Abstract
Background:
Preeclampsia is a pregnancy-specific disease associated with significant maternal and perinatal mortality and morbidity. Lipid abnormality and elevated serum uric acid have been reported as early features of the disease. We aimed to detect the level of serum lipid profile and uric acid abnormalities in severe preeclamptics in Benin City and to measure their clinical significance.
Materials and Methods:
A prospective case-control study was conducted with subjects presenting with severe preeclampsia to the Obstetric Unit of the UBTH, Benin City. Fasting serum lipid profile and uric acid levels of 40 severe preeclamptic subjects and 80 gestation-matched normotensive controls were done at recruitment. The preeclamptic subjects were managed according to our departmental protocol which included stabilisation and delivery. Their sociodemographic and clinical characteristics were used to generate a database for analysis.
Results:
The mean serum uric acid level was 28% higher in severe preeclamptics than normotensive women (5.96 ± 2.54 mg/dl versus 4.30 ± 0.85; P = 0.005). There were statistically significant differences in levels of triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) between the preeclamptics and their normotensive controls (P = 0.006, P = 0.000, P = 0.000, respectively). Abnormal serum uric acid was associated with advanced maternal age (P = 0.000), early-onset preeclampsia (P = 0.000) and abnormal body mass index (BMI; P = 0.000). Low birth weight was more likely in preeclamptics with elevated serum uric acid levels (P = 0.041).
Conclusion:
Abnormality of serum uric acid in preeclampsia was significantly associated with increased frequency of complications but lipid profile abnormalities were not shown in the subjects studied. We recommend a larger scale study to determine lipid profile in normal and complicated pregnancies in our environment.
Keywords: Lipid profile, serum uric acid, severe preeclampsia, University of Benin Teaching Hospital
INTRODUCTION
Preeclampsia is a pregnancy-specific disease with a unique pathogenesis. It remains unclear what triggers the occurrence in any particular individual, but various risk factors have been identified.1 Evidence seems to suggest the pathology of preeclampsia originates early in pregnancy in the placenta, but its manifestation as a clinical entity, which remains largely unpredictable, is seen later in the course of pregnancy. A possible determinant of disease expression is the level of exaggeration of physiologic adaptation in pregnancy.2,3,4 In our environment, preeclampsia is noted to occur late in pregnancy5,6 but evolves rapidly to severe disease; hence, early identification of the maladaptive changes in pregnancy may assist in predicting disease severity.
Various agents have been implicated in the pathogenesis of preeclampsia. Abnormal lipid metabolism and deranged total antioxidant status are some of the observed pathophysiologic abnormalities seen in patients with preeclampsia.2,3,4 Several studies have shown that oxidative stress is predominant in preeclampsia and as such the attempt to overcome this allows the body to overwhelm its antioxidant capacity.7,8 Endothelial dysfunction, which is central to the pathology of preeclampsia, has also been linked to hyperlipidemia; and abnormal lipid peroxidation in preeclampsia, especially with respect to triglycerides, has been consistently reported in the literature.9,10 Similarly, the excessive cellular activity associated with the process of placental ischemia also leads to overproduction of uric acid which serves as a marker of the disease,11,12 with abnormal levels seen much earlier than the detection of proteinuria.
It is likely that an imbalance between lipid peroxidation and antioxidant mechanisms may impair endothelial function leading to the manifestation of preeclampsia. Although the modification of lipid metabolism in normal and preeclamptic pregnancies has been widely studied in Europe and USA, very few local studies have focused on this important subject, and very little attention has been paid to their clinical correlation. In the present study, we aimed to determine the lipid profile and uric acid levels in patients presenting with severe preeclampsia and to correlate these with clinical severity of the disease.
PATIENTS AND METHODS
A prospective case-control study was conducted between June 2012 and December 2012 at the Obstetrics and Gynecology Department of University of Benin Teaching Hospital (UBTH), Benin City with approval of the Institutional Review Board. The subjects were patients managed for severe preeclampsia. To achieve a uniform subject population, only patients with severe preeclampsia who booked in UBTH for antenatal care and presented to hospital in a fasting state (at least 8 hours) were included in this study. Postpartum cases of severe preeclampsia, patients with intrauterine fetal death, renal failure or those who had any treatment administered elsewhere before admission to UBTH were excluded.
