Abstract
Background:
Abnormal lipid homeostasis has been reported in sickle cell anaemia (SCA) as well as in other haematological disorders. However, there is little information on the lipid profile of SCA subjects in vaso-occlusive crisis (VOC). This study determined the lipid profile of adult SCA subjects in VOC and in steady state (SSCA).
Materials and Methods:
Fifty-eight (58) adults with HbSS (30 in steady state and 28 in vaso-occlusive crisis) and 24 age-matched healthy individuals with HbAA genotype were recruited into this study. Standard methods were used for the determination of blood pressure (BP), packed cell volume (PCV), total white blood cell count (WBC) and haemoglobin phenotype. After an overnight fast, 5 ml of venous blood was obtained from each SSCA and the controls while samples were collected upon admission in the VOC group. Plasma lipid profile was determined using enzymatic method. Differences between two groups were determined using independent Student's t-test or Man-Whitney U as appropriate. P-values less than 0.05 were considered significant.
Results:
Plasma total cholesterol (TC) and high density lipoprotein (HDL) were significantly lower while the ratio of triglyceride (TG) to HDL (TG/HDL) was significantly higher in SSCA compared with the controls. Low density lipoprotein (LDL) and TC were significantly lower in SCA subjects in VOC compared with controls. However, TC, TG, LDL and TG/HDL were significantly lower while HDL was significantly higher in VOC compared with SSCA.
Conclusion:
Sickle cell anaemia subjects have defective fasting lipid metabolism which becomes pronounced with VOC.
Keywords: Lipid profile, sickle cell anemia, steady state, vaso-occlusive crisis
INTRODUCTION
Sickle cell anaemia (SCA) is a monogenic disorder resulting from substitution of glutamic acid with valine in position 6 of the β-chains of haemoglobin (Hb). It is characterised by the production of abnormal Hb referred to as sickle Hb or HbS.1,2,3 The prevalence of SCA is high in sub-Saharan Africa with Nigeria having the highest burden.4,5
SCA has been associated with hyperhaemolysis, cerebrovascular disease, acute chest syndrome, vaso-occlusive crisis, pulmonary hypertension and premature death among others.6,7 Relatively, individuals with SCA enjoy a compensated state of ill health interspersed with periods of acute exacerbation characterised by hyperhaemolytic (anaemic) or vaso-occlusive (VOC; painful crisis) with infection, tissue hypoxia and micro-vascular occlusion as important predisposing events.6,8
Abnormal lipid homeostasis has been reported in SCA as well as other haematological disorders such as β-thalassemia and this has been suggested to have the potential to alter membrane fluidity and function of red blood cell (RBC) in individuals with SCA.9,10,11 Earlier studies reported significant increase in plasma triglyceride (TG) levels and concurrent significant decrease in plasma levels of total cholesterol (TC), high-density lipoprotein-cholesterol (HDL) and low-density lipoprotein-cholesterol (LDL) in SCA subjects.9,11,12 Several inconclusive mechanisms such as heightened erythropoiesis (causing increased cholesterol utilization), defective liver function (due to iron overload) and defects in postabsorptive plasma homeostasis of fatty acids have been put forward to explain the pathogenesis of this SCA-associated lipid abnormalities.9,13 However, it is worthy of note that this lipid phenotype is generally recognized as a risk factor for cardiovascular diseases. Zorca et al.11 reported that elevated plasma TG is a potential risk factor for pulmonary hypertension (PH) in SCA subjects.
The impact of disordered lipid metabolism on the course of SCA and its numerous complications are not yet clearly defined. Also, there is little information on the lipid profile of SCA subjects in VOC. Due to the present dearth of knowledge; this study determined the lipid profile of adult Nigerians with SCA in vaso-occlusive crisis (VOC) and in steady state (SSCA).
MATERIALS AND METHODS
Eighty-two participants comprising 58 adults with SCA (30 in steady state and 28 in VOC) and 24 age-matched healthy individuals with HbAA genotype were recruited into this study. The SCA (HbSS) subjects were recruited from the Hematology Day Care Unit, Department of Hematology, University College hospital, Ibadan after approval by University College Hospital (UI/UCH) Joint Ethics Review Committee, and informed consent by participant.
Steady state (SSCA) and vaso-ooclusive crisis (VOC) were defined as earlier reported.14 Subjects with other forms of genotype apart from HbSS and HbAA, diabetes mellitus, hypertension, human immunodeficiency virus (HIV), hepatitis, cancer and with established endocrine dysfunctions were excluded from the study.
Blood pressure (BP) was obtained using a Mercury Sphygmomanometer after at least 10 minutes of rest.
