Abstract
Insulin treatment of mouse ATDC5 chondroprogenitors induces these cells to differentiate into mature chondrocytes. To identify novel factors that are involved in this process, we carried out mutagenesis of ATDC5 cells through retroviral insertion and isolated two mutant clones incapable of differentiation. Inverse PCR analysis of these clones revealed that the retroviral DNA was inserted into the promoter region of the Rab23 gene, resulting in increased Rab23 expression. To investigate whether an elevated level of Rab23 protein led to inhibition of chondrogenic differentiation, we characterized ATDC5 cells that either overexpress endogenous Rab23 or stably express ectopic Rab23. Our results revealed that up-regulation of Rab23 can indeed inhibit chondrogenic differentiation with a concomitant down-regulation of matrix genes such as type II collagen and aggrecan. In addition, stable small interfering RNA knockdown of Rab23 also resulted in inhibition of chondrogenic differentiation as well as down-regulation of Sox9, a master regulator of chondrogenesis. Interestingly, Sox9 expression has recently been linked to Gli1, and we found that Rab23 knockdown decreased Gli1 expression in chondrocytes. Because the phenotypes of Rab23 mutations in mice and humans include defects in cartilage and bone development, our study suggests that Rab23 is involved in the control of Sox9 expression via Gli1 protein.
Chondrogenesis is a multi-step process that coordinately controls differentiation of mesenchymal cells into mature chondrocytes marked by expression of Type II collagen (Col2) and other extracellular matrix proteins. These mature cells could in turn become hypertrophic chondrocytes marked by expression of Type X collagen. Hypertrophic chondrocytes then undergo apoptosis, leaving behind a scaffold for invasion by osteoblasts/osteoclasts during endochondral ossification. Differentiation of chondrocytes is controlled by a signaling pathway involving Indian hedgehog and by transcription factors such as Sox9 (1).
Chondrocyte differentiation can be mimicked in vitro by insulin treatment of the murine chondroprogenitor cell line ATDC5 (2). When cultured in insulin-containing medium over a period of 3–4 weeks, ATDC5 cells will condense to form nodules, and cells in these nodules will eventually differentiate into mature chondrocytes to produce a cartilage-like extracellular matrix that can be stained with Alcian blue. After prolonged culture in insulin medium, mature ATDC5 chondrocytes will enter into the hypertrophic phase characterized by the synthesis of Type X collagen and eventually die by apoptosis.
Utilizing these properties of ATDC5 cells, we carried out a large scale screening of random retroviral insertions to identify novel regulators of chondrocyte differentiation. By random integration of a retroviral vector into the genome of ATDC5 cells, genes that are critical to chondrogenesis could potentially be disrupted or conversely activated. Alterations of these genes will lead to either spontaneous differentiation of ATDC5 cells or failure of ATDC5 cells to differentiate in insulin medium. ATDC5 cells harboring these activating or inactivating mutations can then be screened by Alcian blue staining. Amplification and sequencing of genomic DNAs from these mutant ATDC5 clones will then lead to identification of the exact retroviral integration sites within these ATDC5 clones and subsequently the affected genes.
In this study we report the results of our random mutagenesis screening and describe the characterization of two mutant ATDC5 clones that were unable to undergo chondrogenic differentiation in insulin medium. Sequencing of retroviral integration sites in these two clones revealed that the retroviral DNA was inserted into the promoter region of Rab23 (Ras-related in brain 23), a gene encoding a member of the Rab family of small GTPases (3). Both overexpression and siRNA2 knockdown of Rab23 in ATDC5 cells lead to inhibition of chondrogenic differentiation as well as down-regulation of genes encoding type II collagen and aggrecan. In addition, we showed that a proper level of Rab23 protein is necessary for the expression of Sox9, a well known master regulator of chondrocyte differentiation. Interestingly, we also found that expression of the Gli1 transcription factor (a major downstream target of the hedgehog signaling pathway) is dependent on Rab23 in ATDC5 cells. Rab23 mutations have been identified both in mice and human, and the mutant phenotypes include defects in bone development and limb patterning. Our study provides evidence for the first time that these Rab23 mutations may exert their effects on the skeletal system through down-regulation of the essential transcription factor Sox9 via Gli1.
EXPERIMENTAL PROCEDURES
Cell Culture and Alcian Blue Staining—ATDC5 cells, derived from mouse embryonal carcinoma (2), were cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F-12 (Cambrex Bio Science Inc, Walkersville, MD) supplemented with 2% fetal bovine serum (Invitrogen), 10 μg/ml human transferrin (Sigma-Aldrich), 3 × 10−8m sodium selenite (Sigma-Aldrich) at 37°C under 5% CO2. Upon reaching confluence, the cells were stimulated with 10 μg/ml of bovine insulin (Sigma-Aldrich), and the medium was changed every other day. Alcian blue (Sigma-Aldrich), which stains cartilage-like extracellular matrix produced by mature chondrocytes, was used to evaluate expression of extracellular matrix by ATDC5 cells. The staining was done after the cells were rinsed with phosphate-buffered saline, incubated with 0.1% Alcian blue solution in 0.1 n HCl (pH 1.0) overnight, and washed three times with phosphate-buffered saline before macroscopic examination.
Inverse PCR Analysis of Retroviral Integration Sites—The LXSN retrovirus-infected ATDC5 cells were selected for stable integration with G418 at 0.5 mg/ml. G418-resistant single clones were picked individually and expanded in triplicate 96-well plates. After reaching confluence, the cells in one plate were stimulated with insulin at a final concentration of 10 μg/ml. After 3 weeks, 0.2% Alcian blue was used to stain cells in the insulin-stimulated plate as well as one unstimulated plate.
Genomic DNAs were isolated from ATDC5 cells after expansion of interesting clones. 5 μg of genomic DNA was digested with SacII and circularized with T4 DNA ligase. DNA fragments containing the retroviral insertion sites were amplified using the long template PCR kit (Roche Applied Science) with primers NEO-U3 (5′-agc gca tcg cct tct atc-3′) and NEO-D3 (5′-cca gtc ata gcc gaa tag-3′). The amplified DNAs were isolated and sequenced using primer NEO-U5 (5′-aga gaa cca tca gat gtt-3′). The mouse genomic sequences identified at the sites of retroviral insertion were then used to query the mouse genome data base in the GenBank™ using BLAST for identification of the affected genes.
Plasmid Construction and Retroviral Infection—Full-length mouse Rab23 cDNA was amplified from ATDC5 cells by RT-PCR. The amplified cDNA was then cloned into the XbaI-BamHI sites of the pCG vector (4), which tags the N-terminal end of Rab23 protein with a T7 epitope. For LXSN-T7-Rab23 retroviral construct, T7-Rab23 cDNA was amplified by PCR and cloned into the EcoRI-BamHI sites of pLXSN retroviral vector (Clontech Laboratories, Inc., Mountain View, CA).
Retrovirus was generated by transfection of LXSN-T7-Rab23 into PT67 packaging cells with the calcium phosphate method. After retroviral infection and G418 selection, T7-Rab23 expressing ATDC5 clones were pooled and confirmed by Western blotting with a monoclonal anti-T7 antibody.
Lentivirus-mediated siRNA Knockdown—Lentiviral siRNA construct was obtained by annealing the predesigned primers and subsequent cloning into the HpaI-XhoI sites of the pLL3.7 lentiviral vector (5) that also co-expresses green fluorescent protein as a selection marker. Primers targeting nucleotides 668–686 of mouse Rab23 mRNA are 5′-tcg atg aag atg taa ggc tat tca aga gat agc ctt aca tct tca tcg ttt ttt c-3′ (sense) and 5′-tcg aga aaa aac gat gaa gat gta agg cta tct ctt gaa tag cct tac atc ttc atcga-3′ (antisense). Primers used for generation of nonspecific siRNA are 5′-gaa aca tac tgt tac aag att caa gag atc ttg taa cag tat gtt tct ttt ttc-3′ (sense) and 5′-tcg aga aaa aag aaa cat act gtt aca aga tct ctt gaa tct tgt aac agt atg ttt c-3′ (antisense).