All eligible patients were counseled and their written consent obtained before recruitment. Severe preeclampsia was defined as diastolic blood pressure (DBP) of ≥110 mmHg and/or systolic blood pressure (SBP) of ≥160 mmHg associated with proteinuria of at least 1+ on dipstick examination. Abnormalities of triglycerides, total cholesterol, low-density lipoprotein (LDL)-cholesterol and high-density lipoprotein (HDL)-cholesterol were defined as levels ≥150 mg/dl, ≥200 mg/dl, ≥100 mg/dl and <40 mg/dl, respectively. Abnormal lipid profile for each subject was defined as abnormality in any one of the four parameters in the panel. Abnormal serum uric acid was defined as values >5mg/dl.
At admission, the patients were stabilised and managed according to the departmental protocol, with preparation for delivery. Intravenous access was secured; blood was drawn from the antecubital vein for lipid profile, serum uric acid, serum magnesium assay, electrolytes, urea and creatinine estimation, full blood count with platelets, liver function testand bedside clotting time. Seizure prophylaxis was instituted with intravenous magnesium sulphate and blood pressure control was achieved with intravenous labetalol.
A control group of normotensive patients matched for gestational age with the cases of severe preeclampsia was recruited from the antenatal clinic after obtaining an informed written consent. Due counseling on the nature of the study and the quantity of blood to be taken was then given. The eligible patients had their venous blood taken for laboratory assessment of lipid profile and uric acid estimation. The main outcome measure was the relationship between clinical and laboratory parameters in each group, as well as the comparison between preeclamptic and normotensive subjects.
The subjects’ socio-demographic, clinical and laboratory parameters were used to generate a database for analysis. The information retrieved was coded and entered into a personal computer. Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS) for Windows version 15.0 and GraphPad InStat 3 software. Categorical variables were expressed as absolute numbers and percentages and significant differences determined using the Chi-square test or Fisher exact test where appropriate while continuous variables were presented as means with standard deviations and significant differences determined with the Student t test. The level of significance was set as P < 0.05.
Laboratory assay of lipid profile and uric acid
About 10 ml of fasting venous blood sample was collected from the antecubital vein into plain tubes and lithium heparin tubes, 6 ml and 4 ml, respectively. Immediately after collection, adequate anticoagulation was ensured in the lithium heparin sample by gentle inversion of the bottle while the plain samples were left standing to allow clot retraction. Thereafter, the specimens were centrifuged for 5 min at 3000 rpm to separate the plasma and serum components which were then collected in plain tubes, labeled and stored at −80o C. The stored samples were analysed within two weeks of collection.
The stored serum sample was analysed for total cholesterol, HDL-cholesterol and triglycerides using the direct enzymatic method.13,14 The LDL-cholesterol component was thereafter derived using the Friedwald formula.15 The plasma sample was also analysed for uric acid by standard spectrophotometric methods.
RESULTS
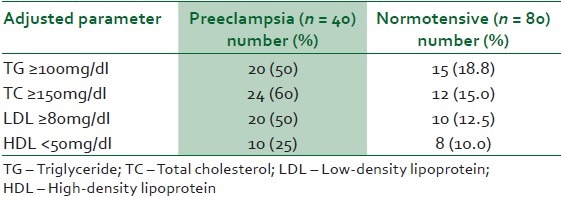
A total of 40 patients with severe preeclampsia were recruited for this study. The control group of gestational age-matched women had 80 subjects. The mean age, parity, gestational age (GA) and body mass index (BMI) were not significantly different between the two groups [Table 1]. The mean triglycerides (TG), total cholesterol (TC), LDL-cholesterol (LDL) and serum uric acid (UA) levels were 13%, 8%, 28% and 28%, respectively higher in the preeclamptic group than the normotensive women [Table 2]. The mean HDL-cholesterol (HDL) level was 12% lower in the women with severe preeclampsia than the normotensive women [Table 2]. Despite this observed difference in mean lipid profile between preeclamptics and normotensive patients, the mean levels in preeclamptic and normotensive patients failed to reach values accepted for abnormality [Table 2]. An adjusted reference range for the lipid panel resulted in the observation represented in Table 3. The mean arterial blood pressure (MABP) of the preeclamptics was 25% higher than the normotensive women (126 ± 18.073 mmHg versus 95 ± 10.156, P = 0.000).
