signal transduction pathways and play diverse and pivotal roles in the physiology of insects, however, the precise function of GPCRs in insecticide resistance remains unclear. Using quantitative RT-PCR and functional genomic methods, we, for the first time, explored the function of GPCRs and GPCR-related genes in insecticide resistance of mosquitoes, Culex quinquefasciatus. A comparison of the expression of 115 GPCR-related genes at a whole genome level between resistant and susceptible Culex mosquitoes identified one and three GPCR-related genes that were up-regulated in highly resistant Culex mosquito strains, HAmCqG8 and MAmCqG6, respectively. To characterize the function of these up-regulated GPCR-related genes in resistance, the up-regulated GPCR-related genes were knockdown in HAmCqG8 and MAmCqG6 using RNAi technique. Knockdown of these four GPCR-related genes not only decreased resistance of the mosquitoes to permethrin but also repressed the expression of four insecticide resistance-related P450 genes, suggesting the role of GPCR-related genes in resistance is involved in the regulation of resistance P450 gene expression. This results help in understanding of molecular regulation of resistance development in Cx. quinquefasciatus.
The development of insecticide resistance is a serious practical problem associated with the chemical control of disease-borne vector insects. One such insect is the mosquito, Cx. quinquefasciatus, which is a primary vector of the pathogens responsible for West Nile encephalitis, eastern equine encephalitis, Saint Louis encephalitis, and lymphatic filariasis and inhabits tropical and subtropical regions worldwide1,2,3. Pyrethroids are particularly suitable for controlling mosquito vectors and, consequently, for controlling against mosquito-borne diseases. However, the development of resistance to pyrethroids in mosquitoes has become an urgent and widespread problem for mosquito-borne disease control efforts worldwide4,5,6. Pyrethroid resistance has been found in a number of Culex mosquito species7,8, so a better understanding of the molecular basis of resistance mechanisms should provide new strategies for mosquito control. Cytochrome P450s play a pivotal role in insecticide detoxification in mosquitoes via an increased expression level in resistant mosquitoes9,10,11,12. Studies on P450 gene up-regulation or induction in response to xenobiotics have indicated that cis- or trans-acting regulators are involved in transcriptional regulation in the house fly13,14,15. Transcriptional element regulated xenobiotic induction of P450 genes has been functionally characterized in several other insect species. These includ EcRE/ARE/XRE-xan elements of Cyp6b promoters in tiger swallowtail16 and black swallowtail17 butterflies, the EcRE element of Cyp6b in black swallowtail butterflies18, the CuRE element of Cyp9m10 in mosquitoes19, and the XRE-Fla element of Cyp321a1 in corn earworms20, and the CncC element of Cyp6a has been shown to be involved in phenobarbital (PB) induction in fruit fly, Drosophila melanogaster21. Bhaskara et al.22 reported that the caffeine-inducible promotion of Cyp6a genes was probably regulated through the cAMP pathway in D. melanogaster. However, the up-stream regulatory pathway of P450 up-regulation or induction in insects remains largely unknown.
G-protein-coupled receptors (GPCRs) are integral membrane proteins with seven α-helical hydrophobic domains that carry out specific physiological functions23. They are divided into several subfamilies based on sequence similarity24. The function or dysfunction of GPCRs may cause various changes in cellular response, so GPCRs are major drug targets for a wide range of diseases and play a critical role in the development of new medical treatments for humans25,26. In insects, GPCRs have been shown to regulate physiological pathways27 and affect insect behavior28, reproduction29, development30,31, and metabolism32. Moreover, recent studies have indicated that GPCRs could also serve as targets for the development of new insecticides33; a dopamine receptor, AaDOP2, has been identified as the target for new larvicide development in Aedes aegypti by cell-based chemical screen34,35. Rediocides, found in a natural terpenoid-based plant extract with insecticidal properties, could act to inhibit Drosophila GPCR by decreasing the calcium response and thus leading to GPCR insensitivity via protein kinase C activation in HEK 293 cells36. Interestingly, up-regulated GPCRs have also been reported in resistant Culex mosquitoes37,38. Multiple gene interaction and regulation play pivotal roles in insecticide resistance37,39,40. Thus, the transcriptionally up-regulated gene expression of GPCRs in insecticide resistance offers a promising new avenue for research. To address the potential function of GPCRs and GPCR-related genes in the development of insecticide resistance in the mosquito, Cx. quinquefasciatus, we therefore characterized the expression level of a total of 115 GPCR and GPCR-related genes in a series of susceptible to highly resistant Culex mosquito strains. Double-stranded RNA interference (RNAi) was utilized to silence the up-regulated GPCR-related genes in the resistant mosquito strains in order to investigate their function in insecticide resistance by regulating the expression of resistance-related P450 genes. Our findings suggest that the resulting interference with GPCR and GPCR-related gene expression could provide a new opportunity for resistance prevention in mosquitoes and other insect pests.
Results
GPCR genes in Cx. quinquefasciatus genome
Based on the GPCR annotation information in the GPCRDB and the Cx. quinquefasciatus genome, we tested 68 GPCR genes in this study. These consisted of 52 annotated GPCR genes in the class A, rhodopsin-like family, with 15 amine receptors, 25 peptide receptors, 1 adenosine A2 receptor, and 11 opsin receptors; 4 GABAB receptors; and 12 orphan GPCR genes (Table 1). Given the focus of this study is on GPCR-biological process analysis, 47 GPCR-related genes were also identified as potential GPCRs regulatory pathways in insecticide resistance regulation. The predominance of rhodopsin-like GPCRs in the Culex genome suggests that this cluster of GPCR genes is likely to perform a critical function in various signal transduction pathways in Cx. quinquefasciatus.
Table 1. Summary of GPCRs identified in Culex quinquefasciatus.
