Abstract
OBJECTIVE
Type 2 diabetes is increasingly common and associated with substantial morbidity and mortality. This study examines trends in the patterns and costs of drug treatment of type 2 diabetes from 1997 to 2012.
RESEARCH DESIGN AND METHODS
We conducted descriptive analyses of cross-sectional data using the IMS Health National Disease and Therapeutic Index, a nationally representative audit of ambulatory physician practices in the U.S. We focused on visits for diabetes among patients 35 years of age or older. We used the IMS Health National Prescription Audit of pharmacy dispensing to derive information about drug expenditures.
RESULTS
Ambulatory diabetes visits increased from 23 million treatment visits in 1997 (95% CI 21–25) to 35 million (32–37) in 2007 and declined to 31 million visits by 2012 (27–31). Between 1997 and 2012 biguanide use increased, from 23% (20–26) to 53% (50–56) of treatment visits. Glitazone use grew from 6% (4–8) in 1997 (41% [39–43] of all visits in 2005), but declined to 16% (14–18) by 2012. Since 2005, dipeptidyl peptidase-4 (DPP-4) inhibitor use increased steadily, representing 21% (18–23) of treatment visits by 2012. Glucagon-like peptide 1 (GLP-1) agonists accounted for 4% of treatment visits in 2012. Visits where two or more drug compounds were used increased nearly 40% from 1997 to 2012. Between 2008 and 2012, drug expenditures increased 61%, driven primarily by use of insulin glargine and DPP-4 inhibitors.
CONCLUSIONS
Declining sulfonylurea and glitazone use has been offset by increases in DPP-4 inhibitor use and, to a lesser degree, use of GLP-1 agonists. Treatment of diabetes has grown in complexity while older treatments continue to be replaced or supplemented by newer therapies.
Introduction
Diabetes is a common chronic disease that affects millions of Americans. As of 2011, 11.3% of people 20 years or older had diagnosed and undiagnosed diabetes (1). Forecasts suggest a continued increase in the population burden of diabetes during the next few decades, with 1 in 3 adults in the U.S. at risk for developing the disease by 2050 (1). This disease is also associated with considerable economic burden, with annual direct medical expenditures for diabetes treatment and management totaling nearly $250 billion in 2012, representing a 41% increase since 2007 (2,3). Although the majority of medical expenditures for diabetes are attributable to hospitalization and physician services, the costs associated with prescription therapies are not trivial, particularly for millions of individuals living on fixed incomes or otherwise burdened by their out-of-pocket prescription costs (4).
The prevalence and burden of diabetes have made it a target ripe for pharmaceutical development, and during the past decade several important changes in the marketplace have occurred (5). Early in the 2000s, glitazones were rapidly adopted for use, although subsequent evidence of cardiovascular risks, particularly with rosiglitazone, led to substantial declines in their use during the latter half of the decade (6). Second, the development and expansion of long-acting insulins that allow patients to take just one injection a day has provided enhanced convenience for patients while achieving more stable glucose control (7). Additionally, during the past decade, the U.S. Food and Drug Administration (FDA) has approved several new classes of therapies for the treatment of type 2 diabetes, including injectable incretin mimetics (glucagon-like peptide 1 [GLP-1] agonists), dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium glucose cotransporter 2 (SGLT-2) inhibitors. GLP-1 receptor agonists first became available in 2005, followed by DPP-4 inhibitors in 2006. Both classes work via the incretin hormone GLP-1, which increases insulin secretion, delays gastric emptying, and decreases blood glucose levels (8,9). Despite their high cost, these therapies have been met with interest, particularly because their novel mechanisms of action allows for their use in combination with other therapies. In addition, GLP-1 agonists have the potential to induce weight loss (10) and both GLP-1 and DPP-4 reduce hypoglycemic risk, though concerns regarding their carcinogenicity and pancreatitis risk have also been raised (11).
A variety of investigations have examined changes in the treatment of diabetes over the past few decades. These studies have shown evidence of large declines in sulfonylurea use, increases in biguanides, vast fluctuations among glitazones, and evidence of increased costs and complexity of treatment (12–14). However, many of these investigations took place only shortly after regulatory communications and prominent scientific reports about the potential cardiovascular risks associated with glitazones and prior to the market diffusion of DPP-4 inhibitors and GLP-1 agonists.
We examined treatment patterns for type 2 diabetes between 1997 and 2012 among office-based physicians in the U.S. In addition to updating prior trends, we were particularly interested in the adoption of DPP-4 inhibitors and GLP-1 agonists as well as how changes in the use of long-acting insulins may have impacted the utilization of oral therapies. We also examined treatment patterns with specific combinations of drugs, treatment complexity, and the aggregate cost of different classes of agents.