Table 1.
Maternal socio-demographic characteristics
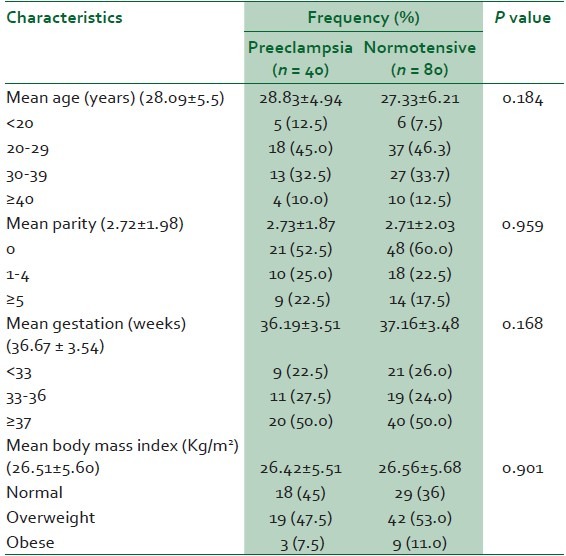
Table 2.
Maternal mean levels of lipid profile and serum uric acid
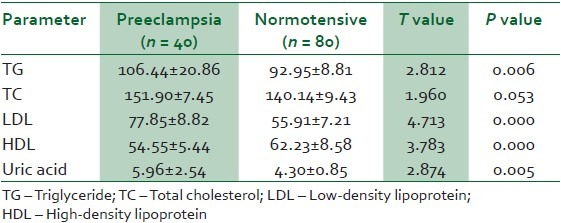
Table 3.
The proportion of patients with abnormalities of lipid profile using adjusted reference ranges
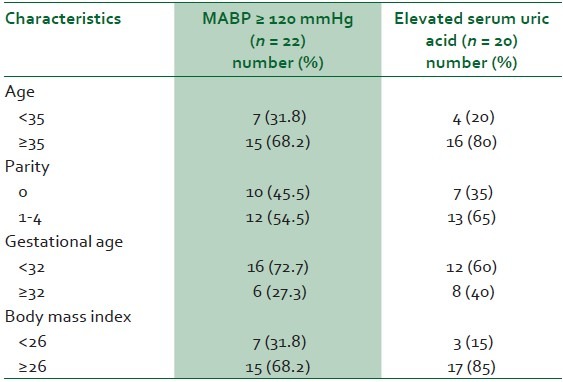
The median age of the preeclamptics was 30 years (range of 19-41), and almost 70% of the women with MABP ≥120 mmHg were 35 years or older [Table 4]. Women older than 35 years were four times more likely to have elevated UA levels than younger women. The median parity was two (range 0-5). Over 45% of the patients with MABP ≥120mmHg were nulliparas, but 65% of those who had elevated serum UA were multiparas [Table 4]. The median GA at diagnosis of severe preeclampsia was 34 weeks (range of 27-41). All patients with MABP ≥140mmHg also had abnormal serum UA levels and were at GA <34 weeks (Information is not in table). The median BMI of the preeclamptic patients was 26.7 kg/m2 ; (range 19.4-35.5). Over 80% of cases of elevated serum UA were seen in preeclamptics with BMI > 26 kg/m2 [Table 4].
Table 4.
Determinants of mean arterial blood pressure (MABP) and elevated serum uric acid level in preeclamptic patients
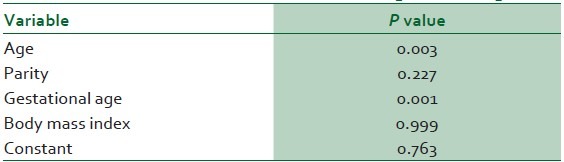
A logistic regression analysis was conducted to predict the roles of age, gestational age, parity and BMI in the determination of abnormal serum UA levels in the preeclamptic group. A test of the full model was statistically significant (Chi-square 51.419, P = 0.000 with df = 4), and prediction success overall was 87.8%. Maternal age and gestational age were found to be significant contributors [Table 5].
Table 5.