For generation of lentivirus, 293FT packaging cells were transfected with 6 μg of pLL3.7-siRNA construct plus 9 μg of ViraPower DNA Mixture (Invitrogen) by the Lipofectamine 2000 method according to the manufacturer’s instructions. After 48 h, supernatants the containing lentivirus were collected and used to infect ATDC5 cells in the presences of 6 μg/ml polybrene. Infected green fluorescent protein-positive cells were first enriched through sorting by flow cytometry then expanded for further analysis.
Western Blot Analysis—To examine changes in protein expression, 2 × 106 ATDC5 cells were collected and lysed with 75 μl of Buffer X (50 mm Tris, pH 7.4, 270 mm NaCl, 0.5% Triton X-100) supplemented with protease and phosphatase inhibitors (Sigma-Aldrich). After separation by SDS-PAGE, the proteins were transferred onto a polyvinylidene difluoride membrane (Bio-Rad), and the membrane was blotted with a rabbit polyclonal anti-Rab23 antibody (6) or a horseradish peroxidase-conjugated mouse monoclonal anti-T7 antibody (EMD Biosciences, Inc., San Diego, CA). Rabbit polyclonal antibodies used for detection of Sox9 (clone H-90), Gli1(clone H-300), and Gli2 (clone H-300) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The protein bands were visualized using the ECL Plus Western blotting detection reagents (Amersham Biosciences).
Extraction of RNA and RT-PCR—Total RNA was extracted from 5 × 105 ATDC5 cells using the RNeasy mini kit (Qiagen) and eluted in 40 μl of H2O. For RT-PCR, 2 μl of RNA was analyzed in a total volume of 25 μl using the SuperScript OneStep RT-PCR kit (Invitrogen). The primers for mouse Col2 mRNA were 5′-cag gcc tcg cgg tga gcc atg at-3′ (sense) and 5′-gtt ctc cat ctc tgc cac g-3′ (antisense). Primers for mouse aggrecan were 5′-gaa atg aca acc cca agc ac-3′ (sense) and 5′-tct ccg ctg att tca gtc ct-3′. Primers for mouse glyceraldehyde-3-phosphate dehydrogenase were 5′-gtg gat att gtt gcc atc att-3′ (sense) and 5′-tga tgg caa caa tat cca ctt-3′ (antisense).
RESULTS
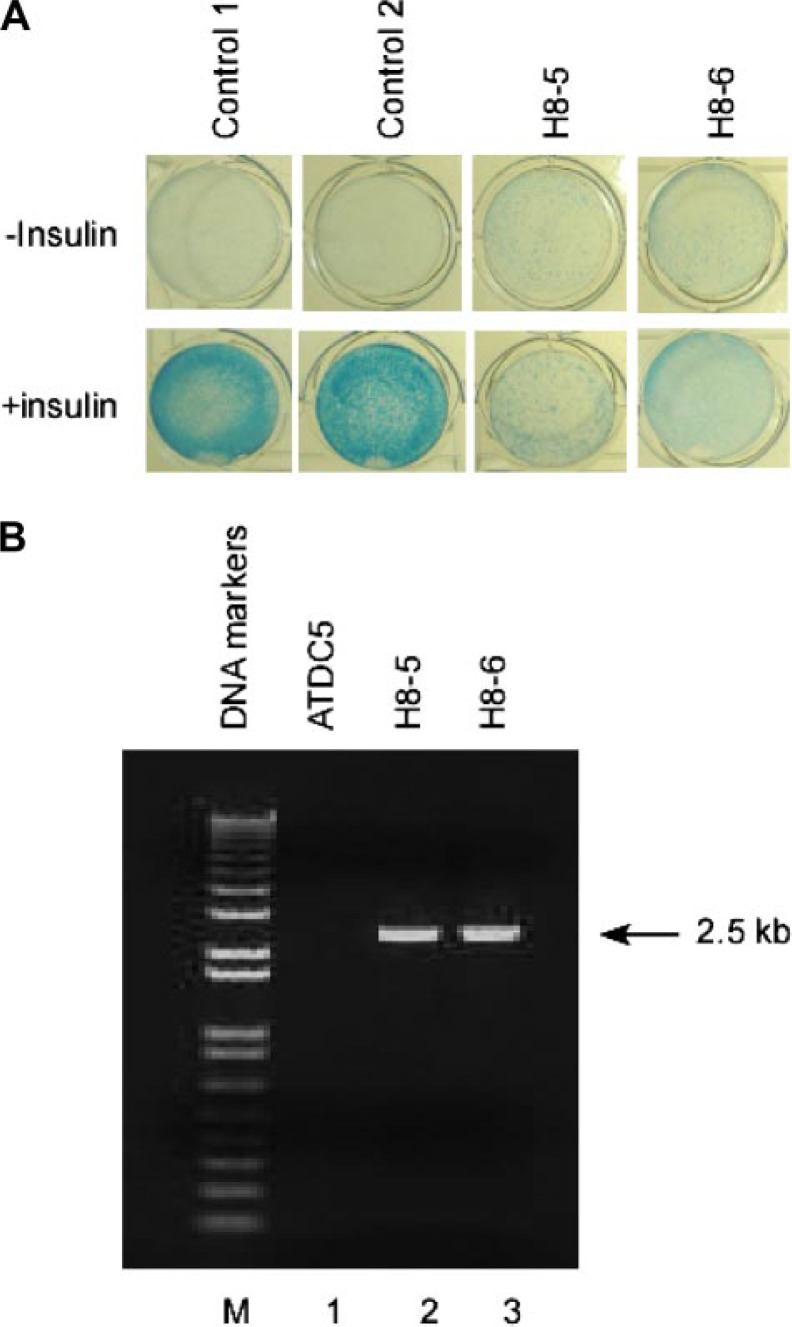
Retroviral Insertion Leads to Failure of ATDC5 Cells to Differentiate in Response to Insulin—Mouse ATDC5 chondroprogenitor cells normally undergo rapid growth until reaching confluence. When stimulated with insulin, confluent ATDC5 cells will first condense to form nodules, and cells within these nodules then initiate chondrogenic differentiation. After 2–3 weeks, these cells can generate a cartilage-like extracellular matrix that stains positive with Alcian blue (Fig. 1A, left two panels).
FIGURE 1.
Failure of chondrogenic differentiation in two mutant ATDC5 clones. A, normal ATDC5 clones (left two panels), and clones H8-5 and H8-6 (right two panels) were seeded into a 24-well plate, cultured in medium with or without insulin for 21 days, and then stained with 0.1% Alcian blue for detection of a cartilage-like extracellular matrix. B, inverse PCR products from plain ATDC5 cells (lane 1) or from clones H8-5 and H8-6 (lanes 2 and 3) were separated on a 1% agarose gel. Note both the H8-5 and H8-6 clones produced 2.5-kb bands.
To identify novel regulators of chondrocyte differentiation, we infected ATDC5 cells with a LXSN retroviral stock that was sufficiently dilute to favor integration of a single copy of retroviral DNA per cell. Retroviral integration was then selected by culture in G418 medium, and G418-resistant colonies were isolated for screening in duplicate 96-well plates. Plates stimulated with insulin medium and those not stimulated were then stained with Alcian blue. We found that the majority of the 10 × 103 clones evaluated showed no change in their phenotype (Fig. 1A, left two panels). In contrast, two clones (H8-5 and H8-6) failed to undergo differentiation after 21 days of incubation with insulin and did not stain with Alcian blue (Fig. 1A, right two panels). We speculated that the retroviral infection had caused changes in the phenotype in these two clones either through inactivation of genes important for chondrogenic differentiation or through activation of genes critical for inhibition of chondrogenic pathways.