Research Design and Methods
Data
We used the IMS Health National Disease and Therapeutic Index (NDTI) to obtain nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the continental U.S. The NDTI sample consists of office-based physicians selected from the master lists of the American Medical Association and the American Osteopathic Association, which include both members and nonmembers of these organizations. About 3,500 physicians participate in data collection on two randomly assigned consecutive workdays in each calendar quarter. IMS Health uses a two-stage stratified cluster design, based on geographic location and physician specialty, to generate national projections from these data.
During each day of participation, physicians complete a form for each consecutive patient encounter. Each encounter form includes basic patient demographic information as well as information about patient diagnoses and treatments. Diagnostic information is captured using a six-digit taxonomic code that is similar to information contained within the ICD-9. We focused on visits for type 2 diabetes, and limited our analyses to individuals 35 years or older to increase the specificity of our method of identification as well as to replicate our prior approach (11). Our primary outcome was a “treatment visit,” defined as a visit for a patient where one or more pharmacotherapy was used for treatment of diabetes. A single patient encounter may generate multiple treatment visits, since each product dispensed generates a separate treatment visit. Although NDTI includes encounters that occur outside of ambulatory settings, we excluded the approximately 15% of encounters that occurred by telephone or took place in inpatient or other institutional settings.
We also analyzed data from the IMS Health National Prescription Audit (NPA) from 2008 through 2012. The NPA is an industry standard source of national prescription dispensing activity for all pharmaceutical products and measures what providers prescribe and what is ultimately dispensed to consumers. From the selected pharmacies, IMS collects new and refilled prescriptions for every day of the month. The NPA is derived from a national random computerized sample that as of 2012 was based on a universe of approximately 57,000 retail pharmacies, 327 nongovernmental mail service pharmacy outlets, and 3,000 long-term care pharmacies, including nursing homes and nursing home providers. Information on estimated total expenditures (consumer plus insurance) for these dispensed medications is reported as part of the administrative systems used by pharmacies to bill consumers and health insurers for these products. Because these data do not provide diagnostic information about the patient receiving the prescription, the cost information generated applies to all possible uses of these medications, including type 1 and type 2 diabetes, as well as any off-label medication uses. We accounted for inflation by adjusting dollars to reflect 2008 values using the consumer price index inflation calculator provided by the U.S. Bureau of Labor Statistics (15).
Analyses
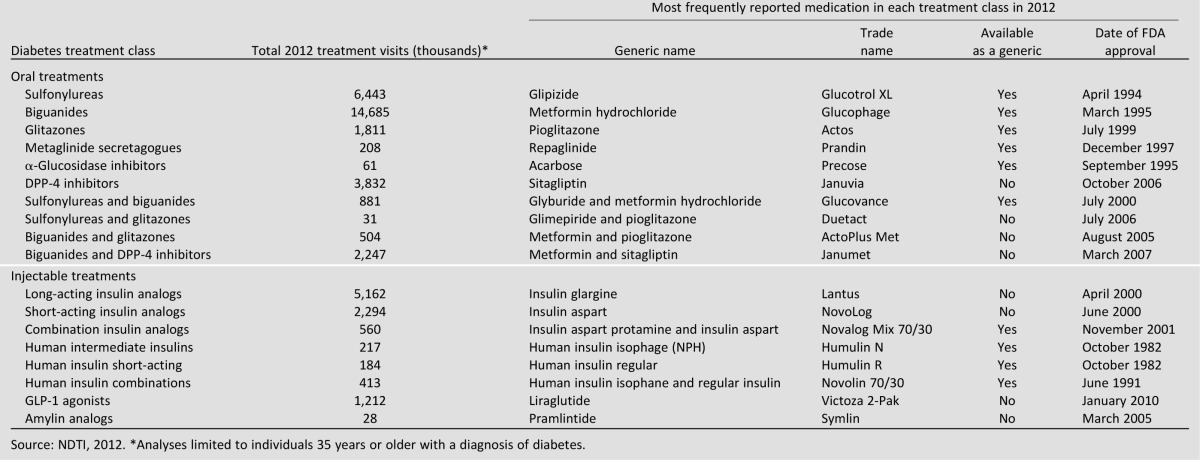
We used descriptive statistics to evaluate trends in the utilization of each therapy of interest. We focused on eight medication classes depicted in Table 1: sulfonylureas (glucotrol XL), biguanides (metformin HCl), glitazones (pioglitazone), DPP-4 inhibitors (sitagliptin), incretins (liraglutide), meglitinides (repaglinide), α-glucosidase inhibitors (acarbose), insulins (insulin glargine), and amylin analogs (pramlintide). We classified drugs within these therapeutic groups based on their chemical composition, using the IMS Health Universal System of Classification (USC) codes. We counted fixed-dose combination products as contributing to each of their constituent classes when computing total compounds; for example, a combination product such as Janumet (sitagliptin and metformin) was counted as contributing once to biguanides and once to DPP-4 inhibitors. Thus, our analysis of trends in DPP-4 use includes visits where they were used as fixed-dose combination products containing DPP-4 inhibitors as well as where they were used as an individual product. We also calculated therapeutic intensity, which we assessed by dividing total number of compounds used by the total number of unique treatment visits in a given year. For these calculations, a visit with a fixed-dose combination would be considered as similarly intense as a treatment where two separate products were used, since both would similarly reflect the use of two compounds during a single visit. For estimates from the NDTI, we calculated 95% CIs using tables of relative standard errors that are derived accounting for the survey’s complex sampling design. Because the NPA is based on such a large sample of pharmacies, the uncertainty surrounding estimates of national prescription expenditures is small.
