Abstract
Women of European ancestry are more likely to harbour a Lactobacillus-dominated microbiome, whereas African American women are more likely to exhibit a diverse microbial profile. African American women are also twice as likely to be diagnosed with bacterial vaginosis and are twice as likely to experience preterm birth. The objective of this study was to further characterize and contrast the vaginal microbial profiles in African American versus European ancestry women. Through the Vaginal Human Microbiome Project at Virginia Commonwealth University, 16S rRNA gene sequence analysis was used to compare the microbiomes of vaginal samples from 1268 African American women and 416 women of European ancestry. The results confirmed significant differences in the vaginal microbiomes of the two groups and identified several taxa relevant to these differences. Major community types were dominated by Gardnerella vaginalis and the uncultivated bacterial vaginosis-associated bacterium-1 (BVAB1) that were common among African Americans. Moreover, the prevalence of multiple bacterial taxa that are associated with microbial invasion of the amniotic cavity and preterm birth, including Mycoplasma, Gardnerella, Prevotella and Sneathia, differed between the two ethnic groups. We investigated the contributions of intrinsic and extrinsic factors, including pregnancy, body mass index, diet, smoking and alcohol use, number of sexual partners, and household income, to vaginal community composition. Ethnicity, pregnancy and alcohol use correlated significantly with the relative abundance of bacterial vaginosis-associated species. Trends between microbial profiles and smoking and number of sexual partners were observed; however, these associations were not statistically significant. These results support and extend previous findings that there are significant differences in the vaginal microbiome related to ethnicity and demonstrate that these differences are pronounced even in healthy women.
Introduction
Bacterial vaginosis (BV) is characterized by a shift in the vaginal microflora away from a low-diversity profile predominated by lactic acid-producing acidophiles to a high-diversity profile in which acidophiles are the minority (Goldenberg et al., 1996). The National Health and Nutrition Examination Survey, conducted through the Centers for Disease Control, found that of 3739 women, 29.2 % were BV-positive according to Nugent’s scoring, making BV the most prevalent vaginal disorder (Koumans et al., 2007; Nugent et al., 1991). This study reported that non-Hispanic African Americans were more than twice as likely (prevalence 51.4 %) as non-Hispanic Caucasians (prevalence 23.2 %) to have BV. Importantly, BV predisposes women to serious health issues including pelvic inflammatory disease (Ness et al., 2004), increased risk for acquisition and transmission of HIV and other sexually transmitted diseases (Cherpes et al., 2003; Coleman et al., 2007; Martin et al., 1999; Schwebke, 2003; Wiesenfeld et al., 2003), and increased risk for adverse pregnancy outcomes, including intrauterine infection, early miscarriage, premature rupture of membranes and preterm birth (Hillier et al., 1995; Nelson et al., 2009).
African Americans are more frequently affected by BV, and they also suffer from a more than twofold increased risk of preterm birth (<37 weeks of gestation), and a threefold greater risk of very preterm birth (<32 weeks) relative to European ancestry women (Kramer & Hogue, 2008; Paige et al., 1998). The bases for racial differences in the rates of BV and adverse pregnancy outcome are unclear, but the disparity cannot be explained by demographic factors or lifestyle factors alone (Culhane et al., 2006; Goldenberg et al., 1996; Ness et al., 2003) and it is likely that the composition of the microbial community of the urogenital tract (e.g. the vaginal microbiome) plays a significant role. Previous studies of the vaginal microbiome reveal significant differences between African American and European ancestry women. Earlier studies using microscopy to assess the microbial profiles by morphotype found that African American women have higher Nugent scores and are less likely to be colonized by lactobacilli than women of European ancestry (Fiscella & Klebanoff, 2004; Ness et al., 2003; Nugent et al., 1991; Royce et al., 1999). These studies were extended by terminal RFLP and shallow 16S rRNA gene profiling (Zhou et al., 2004, 2007, 2010). More recently, a 16S rRNA gene survey using deep next-generation sequencing performed on vaginal samples from 98 European ancestry and 104 African American women (Ravel et al., 2011), similar to Zhou et al. (2010), found that vaginal microbiome profiles typically fit into one of five major groups. Four of these groups were dominated by lactobacilli: group I, Lactobacillus crispatus; group II, Lactobacillus gasseri; group III, Lactobacillus iners; group V, Lactobacillus jensenii. Group IV was a heterogeneous group of strict anaerobes (Ravel et al., 2011). Group I was the most common group amongst European ancestry women whereas group IV was the most common in African American women.
Lactobacilli and related organisms appear to help maintain vaginal health. Oestrogen triggers the accumulation of glycogen in vaginal epithelial cells, which leads to the production of lactic acid by lactobacilli, lowering the vaginal pH to <4.5, thereby preventing growth of ‘unhealthy’ neutralophiles. Some species of Lactobacillus also produce hydrogen peroxide and/or bacteriocins, which may contribute to the suppression of other bacterial species. There are six species of Lactobacillus that commonly colonize the vagina: L. crispatus, L. gasseri, L. jensenii, Lactobacillus johnsonii, Lactobacillus vaginalis and L. iners. Women are frequently colonized by multiple species (Zhou et al., 2010). There is also debate about whether Atopobium vaginae, another lactic acid-producing bacterial species, may be a healthy vaginal component, at least under certain circumstances (Zhou et al., 2004, 2007). The lactic acid-producing species vary in both their stability and their capacity to protect the vagina from colonization by BV-associated anaerobes (Tamrakar et al., 2007). These are both key traits. Stability ensures that these species will not be easily displaced by changes in their environment that may be triggered by hormonal changes, menstruation, semen deposition, transient fluctuations in pH and non-resident bacterial species. Protection reflects the capacity of the species to prevent other bacteria from colonizing the vagina. L. crispatus is highly stable and apparently protective against BV-associated bacteria, and women colonized with L. crispatus have been shown to have a fivefold decreased risk for developing BV (Verstraelen et al., 2009). Conversely, L. iners appears to be the least stable, and the least protective, and women colonized with this species appear to have a significantly greater risk for developing BV relative to women colonized with L. crispatus (Verstraelen et al., 2009).
The first goal of the present study was to compare the vaginal microbiomes of African American women with and without a diagnosis of BV with those of women of European ancestry with and without a diagnosis of BV. The second goal was to investigate the hypothesis that differences in the microbiome may contribute to increased preterm birth risk in African American women. The third goal was to determine whether specific intrinsic and extrinsic factors, including body mass index (BMI), diet, smoking and alcohol use, number of sexual partners, and socioeconomic status, could account for the differences in the microbiomes of these two racial groups.
Methods
Participant recruitment.