| Class A | Amines | Dopamine | 3 |
| Adrenergic | 4 | ||
| Muscarinic acetylcholine | 1 | ||
| Octopamine | 4 | ||
| 5-hydroxytryptamine | 2 | ||
| FMRFamide | 1 | ||
| Peptides | Neuromedin U like | 1 | |
| Myokinin | 1 | ||
| Sulfakinin | 2 | ||
| Endothelin B | 1 | ||
| Cardioacceleratory peptide | 2 | ||
| Cholecystokinin CCK | 1 | ||
| Neuropeptide Y | 2 | ||
| Calcitonin | 3 | ||
| Somatostatin | 2 | ||
| Substance P | 1 | ||
| Pyrokinin | 2 | ||
| Cap2b | 1 | ||
| Diuretic hormone | 1 | ||
| Corazonin | 1 | ||
| Allatostatin | 3 | ||
| Gonadotropin releasing hormone | 1 | ||
| Opsin | 11 | ||
| Adenosine A2 | 1 | ||
| Class C | GABAB | 4 | |
| Orphan | 12 | ||
| Other GPCR-related genes (by biological process relationship) | 47 | ||
| Total | 115 |
Relative expression of GPCR-related genes in the Cx. quinquefasciatus strains
Understanding the changes in the GPCR-related gene expression in resistant and susceptible mosquito strains could shed light on the potential roles of these genes in the development of insecticide resistance in mosquitoes. In this study, we characterized a total of 115 GPCR and GPCR-related genes by examining their expression levels in both larvae and adults of five mosquito strains using qRT-PCR with 115 uniquely designed primer pairs (Table S1). The five strains exhibit different resistance profiles in response to permethrin, ranging from the most susceptible strain, S-Lab, through the intermediately resistant strains, HAmCqG0 and MAmCqG0, to the highly resistant strains, HAmCqG8 and MAmCqG6 8. The GPCR-related gene expression profiles revealed that four GPCR and GPCR-related genes were significantly up-regulated and 11 genes were down-regulated in the highly resistant mosquitoes compared to the intermediately resistant and susceptible strains. However, the remaining 100 genes were equally expressed in all strains (Table S2). These results suggest the possibility that these up-regulated GPCR-related genes are indeed involved in permethrin resistance in Culex mosquitoes.
Up-regulated GPCR genes in both larvae and adults of the resistant strains HAmCqG8 and MAmCqG6
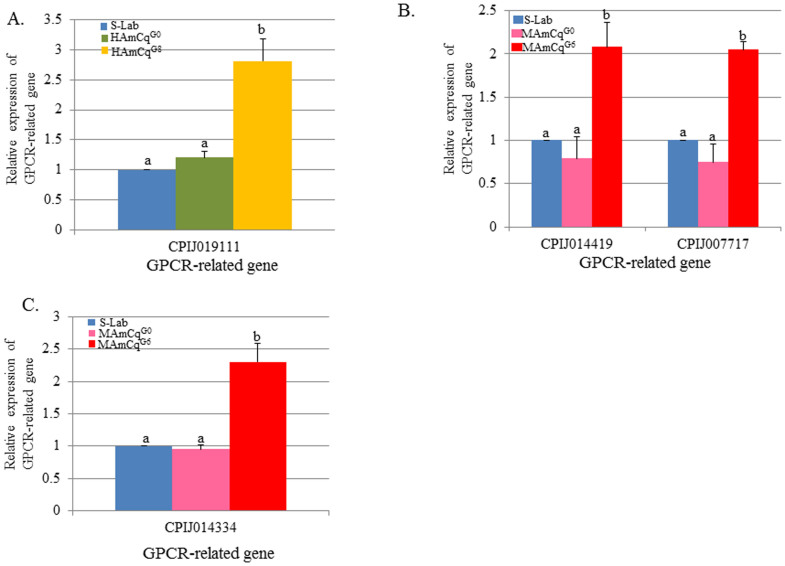
We found that a GPCR-related gene (CPIJ019111, conserved hypothetical protein) was significantly up-regulated (~3-fold) in the 4th instar of HAmCqG8 compared with S-Lab and HAmCqG0 (P ≤ 0.001) (Fig. 1A). A calcitonin receptor (CPIJ014419) was significantly up-regulated (~2-fold) in the 4th instar of MAmCqG6 compared with S-Lab and MAmCqG0 (P = 0.002). A similar expression pattern was also found for a GPCR-related gene coding for a conserved hypothetical protein (CPIJ007717); the expression of the gene was ~2-fold higher in the 4th instar of MAmCqG6 compared with S-Lab and MAmCqG0 (P ≤ 0.001) (Fig. 1B). A pteropsin gene (CPIJ014334) was expressed at a significantly higher rate, ~2.5-fold higher, in adult MAmCqG6 mosquitoes than in MAmCqG0 and S-Lab adults (P ≤ 0.001) (Fig. 1C). All these up-regulated GPCR-related genes in HAmCqG8 and MAmCqG6 adults have similar expression levels in both S-Lab and HAmCqG0 adults (Fig. 1). These results indicate the importance of the up-regulated GPCR-related genes in permethrin resistance following permethrin selection.
Figure 1. Relative expression of 4 GPCR-related genes analyzed by qRT-PCR in mosquito strains of Cx. quinquefasciatus.
A. Relative expression of a GPCR-related gene, CPIJ019111, in 4th instar larvae of S-Lab, HAmCqG0 and HAmCqG8. B. Relative expression of 2 GPCR-related genes, CPIJ014419 and -007717, in 4th instar larvae of S-Lab, MAmCqG0 and MAmCqG6. C. Relative expression of a GPCR-related gene, CPIJ014334, in 3-day-old adult S-Lab, MAmCqG0 and MAmCqG6. The relative gene expression shown along the Y axis is the ratio of the gene expression in each resistant strain compared with the susceptible strain. The results are shown as the mean ± S.E. There was significant difference (P ≤ 0.05) in the levels of P450 gene expression among the samples with the different alphabetic letter (i.e., a, b, or c).