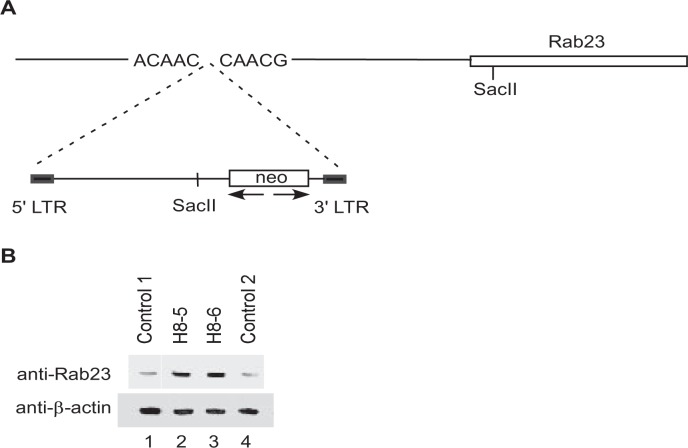
Retroviral Insertion Increases Expression of Rab23 in Mutant ATDC5 Clones That Fail to Undergo Chondrogenic Differentiation—To determine the sites of retroviral insertion and the affected gene(s) in clones H8-5 and H8-6, we expanded these two clones for isolation of genomic DNA. After digestion with the restriction enzyme SacII that allows for only a single cut within the retroviral vector but will cut multiple sites in the surrounding genomic DNA, the digested genomic DNA fragments were circularized with T4 ligase. The resultant circular DNA containing the retroviral insertion site was amplified by PCR with specific Neo primers to produce a single band at 2.5 kb in size for both H8-5 and H8-6 (Fig. 1B). Sequencing of this band from H8-5 and H8-6 confirmed that they share an identical sequence at the retroviral integration site. BLAST search of this sequence within the GenBank™ mouse genome data base revealed that the retrovirus had inserted into the 5′ promoter region of Rab23 around 0.6 kb upstream of the transcription initiation site (Fig. 2A).
FIGURE 2.
Characterization of mutant clones H8-5 and H8-6. A, orientation of the retroviral insertion in both H8-5 and H8-6 was shown in relation to the Rab23 gene. B, total lysates from normal clones C1 and C2 or from mutant clones H8-5 and H8-6 were separated on a 12.5% gel and blotted with a rabbit anti-Rab23 antibody (top panel). The same blot was stripped and blotted with a mouse monoclonal anti-β-actin to show similar loading of proteins in each lane (bottom panel). LTR, long terminal repeat.
To examine how the retroviral insertion affected Rab23 expression, we carried out Western blotting analysis with total lysates from H8-5 and H8-6 as well as from two other normal ATDC5 clones that were capable of chondrogenic differentiation. We noticed that the levels of Rab23 protein in the two normal ATDC5 clones were lower that those in clones H8-5 and H8-6 (Fig. 2B), indicating that retroviral insertion into its promoter activated the Rab23 gene in clones H8-5 and H8-6.
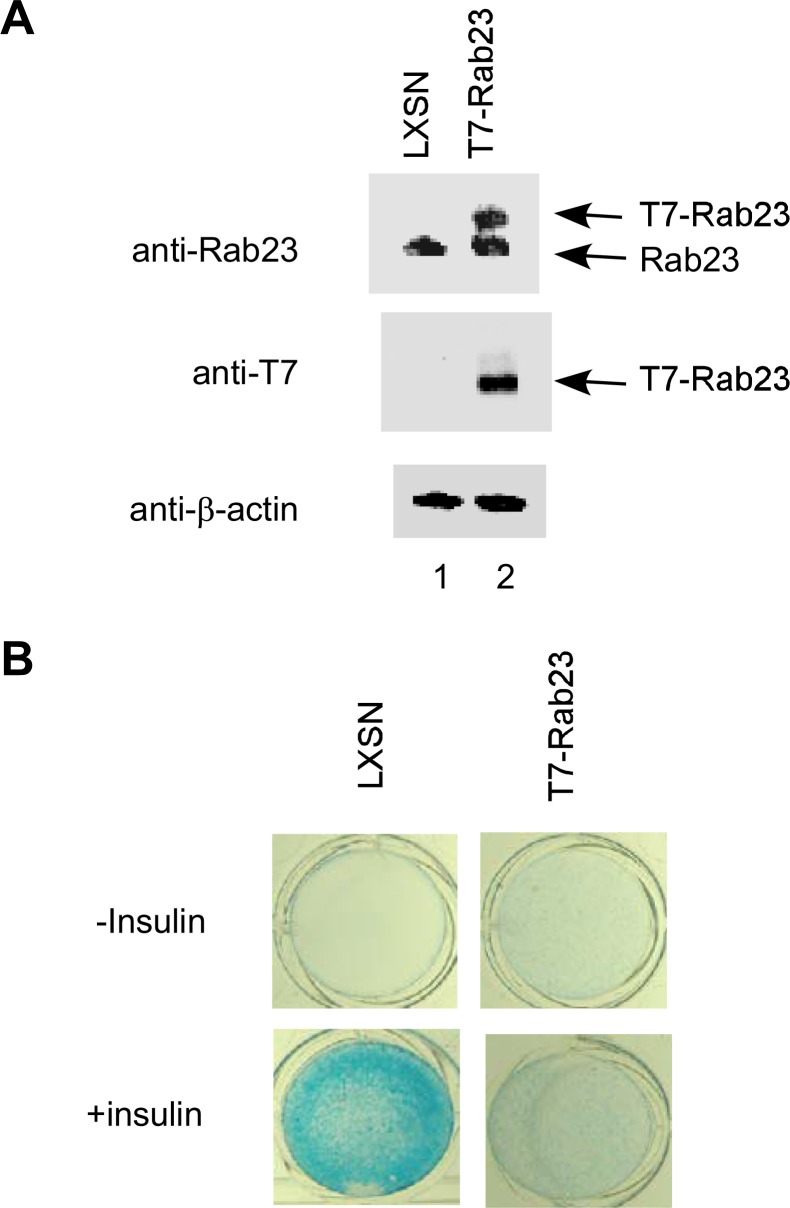
Ectopic Expression of Rab23 Protein Inhibits Differentiation of ATDC5 Cells in the Presence of Insulin—To determine whether overexpression of Rab23 is responsible for the failure of clones H8-5 and H8-6 to differentiate in response to insulin treatment, we amplified the mouse Rab23 cDNA by RT-PCR and then cloned the full-length cDNA into LXSN retroviral vector for infection of ATDC5 cells. To differentiate ectopically expressed Rab23 protein from endogenous Rab23, the Rab23 cDNA in the retroviral vector was tagged with a T7 epitope that can be easily detected by Western blotting. After retroviral infection, individual clones were selected by G418 and pooled to avoid bias because of clonal variations. As a control for our analysis, ATDC5 cells harboring the empty retroviral vector LXSN were also prepared.
As shown in Fig. 3A, the rabbit polyclonal anti-Rab23 antibody was able to detect both endogenous Rab23 and ectopic T7-tagged Rab23. In contrast, the mouse monoclonal anti-T7-antibody detected only T7-Rab23. After confirmation of retroviral T7-Rab23 expression, ATDC5 cells expressing T7-Rab23 were cultured for 21 days in medium with or without insulin. Cells harboring the empty LXSN retroviral vector were treated in a similar manner for use as a positive control. We found that in cells expressing ectopic T7-Rab23, insulin failed to produce a cartilage-like extracellular matrix (Fig. 3B), suggesting that ectopic expression of Rab23 inhibited insulin-induced chondrogenic differentiation.
FIGURE 3.
Inhibition of chondrogenic differentiation by ectopic Rab23 protein. A, ATDC5 cells were infected with an empty retrovirus or with one expressing T7-tagged Rab23. Total lysates from the two populations were first blotted with an anti-Rab23 antibody for comparison of ectopic T7-Rab23 with endogenous Rab23 protein (top panel). The same lysates were also blotted with an anti-T7 antibody to detect ectopic T7-Rab23 (middle panel). The membrane was stripped and blotted against β-actin to show similar protein loading (bottom panel). B, pooled ATDC5 clones harboring the LXSN empty retroviral vector (left panels) or expressing T7-Rab23 (right panels) were cultured in 24-well plates in medium with or without insulin for 21 days and then stained with Alcian blue.