Participants were recruited in 2009–2013 from outpatient clinics at the Virginia Commonwealth University (VCU) Medical Center and the Virginia Department of Health following written, informed consent. Inclusion criteria included women age 18–44 years who were able to provide informed consent and who were willing or already scheduled to undergo a vaginal examination using a speculum. The Institutional Review Boards for Human Subjects Research at VCU (Panel B) and the Virginia Department of Health reviewed and approved this study. Participants filled out a detailed questionnaire that included questions about ethnicity, education, employment, health habits, dietary habits and sexual history. Participants who self-reported African American (black) race and not Latino ethnicity are referred to as African American. Women who self-reported race as Caucasian (white) and not Latino ethnicity are referred to as women of European ancestry. Clinicians also filled out a diagnosis form at the time of each visit that included information about the purpose of each visit, and any diagnoses. Subjects were considered ‘healthy’ at the time of a visit if the purpose of the visit was for an annual examination, they received no diagnosis and were asymptomatic (e.g. no abnormal discharge). BV testing was performed only when indicated, and was based solely on Amsel’s criteria (Amsel et al., 1983). Yeast infection was diagnosed by wet mount microscopy.
Sampling and sample processing.
Samples were taken by a physician using CultureSwab EZ (Becton Dickinson) from the mid-vaginal wall during a speculum examination. DNA was extracted from the swabs within 4 h of collection using the Powersoil kit (MoBio). The swabs were swirled directly in the Powerbead tubes supplied with the kit and processing was performed according to the manufacturer’s instructions.
16S rRNA gene survey.
The V1–V3 hypervariable regions of the bacterial 16S rRNA gene were amplified by PCR using barcoded primers. The 16S primers contain the A or B Titanium sequencing adaptor (shown in italics), followed immediately by a unique variable (6–9 base) barcode sequence and finally the 5′ end of the primer. The forward primer was a mixture (4 : 1) of primers Fwd-P1 (5′-CCATCTCATCCCTGCGTGTCTCCGACTCAGBBBBBBAGAGTTYGATYMTGGCTYAG) and Fwd-P2 (5′-CCATCTCATCCCTGCGTGTCTCCGACTCAGBBBBBBAGARTTTGATCYTGGTTCAG). The reverse primer was Rev1B (5′-CCTATCCCCTGTGTGCCTTGGCAGTCTCAG ATTACCGCGGCTGCTGG). PCR products were sequenced using the Roche 454 GS FLX Titanium platform. These data were generated as part of the Vaginal Human Microbiome Project (Fettweis et al., 2011). Raw sequence data from the project are available from the Short Read Archive at NCBI (project ID phs000256; Fettweis et al., 2011). We used a deep sequencing approach with a median 24 030 reads per sample. All processed samples were represented by >5000 reads.
Reads that met the following criteria were processed: (1) valid primer and multiplex identifier sequences were observed; (2) less than 10 % of base calls had a quality score less than 10; (3) the average quality score was greater than Q20; and (4) the read length was between 200 and 540 bases. Sequences were classified using a local installation of the RDP classifier (0.8 cut-off) and using STIRRUPS, an analysis platform that employs the USEARCH algorithm combined with a curated vaginal 16S rRNA gene database (Fettweis et al., 2012; Wang et al., 2007).
Statistical analyses.
Read counts were converted to proportions for all samples. Alpha diversity was measured using the inverse Simpson’s index. The mean beta diversity and variance were estimated by sampling n distances from all (n choose 2) pairwise distances, where n is the number of samples. Distance was measured using the Bray–Curtis method. Differences in diversity between groups of samples were tested using a two-sided t-test.
Fig. 1 is a contour plot of the decision function for a support vector machine with the Gaussian kernel (SVM). All 416 European ancestry women and a random sample of 416 African American women were used to train an SVM model predicting ethnicity based on the proportion of lactobacilli and diversity (measured using the log of the inverse Simpson’s index). The R package kernlab (Karatzoglou, 2004) was used to generate the SVM model.
Fig. 1.
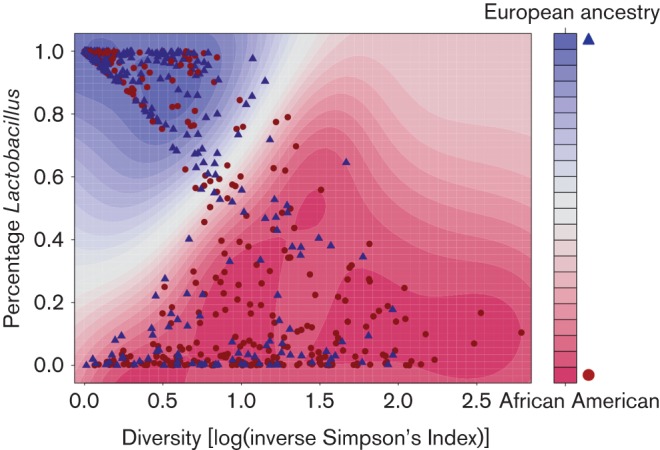
Proportion of lactobacilli, alpha diversity and ethnicity. Circles denote African American (AA) subjects and triangles denote European ancestry (EA) subjects. The model and plot were generated using a random sample of 416 AA subjects and all 416 EA subjects for whom data were available. Diversity is shown on the x-axis and the percentage of the vaginal microbiome belonging to the genus Lactobacillus is on the y-axis. For the subjects who the model indicated are representative of EA (blue triangles in the blue shaded regions), diversity appears to increase as the proportion of Lactobacillus decreases. For the subjects who the model indicated are representative of AA (red circles in the red shaded regions), as diversity increases, so does the proportion of Lactobacillus.
The barplots indicating the effect size of bacterial species that correlate with ethnicity were created using LEfSe (Segata et al., 2011). LEfSe uses the Kruskal–Wallis rank sum test to detect taxa that distinguish groups of subjects, and uses linear discriminant analysis (LDA) to calculate an LDA score for the effect size, as described by Segata et al. (2011).
Logistic regression was used for the multivariate analysis of the differences between healthy subjects and those with a BV diagnosis. Multiple regression was used for the analysis of the relationship of percentage BV-associated bacteria with intrinsic and extrinsic factors.
The boxplot of BV-associated bacteria has whiskers that extend to the highest/lowest value within 1.5 times the interquartile range. Data beyond the end of the whiskers are outliers and are plotted as points. A Wilcoxon rank sum test with continuity correction was used to test whether the proportion of BV-associated bacteria followed the same distribution for groups of subjects (pregnant/non-pregnant, African/European ancestry). Analysis was conducted and plots were created using the R language for statistical computing (Team, 2013) and packages ggplot2 (Wickham, 2009), kernlab (Kratzoglou et al., 2004) and vegan (Oksanen et al., 2013).