Table 1.
Leading ambulatory type 2 diabetes medications by treatment class, 2012
Results
Trends in Treatment Visits
There was a steady rise in ambulatory treatment visits for type 2 diabetes, from 23.6 million (95% CI 20.8–25.3) to a peak of 35.3 million visits in 2007 (32.2–37.3), then a modest decline to 31.2 million visits in 2012 (28.6–34.0).
Changes in Medication Classes Used as Diabetes Therapy
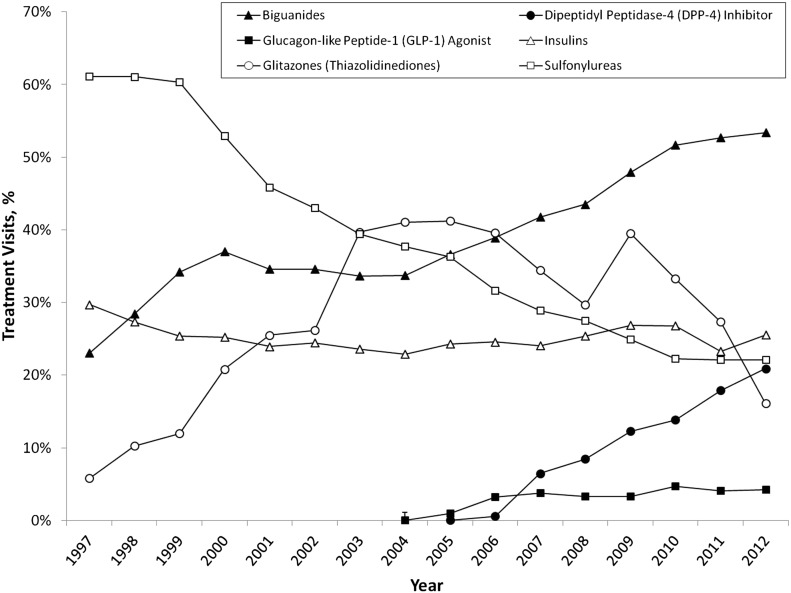
Use of sulfonylureas has declined continuously since 1997, when they were the most prescribed class of medicines accounting for about 61% (95% CI 57–69) of all treatment visits; by 2012, sulfonylureas were used in just 22% (20–26) of diabetes treatment visits (Fig. 1). Glitazone treatment visits increased from 1997 to 2005, peaking at 41% of all visits, then declined to 16% (14–18) in 2012, at which point almost 96% of glitazone treatment visits involved pioglitazone products. Metformin use has increased steadily from 24% (21–27) of treatment visits in 1997 to 53% (51–55) in 2012.
Figure 1.
National trends in the ambulatory treatment of type 2 diabetes, 1997–2012. Source: NDTI, 1997–2012.
Since the approval of the first DPP-4 inhibitor, sitagliptin (Januvia), by the FDA in 2006, DPP-4 inhibitors have grown to account for approximately one in five treatment visits for diabetes. Over a similar time period, the injectable GLP-1 agonists have grown to just 4% of all treatment visits.