Down-regulated GPCR genes in both larvae and adults of the resistant strains HAmCqG8 and MAmCqG6
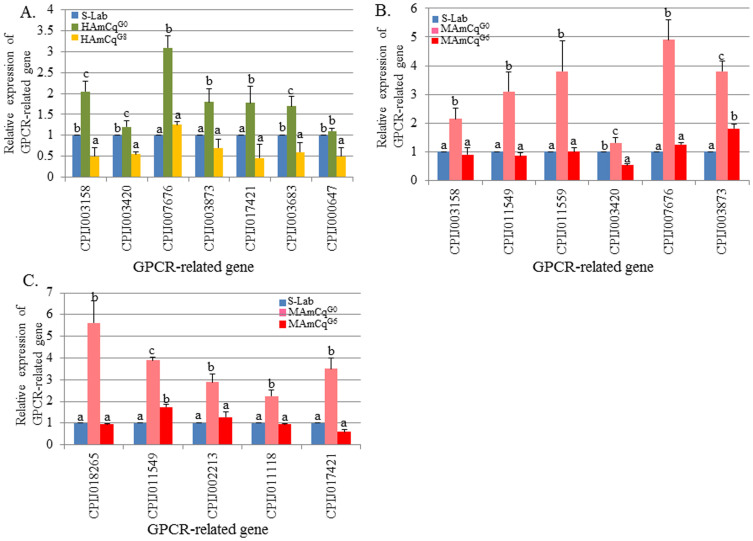
With regard to GPCRs that were down-regulated in the permethrin selected strains using qRT-PCR, we found several GPCR and GPCR-related genes were down-regulated (≤-2-fold) in HAmCqG8 and MAmCqG6 compared with their parental strains. Seven genes were significantly down-regulated in the larvae of HAmCqG8 compared to the parental strain HAmCqG0, including CPIJ003158 (GPCR) (P = 0.003), -003873 (beta adrenergic receptor) (P = 0.028), -003683 (5-hydroxytryptamine receptor 2B) (P = 0.015), and 4 GPCR-related genes as following -003420 (P = 0.007), -007676 (P ≤ 0.001), -017421(P = 0.042), and -000647(P = 0.035) (Fig. 2A). The expression of the majority of these genes in HAmCqG8 was at a lower level than that observed in the susceptible S-Lab mosquitoes, even though most were expressed at higher levels in HAmCqG0 than in S-Lab (Fig. 2A). We also found a similar down-regulation pattern in MAmCqG6 larvae compared with that in MAmCqG0 and S-Lab larvae, with a GPCR gene (CPIJ003158) (P = 0.031), a calcitonin receptor (-011559) (P = 0.04), a beta adrenergic receptor (-003873) (0.002), and 3 GPCR-related genes as following -011549 (P = 0.018), -003420 (P = 0.01), and -007676 (P = 0.002) all having ~2-fold significantly lower expression than in MAmCqG0 (Fig. 2B). Most of these genes exhibited similar expression levels in MAmCqG6 larvae compared with those in S-Lab larvae, even though they were expressed at higher levels in MAmCqG0 than in S-Lab (Fig. 2B). Moreover, given the study of down-regulated genes in adult MAmCqG6 mosquitoes compared with MAmCqG0 adults, 5 genes were significantly down-regulated (≤2-fold): a GPCR gene (CPIJ018265) (P = 0.003), an allatostatin receptor (-011118) (P = 0.004), and 3 GPCR-related genes as following -011549 (P = 0.011), -002213 (P = 0.011), -017421 (P = 0.046) (Fig. 2C). Again, the expression levels of these down-regulated genes were similar in adult MAmCqG6 mosquitoes compared with S-Lab adults, even though all were at higher levels in MAmCqG0 compared with S-Lab (Fig. 2C). In summary, the GPCR gene (CPIJ003158), a beta-adrenergic receptor (-003873), and 2 GPCR-related genes (-003420, -007676) were down-regulated in the larvae of both HAmCqG8 and MAmCqG6, and CPIJ011549 was down-regulated in both larvae and adults MAmCqG6 mosquitoes. The GPCR-related gene (CPIJ017421) was down-regulated in both HAmCqG8 larvae and MAmCqG6 adults. The down-regulated GPCR-related genes represented in the resistant mosquito strains probably shut down after permethrin selection in order to conserve energy, which could then be used for the production of resistance-related gene expression.
Figure 2. Relative expression of 11 GPCR-related genes analyzed by qRT-PCR in mosquito strains of Cx. quinquefasciatus.
(A). Relative expression of 7 GPCR-related genes in 4th instar larvae of HAmCqG0 and HAmCqG8. (B). Relative expression of 6 GPCR-related genes in 4th instar larvae of MAmCqG0 and MAmCqG6. (C). Relative expression of 5 GPCR-related genes in 3-day-old adult MAmCqG0 and MAmCqG6. The Y-axis represents the fold change of the expression of each gene in resistant strains compared with the susceptible S-Lab strain. The results are shown as the mean ± S.E. There was significant difference (P ≤ 0.05) in the levels of P450 gene expression among the samples with the different alphabetic letter (i.e., a, b, or c).
Microinjection of CPIJ019111 dsRNA of in HAmCqG8
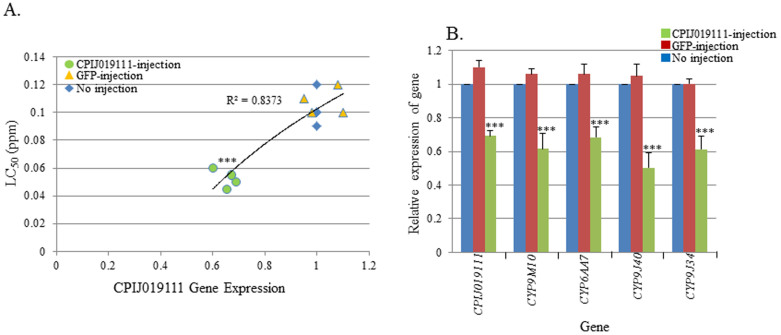
To investigate the function of the up-regulated GPCR-related genes in Culex mosquitoes, we injected CPIJ019111 dsRNA into HAmCqG8 embryos to knockdown the target gene in HAmCqG8 larvae. Comparing the relative gene expression and larval sensitivity to permethrin in the CPIJ019111 dsRNA injected HAmCqG8 larvae with those of GFP-injected or no-injected larvae revealed a strong correlation between the gene expression of CPIJ019111 and sensitivity to permethrin (R2 = 0.8373). Knockdown of CPIJ019111 (1.6-fold decrease in gene expression, P ≤ 0.001) significantly correlated with the decrease of the LC50 of permethrin in CPIJ019111 dsRNA-injected larvae compared to GFP-injected and non-injected larvae (Fig. 3A). To confirm the involvement of CPIJ019111 dsRNA in the regulation of resistance-related P450 gene expression, we tested changes in the expression of 4 P450 genes (CYP9M10, -9J34, -9J40, -6AA7) known to be involved in permethrin resistance in Cx. quinquefasciatus11,12 in CPIJ019111 knockdown mosquitoes. The results showed that suppression of CPIJ019111 expression also significantly decreased the expression of all four P450 genes (Fig. 3B, P ≤ 0.001).
Figure 3. Functional study of the GPCR019111 gene in the permethrin resistant HAmCqG8 strain using RNAi.
(A). The correlation between GPCR019111 expression and susceptibility to permethrin was determined by dsRNA knockdown of the GPCR019111 gene in HAmCqG8 mosquitoes compared to non-injected and GFP-injected mosquitoes. The Y-axis represents the LC50 of permethrin in dsRNA-injected and non-injected mosquitoes. (B). Relative expression of 4 cytochrome P450 genes in dsRNA of GPCR019111-injected, GFP-injected and non-injected mosquitoes. The relative gene expression shown along the Y-axis is the ratio of the gene expression in dsRNA-injected mosquitoes compared with that in non-injected mosquitoes. The results are shown as the mean ± S.E. Significant differences are indicated by *(P ≤ 0.05), **(P ≤ 0.01), and ***(P ≤ 0.001).