Results
Vaginal microbial diversity is significantly greater in African Americans
We analysed 1268 vaginal samples from African American women and 416 from women of European ancestry. Subjects 18–44 years of age who were scheduled for a pelvic examination were recruited from the Women’s Health outpatient clinics of the VCU Medical Center and from the Women’s Health Clinics of the Virginia Department of Health. Demographic and health history information for this cohort is given in Table 1.
Table 1. Demographic information and health habits.
| African American | European ancestry | |
| Number of subjects | 1268 | 416 |
| Median age (years) | 28 | 29 |
| BV diagnosis (n)* | 22.8 % (289) | 6.5 % (27) |
| Median BMI | 29.3 | 24.1 |
| Yeast infection† (n) | 8.4 % (106) | 4.1 % (17) |
| Current smokers (n) | 25.7 % (326) | 16.6 % (69) |
| Income <$20k (n) | 65.4 % (829) | 23.3 % (97) |
| Yogurt >1 per week (n) | 41.8 % (530) | 64.2 % (267) |
| Alcohol >0 past week (n) | 24.4 % (309) | 41.1 % (171) |
| Healthy‡ (n) | 48.3 % (612) | 75.0 % (312) |
| Sexual partners >1 in last year (n) | 32.6 % (413) | 20.0 % (83) |
| Pregnant self-reported (n) | 19.4 % (246) | 18.3 % (76) |
| Median number of days until due date (interquartile range) | 188.5 (128.8–216.0) | 158.2 (166.2–219.8) |
| Vaginal douching in past month (n) | 14 % (177) | 3.8 % (16) |
BV was diagnosed by a physician using Amsel’s criteria (Amsel et al., 1983).
Yeast infection was diagnosed by a physician by wet mount microscopy.
Healthy was defined as women who visited the clinic for an annual examination and did not receive a diagnosis of BV, yeast infection or sexually transmitted disease.
We analysed both the alpha diversity (i.e. diversity of bacterial species within individuals) and the beta diversity (i.e. differences between different subjects) in the vaginal microbiomes of women of African and European ancestry. We compared the microbiome profiles of 960 women of African ancestry with 330 women of European ancestry who were self-reported as non-pregnant. As previously reported (Ravel et al., 2011; Zhou et al., 2007, 2010), the mean (±sd) alpha diversity of microbiomes of African Americans was significantly greater than the mean alpha diversity of microbiomes of women of European ancestry (2.7±1.9 versus 1.8±1.1, P<0.0001), but the mean beta diversity was not significantly different between the two groups (0.69±0.23 versus 0.79±0.23, P = 0.10).
We also analysed the relationship between the proportion of lactobacilli and diversity. When lactobacilli were present in the vaginal microbiomes of women of European ancestry, they tended to dominate the microbial population and these microbiomes exhibited low diversity (Fig. 1). In contrast, the microbiome profiles of African American samples exhibited higher diversity even when they contained lactobacilli. When lactobacilli were absent or present in very low numbers, microbial diversity ranged widely in both groups (Fig. 1).
Prevalence of microbiome profiles among ethnicities
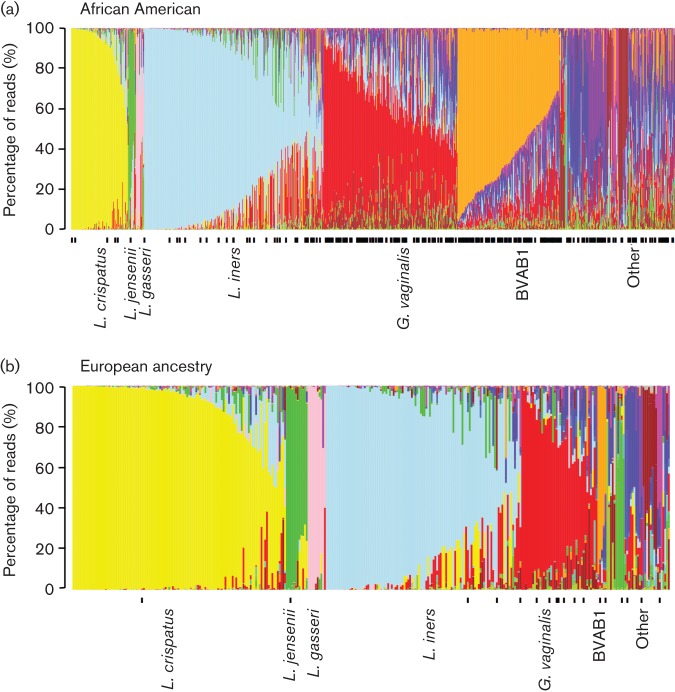
When grouped according to the predominating bacterial species, the samples from non-pregnant subjects analysed in this study fell into six distinct microbiome profiles: those predominated by L. crispatus, L. jensenii, L. gasseri, L. iners, Gardnerella vaginalis or bacterial vaginosis-associated bacterium-1 (BVAB1). BVAB1 is an uncultivated bacterium that appears to be related to the family Lachnospiraceae and is associated with BV (Fredricks et al., 2005; Marrazzo et al., 2008). A significant number of samples did not fit into any common profile and were grouped together as ‘Other’. In African American women, the most common profile was L. iners, followed by Gardnerella vaginalis, BVAB1, ‘Other’ and L. crispatus (Fig. 2; the colour code for bacterial taxa is shown in Fig. S1, available in the online Supplementary Material). In contrast, the most common profile in women of European ancestry was L. crispatus, followed by L. iners and Gardnerella vaginalis. The BVAB1 microbial profile was only found in five samples from women of European ancestry.
Fig. 2.
Microbiome profiles of women of African American or European ancestry. Stacked bar plots showing microbiome profiles from (a) 960 African American women and (b) 330 European ancestry women. The profiles are grouped by the dominant species into different profile types and are ordered by decreasing proportion of the dominant bacterium. Black ticks below the x-axis denote subjects with BV. Colour codes for bacterial taxa appear in Figs S1 and S2.