Treatment Patterns Using Insulins
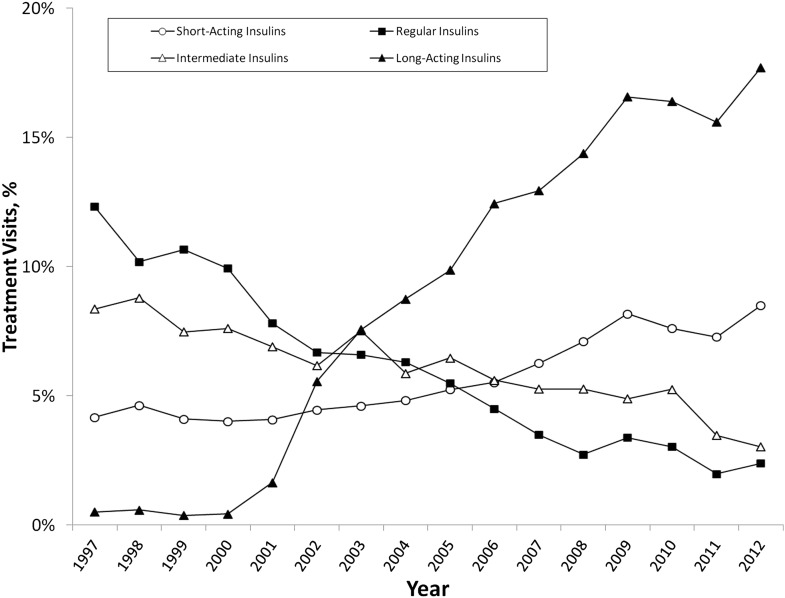
As a class, insulin use has remained relatively stable, accounting for 27% (95% CI 25–29) of treatment visits in 1997 and 26% (23–27) in 2012. However, since 1997, regular and NPH insulin treatment visits have declined to account for only 2% and 3% of all treatment visits, respectively (Fig. 2). Conversely, short-acting insulin use has doubled over that same time period, while use of long-acting insulin has increased from less than 1% of treatment visits (1997) to almost 18% of all treatment visits (2012).
Figure 2.
National trends in the ambulatory treatment of type 2 diabetes with insulins, 1997–2012. Source: NDTI, 1997–2012.
Mono- and Dual-Therapy and Intensity of Treatment
Since 1997, the overall proportion of visits where a single treatment, including a single fixed-dose combination treatment, was used has declined by almost 30%, representing 42% of treatment visits in 2012 (Table 2). When combination products are excluded, this decreased further to only 35% of treatment visits. Of the 14.9 million treatment visits where a single compound was used in 2012, biguanide (53% [95% CI 51–55]) and insulin (15% [13–17]) visits were most common, with fewer accounted for by sulfonylureas (13% [12–14]), DPP-4 inhibitors (10% [9–11]), or other products (Table 3).
Table 2.
National trends in ambulatory type 2 diabetes treatment intensity, 1997–2012
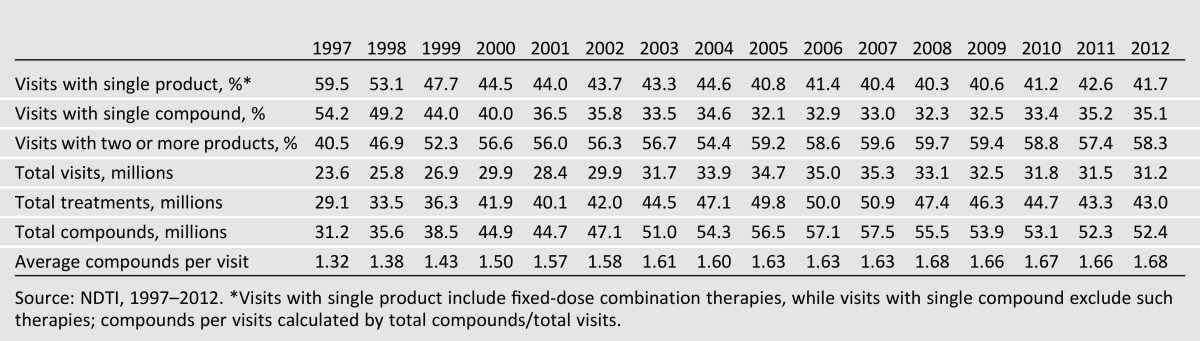
Table 3.