Logistic regression analysis to predict abnormal serum uric acid levels in preeclampsia
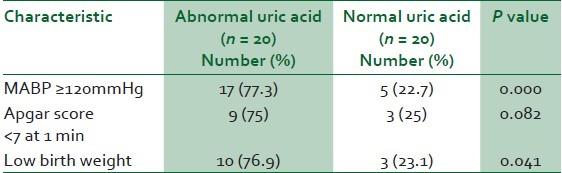
Preeclamptics with abnormal serum UA levels were 55% more likely to have MABP ≥120 mmHg (77.3% vs 22.7, P = 0.000; Table 6), 50% more likely to have babies with birth asphyxia (75% vs 25, P = 0.082; Table 6), and 53% more likely to have low birth weight babies (76.9% vs 23.1, P = 0.041; Table 6). And further logistic regression analysis confirmed the correlation of abnormal serum uric acid levels with low birth weight (P = 0.018).
Table 6.
Clinical correlates of elevated serum uric acid levels in preeclampsia
DISCUSSION
This study showed that serum uric acid levels were elevated in severe preeclampsia but lipid profile abnormality was not seen in this group of preeclamptic patients. However, there were significant differences between the mean triglycerides, LDL-cholesterol, HDL-cholesterol and total cholesterol of the preeclamptics and the normotensive patients studied. Severe preeclampsia was more likely in young, nulliparous women at advanced gestational ages. Abnormal serum uric acid levels were associated with advanced maternal age, multiparity, early-onset disease and abnormal BMI.
The sample size of this study was adequate considering the homogeneity of the group. The case-control design of this study, the established sensitivity and reproducibility of the methods of analyses employed and ready availability of the reagents used are important in the validity of our results. This hospital-based cross-sectional study may be representative of the general obstetric population because of the referral status of our hospital.
Our sample size of 40 severe preeclamptics is similar to the sample size of previous studies,16,17,18 but larger than the study population in the work by Var et al.19 A larger sample size would probably improve the external validity of the results but the homogenous population and gestational age-matched controls utilized in this study ensured the reliability of our results.
Uric acid levels have been consistently reported to be elevated in preeclampsia with highest levels seen to mirror the severity of the condition.20,21 Our results confirmed this observation with half of the patients with severe preeclampsia already having abnormal serum uric acid levels at the time of diagnosis. Serum uric acid levels have been employed as discriminatory to diagnosis of severe preeclampsia because abnormal levels may predate proteinuria by several weeks.11,20,21,22,23 Advanced maternal age, multiparity and abnormal BMI which were associated with elevated serum uric acid in this study probably reflected the role of age and BMI in hypertension and renal status that could be unmasked by the superimposition of preeclampsia. Further logistic regression analysis also revealed an association between uric acid abnormality and maternal age.
Complications of severe preeclampsia noted in this study included birth asphyxia and low birth weight which are in agreement with the reports of previous authors.5,6,24,25 It is instructive to note our finding that all patients with MABP ≥140mmHg had abnormal serum uric acid levels. The occurrence of birth asphyxia and presence of low birth weight were also significantly correlated with abnormal serum uric acid levels.
Applying the current reference range for diagnosis of abnormal lipid profile will appear to exclude all of the subjects in the present study. It is likely that pregnancy or the presence of preeclampsia in our women does not significantly alter the metabolism of lipids; hence to define the modest abnormalities in lipid profile associated with severe preeclampsia in our environment, the reference range for pregnant women will have to be ascertained. We found only 50% of our preeclamptics had elevated triglycerides when the level of abnormality was relaxed to 100 mg/dl from 150 mg/dl. Similarly, reducing the reference range of normal for total cholesterol from 200 mg/dl to 150 mg/dl resulted in 60% abnormality. Thus our findings, using the accepted reference range for lipid profile, do not support the report of many authors that abnormal lipid profile is associated with preeclampsia with worsening levels shown as pregnancy advanced.26,27 It is also likely that the physiologic adaptations to the cardiovascular changes in pregnancy in our women are more pronounced and so result in physiologic dilution of many blood constituents including lipids.