Healthy versus BV profiles in African American and European ancestry women
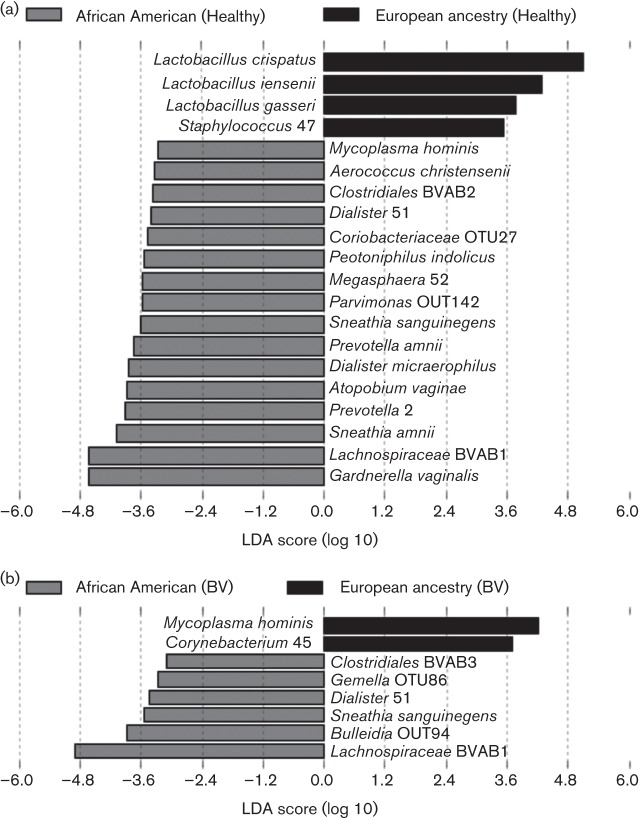
The findings that alpha diversity and prevalence of G. vaginalis and BVAB1-dominated microbiome profiles were significantly greater in African American women were striking. We wanted to determine whether these differences occurred in healthy women or were evident primarily in women with a diagnosis of BV. Of the participants in the study, 419 non-pregnant African American women and 243 non-pregnant women of European ancestry who did not receive a diagnosis of a vaginal disorder (BV, yeast infection or sexually transmitted infection) were selected to represent the ‘healthy’ population. In addition, 233 samples from non-pregnant African American women and 18 samples from non-pregnant women of European ancestry were selected for analysis based on a positive diagnosis for BV. Among healthy subjects, women of European ancestry were more likely to be colonized with L. crispatus, L. jensenii, L. gasseri and Staphylococcus. African American women were more likely to be colonized by Mycoplasma hominis, L. iners and Aerococcus and a variety of strict anaerobes, including Anaerococcus, BVAB1 and BVAB2, Dialister, Peptoniphilus, Coriobacteriaceae, Parvimonas, Megasphaera, Sneathia, Prevotella amnii, Atopobium and G. vaginalis (Fig. 3a). Comparison of subjects with BV revealed that African Americans are more likely to be colonized by BVAB1 and BVAB3, Gemella, Bulleidia, Dialister and Sneathia, and women of European ancestry were more likely to be colonized by M. hominis and Corynebacterium (Fig. 3b).
Fig. 3.
Bacterial species that correlate significantly with ethnicity. Barplot of the LDA score for bacterial species that are more prevalent in (a) healthy African American women and healthy European ancestry women and (b) those diagnosed with BV. The healthy (a) cohort includes 662 women (419 African American, 243 European ancestry) and the BV cohort (b) includes 251 women (233 African American, 18 European ancestry).
Prevalence of preterm birth-associated species
Preterm birth rates are more than twofold higher in African Americans. We hypothesized that taxa associated with preterm birth would be more prevalent in the vaginal microbiomes of pregnant African American women. We analysed the microbiomes of 246 pregnant African Americans and 76 pregnant women of European ancestry. Ureaplasma, Mycoplasma, Fusobacterium, Sneathia, Gardnerella, Streptococcus, Prevotella and Bacteroides have all been detected in amniotic fluid from pregnancies that resulted in preterm birth by culture or molecular techniques (DiGiulio et al., 2010; Han et al., 2009). Fig. 4 shows that the prevalence of Prevotella and Sneathia was higher in samples from pregnant African American women. However, vaginal microbiomes of pregnant women of European ancestry actually had higher levels of Gardnerella.
Fig. 4.
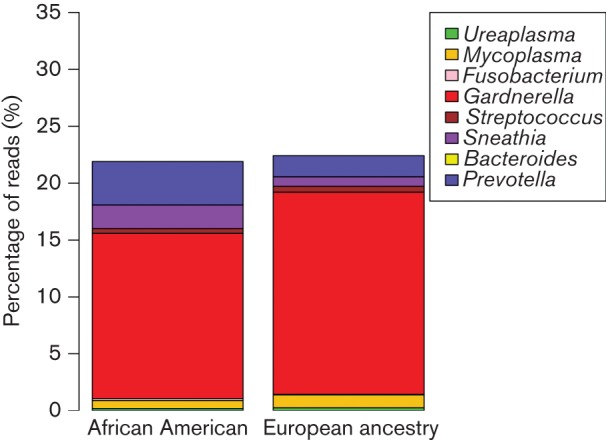
Prevalance of preterm birth-associated species in samples from pregnant subjects. Stacked barplot of the percentage of reads from preterm birth-associated bacterial taxa in African Americans versus women of European ancestry. The plot is based on 246 pregnant African Americans and 76 pregnant women of European ancestry. Of these subjects, 33 African Americans and five European ancestry women were diagnosed with BV and 137 African Americans and 45 women of European ancestry were healthy.
Relationship between intrinsic and extrinsic factors and racial disparities
We investigated the differences between ‘healthy’ women and those receiving a diagnosis of BV based on various attributes including ethnicity (African American, European ancestry), self-reported pregnancy status (yes, no; subjects unsure of pregnancy status were excluded), BMI, yogurt consumption per week (0, >0 servings), smoking status (no, yes), income (<$20k, ≥$20k), alcohol use per week (0, >0 servings), number of sexual partners in the last year (0, 1, >1), and time since last douche [≤1 month ago, >1 month ago (including never)]. This analysis included 237 healthy women and 76 women diagnosed with BV for whom complete data were available and the results are shown in Table 2. The statistically significant predictors were pregnancy status (P = 0.0270) and ethnicity (P = 0.0110). It was estimated that African Americans are 2.9 times as likely to be diagnosed with BV relative to women of European ancestry, and non-pregnant women are 3.1 times as likely to be diagnosed with BV relative to pregnant women. BV diagnosis also seemed to be associated with smoking, more sexual partners per year, less yogurt consumption and lower income, although the relationships did not achieve statistical significance.
Table 2. Odds ratios from a logistic regression model for predicting a diagnosis of BV.