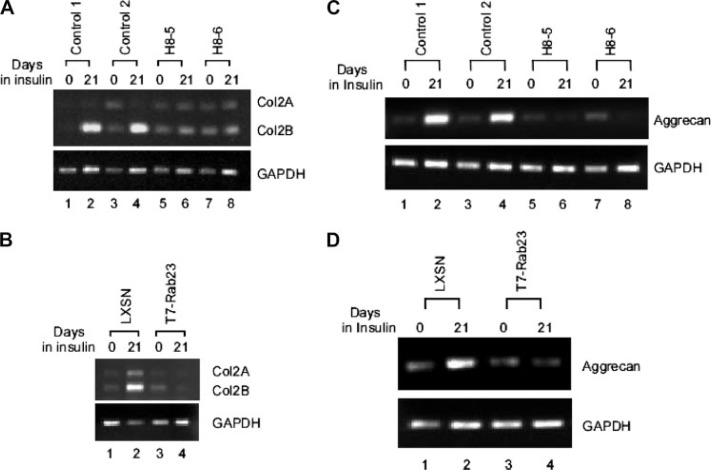
Rab23 Down-regulates Expression of Type II Collagen and Aggrecan in ATDC5 Cells—A hallmark of the chondrogenic differentiation is the production of Type II collagen, the major component of cartilage produced by mature chondrocytes (7). We have shown in the above experiments that ectopic Rab23 was able to inhibit production of a cartilage-like matrix in ATDC5 cells. To further investigate whether such inhibition is accompanied by down-regulation of the Col2 gene, we isolated total RNA from normal ATDC5 clones and from clones H8-5 and H8-6 for RT-PCR analysis. As shown in Fig. 4A, insulin treatment of normal clones induced expression of Col2 with the splicing isoform Col2B as the major product (lanes 1–4). In contrast, insulin treatment of Rab23-overexpressing clones H8-5 and H8-6 did not induce Col2 expression (lanes 5–8). We also examined Col2 expression in ATDC5 cell harboring the empty LXSN retroviral vector or expressing T7-Rab23 after 21 days of insulin treatment. As shown in Fig. 4B, insulin was able to induce Col2 expression in ATDC5 cells harboring the empty retroviral vector (lanes 1 and 2) but failed to do so in cells expressing T7-tagged Rab23 (lanes 3 and 4).
FIGURE 4.
Repression of Col2 and aggrecan by Rab23 overexpression. A, expression of Col2 gene was analyzed by RT-PCR using total RNAs from normal ATDC5 clones (lanes 1– 4) or Rab23 overexpressing clones H8-5 and H8-6 (lanes 5– 8). Induction of the Col2 gene was achieved by treatment with insulin for 21 days. Two splicing isoforms of Col2 transcripts are indicated. RT-PCR analysis of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was performed to show the amounts of total RNAs used in the analysis. B, expression of the Col2 gene in ATDC5 cells harboring the LXSN empty retroviral vector (lanes 1 and 2) or in those expressing T7-Rab23 (lanes 3 and 4). C, expression of the aggrecan gene in normal ATDC5 clones (lanes 1– 4) or Rab23 overexpressing clones H8-5 and H8-6 (lanes 5– 8). D, expression of the aggrecan gene in ATDC5 cells harboring the LXSN empty retroviral vector (lanes 1 and 2) or in those expressing T7-Rab23 (lanes 3 and 4).
To investigate whether the inhibitory effect of Rab23 is Col2-specific or whether it can also down-regulate other genes encoding additional matrix proteins, we assessed the expression of aggrecan. Aggrecan is the major proteoglycan expressed in mature cartilage (8). After assembly into its supramolecular form, aggrecan contributes to resistance to compressive forces in cartilage through its osmotic resistance. Similar to type II collagen, expression of aggrecan is also thought to be the hallmark of chondrocyte maturation and chondrogenesis (9). When analyzed by RT-PCR, insulin treatment of normal clones induced expression of aggrecan (Fig. 4C, lanes 1–4). In contrast, insulin treatment of Rab23-overexpressing clones H8-5 and H8-6 did not induce aggrecan expression (Fig. 4C, lanes 5–8). We also examined aggrecan expression in ATDC5 cells harboring the empty LXSN retroviral vector or expressing T7-Rab23 after 21 days of insulin treatment. Expression of aggrecan was also induced by insulin in ATDC5 cells harboring the empty retroviral vector (Fig. 4D, lanes 1 and 2) but was not inducible in cells expressing T7-tagged Rab23 (Fig. 4D, lanes 3 and 4).
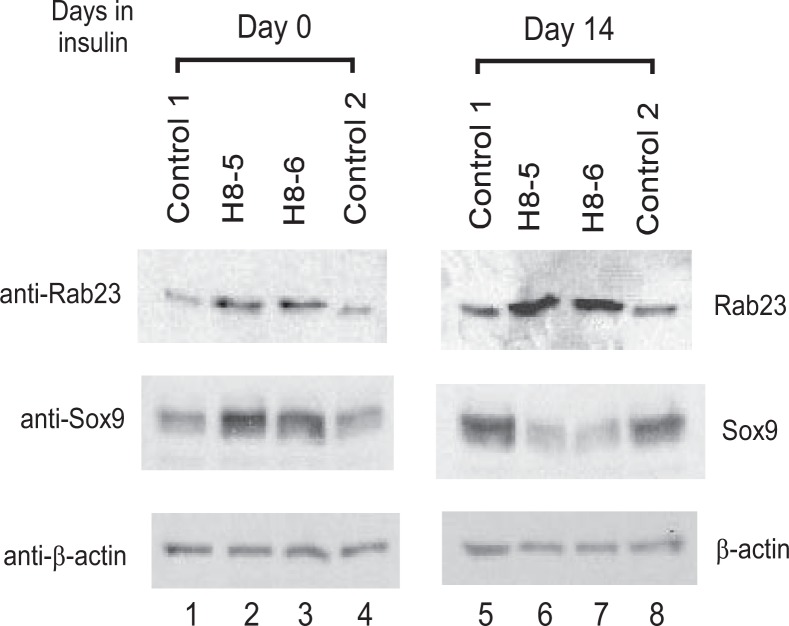
Insulin Down-regulates Sox9 Expression in Rab23-overexpressing ATDC5 Cells—The above observation that overexpression of Rab23 results in inhibition of chondrogenic differentiation prompted us to investigate the status of Sox9 expression in Rab23-overexpressing clones H8-5 and H8-6. We reasoned that a proper level of Rab23 might be critical for the functions of chondrocyte-regulatory genes such as Sox9, which is known to control Col2 and aggrecan expression and cartilage formation (10, 11). Before insulin treatment, the levels of Sox9 protein in clones H8-5 and H8-6 were higher than in the two normal control clones (Fig. 5, lanes 1–4). After insulin treatment for 2 weeks, however, the levels of Sox9 protein in clones H8-5 and H8-6 were instead significantly lower than in the two normal control clones (Fig. 5, lanes 5–8). This insulin-dependent down-regulation of Sox9 in clones H8-5 and H8-6 helps to explain why expression of Col2 and aggrecan fails to be induced following insulin treatment in Rab23-overexpressing ATDC5 cells. It is possible that because of the lack of sufficient Sox9 protein after insulin treatment of clones H8-5 and H8-6, chondrogenic differentiation could not take place in these Rab23-overexpressing cells.
FIGURE 5.
Insulin-dependent down-regulation of Sox9 in Rab23 overexpressing ATDC5 cells. Normal ATDC5 control clones 1 and 2 or mutant clones H8-5 and H8-6 were lysed before (lanes 1– 4) or after insulin treatment for 14 days (lanes 5– 8). The lysates were blotted with the anti-Rab23 antibody (top panel), the anti-Sox9 antibody (middle panel), or a mouse monoclonal anti-β-actin to show similar loading of proteins in each lane (bottom panel).
siRNA Knockdown of Rab23 Inhibits Chondrocyte Differentiation and Down-regulates Sox9 Gene Expression—A concern with protein overexpression is that the phenotype change may be artificial and not physiologically relevant. If Rab23 indeed plays a critical role in the regulation of chondrogenesis, depletion of Rab23 protein from the cells should also exert some effects on chondrocyte differentiation. To this end, we constructed a lentiviral vector for delivery of siRNA to achieve stable and long term knockdown of Rab23 in ATDC5 cells. Because the lentiviral vector also co-expresses green fluorescence protein, infected cells can also be enriched first through fluorescence-activated cell sorter sorting and then expanded for analysis.