After an overnight fast of about 10 hr, 5 ml of venous blood was obtained from each SCA subject in steady state (SSCA) and the controls. Samples were collected upon admission in the VOC group as VOC is an acute clinical condition hence; could not have been predicted for possible overnight fast. Most subjects in VOC would probably be anorexic because of the acute pain they were going through. Blood samples were dispensed into EDTA-containing samples bottles and after determining the packed cell volume (PCV) and total white blood cell count (WBC), plasma was appropriately obtained and stored at −20°C until analyses were done.
Haemoglobin phenotype of each subject was determined using standard electrophoretic method at pH 6.8 while PCV and WBC were determined as described by Cheesbrough.15 Plasma lipid profile was determined using enzymatic method while LDL was calculated using Friedwald equation.16
Statistical analysis
The distribution of the data was assessed using histogram with normal curve. Results are presented as mean ± standard deviation or as median (interquartile range) for Gaussian and non-Gaussian distributed data, respectively. Analysis of Variance (ANOVA) or Kruskal-Wallis test was used to compare all the three groups while differences between two groups were determined using independent Student's t-test or Man-Whitney U as appropriate. P-values less than 0.05 were considered significant.
RESULTS
Table 1 shows the characteristics of all the subjects. PCV, TC, HDL and LDL were significantly lower while WBC was significantly higher in SCA compared with the control subjects. There was slight, but insignificant elevation of TG in SCA compared with the control subjects.
Table 1.
Characteristics of the subjects

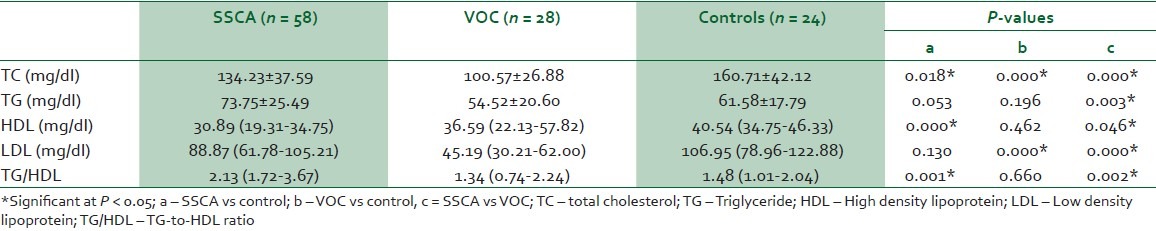
In Table 2, all the components of the lipid profile between the three groups (SSCA, VOC and controls) were significantly different. There was progressive decrease in TC and LDL from controls through VOC. Other components of the lipid profile had no specific pattern of differences.
Table 2.
Comparison of lipid profile in SSCA, VOC and control subjects using ANOVA
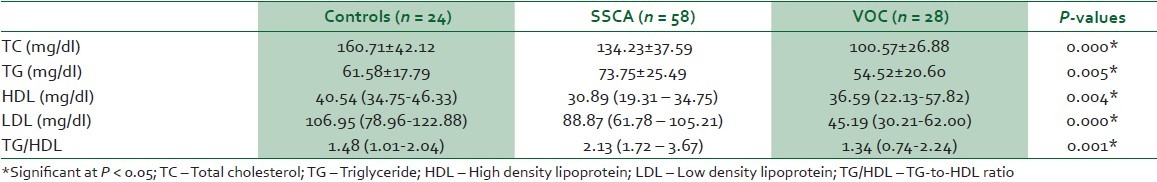
In Table 3, TC and HDL were significantly lower while TG/HDL was significantly higher in SCA subjects in steady state (SSCA) compared with the control subjects. Similarly, TC and LDL were significantly lower in SCA subjects in vaso-occlusive crisis (VOC) compared with controls. However, TC, TG, LDL and TG/HDL were significantly lower while HDL was significantly higher in VOC compared with SSCA.
Table 3.
Lipid profile in SSCA, VOC and control subjects
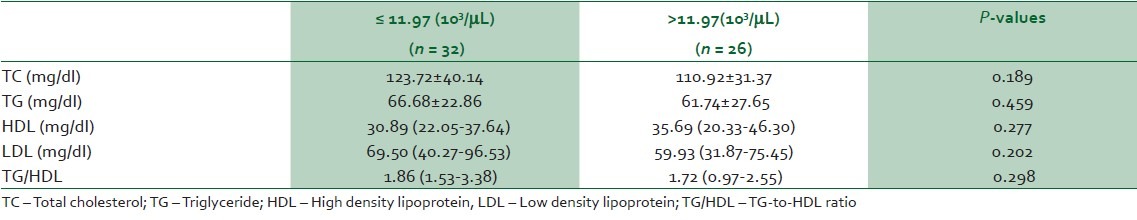
To find out if there is any interaction between WBC and lipid profile, SCA subjects were classified into two groups based on the mean WBC value; 11.97 (103/μL) [Table 1] into ≤11.97 (103/μL) and >11.97 (103/μL) groups. As shown in Table 4, the two groups had similar lipid profile but they exhibited a similar pattern to that observed when SSCA were compared with VOC. There was insignificant reduction in the levels of TC, TG, LDL, TG/HDL and insignificant elevation of HDL level in SCA subjects with >11.97 (103/μL) WBC compared with SCA subjects with ≤11.97 (103/μL)
Table 4.