The model is based on data from 237 healthy subjects (annual examination, no diagnosis) and 76 subjects diagnosed with BV. ‘Ref’ indicates that the variable served as the reference and the odds ratio is fixed at 1.0. Demographic information about the included subjects is listed in Table S1.
| Attribute | Odds ratio | Confidence interval | P | |
| Race/ethnicity | European ancestry | 1.0 | Ref | |
| African American | 3.1 | 1.3–7.7 | 0.011 | |
| BMI | 0.98 | 0.94–1.0 | 0.26 | |
| Yogurt past week | >0 per week | 1.0 | Ref | |
| 0 per week | 1.7 | 0.98–3.2 | 0.060 | |
| Current smoker | No | 1.0 | Ref | |
| Yes | 1.3 | 0.7–2.3 | 0.42 | |
| Income | ≥$20k | 1.0 | Ref | |
| <$20k | 1.2 | 0.62–2.4 | 0.58 | |
| Alcohol in past week | 0 | Ref | 1.0 | |
| >0 servings | 0.95 | 0.51–1.8 | 0.88 | |
| Sexual partners in last year | 0 | Ref | 1.0 | |
| 1 | 3.1 | 0.5–59.6 | 0.30 | |
| >1 | 4.5 | 0.73–87.8 | 0.17 | |
| Pregnant | Yes | Ref | 1.0 | |
| No | 2.4 | 1.1–5.6 | 0.027 | |
| Time since douche | ≤1 month | Ref | 1.0 | |
| >1 month | 1.1 | 0.5–2.3 | 0.86 |
Relationship between intrinsic and extrinsic factors and BV-associated bacteria
We analysed the prevalence of a group of bacteria that have been previously reported to have a strong association with BV, including Ureaplasma, Mycoplasma, Fusobacterium, Leptotrichia, Gardnerella, Sneathia, Prevotella, BVAB1, BVAB2, BVAB3, Atopobium, Mobiluncus and Megasphaera (Brotman, 2011; Fredricks et al., 2005). We investigated the relationship between the percentage of reads of these BV-associated bacteria and African American versus European ancestry ethnicity, pregnancy status (no, yes), household income (<$20k, ≥$20k), smoking status (no, yes), BMI, alcohol use in the past week (0, >0 servings), yogurt consumption per week (0, >0 servings) and number of sexual partners in the last year (0, 1, >1). Table 3 contains the coefficients of the linear model. All variables except for BMI and number of sexual partners were categorical with two levels. BMI is measured on a continuous scale, and the number of sexual partners has three levels (0, 1, >1). Within the cohort studied, ethnicity was not independent of alcohol consumption, BMI, income, number of sexual partners in the past year, smoking status, yogurt consumption or douching practices. Nevertheless, ethnicity is a significant predictor of BV-associated bacteria. European ancestry women had 25.8 % less BV-associated bacteria than African American women (P<0.0001) as indicated in Table 3. Those who had consumed alcohol in the past week had 11.3 % less BV-associated bacteria (P = 0.0079). Non-pregnant women had 10.3 % more BV-associated bacteria than pregnant women (P = 0.0047). The proportion of BV-associated bacteria increased with smoking and number of sexual partners, but did not achieve statistical significance at 5 %.
Table 3. Coefficients for multiple linear regression model of BV-associated bacteria and attributes.
Included in the analysis are data from 418 subjects: 296 African American and 122 European ancestry. Baseline levels for categorical variables are excluded. Demographic information about the included subjects is listed in Table S2.
| Attribute | Coefficient estimate | t statistic | P |
| Ethnicity (European ancestry) | −25.8 | −5.29 | <0.0001 |
| BMI | −0.321 | −1.33 | 0.18 |
| Yogurt past week (<1) | −0.287 | −0.074 | 0.94 |
| Current smoker (Yes) | 6.16 | 1.53 | 0.13 |
| Income (<$20k) | −2.16 | −0.491 | 0.62 |
| Alcohol past week (>0) | −11.3 | −2.67 | 0.0079 |
| Sexual partners in last year = 1 | 2.82 | 0.25 | 0.80 |
| Sexual partners in last year >1 | 10.9 | 0.942 | 0.35 |
| Pregnant (No) | 7.61 | 1.52 | 0.13 |
| Time since douche <1 month | 0.522 | 0.096 | 0.92 |
Fig. 5 is a boxplot of the proportion of BV-associated bacteria for subjects by race and pregnancy status. Among both pregnant and non-pregnant subjects, African American women had higher proportions of BV-associated bacteria (P = 0.0086 and P<0.0001, respectively). Pregnant women had lower median proportions of BV-associated bacteria within each ethnic group, but the differences in proportion from non-pregnant women did not achieve statistical significance (P = 0.22 and P = 0.44, respectively).
Fig. 5.
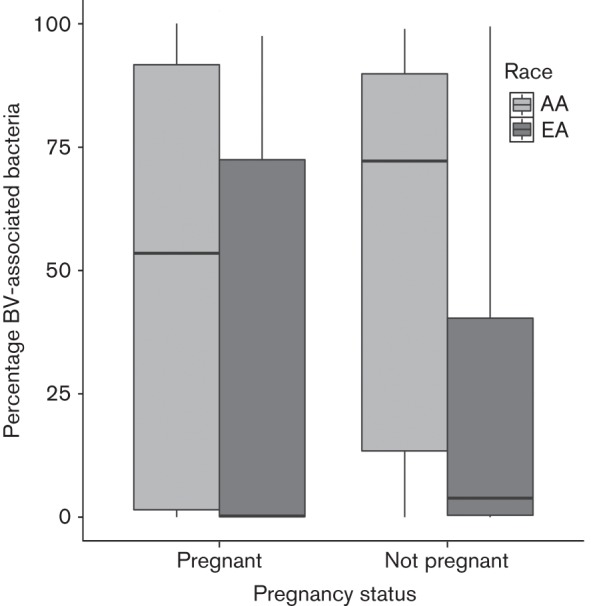
Relationship between the percentage of BV-associated bacteria and pregnancy and ethnicity. The analysis includes healthy women and women with BV. Subjects were grouped based on self-reported pregnancy status. Within each group, the proportion of BV-associated bacteria was plotted for women of African ancestry (AA) and women of European ancestry (EA). The boxes indicate the interquartile range, and the horizontal line in each box indicates the median. The plot reflects data from 418 women.