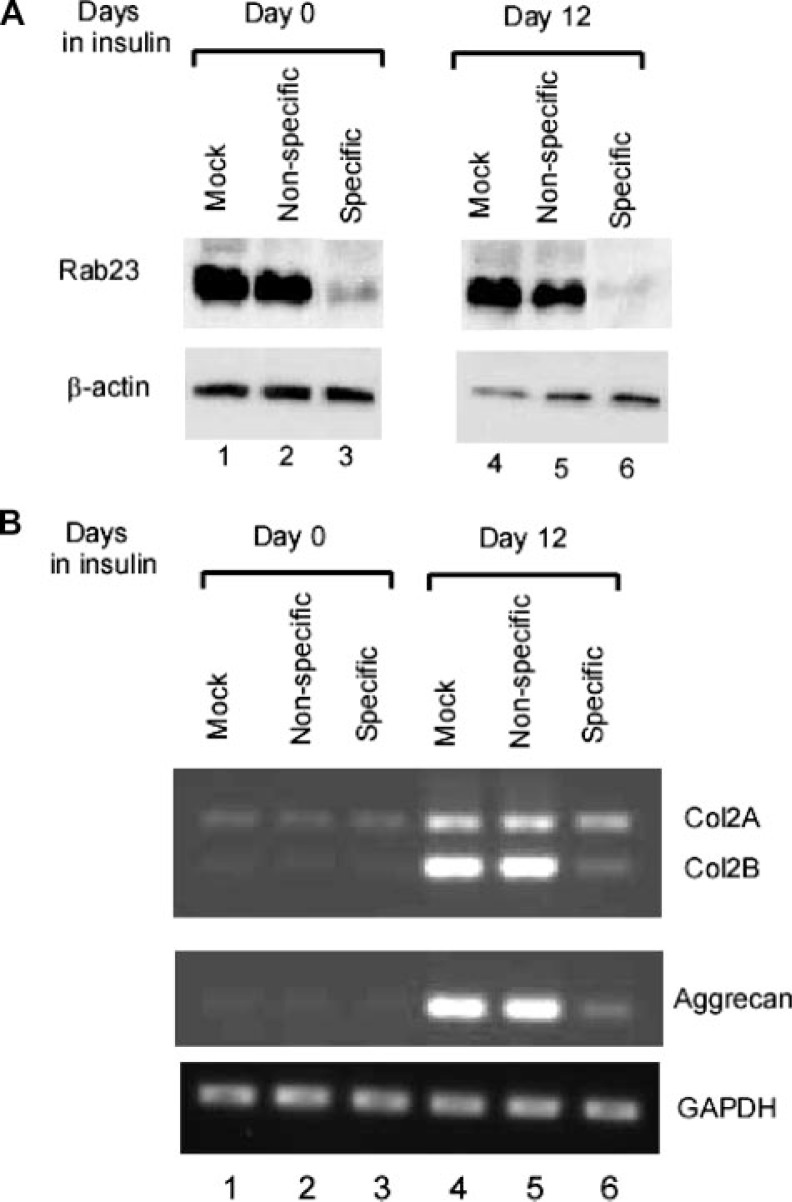
Although ATDC5 cells infected with a nonspecific siRNA had no effect on Rab23 expression when compared with mock infected cells, lentivirus-delivered siRNA against nucleotides 668–686 of Rab23 mRNA was able to significantly knock down Rab23 in ATDC5 cells (Fig. 6A, lanes 1–3). Such knockdown of Rab23 appeared to be sustainable because similar effect was observed in cells after treatment with insulin for 12 days. To analyze the effects of Rab23 knockdown on type II collagen expression, total RNAs were prepared from lentivirus-infected ATDC5 cells before or after insulin treatment. RT-PCR analysis revealed that knockdown of Rab23 was accompanied by a significant inhibition of Col2 expression when compared with mock or nonspecific siRNA samples (Fig. 6B, lanes 1–6, top panel). This inhibitory effect on matrix genes was not limited to type II collagen because expression of aggrecan was also down-regulated following Rab23 knockdown. However, expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase was not affected, suggesting a specific effect of Rab23 knockdown on genes encoding proteins involved in the production of the extracellular matrix.
FIGURE 6.
Down-regulation of Col2 and aggrecan following siRNA knockdown of Rab23. A, ATDC5 cells that were mock-infected, infected with a lentivirus that generates either nonspecific siRNA or specific siRNA against Rab23 were lysed for Western blotting with the anti-Rab23 antibody before (lanes 1–3) or after insulin treatment for 12 days (lanes 4 – 6). B, expression of Col2 and aggrecan was analyzed by RT-PCR using total RNAs from ATDC5 before (lanes 1–3) or after treatment with insulin for 12 days (lanes 4 – 6). RT-PCR analysis of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was performed to show specific down-regulation of Col2 and aggrecan following Rab23 knockdown.
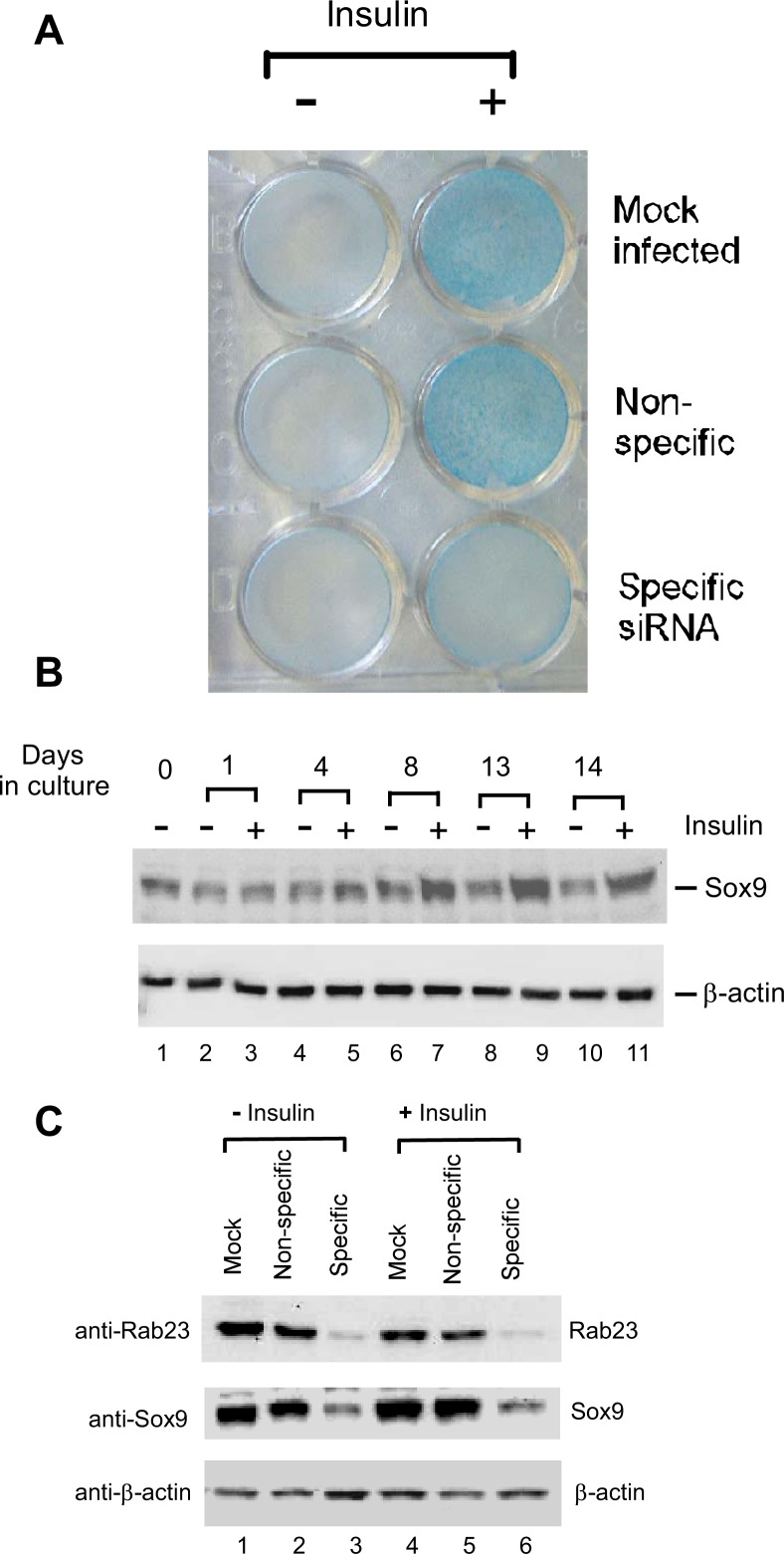
To further confirm that production of the cartilage-like extracellular matrix was negatively impacted by Rab23 knockdown, ATDC5 cells in a 24-well plate were first infected with lentivirus and then cultured in insulin medium for 12 days before staining with Alcian blue. The intensity of Alcian blue staining did not change between mock infected cells and cells with nonspecific siRNA. However, the intensity of Alcian blue staining was significantly diminished in cells with Rab23 knockdown (Fig. 7A), reflecting a reduced production of the cartilage-like extracellular matrix by these cells.