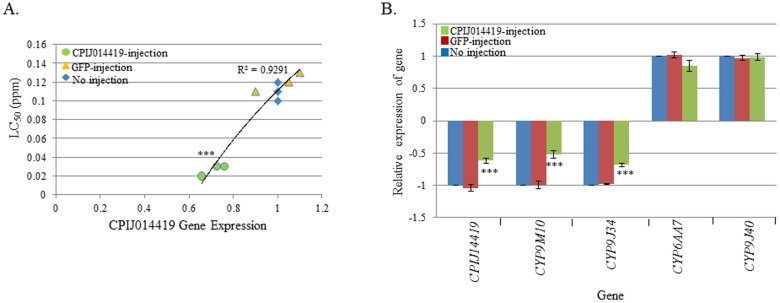
Microinjection of CPIJ007717 and -014419 dsRNAs into MAmCqG6
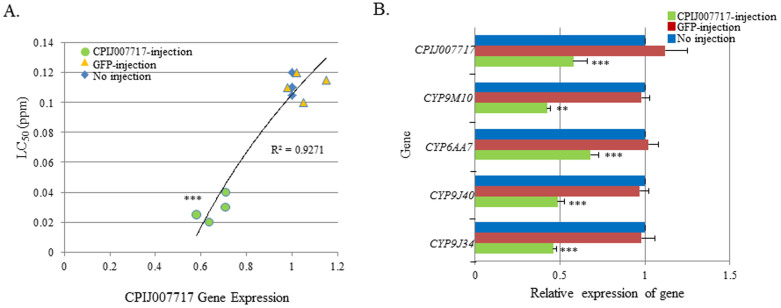
Two of the up-regulated GPCR-related genes, CPIJ007717 and CPIJ014419, may play critical roles in permethrin resistance in MAmCqG6. Thus, CPIJ007717 and -014419 dsRNAs were each injected into early embryos of MAmCqG6 to investigate their function in insecticide resistance. The results revealed that knockdown of the CPIJ007717 gene (with 1.9-fold decrease in expression, P ≤ 0.001) was significantly correlated with an increased sensitivity to permethrin and decreased LC50 (R2 = 0.9271, Fig. 4A). The regulatory function of CPIJ007717 on resistance-related P450 gene expression was also confirmed, as repression of the CPIJ007717 gene significantly decreased the expression of 4 P450 genes (P = 0.003 for CYP9M10 or P ≤ 0.001, Fig. 4B). We also found a similar result in MAmCqG6 larvae with dsRNA interference of CPIJ014419, where a strong correlation was observed between the expression of CPIJ014419 and sensitivity to permethrin (R2 = 0.9291). Decreased expression of CPIJ014419 (1.7-fold) significantly decreased the LC50 of permethrin in larvae of CPIJ014419 injected MAmCqG6 mosquitoes compared with GFP-injected and non-injected larvae (P ≤ 0.001, Fig. 5A). Examining the regulatory function of CPIJ014419 on P450 gene expression revealed that the expression two P450 genes, CYP9M10 and -9J34, was significantly decreased in CPIJ014419 asRNA-injected mosquitoes (P ≤ 0.001, Fig. 5B). However, two other P450 genes, CYP6AA7 and -9J40, exhibited no change in gene expression following the CPIJ014419 expression repression (Fig. 5B). These results confirmed the importance of up-regulated GPCR-related genes in permethrin resistance and the involvement in the regulation of some of insecticide resistance-related P450 genes in MAmCqG6 mosquitoes.
Figure 4. Functional study of the GPCR007717 gene in the permethrin resistant MAmCqG6 strain using RNAi.
(A). The correlation between GPCR007717 and susceptibility to permethrin was characterized by dsRNA knockdown of the GPCR007717 gene in MAmCqG6 mosquitoes compared to non-injected and GFP-injected mosquitoes. The Y-axis represents the LC50 of permethrin in dsRNA-injected and non-injected mosquitoes. (B). Relative expression of 4 cytochrome P450 genes in dsRNA of GPCR007717-injected, GFP-injected and non-injected mosquitoes. The relative gene expression shown along the X-axis is the ratio of the gene expression in dsRNA-injected mosquitoes compared with that in non-injected ones. The results are shown as the mean ± S.E. Significant differences are indicated by *(P ≤ 0.05), **(P ≤ 0.01), and ***(P ≤ 0.001).
Figure 5. Functional study of the GPCR014419 gene in the permethrin resistant MAmCqG6 strain using RNAi.
(A). The correlation between GPCR014419 and susceptibility to permethrin was characterized by dsRNA knockdown of the GPCR014419 gene in MAmCqG6 mosquitoes compared to non-injected and GFP-injected mosquitoes. The Y-axis represents the LC50 of permethrin in dsRNA-injected and non-injected mosquitoes. (B). Relative expression of 4 cytochrome P450 genes in dsRNA of GPCR014419-injected, GFP-injected and non-injected mosquitoes. The relative gene expression shown along the Y-axis is the ratio of the gene expression in dsRNA-injected mosquitoes compared with that in non-injected ones. The results are shown as the mean ± S.E. Significant differences are indicated by *(P ≤ 0.05), **(P ≤ 0.01), and ***(P ≤ 0.001).
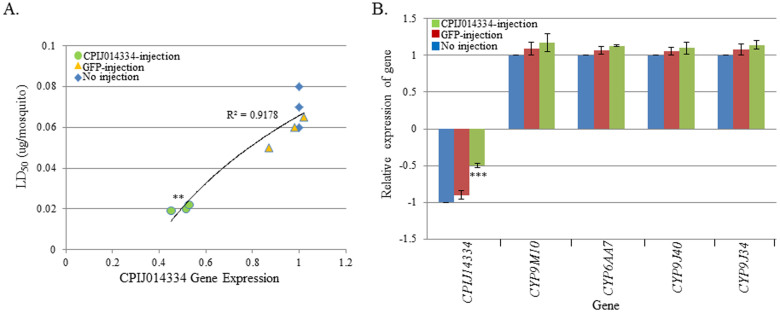
Microinjection of CPIJ014334 dsRNA into adults of MAmCqG6
As only one GPCR-related gene, CPIJ014334, was found to be up-regulated in adults of the highly resistant mosquito strain, MAmCqG6, we injected CPIJ014334 dsRNA into adult MAmCqG6 mosquitoes. A 2-fold decrease in the expression of CPIJ014334 gene was significantly correlated with a decreased LD50 of permethrin in 3-day post-injection female mosquitoes compared to their non-injected and GFP-injected counterparts (R2 = 0.9178, P = 0.008, Fig. 6A). However, testing the expression of four P450 genes in CPIJ014334 knockdown mosquitoes revealed no changes in the expression of the P450 genes (Fig. 6B), indicating that the CPIJ014334 gene may perform a regulatory function on the expression of another resistance-related gene in adult MAmCqG6 mosquitoes.
Figure 6. Functional study of the GPCR014334 gene in female adults of the permethrin resistance strain MAmCqG6 using RNAi.
(A). The correlation between GPCR014334 and susceptibility to permethrin was characterized by dsRNA knockdown of the GPCR014334 gene in MAmCqG6 mosquitoes compared to non-injected and GFP-injected mosquitoes. The Y-axis represents the LC50 of permethrin in dsRNA-injected and non-injected mosquitoes. (B). Relative expression of 4 cytochrome P450 genes in dsRNA of GPCR014334-injected, GFP-injected and non-injected mosquitoes. The relative gene expression shown along the Y-axis is the ratio of the gene expression in dsRNA-injected mosquitoes compared with that in non-injected mosquitoes. The results are shown as the mean ± S.E. Significant differences are indicated by *(P ≤ 0.05), **(P ≤ 0.01), and ***(P ≤ 0.001).