Discussion
Previous studies that used microscopy to assess microbial profiles by morphotype found that African American women have higher Nugent scores and are less likely to be colonized by lactobacilli than women of European ancestry (Fiscella & Klebanoff, 2004; Ness et al., 2003; Nugent et al., 1991; Royce et al., 1999). These studies were later supported by terminal RFLP analysis and shallow traditional 16S rRNA gene sequencing (Zhou et al., 2004, 2007, 2010). More recently, a 16S rRNA gene survey using deep next-generation sequencing was performed on vaginal samples from 98 women of European ancestry and 104 African American women (Ravel et al., 2011). The authors found that vaginal microbial profiles typically fit into one of five major groups. Four of these groups were dominated by lactobacilli: group I, L. crispatus; group II, L. gasseri; group III, L. iners; group V, L. jensenii. Group IV was a heterogeneous group of strict anaerobes (Ravel et al., 2011). We detected these groups as well but we also found two additional major microbiome profiles, one dominated by G. vaginalis and the other dominated by BVAB1. A high proportion of samples from non-pregnant African American women in our study exhibited G. vaginalis and BVAB1 community profiles (22.1 and 16.9 %, respectively), and together these two profiles were more common than the L. iners microbiome profile (29.7 %). It is unclear why these common vaginal species appear to have been underreported in prior studies, but strengths of this study that support the validity of the finding include the size of the cohort and the custom design and validation of 16S rRNA primers used for the 16S rRNA gene survey. This study was based on the largest African American cohort to date: 1268 total participants of whom 960 were not pregnant. Primer mixes were specifically designed not only to universally amplify bacterial 16S rRNA genes but to target species known to be present in the vaginal microbiome as well, and they were carefully validated using a standardized mock sample consisting of a number of bacteria, including species of Chlamydia, that are commonly found in the vagina. All samples were carefully and specifically collected from the mid-vaginal wall of each participant, avoiding contact with and possible contamination by bacteria from the introitus, or other vaginal sites, during speculum examinations by qualified clinicians during standard practice of care to ensure the quality and integrity of the materials collected and the data derived from it. Furthermore, even less abundant bacteria were easily detected because of the depth of the sequencing performed (the median number of reads per sample was over 24 000).
Taxonomic identification of the 16S rRNA gene reads to the species level was performed using an analysis pipeline specifically designed for vaginal bacteria (Fettweis et al., 2012). Species-level classification of lactobacilli confirmed prior reports that L. iners is the most common species in the vaginal microbiomes of African American women (Ravel et al., 2011). L. iners appears to be less exclusive relative to other lactobacilli such as L. crispatus, and L. jensenii (Jakobsson & Forsum, 2007; Tamrakar et al., 2007; Verstraelen et al., 2009). A recent report suggests that the failure of L. iners to effectively inhibit the growth of other species is a consequence of its low d-lactic acid production relative to other vaginal lactobacilli (Witkin et al., 2013). Indeed, a number of anaerobic, BV-associated bacteria, including G. vaginalis, BVAB1, BVAB2, Atopobium vaginae, Megasphaera, Sneathia and Prevotella, were also more prevalent in healthy (i.e. no BV diagnosis) African American women. Thus, colonization by L. iners is probably responsible, at least in part, for the higher microbial diversity in African American women. It remains unclear why African American women would be more likely to be colonized by L. iners than the other Lactobacillus species. In contrast, healthy women of European ancestry were more likely to be colonized with three health-associated Lactobacillus species, namely L. crispatus, L. jensenii and L. gasseri, and exhibited significantly lower bacterial diversity. However, they were also more likely to be colonized by two ‘unhealthy’ taxa, including Prevotella bivia, which is common in BV, and Ureaplasma, which has been associated with preterm birth and neonatal infections (Viscardi, 2014). African Americans with BV were more likely to be colonized with BVAB1 and Sneathia, which have been associated with preterm birth (Nelson et al., 2014), and several other species including BVAB3, Gemella, Bulleidia and Dialister. In contrast, women of European ancestry were more likely to be colonized by M. hominis, which is also associated with preterm birth (Foxman et al., 2014), and Corynebacterium, which are normal inhabitants of skin and mucous membranes. However, a weakness in this study was the small size (18) of the subset of women of European ancestry with a BV diagnosis. A larger sample size would make these conclusions more definitive.
Statistical analysis revealed associations between BV-related species and ethnicity, pregnancy, alcohol consumption, smoking status and number of sexual partners. Even when considering these and other factors, ethnicity remained a statistically significant predictor. Prior studies have shown a link between vaginal douching and BV (Brotman et al., 2008; Cottrell, 2010). However, the number of women of European ancestry who reported douching within the past month was so low (10) that we were not able to determine whether the practice had an additional effect over ethnicity. There was a lower chance of diagnosis of BV during pregnancy, and BV-associated bacteria are less prevalent during pregnancy. This observation is in agreement with a recent study that found increased abundance of healthy lactobacilli and lower bacterial diversity in pregnant women (Romero et al., 2014). The variables were not independent within the cohort used in this study. Smoking status, number of sexual partners, income, alcohol consumption and yogurt consumption were all correlated with ethnicity. Smoking has been implicated as a risk factor for BV in numerous studies (Cherpes et al., 2008; Ryckman et al., 2009). Smoking may be compounding the risk for BV in conjunction with other factors related to socioeconomic status, rather than directly increasing it. It is likely that numerous confounding factors associated with socioeconomic status influence the development of BV. Other factors that we analysed, including BMI and yogurt consumption, did not correlate significantly in the analysis of our large cohort, although there was a trend towards a negative correlation with BV-associated bacteria with greater yogurt consumption (P = 0.21). A previous study suggested a trend between BV and BMI, but this trend did not reach statistical significance in multivariate analysis (Koumans et al., 2007).
These findings are clinically relevant because African American women suffer more frequently from BV and the basis for this disparity is not understood. Besides being an annoyance and an added healthcare expense, the increased BV rates translate into higher rates of acquisition and transmission of HIV and other sexually transmitted infections (Allsworth et al., 2008; Atashili et al., 2008; Myer et al., 2005). Not only do African American women suffer from high rates of BV, but African women have high rates of BV as well. To compound the problem, sub-Saharan Africa has the highest percentage of HIV-infected individuals in the world, and the incidence is particularly high in women; 60–75 % of HIV infections in people aged 15–24 years are in women (D’Cruz & Uckun, 2004; Sibanda et al., 2003). Point prevalence of BV in sub-Saharan Africa may exceed 50 % and therefore contributes substantially to the rampant spread of HIV (Aboud et al., 2008). Thus, understanding the variables associated with African ethnicity that contribute to BV is very important. Moreover, preterm birth is thought to be associated with BV and is known to be associated with African American ethnicity. This study revealed that certain bacteria that have been linked to preterm birth, including Sneathia, and species of Prevotella, are more prevalent in pregnant African American women. However, pregnant women of European ancestry were more likely to be colonized by M. hominis and G. vaginalis, which can also be detected in amniotic fluid. Therefore, other factors, in addition to differences in vaginal microbiota, probably contribute to the racial disparity in rates of preterm birth.
In summary, the data presented in this study add to the emerging evidence that a gradual spectrum of diversity in vaginal flora exists rather than the previous distinction of ‘normal’ and BV. This has possible clinical importance in that treatment of specific organisms has not proven effective for the prevention of preterm birth, but addressing abnormal vaginal flora with clindamycin as detected by Gram staining has been reported to have favourable results (Lamont et al., 2003; Ugwumadu, 2007). Thus, understanding the variables associated with African ethnicity that contribute to vaginal flora has important implications regarding gynaecological, reproductive and overall health.