FIGURE 7.
Rab23-dependent chondrogenic differentiation and Sox9 expression in ATDC5 cells. A, ATDC5 cells that were mock-infected or infected with a lentivirus that generates either nonspecific siRNA or specific siRNA against Rab23 were plated onto a 24-well plate. The cells were cultured in medium with or without insulin for 12 days and then stained with 0.1% Alcian blue to detect production of the cartilage-like extracellular matrix. B, confluent ATDC5 cells were cultured in medium with or without insulin, and the cells were lysed at different time points for Western blotting with antibodies against Sox9 (top panel) or β-actin (bottom). C, mock-infected ATDC5 cells and those harboring nonspecific siRNA or specific siRNA against Rab23 were lysed for Western blotting with anti-Rab23 antibody (top panel), anti-Sox9 antibody (middle panel), or anti-β-actin antibody (bottom panel). The lysates were prepared from cells before (lanes 1–3) or after insulin treatment for 12 days (lanes 4 – 6).
How could a knockdown in Rab23 protein lead to down-regulation of matrix genes and reduction of cartilage-like extra-cellular matrix? We again examined the expression of Sox9 in these cells. As shown by Western blotting analysis, Sox9 protein is indeed abundant in uninfected ATDC5 cells. When cultured in insulin medium for a longer period of time, Sox9 level was increased in comparison with similarly cultured cells in medium without insulin (Fig. 7B). Interestingly, the level of Sox9 protein decreased following Rab23 knockdown both in unstimulated ATDC5 cells (Fig. 7C, lanes 1–3) and in insulin-stimulated cells (Fig. 7C, lanes 4–6). Because the level of Sox9 protein is critical to chondrogenesis (12), the inhibitory effects of Rab23 knockdown on Col2 and aggrecan expression are therefore most likely to be mediated through a concomitant down-regulation of Sox9.
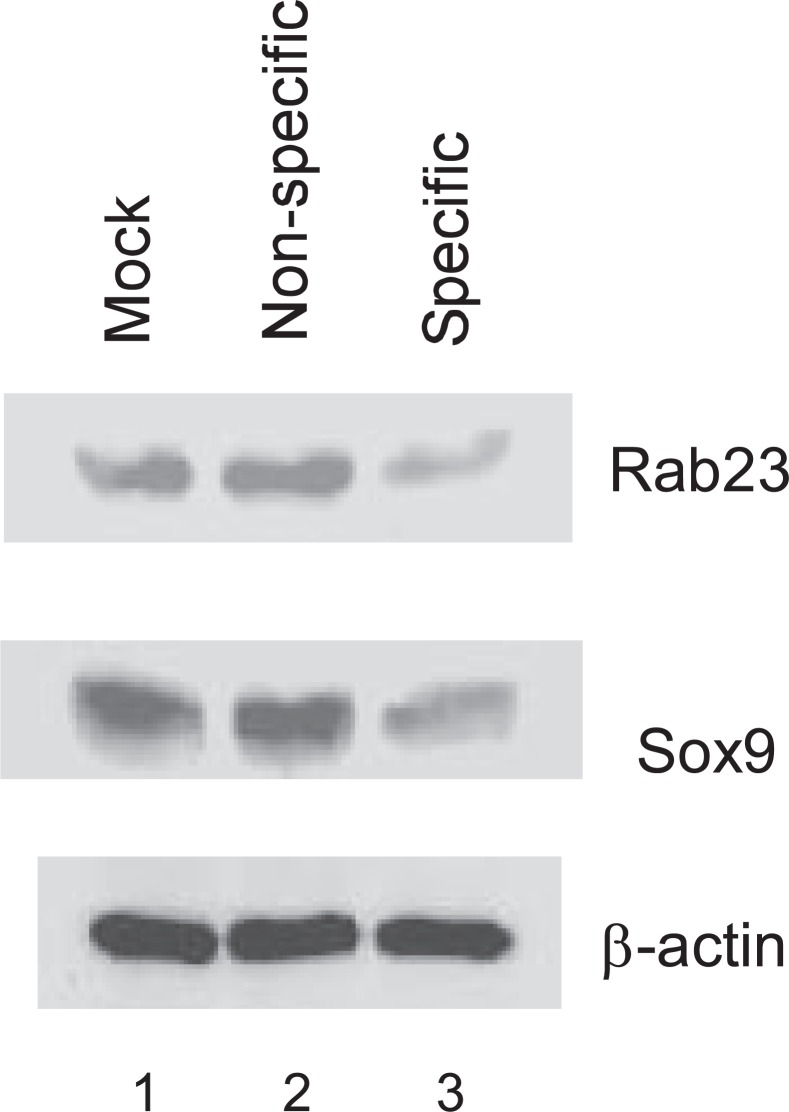
To confirm that Rab23-dependent expression of Sox9 is a common phenomenon and not specific to ATDC5 cells, we also infected the mouse mesenchymal stem cell line C3H10T1/2 with lentivirus for delivery of siRNA. It is well known that, depending on both the concentration and continuous presence of BMP in the growth medium, these cells can undergo a sequential pattern of chondrogenic followed by osteogenic differentiation (13). Like in ATDC5 cells, Rab23 expression was knocked down by a specific siRNA against Rab23 (Fig. 8, top panel). Although cells with the specific Rab23 siRNA had a decreased level of Sox9 protein in comparison with mock infected cells or cells harboring the nonspecific siRNA (middle panel), the levels of β-actin were not affected by different treatments in these C3H10T1/2 cells (bottom panel).
FIGURE 8.
Rab23-dependent Sox9 expression in C3H10T1/2 cells. Mouse mesenchymal stem cell line C3H10T1/2 was mock-infected, infected with a lentivirus that generates either nonspecific or specific siRNA against Rab23. Four days after infection, the cells were lysed for Western blotting with antibodies against Rab23 (top panel), Sox9 (middle panel), and β-actin (bottom panel).