Discussion
Given the critical function of GPCRs in insect development, reproduction, and metabolism, it is not surprising that GPCRs have been characterized in a wide range of insect species, with 276 GPCRs being identified in Anopheles gambiae41, 135 non-sensory and opsin GPCRs in Aedes aegypti33, ~70 neurohormone GPCRs in the red flour beetle42,43, 56 neurohormone receptor genes in Apis mellifera and 69 in Drosophila melanogaster30, as well as 107 GPCRs in the Pediculus humanus whole genome44. Cx. quinquefasciatus genome sequencing allowed us to select 68 annotated GPCR genes and 47 GPCR biological processes that are known to be pathway-related genes for inclusion in the current study. Most of the GPCRs in Cx. quinquefasciatus belong to class A (Rhodopsin-like GPCR), which comprises the largest family of GPCRs and could be transducers that bind a striking and extraordinary range of ligands, including light, peptides, lipids, and nucleotides, in both vertebrates and invertebrates45, indicating the importance of the rho-like GPCRs in Culex mosquitoes.
Our previous studies demonstrated that GPCR and the GPCR-regulatory pathway may be involved in the development of insecticide resistance in Cx. quinquefasciatus37. Moreover, GPCRs have also been identified as a promising new target for future insecticide development31,41. Thus, a better understanding of the precise role of GPCR and GPCR-related genes in insecticide resistance will support the development of innovative strategies to control mosquitoes and mosquito-borne diseases. To adapt to xenobiotic exposure, insects employ a coordinated transcriptional response, an example of which is gene up-regulation in insecticide resistant insects. Thus, we expected that GPCR-related genes, which may play a role in permethrin resistance, would be up-regulated in resistant mosquitoes following permethrin selection. In the research reported here, we have, for the first time, examined the expression profiles of 115 GPCR and GPCR-related genes in larvae and adult Cx. quinquefasciatus mosquitoes by comparing the different expression levels among two highly resistant mosquito strains, HAmCqG8 and MAmCqG6, two intermediately resistant strains, HAmCqG0 and MAmCqG0, and a susceptible strain, S-Lab. Our results reveal a dynamic change in the expression of these 115 GPCR-related genes between the permethrin resistant and susceptible strains. Interestingly, the genes that were up-regulated following permethrin selection in the mosquito larval stage were not found in the adult stage, and vice versa. Additionally, comparing the up-regulated GPCR-related genes in the two highly resistant mosquito strains revealed different gene sets to be involved in their response to permethrin selection, indicating the possibility of different regulation pathways for the GPCRs in different mosquito strains.
Our study also revealed the existence of down-regulated GPCR expression following permethrin selection in Cx. quinquefasciatus. Although the mechanisms by which down-regulated GPCRs could contribute to insecticide resistance are still unclear, a number of mechanisms to explain this effect have been proposed in other insects: 1) down-regulation of the odorant receptor gene (putative GPCR) after blood feeding in A. gambiae could modify the organism's odorant response profile46; 2) down-regulation of GPCR genes was thought likely to be a response to low-intensity malarial parasites in A. gambiae47; and 3) down-regulated GPCR (PBAN-receptor) in the 4th instar to 1st and 2nd pupa of Ae. aegypti compared to other stages corresponded to the up-regulation of another GPCR (diapause hormone receptor)48. The evidence and hypotheses reported in these studies all suggest reasonable explanations for the potential function of down-regulated GPCRs corresponding to different biological pathways in insects. Taken together, the findings of the current study may indicate that the down-regulated GPCR and GPCR-related genes were involved in the response to permethrin selection by homeostatically reacting to balance the up-regulated GPCR-related gene expression.
The use of the RNAi technique for this functional study of up-regulated GPCR-related genes in highly resistant mosquitoes provides a powerful tool for silencing gene expression post-transcriptionally49,50. For example, Bai et al. used the RNAi technique to determine the precise functions of non-sensory GPCR genes in the red flour beetle, Tribolium castaneum, including 6 GPCRs that had critical effects on larval and pupal molting and mortality31. In the current study, we used the RNAi method to repress the GPCR gene expression in resistant mosquitoes. Our findings revealed that knockdown of the up-regulated GPCR-related genes (CPIJ019111,-007717, -014419, -14413) was strong correlated with decreased resistance to permethrin, signifying the important function of these GPCR-related genes in the permethrin resistance of Cx. quinquefasciatus.
Cytochrome P450 gene up-regulation is known to be involved in insecticide resistance in insects11,51,52, but the regulatory mechanisms of P450 up-regulation are still unclear. GPCRs play essential roles in switching on chemical signals in response to environmental stimuli and in triggering several specific downstream signaling pathways with targets in the cell membrane, cytoplasm, and nucleus53,54. It has also been shown that GPCRs could be regulatory factors for resistance-related P450 gene regulation in the housefly15. Taken together, we think it likely that the regulatory function of these up-regulated GPCR genes in insecticide resistance is also linked to the regulation pathway of resistance-related P450 gene expression. The results demonstrated the decreased expression of P450 genes following the knockdown of GPCR-related genes (CPIJ019111, -007717, and -14419), suggesting that these up-regulated GPCR-related genes could be critical for P450 gene expression. However, knockdown of the GPCR-related gene (CPIJ014413) in adult MAmCqG6 mosquitoes lowered resistance to permethrin but had no effect on the expression of any of these four P450 genes, suggesting that it either regulates the expression of another resistance-related P450 gene besides that is not one of the four genes tested or is involved in a completely separate regulatory pathway for resistance-related gene expression. The mechanisms by which the GPCR regulation pathway in permethrin resistant Culex mosquitoes appears to operate also suggest that the importance of the up-regulation of GPCR-related gene expression lies in its ability to regulate the resistance-related gene expression beyond the signal stimulation that caused the original cell response.
This study has shed new light on the potential function of GPCR and GPCR-related genes in insecticide resistance and their regulatory function on resistance-related P450 gene expression. However, the entire regulatory pathway remains largely unclear. Thus, future research will focus on the downstream factors in the GPCR regulatory pathway in order to characterize their involvement in insecticide resistance and, consequently, support the effort to identify new insecticide targets and strategies with which to control mosquitoes and mosquito-borne diseases, as well as helping to build a better understanding of the regulatory pathway of insecticide detoxification and evolutionary insecticide selection in mosquitoes.