Acknowledgements
This work was supported by National Institutes of Health grant 4UH3AI083263 (The Vaginal Microbiome: Disease, Genetics and the Environment) and P60 MD002256, and by the VCU NIMHD Comprehensive Center of Excellence. Sequence analysis was performed in the Nucleic Acids Research Facilities at VCU. Bioinformatics analysis was provided by the staff of the Bioinformatics Computational Core Laboratories at VCU. The authors report no conflict of interest.
Abbreviations:
- BMI
body mass index
- BV
bacterial vaginosis
- LDA
linear discriminant analysis
Footnotes
Two supplementary figures and two supplementary tables are available with the online Supplementary Material.
References
- Aboud S., Msamanga G., Read J. S., Mwatha A., Chen Y. Q., Potter D., Valentine M., Sharma U., Hoffmann I. & other authors (2008). Genital tract infections among HIV-infected pregnant women in Malawi, Tanzania and Zambia. Int J STD AIDS 19, 824–832. 10.1258/ijsa.2008.008067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsworth J. E., Lewis V. A., Peipert J. F. (2008). Viral sexually transmitted infections and bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Sex Transm Dis 35, 791–796. 10.1097/OLQ.0b013e3181788301 [DOI] [PubMed] [Google Scholar]
- Amsel R., Totten P. A., Spiegel C. A., Chen K. C., Eschenbach D., Holmes K. K. (1983). Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74, 14–22. 10.1016/0002-9343(83)91112-9 [DOI] [PubMed] [Google Scholar]
- Atashili J., Poole C., Ndumbe P. M., Adimora A. A., Smith J. S. (2008). Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22, 1493–1501. 10.1097/QAD.0b013e3283021a37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman R. M. (2011). Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest 121, 4610–4617. 10.1172/JCI57172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman R. M., Klebanoff M. A., Nansel T. R., Andrews W. W., Schwebke J. R., Zhang J., Yu K. F., Zenilman J. M., Scharfstein D. O. (2008). A longitudinal study of vaginal douching and bacterial vaginosis–a marginal structural modeling analysis. Am J Epidemiol 168, 188–196. 10.1093/aje/kwn103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherpes T. L., Meyn L. A., Krohn M. A., Lurie J. G., Hillier S. L. (2003). Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 37, 319–325. 10.1086/375819 [DOI] [PubMed] [Google Scholar]
- Cherpes T. L., Hillier S. L., Meyn L. A., Busch J. L., Krohn M. A. (2008). A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis 35, 78–83. 10.1097/OLQ.0b013e318156a5d0 [DOI] [PubMed] [Google Scholar]
- Coleman J. S., Hitti J., Bukusi E. A., Mwachari C., Muliro A., Nguti R., Gausman R., Jensen S., Patton D. & other authors (2007). Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS 21, 755–759. 10.1097/QAD.0b013e328012b838 [DOI] [PubMed] [Google Scholar]
- Cottrell B. H. (2010). An updated review of evidence to discourage douching. MCN Am J Matern Child Nurs 35, 102–107, quiz 108–109. 10.1097/NMC.0b013e3181cae9da [DOI] [PubMed] [Google Scholar]
- Culhane J. F., Rauh V. A., Goldenberg R. L. (2006). Stress, bacterial vaginosis, and the role of immune processes. Curr Infect Dis Rep 8, 459–464. 10.1007/s11908-006-0020-x [DOI] [PubMed] [Google Scholar]
- D’Cruz O. J., Uckun F. M. (2004). Clinical development of microbicides for the prevention of HIV infection. Curr Pharm Des 10, 315–336. 10.2174/1381612043386374 [DOI] [PubMed] [Google Scholar]
- DiGiulio D. B., Romero R., Kusanovic J. P., Gómez R., Kim C. J., Seok K. S., Gotsch F., Mazaki-Tovi S., Vaisbuch E. & other authors (2010). Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 64, 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettweis J., Alves J., Borzelleca J., Brooks J. P., Friedline C. J., Gao Y., Gao X., Girerd P., Harwich M. D. & other authors (2011). The Vaginal Microbiome: Disease, Genetics and the Environment. Nature Preceedings. [Google Scholar]
- Fettweis J. M., Serrano M. G., Sheth N. U., Mayer C. M., Glascock A. L., Brooks J. P., Jefferson K. K., Buck G. A., Vaginal Microbiome Consortium (additional members) (2012). Species-level classification of the vaginal microbiome. BMC Genomics 13 (Suppl 8), S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscella K., Klebanoff M. A. (2004). Are racial differences in vaginal pH explained by vaginal flora? Am J Obstet Gynecol 191, 747–750. 10.1016/j.ajog.2004.03.032 [DOI] [PubMed] [Google Scholar]
- Foxman B., Wen A., Srinivasan U., Goldberg D., Marrs C. F., Owen J., Wing D. A., Misra D. (2014). Mycoplasma, bacterial vaginosis-associated bacteria BVAB3, race, and risk of preterm birth in a high-risk cohort. Am J Obstet Gynecol 210, e1–e7. 10.1016/j.ajog.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredricks D. N., Fiedler T. L., Marrazzo J. M. (2005). Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353, 1899–1911. 10.1056/NEJMoa043802 [DOI] [PubMed] [Google Scholar]
- Goldenberg R. L., Klebanoff M. A., Nugent R., Krohn M. A., Hillier S., Andrews W. W., Vaginal Infections and Prematurity Study Group (1996). Bacterial colonization of the vagina during pregnancy in four ethnic groups. Am J Obstet Gynecol 174, 1618–1621. 10.1016/S0002-9378(96)70617-8 [DOI] [PubMed] [Google Scholar]
- Han Y. W., Shen T., Chung P., Buhimschi I. A., Buhimschi C. S. (2009). Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol 47, 38–47. 10.1128/JCM.01206-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier S. L., Nugent R. P., Eschenbach D. A., Krohn M. A., Gibbs R. S., Martin D. H., Cotch M. F., Edelman R., Pastorek J. G., II & other authors (1995). Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med 333, 1737–1742. 10.1056/NEJM199512283332604 [DOI] [PubMed] [Google Scholar]
- Jakobsson T., Forsum U. (2007). Lactobacillus iners: a marker of changes in the vaginal flora? J Clin Microbiol 45, 3145. 10.1128/JCM.00558-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzoglou A., Smola A., Hornik K., Zeileis A. (2004). kernlab: an S4 package for kernel methods in R. J Stat Softw 11, 1–20. [Google Scholar]
- Koumans E. H., Sternberg M., Bruce C., McQuillan G., Kendrick J., Sutton M., Markowitz L. E. (2007). The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 34, 864–869. 10.1097/OLQ.0b013e318074e565 [DOI] [PubMed] [Google Scholar]