Diabetes treatments used alone and in combination, 2012
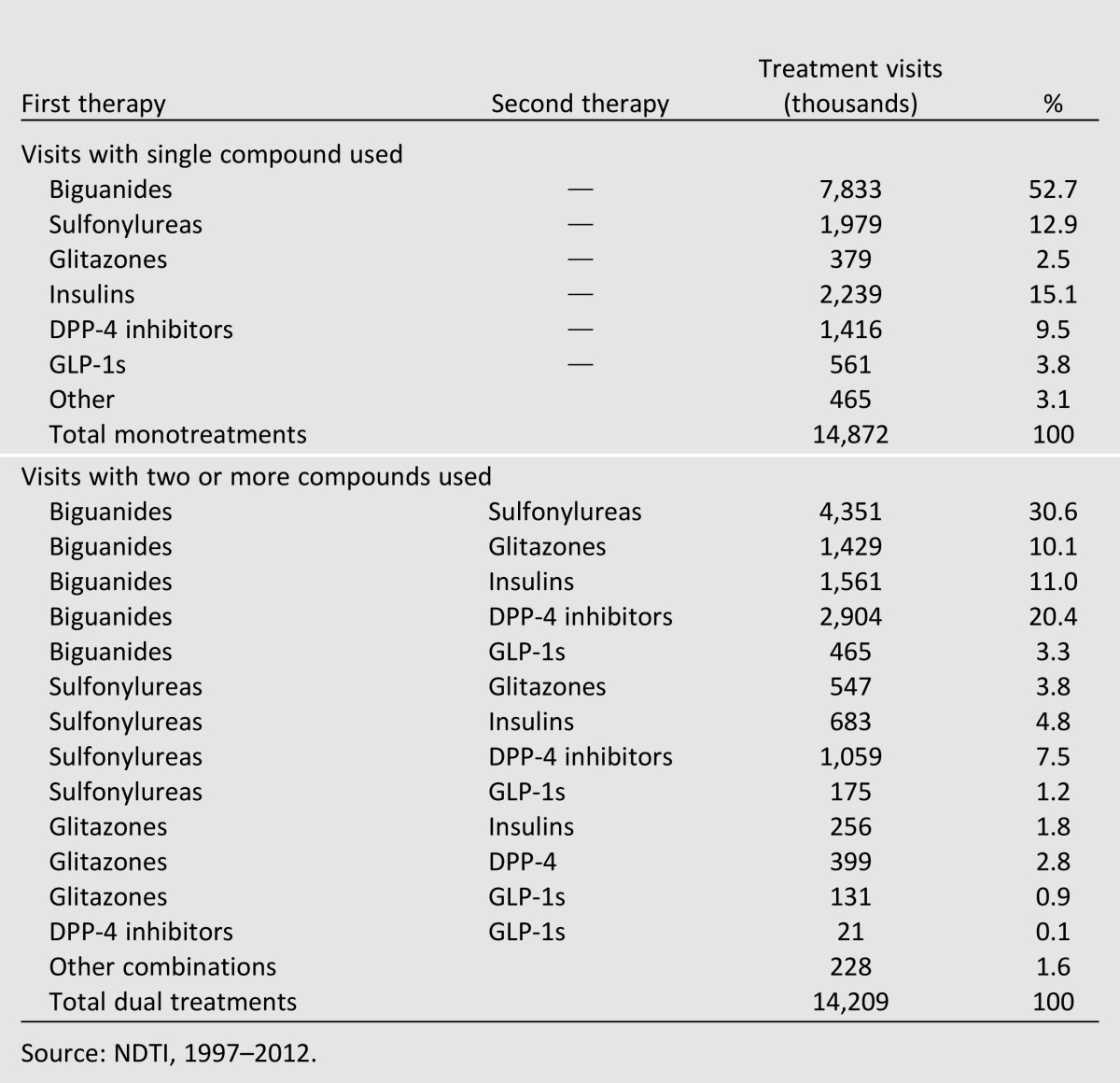
In 1997, 9.6 million treatment visits (41% of all treatment visits) included two or more compounds. By 2012, this had increased to 18.2 million treatment visits, more than 58% of all treatment visits. Approximately one-third of these visits were represented by the combination of biguanides and sulfonylureas (31% [29–33]), while approximately one-fifth were accounted for by biguanides and DPP-4 inhibitors (20% [18–22]). Almost 18% (16–20) of dual-therapy visits included some form of insulin. Nearly 76% (75–77) of all DPP-4 agonists were used in combination therapies.
Therapeutic intensity, defined as the average number of compounds used per treatment visit, has increased steadily over time, from an average of 1.32 compounds per visit in 1997 to 1.68 compounds per visit in 2012, a 27% increase over 15 years.