Methods
Mosquito strains
Five mosquito strains of Cx. quinquefasciatus were used in this study. Two field strains, HAmCqG0 and MAmCqG0, were collected from Madison County and Mobile County, AL, respectively, which are located more than 600 km apart; HAmCqG8 is the eighth generation of permethrin-selection HAmCqG0 offspring; MAmCqG6 is the sixth generation of permethrin-selected MAmCqG0 offspring8,12; and S-Lab is an insecticide susceptible strain kindly provided by Dr. Laura Harrington (Cornell University, Ithaca, NY). All the mosquitoes were reared at 25 ± 2°C under a photoperiod of 12:12 (L:D) h (insectary conditions) and fed blood samples from horses (Large Animal Teaching Hospital, College of Veterinary Medicine, Auburn University, Auburn, AL).
RNA extraction and cDNA preparation
Total RNAs were extracted from 4th instar larvae and 3 day-old adults (collected prior to blood feeding) of each mosquito strain using the acidic guanidine thiocyanate-phenol-chloroform method14. The DNA was removed from total RNA (5 ug) using TURBO DNA-free (Ambion) following the manufacturer's instructions. cDNA was synthesized using the DNA-free RNA as a template and the Transcriptor First Strand cDNA Synthesis kit (Roche), following the manufacturer's instructions. The quantity of cDNA produced was measured by a spectrophotometer prior to qRT-PCR. Each experiment was repeated more than 3 times, with independent RNA preparation and cDNA synthesis.
Quantitative real-time PCR (qRT-PCR)
According to the database maintained by GPCRDB (http://www.gpcr.org/7tm/search) and GPCR biological processes related GPCR genes (https://www.vectorbase.org/search), 68 GPCR genes and 47 GPCR-related gene full length were exported from the Cx. quinquefasciatus whole genome sequence database (https://www.vectorbase.org/organisms/culex-quinquefasciatus). The qRT-PCR was performed with the FastStart Universal SYBR Green master mix Kit (Roche) and ABI 7500 Real Time PCR system (Applied Biosystems). Each qRT-PCR reaction was run in 3 replicates, for a total reaction volume of 25 μl, and contained SYBR Green master mix, specific primer pairs of GPCR, and GPCR-related genes at a final concentration of 3–5 μM that were designed based on each of the GPCR-related gene sequences (HYPERLINK “http://cquinquefasciatus.vectorbase.org/”), as listed in Table S1 with the accession number for each GPCR-related gene, as well as a 1 µg cDNA template from each mosquito sample. A ‘no-template’ negative control was also performed for each. The reaction cycle consisted of a melting step of 50°C for 2 min, then 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Specificity of the GPCR-related gene PCR reactions was assessed by a melting curve analysis using Dissociation Curves software55. Relative expression levels of GPCR-related genes were calculated by the 2−ΔΔCT method using SDS RQ software56. The 18S ribosomal RNA (rRNA) gene served as an endogenous control11,12. Each experiment was repeated 3 times with 3 independently isolated RNA mosquito samples. The statistical significance of the gene expression was calculated using a Student's t-test for all 2-sample comparisons and a one-way analysis of variance (ANOVA) for multiple sample comparisons using Statistical Package for the Social Sciences (SPSS) software with both Least Significant Difference (LSD) and Tukey tests to analyze the significance of means. A value of P ≤ 0.05 was considered statistically significant. Significant up-regulation or down-regulation was determined using a cut-off value of a “2-fold change in expression”10.
Double-stranded RNA (dsRNA) synthesis
The synthesis of the dsRNA (~200 bp-650 bp based on the full length of GPCR-related genes) was performed in vitro using the MEGAscrip T7 High Yield Transcription kit (Ambion). The specific primers designed with the T7 promoter to amplify the up-regulated GPCR-related genes (CPIJ019111, -014419, -007717, and -014334) are listed in Table S3. For dsRNA purification, a phenol/chloroform extraction was followed by ethanol precipitation. A dsRNA of a green fluorescent protein gene was generated that was complementary to a pMW1650 plasmid (a generous gift from Dr. Zhiyong Xi, Department of Microbiology and Molecular Genetics, Michigan State University) and served as a negative control for the dsRNA injection. Non-injected mosquitoes from the same strain reared under the same rearing conditions were used for calibration.
Adult injection with dsRNA of GPCR-related genes
To investigate the precise role of the up-regulated GPCR-related genes in resistant mosquitoes, we used the dsRNA interference (RNAi) technique to knockdown an adult up-regulated GPCR-related gene (CPIJ014334) in the resistant strain MAmCqG6 based on the mosquito adult injection method57,58. The microinjection glass needle was pulled from a borosilicate glass capillary tube (1 mm OD x 0.58 ID mm, 100 mm length, World Precision Instruments, Inc.) using a Model P-97 Flaming/Brown micropipette puller (Sutter Instrument Co.), following the program: Heat 454, Vel 120, Time 125, and Pull 30. Forceps were used to open the tip of the needle and the Nanoject II injector (Drummond Scientific Company) to draw dsRNA into the glass needle for injection. Approximately 138 nl of dsRNA (with a dsRNA concentration of 3.5 µg/µl in distilled water) was injected into the thorax of CO2-anesthetized 1-d-old female mosquitoes using the Nanoject II injector and Drosophila CO2 Fly Pads (Tritech Research, Inc.). To determine if there is any non-specific effect of dsRNA injection, we injected dsRNA of the GFP gene as a negative control and non-injected mosquitoes were used for the calibration, as noted above. Both injected female mosquitoes (~100 individuals) and an equal number of non-injected mosquitoes were transferred and provided with 10% sugar solution under insectary conditions (25 ± 2°C and a photoperiod of 12:12 (L:D) h) for 3 days. The involvement of the gene in resistance against permethrin was investigated by using a topical application assay with a series concentration of permethrin designed to result in >0 and <100% mortality for each of the five strains, as previously described8. Total RNA was then extracted from the dsRNA-injected and non-injected female mosquitoes and the relative expression of CPIJ014334 determined using qRT-PCR with primer pairs of this gene (Table S3).
Since the GPCR-related genes are up-stream and function to regulate the downstream gene expression, we considered it possible that they could be involved in resistance-related P450 gene expression. We therefore chose 4 P450 genes (CYP9M10, -9J34, -9J40, and -6AA7) that are known to play very important roles in the permethrin resistance of Cx. quinquefasciatus11,12, and tested their gene expression to identify the regulatory function of CPIJ014334. Each experiment was repeated at least 3 times with independent microinjection and RNA isolation.