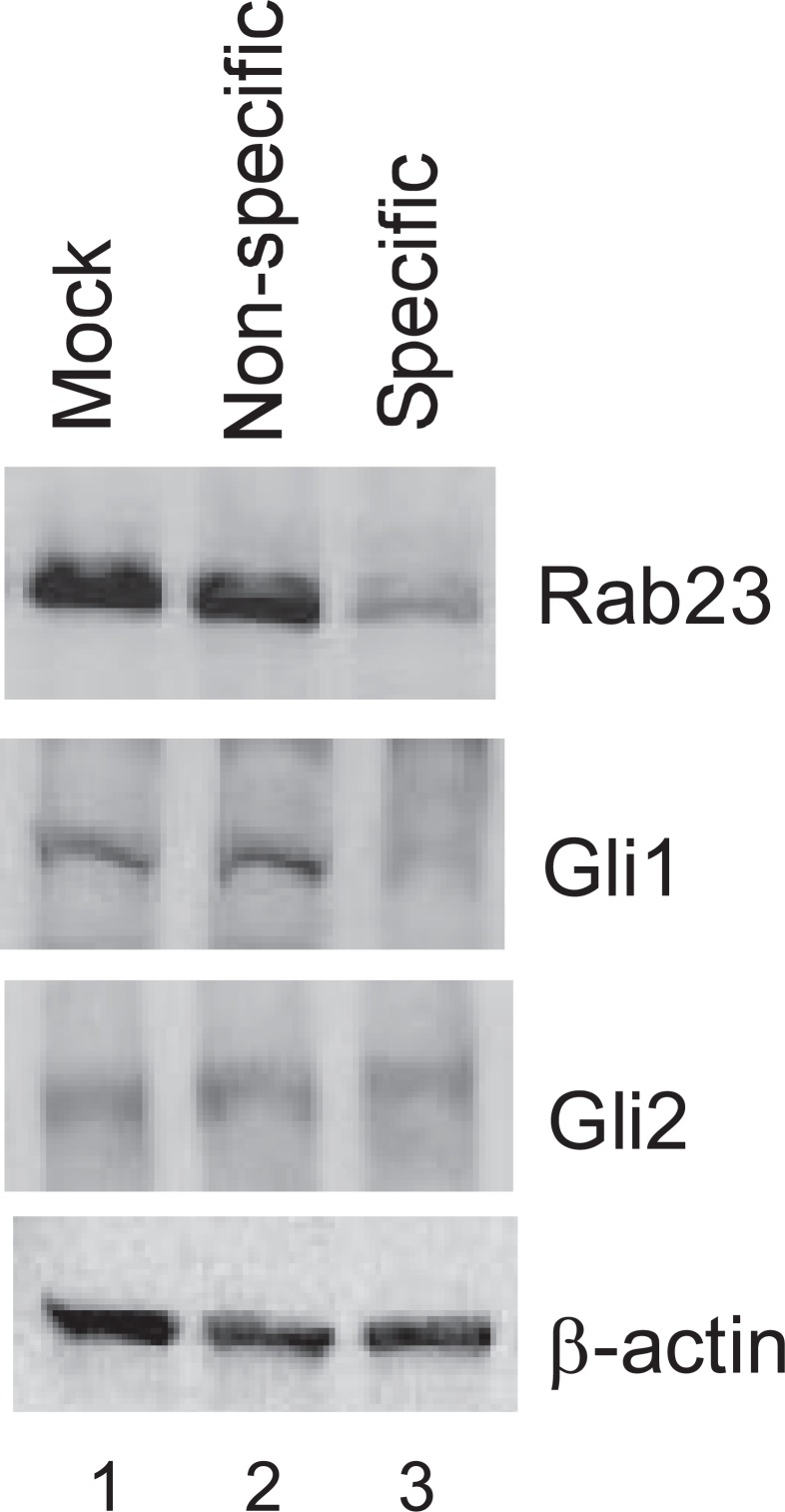
siRNA Knockdown of Rab23 Results in a Decrease in Gli1 Protein—How could a decrease in Rab23 lead to down-regulation of Sox9 in chondrogenic cells? In neuronal cells, Rab23 is known to antagonize signaling of the Sonic hedgehog pathways. Because Indian hedgehog pathway plays an important role in the differentiation of chondrocytes, we next examined in ATDC5 cells whether changes in Rab23 expression had any effect on the common targets of the hedgehog pathways such as Gli proteins. For this, the cells were again infected with lentivirus for delivery of siRNA. In cells expressing specific siRNA against Rab23, we observed knockdown of Rab23 and a concomitant decrease in Gli1 expression, whereas Gli2 was not affected (Fig. 9). Transcription of the Gli1 gene has been known to be under the control of hedgehog, and up-regulation of Gli1 is diagnostic of hedgehog signaling (14). Because expression of Gli1 but not Gli2 is dependent on Rab23 in chondrogenic cells, our data suggest that Rab23 is able to selectively affect component(s) of the hedgehog pathway even in the absence of active hedgehog signaling.
FIGURE 9.
Effects of Rab23 knockdown on Gli proteins in ATDC5 cells. Mock-infected ATDC5 cells and those harboring nonspecific or specific siRNA against Rab23 (lanes 1–3) were lysed for Western blotting with antibodies against Rab23 (top panel), Gli1 (second panel), Gli2 (third panel), or β-actin (bottom panel).
DISCUSSION
Mutations in Rab23 were originally found to be associated with the mouse open brain phenotype (opb) (15, 16). These mutations are single-nucleotide substitutions in both mutant alleles that introduce early stop codons into the open reading frame. In addition to neural tube defects noted in opb, giving the phenotype its name, Rab23 mutations are also associated with malformations in axial skeleton and limbs. In opb embryos, the anlagen of the vertebral bodies were irregular in shape and appeared to be split in half. Defects in the developing ribs include fusions of ribs, split ribs, and holes in the rib anlagen. Limb malformations in opb mutant embryo include shortening of the fibula, greatly reduced tibia anlagen, and duplication of digit I or digit II (polydactyly) (15).
In human, homozygous nonsense mutations in Rab23 have been recently linked to Carpenter syndrome (17), a rare genetic disorder characterized by premature closure of the fibrous joints (cranial sutures) between certain bones of the skull. Individuals with Carpenter syndrome may also have unusually short fingers and toes, partial fusion of the soft tissues between certain digits, the presence of extra toes or additional fingers, or physical abnormalities such as short stature.
How does Rab23 exert its effects on development? The extra-cellular signaling protein Shh (Sonic hedgehog) is essential for many aspects of mammalian embryogenesis such as patterning of the neural tube and limbs. Mutations in Shh and Rab23 are known to cause opposing transformations in neural cell fate; Shh mutant embryos lack ventral cell types, whereas Rab23 mutants lack dorsal cell types. Because ventral cell types that are absent in Shh mutants can be partially restored in Shh Rab23 double mutants (18), it has been thought that Rab23 acts down stream of Shh and functions as an essential negative regulator of the Sonic hedgehog signaling pathway.
Shh signaling is mainly mediated through the Patched and Smoothened transmembrane proteins, and the information is eventually decoded in the nucleus by the action of three zinc finger transcription factors Gli1, Gli2, and Gli3 (19). In general, Gli1 and Gli2 are activators of Shh-responsive genes, whereas Gli3 functions as a repressor. Genetic studies have indicated that Rab23 inhibits the formation of activator Gli2 and that inappropriate activation of Gli2 is responsible for most neural patterning defects of the Rab23 mutant phenotype (20). In addition, Rab23 has also been shown to promote the production of Gli3 repressor (20). In this study, we found that Gli2 is not affected by a decrease of Rab23 in chondrogenic cells, but expression of Gli1 appears to be dependent on Rab23.
Ihh (Indian hedgehog), one of the three mammalian hedgehog proteins, coordinates proliferation and differentiation of chondrocytes during endochondral bone development (21). Because Sonic hedgehog and Indian hedgehog share a similar signaling pathway, it is possible that Rab23 protein may affect chondrogenesis as an antagonist of Indian hedgehog signaling in chondrocytes. The role of Rab23 on chondrocyte differentiation revealed in this study may help to explain why mutations in Rab23 can negatively impact chondrogenesis and bone development but is difficult to reconcile with the fact that the antagonistic effects of Rab23 are cell autonomous and do not need Patched or Smoothened protein (20). It is therefore more likely that Rab23 acts in a hedgehog-independent manner by regulating expression of a specific downstream component of the signaling pathway such as Gli1.
In this study, we report that either overexpression or siRNA knockdown of Rab23 results in inhibition of chondrogenic differentiation and a loss of cartilage-like extracellular matrix by ATDC5 cells. In Rab23 overexpressing ATDC5 cells, we noticed that insulin failed to induce differentiation while causing a down-regulation of Sox9. It is not clear at the present time whether this effect of Rab23 overexpression on Sox9 is physiologically relevant. The major concern is that such an effect of Rab23 overexpression is insulin-dependent and may vary from cell type to cell type. On the other hand, siRNA knockdown of Rab23 was accompanied by a failure of differentiation and a decrease in Sox9 protein regardless of insulin treatment in ATDC5 cells. In addition, a similar effect of Rab23 knockdown on Sox9 was observed in C3H10T1/2 mesenchymal stem cells. Thus, it appears that normal differentiation of chondrogenic cells requires a certain level of Rab23 protein. Too little Rab23 protein can lead to down-regulation of Sox9 expression and subsequent failure of chondrogenic differentiation.