Medication Expenditures
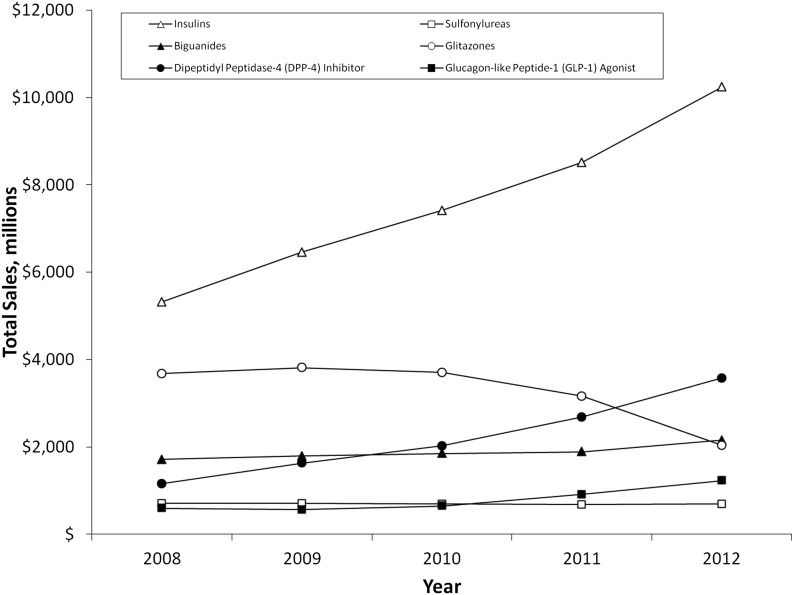
Since 2008, retail pharmacy expenditures increased by 61%, reaching almost $22 billion by 2012 after adjustment for inflation. This increase was driven primarily by insulins and, to a lesser degree, DPP-4 inhibitors (Fig. 3). As a class, insulin’s total retail sales have more than doubled, primarily due to increases in long- and short-acting insulins (data not shown). DPP-4 inhibitor sales have increased by 10% since 2008, reaching almost $4 billion dollars in 2012. GLP-1 agonists and biguanides have experienced modest increases over this same time period, while sulfonylurea sales have remained around $700 million annually. Glitazone sales increased from 2007 to 2008, then decreased to just over $2 billion dollars in 2012.
Figure 3.
National trends in pharmacy expenditures on diabetes drugs, 2008–2012. Adjusted for inflation using 2008 dollars. Source: NPA, 2008–2012.
Conclusions
In this study of a nationally representative audit of ambulatory practice in the U.S., we found substantial changes in treatment of type 2 diabetes during the past decade. These changes include continued declines in the use of sulfonylureas and glitazones, a plateauing of biguanide use after steady increases until 2010, and stable insulin use that is increasingly dominated by long-acting insulin glargine. We also found rapid increases in the use of DPP-4 inhibitors since their market debut, reaching approximately 20% of treatment visits by 2012, and a leveling off of incretin mimetic use after modest uptake between 2005 and 2007. These findings are important because of the economic and clinical burden posed by type 2 diabetes, as well as a rapidly evolving clinical marketplace.
Our findings regarding DPP-4 inhibitors and GLP-1 agonists are noteworthy. Both of these classes of agents produce significant improvements to glycemic control, particularly in patients who are not well controlled with first-line oral agents (16–18), although there is some evidence that GLP-1s are more efficacious than DPP-4 inhibitors with respect to glycemic end points (19). There is also an increasingly amount of scientific information available about their association with cardiovascular outcomes (20,21). Clinical guidelines by professional societies have also supported earlier use of these therapies (11,22,23), and declines in glitazones may have also further facilitated their adoption (12). Despite this, there is ongoing disagreement regarding the degree to which agents acting through the GLP-1 pathway are associated with pancreatitis and pancreatic cancer (24,25).
Biguanides continue to be the cornerstone of diabetes management; by 2012, more than 50% of treatment visits were accounted for by metformin. Conversely, we found continued reductions in glitazones and sulfonylureas. In the case of glitazones, the use of these products steadily increased since the market introduction of rosiglitazone and pioglitazone in 1999, until they peaked in 2005. The ensuing decrease of all glitazone use was strongly correlated with the publication and FDA warning of increased cardiovascular risk with rosiglitazone. Though these risks continue to be debated (26), the product label continues to carry warnings and there is no evidence of any substantial recovery of market share by these products. In contrast to the glitazones, sulfonylureas use began to wane earlier, in the mid-1990s; these declines have occurred for a number of reasons, including their risks of hypoglycemia, association with weight gain, and minimal marketing and promotion (17,27).
Although we did not identify large changes in the proportion of treatment visits where any insulin was used—approximately one-fourth to one-third of visits—over the time period examined, there was substantial change in the type of insulins used, with large increases in long-acting insulins such as insulin glargine and insulin detemir, modest increases in short-acting insulins, and reductions in the use of intermediate and regular insulins. Some of these changes may be due to declining use of premixed insulins, with increases in the use of long-acting and short-acting insulins instead. While there is evidence that long-acting insulins decrease symptomatic and nocturnal hypoglycemic events, there is little evidence of their impact on patient-oriented outcomes such as mortality, morbidity, quality of life, or costs (28,7).