Embryo injection with dsRNA of up-regulated GPCR-related genes
According to the literature, Drosophila embryo injection with dsRNA59, Aedes aegypti embryo injection methods and mosquito transgenic methods60 have all been used to examine the function of up-regulated GPCR genes (CPIJ019111, -7717, -14419). In this study, larvae of the resistant mosquito strains HAmCqG8 and MAmCqG6 were tested by injecting the dsRNA of the target gene into mosquito embryos. Around 1000 fresh grey embryos were collected from HAmCqG8 and MAmCqG6 mosquito cages in which the mosquitoes had been fed with blood three days earlier and reared under insectary conditions. Around 120 embryos were arranged on each piece of paper filter. Embryos were allowed to dry for 2–3 min and transferred to a microscope cover slide (VWR Scientific Products) using double sided tape, and then covered with Halocarbon 700 oil (Halocarbon Products Corp.) for protection against dehydration. As before, a glass capillary tube (borosil 1.0 mm OD × 0.5 mm ID/Fiber, 100 mm length, FHC, Inc) was pulled using the Model P-97 Flaming/Brown micropipette puller (Sutter Instrument Co.), following the program: Heat 525, Vel 50, Time 250, and Pull 50. The glass needle tip was opened using the Model BV-10, K. T. Brown Type micropipette beveler (Sutter Instrument Co.). We injected 0.2–0.5 nl of dsRNA (3.5 ug/ul) into the embryo posterior horizontally using the Picospritzer III injector system (Parker Instrumentation) under the Nikon Eclipse TS100 microscope (Nikon Instruments). Injected embryos were individually transferred by a needle onto another wet filter paper, and then water was used to move all the injected embryos into a clean water container. The water container was kept under insectary conditions for 3–4 days. For each gene, about 500 embryos were injected with the corresponding dsRNA: the CPIJ019111 gene in HAmCqG8, the CPIJ014419 and -007717 genes in MAmCqG6, and the GFP gene in both strains. Hatched 2nd instar larvae were separated into two groups: one group was tested for larva bioassay with a series concentration of permethrin designed to result in >0 and <100% mortality, as previously described8, and the other group was prepared for gene expression identification of the up-regulated GPCR-related gene and of permethrin resistance-related P450 genes (CYP9M10, -9J34, -9J40, and -6AA7) using qRT-PCR. Each experiment was repeated at least 3 times with independent microinjection and RNA isolation.
Author Contributions
Performed the experiments: T.L., L.L. Analyzed the data: N.L., T.L., L.L. L.Z. Contributed reagents/materials/analysis tools: N.L. Wrote the paper: N.L., T.L., L.L. L.Z.
Supplementary Material
Table S1-S3
Acknowledgments
The authors are grateful to Drs. Peter W. Atkinson, Peter Arensburger and the Culex quinquefasciatus genome community for their efforts devoted to determining the genome sequence and making that genome sequence information available in VectorBase. We also thank Jan Szechi for editorial assistance. This study was supported by an NIH grant (1R21AI076893) to N.L., AAES Hatch/Multistate Grants ALA08-045 to N.L., and ALA015-1-10026 to N.L.
References
- Sardelis M. R., Turell M. J., Dohm D. J. & O'Guinn M. L. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 7, 1018–1022 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. C., Morris J., Hill G., Alderman M. & Ratard R. C. St. Louis encephalitis outbreak in Louisiana in 2001. J. La. State. Med. Soc. 154, 303–306 (2002). [PubMed] [Google Scholar]
- Reisen W. K., Fang Y. & Martinez V. M. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis Encephalitis virus transmission. J. Med. Entomol. 42, 367–375 (2005). [DOI] [PubMed] [Google Scholar]
- Ranson H. et al. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 27, 91–98 (2011). [DOI] [PubMed] [Google Scholar]
- Asidi A. et al. Loss of household protection from use of insecticide-treated nets against pyrethroid-resistant mosquitoes, Benin. Emerg. Infect. Dis. 18, 1101–1106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondji C. S. et al. Impact of pyrethroid resistance on operational malaria control in Malawi. Proceed. Natl. Acad. Sci. USA. 109, 19063–19070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel V. et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Tropica. 101, 207–216 (2007). [DOI] [PubMed] [Google Scholar]
- Li T. & Liu N. Inheritance of permethrin resistance in Culex quinquefasciatus. J. Med. Entomol. 47, 1127–1134 (2010). [DOI] [PubMed] [Google Scholar]
- Müller P. et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genetic. 4, e1000286 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strode C. et al. Genomic analysis of detoxification genes in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 38, 113–123 (2008). [DOI] [PubMed] [Google Scholar]
- Liu N., Li T., Reid W. R., Yang T. & Zhang L. Multiple cytochrome P450 genes: their constitutive overexpression and permethrin induction in insecticide resistant mosquitoes, Culex quinquefasciatus. PLoS One. 6, e23403 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. & Liu N. Genome analysis of cytochrome P450s and their expression profiles in insecticide resistant mosquitoes, Culex quinquefasciatus. PLoS One. 6, e29418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N. & Scott J. G. Inheritance of CYP6D1-mediated pyrethroid resistance in house fly (Diptera: Muscidae). J. Econ. Entomol. 90, 1478–81 (1997). [DOI] [PubMed] [Google Scholar]
- Liu N. & Scott J. G. Phenobarbital induction of CYP6D1 is due to a reans acting factor on autosome 2 in house flies, Musca domestica. Insect Mol. Biol. 6, 77–81 (1997). [DOI] [PubMed] [Google Scholar]
- Li M. et al. A whole transcriptomal linkage analysis of gene co-regulation in insecticide resistant house flies, Musca domestica. BMC Genomics. 14, 803 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell C. M., Brown R. P., Berenbaum M. R. & Schuler M. A. Conserved regulatory elements in the promoters of two allelochemical-inducible cytochrome P450 genes differentially regulate transcription. Insect Biochem. Mol. Biol. 34, 1129–1139 (2004). [DOI] [PubMed] [Google Scholar]
- Petersen R. A., Niamsup H., Berenbaum M. R. & Schuler M. A. Transcriptional response elements in the promoter of CYP6B1, an insect P450 gene regulated by plant chemicals. Biochimica Biophysica Acta. 1619, 269–282 (2003). [DOI] [PubMed] [Google Scholar]
- Brown R. P., Berenbaum M. R. & Schuler M. A. Transcription of a lepidopteran cytochrome P450 promoter is modulated by multiple elements in its 5′UTR and repressed by 20-hydroxyecdysone. Insect Mol. Biol. 13, 337–347 (2004). [DOI] [PubMed] [Google Scholar]
- Wilding C. S. et al. A cis-regulatory sequence driving metabolic insecticide resistance in mosquitoes: functional characterisation and signatures of selection. Insect Biochem. Mol. Biol. 42, 699–707 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang C., Lou X., Ni X., Zhang Y. & Li X. Functional characterization of cis-acting elements mediating flavones-inducible expression of CYP321A1. Insect Biochem. Mol. Biol. 40, 898–908 (2010). [DOI] [PubMed] [Google Scholar]