Despite the general observation that lipid profile was not abnormal in both groups of patients in this study, there were statistically significant differences between the mean triglycerides, low density lipoprotein and high density lipoprotein of the preeclamptics and normotensive women. A previous study done in northern Nigeria had reported that none of the 500 subjects in the study population had a plasma total cholesterol level up to 200 mg/dl,28 a level commonly accepted as upper limit of normal in this environment.29 It has been argued by some researchers that the range of plasma cholesterol level derived for the developed countries might have to be modified for developing countries like Nigeria. The worry expressed by such authors is that subjects in developing countries could indeed be prone to developing complications even at lower plasma cholesterol levels.30
Elevated serum uric acid levels in preeclamptic patients were associated with higher MABP, higher risk of birth asphyxia and low birth weights. These observations have also been reflected in the findings of previous authors.11,12,20,21
This study has confirmed the association between severe preeclampsia and elevated serum uric acid. However, abnormalities of lipid profile were not found to be associated with preeclampsia in this study. It appears the reference range of normal lipid profile in pregnancy in our environment may require redefinition considering the low frequency of abnormality found in this study. We recommend a cross-sectional multicentre study to define the reference range of lipid profile in pregnancy as well as the possible role of preeclampsia in lipid metabolism.
In conclusion, serum uric acid levels are elevated in pregnancies complicated by preeclampsia, and this may serve as a marker for early diagnosis of the disease as well as a surrogate for clinical severity of the condition.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lim KH. Preeclampsia. [Last accessed on 2012 Aug 06]. Available from: http://emedicine.medscape.com/.../1476919-
- 2.Ghulmiyyah LM, Sibai BM. Gestational hypertension-preeclampsia and eclampsia. In: Queenan JT, Spong CY, Lockwood CJ, editors. Management of High-Risk Pregnancy: An Evidence-Based Approach. 5th ed. Malden: Blackwell Publishing; 2007. pp. 271–9. [Google Scholar]
- 3.Arias F. Practical Guide to High-Risk Pregnancy and Delivery. A South Asian Perspective. 3rd ed. India: Saunders; 2008. Hypertensive disorders in pregnancy; pp. 397–439. [Google Scholar]
- 4.Lain KY, Roberts JM. Management of preeclampsia. In: Ransom SB, Dombrowski MP, Evans MI, Ginsburg KA, editors. Contemporary Therapy in Obstetrics and Gynaecology. Philadelphia: WB Saunders; 2002. pp. 44–8. [Google Scholar]
- 5.Onuh SO, Aisien AO. Maternal and fetal outcome in eclamptic patients in Benin City, Nigeria. J Obstet Gynaecol. 2004;24:765–8. doi: 10.1080/01443610400009451. [DOI] [PubMed] [Google Scholar]
- 6.Ugwu EO, Dim CC, Okonkwo CD, Nwankwo TO. Maternal and perinatal outcome of severe preeclampsia in Enugu, Nigeria after introduction of magnesium sulphate. Niger J Clin Prac. 2011;14:418–21. doi: 10.4103/1119-3077.91747. [DOI] [PubMed] [Google Scholar]
- 7.Raijmakers MT, Dechend R, Poston L. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension. 2004;44:374–80. doi: 10.1161/01.HYP.0000141085.98320.01. [DOI] [PubMed] [Google Scholar]
- 8.Poston L, Chappell L, Seed P, Shennan A. Biomarkers of oxidative stress in preeclampsia. [Last accessed on 2012 Dec 18];Pregnancy Hypertens. 2011 1(1):22–27. doi: 10.1016/j.preghy.2010.10.009. Available from: www.pregnancyhypertension.org/article/S2210-7789(10)00010-3/abstract . [DOI] [PubMed] [Google Scholar]
- 9.Hubel CA, Shakir Y, Gallaher MJ, McLaughlin MK, Roberts JM. Low-density lipoprotein particle size decreases during normal pregnancy in association with triglyceride increases. J Soc Gynaecol Investig. 1998;5:244–50. doi: 10.1016/s1071-5576(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 10.Punthumapol C, Kittichotpanich B. Comparative study of serum lipid concentrations in preeclampsia and normal pregnancy. J Med Assoc Thai. 2008;91:957–61. [PubMed] [Google Scholar]