Overall, diabetes treatment continues to grow in its complexity with a large number of drugs and drug classes, an increasing use of dual therapies and combination products, and the availability of new medications with novel pathways. Evidence of the improved glycemic control associated with two-drug combinations (29) has likely contributed to the growth in the use of both fixed-dose combination products as well as the combined use of multiple agents in a given patient. The majority of dual-therapy use that we identified, such as the combination of a biguanide with a sulfonylurea or glitazone, is well supported by clinical guidelines. Despite this, we also identified a substantial minority of visits where dual therapies were employed that have less scientific support (e.g., combination of a DPP-4 agent with a sulfonylurea). Such utilization patterns are particularly concerning when combining two drugs that may synergistically increase the risks of hypoglycemia.
As novel agents are developed and complexity grows, so too have the costs associated with treatment of diabetes. From 2008 to 2012, we estimate that pharmacy expenditures for diabetes medications increased by 61% to almost $22 billion, estimates that are similar in scope to those from other reports (2). This increase has primarily been driven by insulin, particularly long-acting products, and DPP-4 inhibitor expenditures, which have increased 105 and 216%, respectively. Although numerous studies have demonstrated improved glycemic control with the use of these therapeutic classes (30–33), information regarding their real-world comparative- and cost-effectiveness continues to accrue.
Our study has several limitations. First, the NDTI provides limited information from which to judge the clinical appropriateness of the therapies that are observed, although some utilization patterns (e.g., use of DPP-4 inhibitors as monotherapy) are noteworthy. Second, because the data are cross-sectional, they preclude the ability to examine prior treatment failures or the safety or effectiveness of the treatments on a variety of important outcomes. Our study design also precludes causal inference regarding what may have accounted for various trends, such as moderate declines in the overall number of visits from 2008 through 2012, although the Great Recession and loosening of some clinical guidelines may have played a role (34,35). Third, the medication reporting reflects physician’s best knowledge of new or continuing medications, and thus these data do not provide insights into patients’ adherence with therapy or other aspects of patients’ medication-taking behaviors. Finally, our analyses of costs of care was limited to retail, mail order, and long-term pharmacy claims and thus does not reflect changes in nondrug expenditures such as ambulatory visits or diabetes-related hospitalizations.
In conclusion, declining sulfonylurea and glitazone use has been offset by increases in DPP-4 inhibitor use and, to a lesser degree, use of GLP-1 agonists. Treatment of type 2 diabetes has grown in complexity and cost while older treatments continue to be replaced or supplemented by newer therapies. It is critical to monitor these patterns as additional evidence is developed regarding the comparative effectiveness as well as potential risks of newer therapies, especially injectable incretin mimetics (GLP-1 agonists), DPP-4 inhibitors, and SGLT-2 inhibitors.
Supplementary Material
Article Information
Funding. G.C.A. is supported by the Agency for Healthcare Research and Quality (RO1-HS-0189960).
The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data, and preparation or final approval of the manuscript prior to publication.
Duality of Interest. G.C.A. is an ad hoc member of the FDA’s Drug Safety and Risk Management Advisory Committee, serves as a paid consultant to IMS Health, and serves on an IMS Health scientific advisory board (this arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies). R.S.S. serves as an unpaid consultant to this same IMS Health scientific advisory committee. No other potential conflicts of interest relevant to this article were reported.
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health, Inc. information service(s): National Disease and Therapeutic Index (January 1997–December 2012), National Prescription Audit (2008–2012).
Author Contributions. L.W.T. researched data, wrote the manuscript, and edited the manuscript. D.N. researched data and contributed to the manuscript. R.S.S. contributed to discussion. S.S. edited the manuscript and contributed to the discussion. G.C.A. reviewed and edited the manuscript and oversaw the project. G.C.A. is the guarantor for this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A slide set summarizing this article is available online.
The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health, Inc. or any of its affiliated or subsidiary entities.