- Misra J. R., Horner M. A., Lam G. & Thummel C. S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes. Dev. 25, 1796–1806 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara S., Chandrasekharan M. B. & Ganguly R. Caffeine induction of Cyp6a2 and Cyp6a8 genes of Drosophila melanogaster is modulated by cAMP and D-JUN protein level. Gene. 415, 49–59 (2008). [DOI] [PubMed] [Google Scholar]
- Tadevosyan A., Vaniotis G., Allen B. G., Hébert T. E. & Nattel S. G protein-coupled receptor signaling in the cardiac unclear membrane: evidence and possible roles in physiological and pathophysiological function. J. Physiol. 590, 1313–1330 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. C. et al. The GPCR network: a large-scale collaboration to determine human GPCR structure and function. Nature Rev. Drug. Disco. 12, 25–34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel P. A. et al. GPCR expression in tissues and cells: are the optimal receptors being used as drug target? B. J. Pharmacolo. 165, 1613–1616 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoichet B. K. & Kobilka B. K. Structure-based drug screening for G-protein-coupled receptors. Trend. Pharmacolog. Sci. 33, 268–272 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caers J. et al. More than two decades of research on insect neuropeptide GPCRs: an overview. Front. Endocrinol. 3, 151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri C. et al. Plant insecticide L-canavanine repels Drosophila via the insect orphan GPCR Dmx. PLoS Bio. 7, e1000147 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet G. et al. Neuroendocrinological and molecular aspects of insect reproduction. J. Neuroendocrin. Vol. 16, 649–659 (2004). [DOI] [PubMed] [Google Scholar]
- Hauser F., Cazzamali G., Williamson M., Blenau W. & Grimmelikhuijzen C. J. P. A review of neurohormone GPCRs present in the fruitfly Drosphila melanogaster and the honey bee Apis mellifera. Prog. Neurobio. 80, 1–19 (2006). [DOI] [PubMed] [Google Scholar]
- Bai H., Zhu F., Shah K. & Palli S. R. Large-scale RNAi screen of G-protein-coupled receptors involved in larval growth, molting and metamorphosis in the red flour beetle. BMC Genomics. 12, 388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spit J. et al. Peptidergic control of food intake and digestion in insects. Can. J. Zool. 90, 489–506 (2012). [Google Scholar]
- Nene V. et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 316, 1718–1723 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. M. et al. A “genome-to-lead” approach for insecticide discovery: pharmacological characterization and screening of Aedes aegypti D1-like dopamine receptors. PLoS Neglect. Tropic. Diseases. 6, e1478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. A. et al. Re-invigorating the insecticide discovery pipeline for vector control: GPCRs as targets for the identification of next gene insecticides. Pest. Biochem. Physiol. 106, 141–148 (2013). [Google Scholar]
- Cui L., Li J. & Xie X. Rediocide A, an insecticide, induces G-protein-coupled receptor desensitization via activation of conventional protein kinase C. J. Nat. Prod. 75, 1058–1062 (2012). [DOI] [PubMed] [Google Scholar]
- Liu N., Liu H., Zhu F. & Zhang L. Differential expression of genes in pyrethroid resistant and susceptible mosquitoes, Culex quinquefasciatus (S.). Gene. 394, 61–68 (2007). [DOI] [PubMed] [Google Scholar]
- Hu X. et al. Cloning and characterization of NYD-OP7, a novel deltamethrin resistance associated gene from Culex pipiens pallens. Pest. Biochem. Physiol. 88, 82–91 (2007). [Google Scholar]
- Feyereisen R. Insect P450 enzymes. Annu. Rev. Entomol. 44, 507–533 (1999). [DOI] [PubMed] [Google Scholar]
- Hemingway J., Hawkes N. J., McCarroll L. & Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 34, 653–665 (2004). [DOI] [PubMed] [Google Scholar]
- Hill C. A., Fox A. N., Pitts R. J., Kent L. B. & Tan P. L. G protein-coupled receptors in Anopheles gambiae. Science. 298, 176–178 (2002). [DOI] [PubMed] [Google Scholar]
- Hauser F. et al. A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum. Fronti. Neuroendocrin. 29, 142–165 (2008). [DOI] [PubMed] [Google Scholar]
- Richards S. et al. The genome of the model beetle and pest Tribolium castaneum. Nature. 452, 949–955 (2008). [DOI] [PubMed] [Google Scholar]
- Kirkness E. et al. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proceed. Natl. Acad. Sci. USA. 107, 12468–12173 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C. E., Schiedel A. C. & Baqi Y. Allosteric modulators of rhodopsin-like G protein-coupled receptors: opportunities in drug development. Pharmacol. Therapeu. 135, 292–315 (2012). [DOI] [PubMed] [Google Scholar]
- Fox A. N., Pitts R. J., Robertson H. M., Carlson J. R. & Zwiebel L. J. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proceed. Natl. Acad. Sci. USA. 98, 14693–14697 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes A. M. et al. Infection intensity-dependent responses of Anopheles gambiae to the African malaria parasite Plasmodium falciparum. Infect. Immun. 79, 4708–4715 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.-Y., Estep A., Sanscrainte N., Becnel J. & Vander Meer R. K. Identification and expression of PBAN/diapause hormone and GPCRs from Aedes aegypti. Mole. Cell. Endocrinol. 375, 113–120 (2013). [DOI] [PubMed] [Google Scholar]
- Montgomery M. K., Xu S. & Fire A. RNA as a target of double-stranded RNA-mediated genetic interference in Caenorhabditis elegans. Proceed. Natl. Acad. Sci. USA. 95, 15502–15507 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C. & Conte,. Jr D. Revealing the world of RNA interference. Nature 431, 338–342 (2004). [DOI] [PubMed] [Google Scholar]
- Zhu F. et al. A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the OTC279 strain of Tribolium castaneum. Proceed. Natl. Acad. Sci. USA. 107, 8557–8562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B. J. et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 41, 492–502 (2011). [DOI] [PubMed] [Google Scholar]
- Rosenbaum D. M., Rasmussen S. G. & Kobilka B. K. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Delaney M. K. & Du X. Inside-out, outside-in, and inside-outside-in: G protein signaling in itegrin-mediated cell adhesion, spreading, and retraction. Current Opin. Cell Biol. 24, 600–606 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer C. T. et al. Continuous fluorescence monitoring of rapid cycle DNA amplification. BioTechniques. 22, 130–131 (1997). [DOI] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Garver L. & Dimopoulos G. Protocol for RNAi assays in adult mosquitoes (A. gambiae). J. Visualized Experiments. 10.3791/230 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X. et al. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proceed. Natl. Acad. Sci. USA. 109, E23–E31 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I. et al. Genes required for Drosophila nervous system development identified by RNA interference. Proceed. Natl. Acad. Sci. USA. 101, 16216–16221 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman Z. N., Anderson M. A. E., Morazzani E. M. & Myles K. M. A transgenic sensor strain for monitoring the RNAi pathway in the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 38, 705–713 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1-S3