Sox9 has essential, nonredundant roles in specifying the commitment and differentiation of mesenchymal cells toward the chondrogenic lineage in all developing skeletal elements (7). Heterozygous mutations in and around the Sox9 gene result in haploinsufficiency and cause campomelic dysplasia (22, 23), a severe form of chondrodysplasia in human. Sox9, together with L-Sox5 and Sox6, constitutes a master chondrogenic regulatory trio (24). Because a sufficient level of Sox9 protein is so important to normal chondrogenesis, we speculate that skeletal defects associated with Rab23 mutations in mice are caused by a down-regulation of the Sox9 protein and defective or incomplete cartilaginous anlagen.
Formation of flat bones of the skull does not require a cartilage template but instead is mediated through intramembranous ossification. In this process, osteochondral progenitor cells bypass chondrocytes and directly differentiate into bone-forming osteoblasts. It is possible that in patients with Carpenter syndrome, Rab23 mutations result in decreased expression of Sox9 in osteochondral progenitor cells that eventually differentiate into flat bones of the skull. Sox9 expression normally favors chondrocyte lineage. A decreased level of Sox9 protein in these osteochondral progenitor cells may lessen the antagonistic effects of Sox9 and accelerate osteoblast differentiation, leading to premature closure of the cranial sutures.
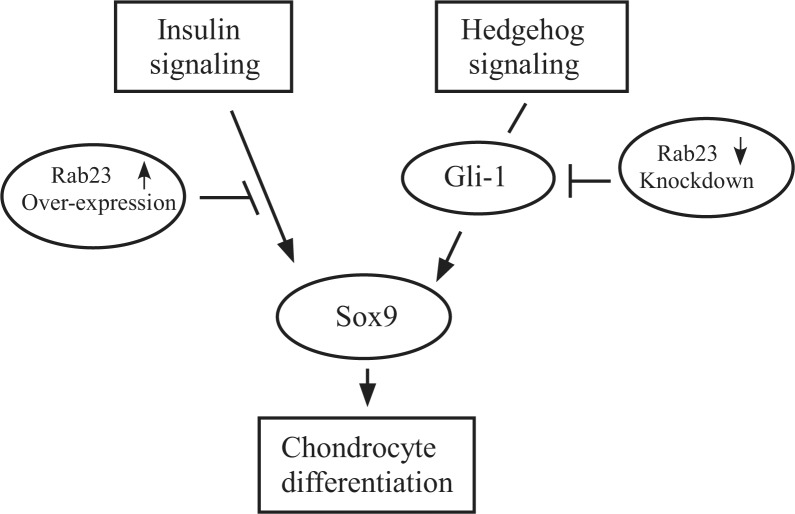
It is not clear at the present time why expression of Sox9 protein requires a proper level of Rab23 protein. We speculate that in Rab23-overexpressing ATDC5 cells, too much Rab23 some how blocks insulin signaling that is required for up-regulation of Sox9 and other chondrogenic factors. In chondrogenic cells with Rab23 knockdown, our study suggests that Rab23-dependent Sox9 expression could be mediated by Gli1. A recent study supports a direct link between the Shh signaling protein Gli1 and Sox9 regulation, wherein Shh stimulates Sox9 through Gli1 binding to an enhancer element 1.1 Mb upstream of the Sox9 gene (25). In this scenario, Gli1 expression itself may require Rab23 protein, and knockdown of Rab23 leads to less Gli1 and subsequent down-regulation of Sox9 (summarized in Fig. 10). Because Rab23 is also thought to be involved in intra-cellular protein trafficking (26, 27), we cannot rule out the possibility that Sox9 expression may require the trafficking of a critical factor by Rab23, and loss of Rab23 will block the supply of such a factor therefore leading to Sox9 down-regulation.
FIGURE 10.
Schematic of Rab23 functions in chondrogenic cells. Chondrogenic cells can be induced to undergo differentiation either through insulin or hedgehog pathway. Overexpression of Rab23 is able to disrupt insulin signaling and thus blocks up-regulation of Sox9. On the other hand, expression of Gli1 is dependent on Rab23 in these cells. Gli1 in turn controls expression of Sox9. Knockdown of Rab23 leads to down-regulation of Gli1, resulting in a decrease in Sox9 and a failure of chondrogenic differentiation.
Footnotes
This work was supported by Public Health Service Grant RO1 AR051455 (to L. Y.) and by a Veterans Affairs Merit Review Award (to H. A. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: siRNA, small interfering RNA; RT, reverse transcription.
REFERENCES
- 1.Kronenberg HM. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 2.Atsumi T, Miwa Y, Kimata K, Ikawa Y. Cell Differ Dev. 1990;30:109–116. doi: 10.1016/0922-3371(90)90079-c. [DOI] [PubMed] [Google Scholar]
- 3.Olkkonen VM, Peterson JR, Dupree P, Lutcke A, Zerial M, Simons K. Gene (Amst) 1994;138:207–211. doi: 10.1016/0378-1119(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 4.Caceres JF, Misteli T, Screaton GR, Spector DL, Krainer AR. J Cell Biol. 1997;138:225–238. doi: 10.1083/jcb.138.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 6.Guo A, Wang T, Ng EL, Aulia S, Chong KH, Teng FY, Wang Y, Tang BL. J Neurosci Res. 2006;83:1118–1127. doi: 10.1002/jnr.20788. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre V, Smits P. Birth Defects Res C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- 8.Doege KJ, Sasaki M, Kimura T, Yamada Y. J Biol Chem. 1991;266:894–902. [PubMed] [Google Scholar]
- 9.Schwartz NB, Pirok EW, 3rd, Mensch JR, Jr, Domowicz MS. Prog Nucleic Acids Res Mol Biol. 1999;62:177–225. doi: 10.1016/s0079-6603(08)60508-5. [DOI] [PubMed] [Google Scholar]
- 10.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 11.Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, Nifuji A, Noda M. J Biol Chem. 2000;275:10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- 12.Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Proc Natl Acad Sci U S A. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. J. Cell. Biochem. 2003;90:1112–1127. doi: 10.1002/jcb.10734. [DOI] [PubMed] [Google Scholar]
- 14.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. J Biol Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 15.Gunther T, Struwe M, Aguzzi A, Schughart K. Development. 1994;120:3119–3130. doi: 10.1242/dev.120.11.3119. [DOI] [PubMed] [Google Scholar]
- 16.Kasarskis A, Manova K, Anderson KV. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7485–7490. doi: 10.1073/pnas.95.13.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, Donnai D, Josifiova D, Mathijssen IM, Morton JE, Orstavik KH, Sweeney E, Wall SA, Marsh JL, Nurnberg P, Passos-Bueno MR, Wilkie AO. Am J Hum Genet. 2007;80:1162–1170. doi: 10.1086/518047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggenschwiler JT, Espinoza E, Anderson KV. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz i Altaba A, Nguyen V, Palma V. Curr Opin Genet Dev. 2003;13:513–521. doi: 10.1016/j.gde.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV. Dev Biol. 2006;290:1–12. doi: 10.1016/j.ydbio.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 21.St-Jacques B, Hammerschmidt M, McMahon AP. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 23.Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 24.Smits P, Dy P, Mitra S, Lefebvre V. J Cell Biol. 2004;164:747–758. doi: 10.1083/jcb.200312045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bien-Willner GA, Stankiewicz P, Lupski JR. Hum Mol Genet. 2007;16:1143–1156. doi: 10.1093/hmg/ddm061. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Ng EL, Tang BL. Traffic. 2006;7:746–750. doi: 10.1111/j.1600-0854.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 27.Evans TM, Ferguson C, Wainwright BJ, Parton RG, Wicking C. Traffic. 2003;4:869–884. doi: 10.1046/j.1600-0854.2003.00141.x. [DOI] [PubMed] [Google Scholar]