- 11.Roberts JM, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46:1263–9. doi: 10.1161/01.HYP.0000188703.27002.14. [DOI] [PubMed] [Google Scholar]
- 12.Lam C, Lim KH, Kang DH, Karumanchi SA. Uric acid and preeclampsia. Semin Nephrol. 2005;25:56–60. doi: 10.1016/j.semnephrol.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Zak B. Cholesterol methodologies: A review. Clin Chem. 1977;23:1201–14. [PubMed] [Google Scholar]
- 14.Klotzsch SG, McNamara JR. Triglyceride measurement: A review of methods and interferences. Clin Chem. 1990;36:1605–13. [PubMed] [Google Scholar]
- 15.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifugation. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Gohil JT, Patel PK, Gupta P. Estimation of lipid profile in subjects of preeclampsia. J Obstet Gynaecol India. 2011;61:399–403. doi: 10.1007/s13224-011-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belo L, Caslake M, Gaffney D, Santos-Silva A, Pereira-Leite L, Quintanilha A, et al. Changes in LDL size and HDL concentration in normal and preeclamptic pregnancies. Atherosclerosis. 2002;162:425–32. doi: 10.1016/s0021-9150(01)00734-1. [DOI] [PubMed] [Google Scholar]
- 18.Bayhan G, Kocyigit Y, Atamer A, Atamer Y, Akkus Z. Potential atherogenic roles of lipids, lipoprotein A and lipid peroxidase in preeclampsia. Gynaecol Endocrinol. 2005;21:1–6. doi: 10.1080/09513590500097382. [DOI] [PubMed] [Google Scholar]
- 19.Var A, Kuscu NK, Koyuncu F, Uyanik BS, Onar E, Yildirim Y, et al. Atherogenic profile in preeclampsia. Arch Gynaecol Obstet. 2003;268:45–7. doi: 10.1007/s00404-002-0317-4. [DOI] [PubMed] [Google Scholar]
- 20.Bellomo G. Serum uric acid and preeclampsia: An update. Expert Rev Cardiovasc Ther. 2012;10:701–5. doi: 10.1586/erc.12.51. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Zhao X, Wang Z, Hu Y. Combination of lipids and uric acid in mid-trimester can be used to predict adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2012;25:2633–8. doi: 10.3109/14767058.2012.704447. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins TL, Roberts JM, Mangos GJ, Davis GK, Roberts LM, Brown MA. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: A retrospective cohort study. BJOG. 2012;119:484–92. doi: 10.1111/j.1471-0528.2011.03232.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Xiong X, Fraser WD, Luo ZC. Association of uric acid with progression to preeclampsia and development of adverse conditions in gestational hypertensive pregnancies. Am J Hypertens. 2012;25:711–7. doi: 10.1038/ajh.2012.18. [DOI] [PubMed] [Google Scholar]
- 24.Ebeigbe PN, Aziken ME. Early onset pregnancy-induced hypertension/eclampsia in Benin City, Nigeria. Niger J Clin Pract. 2010;13:388–93. [PubMed] [Google Scholar]
- 25.Onyiriuka AN, Okolo AA. Perinatal outcome in patients with preeclampsia in Benin City, Nigeria. Trop J Obstet Gynaecol. 2004;21:148–52. [Google Scholar]
- 26.Lippi G, Albiero A, Montagnana M, Salvagno GL, Scevarolli S, Franchi M, et al. Lipid and lipoprotein profile in physiological pregnancy. Clin Lab. 2007;53:173–7. [PubMed] [Google Scholar]
- 27.Chappell LC, Seed PT, Briley A, Kelly FJ, Hunt BJ, Charnock-Jones DS, et al. A longitudinal study of biochemical variables in women at risk of preeclampsia. Am J Obstet Gynaecol. 2002;187:127–36. doi: 10.1067/mob.2002.122969. [DOI] [PubMed] [Google Scholar]
- 28.Youmbissi TJ, Djoumessi S, Nouedoui C. Lipid profile in a group of hypertensive Black African Cameroonians. Medicine d’ Afrique Noir. 2001;31:114–8. [Google Scholar]
- 29.Okesina AB, Oparinde DP, Akindoyin KA, Erasmus RT. Prevalence of some risk factors of coronary heart disease in a rural Nigerian population. East Afr Med J. 1999;76:212–6. [PubMed] [Google Scholar]
- 30.Singh RB, Rastogi V, Niaz MA, Ghosh S, Sy RG, Janus ED. Serum cholesterol and coronary artery disease in a population with low cholesterol levels: The Indian paradox. Int J Cardiol. 1998;65:81–90. doi: 10.1016/s0167-5273(98)00099-0. [DOI] [PubMed] [Google Scholar]