- Kramer M. R., Hogue C. R. (2008). Place matters: variation in the black/white very preterm birth rate across U.S. metropolitan areas, 2002–2004. Public Health Rep 123, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont R. F., Duncan S. L., Mandal D., Bassett P. (2003). Intravaginal clindamycin to reduce preterm birth in women with abnormal genital tract flora. Obstet Gynecol 101, 516–522. 10.1016/S0029-7844(02)03054-5 [DOI] [PubMed] [Google Scholar]
- Marrazzo J. M., Thomas K. K., Fiedler T. L., Ringwood K., Fredricks D. N. (2008). Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Ann Intern Med 149, 20–28. 10.7326/0003-4819-149-1-200807010-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. L., Richardson B. A., Nyange P. M., Lavreys L., Hillier S. L., Chohan B., Mandaliya K., Ndinya-Achola J. O., Bwayo J., Kreiss J. (1999). Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180, 1863–1868. 10.1086/315127 [DOI] [PubMed] [Google Scholar]
- Myer L., Denny L., Telerant R., Souza M., Wright T. C., Jr, Kuhn L. (2005). Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis 192, 1372–1380. 10.1086/462427 [DOI] [PubMed] [Google Scholar]
- Nelson D. B., Hanlon A., Hassan S., Britto J., Geifman-Holtzman O., Haggerty C., Fredricks D. N. (2009). Preterm labor and bacterial vaginosis-associated bacteria among urban women. J Perinat Med 37, 130–134. 10.1515/JPM.2009.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. B., Hanlon A., Nachamkin I., Haggerty C., Mastrogiannis D. S., Liu C., Fredricks D. N. (2014). Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr Perinat Epidemiol 28, 88–96. 10.1111/ppe.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness R. B., Hillier S., Richter H. E., Soper D. E., Stamm C., Bass D. C., Sweet R. L., Rice P. (2003). Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc 95, 201–212. [PMC free article] [PubMed] [Google Scholar]
- Ness R. B., Hillier S. L., Kip K. E., Soper D. E., Stamm C. A., McGregor J. A., Bass D. C., Sweet R. L., Rice P., Richter H. E. (2004). Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol 104, 761–769. 10.1097/01.AOG.0000139512.37582.17 [DOI] [PubMed] [Google Scholar]
- Nugent R. P., Krohn M. A., Hillier S. L. (1991). Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen, J., Blanchet, F. G., Kindt, R. & other authors (2013). Vegan: Community Ecology Package version 2.0–10. http://cran.r-project.org/web/packages/vegan/index.html.
- Paige D. M., Augustyn M., Adih W. K., Witter F., Chang J. (1998). Bacterial vaginosis and preterm birth: a comprehensive review of the literature. J Nurse Midwifery 43, 83–89. 10.1016/S0091-2182(97)00161-4 [DOI] [PubMed] [Google Scholar]
- R Foundation for Statistical Computing (2013). R: A language and environment for statistical computing. http://www.R-project.org/.
- Ravel J., Gajer P., Abdo Z., Schneider G. M., Koenig S. S., McCulle S. L., Karlebach S., Gorle R., Russell J. & other authors (2011). Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108 (Suppl 1), 4680–4687. 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R., Hassan S. S., Gajer P., Tarca A. L., Fadrosh D. W., Nikita L., Galuppi M., Lamont R. F., Chaemsaithong P. & other authors (2014). The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2, 4. 10.1186/2049-2618-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce R. A., Jackson T. P., Thorp J. M., Jr, Hillier S. L., Rabe L. K., Pastore L. M., Savitz D. A. (1999). Race/ethnicity, vaginal flora patterns, and pH during pregnancy. Sex Transm Dis 26, 96–102. 10.1097/00007435-199902000-00007 [DOI] [PubMed] [Google Scholar]
- Ryckman K. K., Simhan H. N., Krohn M. A., Williams S. M. (2009). Predicting risk of bacterial vaginosis: the role of race, smoking and corticotropin-releasing hormone-related genes. Mol Hum Reprod 15, 131–137. 10.1093/molehr/gan081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwebke J. R. (2003). Gynecologic consequences of bacterial vaginosis. Obstet Gynecol Clin North Am 30, 685–694. 10.1016/S0889-8545(03)00086-X [DOI] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W. S., Huttenhower C. (2011). Metagenomic biomarker discovery and explanation. Genome Biol 12, R60. 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibanda E. N., Stanczuk G., Kasolo F. (2003). HIV/AIDS in Central Africa: pathogenesis, immunological and medical issues. Int Arch Allergy Immunol 132, 183–195. 10.1159/000074299 [DOI] [PubMed] [Google Scholar]
- Tamrakar R., Yamada T., Furuta I., Cho K., Morikawa M., Yamada H., Sakuragi N., Minakami H. (2007). Association between Lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women. BMC Infect Dis 7, 128. 10.1186/1471-2334-7-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugwumadu A. (2007). Role of antibiotic therapy for bacterial vaginosis and intermediate flora in pregnancy. Best Pract Res Clin Obstet Gynaecol 21, 391–402. 10.1016/j.bpobgyn.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Verstraelen H., Verhelst R., Claeys G., De Backer E., Temmerman M., Vaneechoutte M. (2009). Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol 9, 116. 10.1186/1471-2180-9-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscardi R. M. (2014). Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed 99, F87–F92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M., Cole J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73, 5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. (2009). ggplot2: Elegant Graphics for Data Analysis. New York: Springer. http://had.co.nz/ggplot2/book.
- Wiesenfeld H. C., Hillier S. L., Krohn M. A., Landers D. V., Sweet R. L. (2003). Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 36, 663–668. 10.1086/367658 [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Mendes-Soares H., Linhares I. M., Jayaram A., Ledger W. J., Forney L. J. (2013). Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. MBio 4, e00460-13. 10.1128/mBio.00460-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Bent S. J., Schneider M. G., Davis C. C., Islam M. R., Forney L. J. (2004). Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology 150, 2565–2573. 10.1099/mic.0.26905-0 [DOI] [PubMed] [Google Scholar]
- Zhou X., Brown C. J., Abdo Z., Davis C. C., Hansmann M. A., Joyce P., Foster J. A., Forney L. J. (2007). Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 1, 121–133. 10.1038/ismej.2007.12 [DOI] [PubMed] [Google Scholar]
- Zhou X., Hansmann M. A., Davis C. C., Suzuki H., Brown C. J., Schütte U., Pierson J. D., Forney L. J. (2010). The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. FEMS Immunol Med Microbiol 58, 169–181. 10.1111/j.1574-695X.2009.00618.x [DOI] [PMC free article] [PubMed] [Google Scholar]