References
- 1.Diabetes Statistics. Data from the 2011 National Diabetes Fact Sheet (released January 26, 2011). Available from http://www.diabetes.org/diabetes-basics/diabetes-statistics/ Accessed 22 August 2013
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams R, Van Gaal L, Lucioni C, CODE-2 Advisory Board Assessing the impact of complications on the costs of type II diabetes. Diabetologia 2002;45:S13–S17 [DOI] [PubMed] [Google Scholar]
- 4.Alexander GC, Tseng CW. Six strategies to identify and assist patients burdened by out-of-pocket prescription costs. Cleve Clin J Med 2004;71:433–437 [DOI] [PubMed] [Google Scholar]
- 5.Grant RW, Pirraglia PA, Meigs JB, Singer DE. Trends in complexity of diabetes care in the United States from 1991 to 2000. Arch Intern Med 2004;164:1134–1139 [DOI] [PubMed] [Google Scholar]
- 6.Cohen A, Rabbani A, Shah N, Alexander GC. Changes in glitazone use among office-based physicians in the U.S., 2003-2009. Diabetes Care 2010;33:823–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;2:CD005613. [DOI] [PubMed] [Google Scholar]
- 8.Ahrén B, Schmitz O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res 2004;36:867–876 [DOI] [PubMed] [Google Scholar]
- 9.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206 [DOI] [PubMed] [Google Scholar]
- 10.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275–286 [DOI] [PubMed] [Google Scholar]
- 11.Boland CL, Degeeter M, Nuzum DS, Tzefos M. Evaluating second-line treatment options for type 2 diabetes: focus on secondary effects of GLP-1 agonists and DPP-4 agonists. Ann Pharmacother 2013;47:490–505 [DOI] [PubMed]
- 12.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994-2007. Arch Intern Med 2008;168:2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah ND, Montori VM, Krumholz HM, Tu K, Alexander GC, Jackevicius CA. Responding to an FDA warning—geographic variation in the use of rosiglitazone. N Engl J Med 2010;363:2081–2084 [DOI] [PubMed] [Google Scholar]
- 14.Rodbard HW, Jellinger PS. Physicians’ prescribing patterns for patients with diabetes are changing for the better. Am J Med 2012;125:e11–e12 [DOI] [PubMed] [Google Scholar]
- 15.U.S. Bureau of Labor Statistics. Consumer price index inflation calculator. http://www.bls.gov/data/inflation_calculator.htm Accessed 9 October 2013
- 16.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 2009;5:262–269 [DOI] [PubMed] [Google Scholar]
- 17.Bergenstal RM, Wysham C, Macconell L, et al. DURATION-2 Study Group Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431–439 [DOI] [PubMed] [Google Scholar]
- 18.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009;52:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aroda VR, Henry RR, Han J, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther 2012;34:1247–1258, e22 [DOI] [PubMed] [Google Scholar]
- 20.Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 21.White WB, Cannon CP, Heller SR, et al. EXAMINE Investigators Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 22.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/ American College of Endocrinology Consensus Panel on Type 2 Diabetes Mellitus: An Algorithm for Glycemic Control. Endocrine Practice 2009 Sept/Oct; 15(6): 540–559. Update notice. Endocr Pract 2009;15:768–770 [DOI] [PubMed] [Google Scholar]
- 23.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–549 [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med 2013;173:534–539 [DOI] [PubMed] [Google Scholar]
- 25.Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab 2011;96:2027–2031 [DOI] [PubMed] [Google Scholar]
- 26.FDA panel urges looser restrictions on diabetes drug Avandia [article online]. Available from: http://www.cnn.com/2013/06/06/health/fda-avandia-restrictions/index.html Accessed 9 October 2013
- 27.Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care 2008;31:2086–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract 2008;81:184–189 [DOI] [PubMed] [Google Scholar]
- 29.Bolen S, Wilson L, Vassy J, et al. Comparative effectiveness and safety of oral diabetes medications for adults with type 2 diabetes. Comparative Effectiveness Review No. 8. (Prepared by Johns Hopkins Evidence-based Practice Center under contract No. 290-02-0018.) Rockville, MD: Agency for Healthcare Research and Quality, July 2007. Available from www.effectivehealthcare.ahrq.gov/reports/final.cfm Accessed 29 October 2013
- 30.Robinson JD, Neumiller JJ, Campbell RK. Can a new ultra-long-acting insulin analogue improve patient care? Investigating the potential role of insulin degludec. Drugs 2012;72:2319–2325 [DOI] [PubMed] [Google Scholar]
- 31.Dicker D. DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care 2011;34(Suppl. 2):S276–S278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson JA. Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors. Cleve Clin J Med 2009;76(Suppl. 5):S28–S38 [DOI] [PubMed] [Google Scholar]
- 33.Sinha A, Rajan M, Hoerger T, Pogach L. Costs and consequences associated with newer medications for glycemic control in type 2 diabetes. Diabetes Care 2010;33:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catalano R. Health, medical care, and economic crisis. N Engl J Med 2009;360:749–751 [DOI] [PubMed] [Google Scholar]
- 35.Ratner RE. Diabetes management in the age of national health reform. Diabetes Care 2011;34:1054–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.