Pattern of lipid profile based on mean total white blood cell count (WBC) in SCA subjects
DISCUSSION
Despite intense research for over 4 decades, mechanism of lipid homeostasis alteration in SCA subjects is not yet fully understood.11 The observed lower levels of TC, HDL and LDL in the combined SCA subjects (SSCA and VOC) are not novel findings. Hypocholesterolemia has been widely reported in SCA subjects11,12 and was thought to be due to increased cholesterol utilization consequent to increased erythropoiesis of SCA. However, the reports of Westerman17 and Ngogang et al.,18 showed that hypocholesterolaemia is a common feature of both haemolytic and non-haemolytic anaemia and that serum cholesterol is in equilibrium with the cholesterol content of total red cell mass. It was, therefore, suggested that SCA-associated hypocholesterolemia is a consequence of anaemia itself and not increased erythropoiesis.11,17
The interaction between SCA complications such as VOC and disturbed metabolic homeostasis in individuals with SCA has been reported.14,19 In this study, TC and LDL decreased progressively from control-to-SSCA-to-VOC. SSCA had lower TC and HDL while VOC had lower TC and LDL compared with the control subjects. This observation further confirms that SCA-associated hypocholesterolemia might be anaemia dependent as intense haemolysis has been associated with various complications of SCA.20 Also, TG/HDL was higher in SSCA than the control groups. The ratio of TG to HDL has been reported to be relevant in determining the risk of clinical vascular disease. It has been used to identify diabetic patients with an atherogenic lipid profile and has been found suitable in selecting patients needing earlier and aggressive treatment of lipid abnormalities.21 Our observation is not surprising as the SSCA group had slightly higher TG with concurrent lower HDL compared with the control subjects. Zorca et al.11 reported that high TG/HDL is associated with endothelial dysfunction and suggested that high TG/HDL is a potential risk factor for pulmonary hypertension. Although LDL is usually low in SCA subjects, Belcher et al.22 showed that LDL from SCA subjects is more susceptible to oxidation and cytotoxicity to endothelium. Our observation, together with earlier reports, indicates that anaemia-associated lipid homeostasis disturbance could predispose SCA subjects to various vascular diseases.
Unfavorable plasma fatty acid composition has been associated with clinical severity of SCA.23 Similarly, Nouraie et al.20 reported that intensity of haemolytic anaemia is an independent risk factor for the development of SCA complications such as PH and hypoxaemia. In this study, plasma levels of TC, LDL and TG/HDL were lower in VOC compared with SSCA. This observation could be as a result of possible intense haemolytic anaemia in VOC which would facilitate the attainment of a new equilibrium between the serum cholesterol and cholesterol content of total red cell mass.11,17
The observed higher HDL in VOC compared with SSCA supports the report of Darbari et al.3 which showed that higher HDL is independently associated with frequent VOC. The observed elevated plasma HDL could be a marker of less marrow activity in SCA subjects since formation of erythroid cell membrane requires cholesterol.11,24 The reason for the observed lower TG in VOC compared with SSCA is presently unclear. However, intake of drugs and/or reduced food intake (due to possible anorexia) before presentation may be responsible for this observation. Further research work is still required to properly understand the disturbance in lipid homeostasis following VOC as standard fasting period could not be ensured in our VOC subjects. Also, the small sample size used in this study could limit proper data interpretation.
CONCLUSIONS
Our study further confirms the widely reported defective lipid homeostasis in adults with sickle cell anaemia. It also showed that the alteration in the lipid metabolism becomes pronounced with vaso-occlusive crisis.
ACKNOWLEDGEMENT
This research work was self-funded. The authors appreciate the cooperation of all the study participants and the support of the entire Resident Doctors of the Departments of Chemical Pathology and Hematology, University College Hospital, Ibadan.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–9. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Serjeant GR. Sickle Cell Disease. 3rd ed. New York: Oxford University Press; 2001. pp. 3–15. [Google Scholar]
- 3.Darbari DS, Wang Z, Kwak M, Hildesheim M, Nichols J, Allen D, et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS One. 2013;8:e79923. doi: 10.1371/journal.pone.0079923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliyu ZY, Suleiman A, Attah E, Mamman AI, Babadoko A, Nouraie M, et al. NT-proBNP as a marker of cardiopulmonary status in sickle cell anaemia in Africa. Br J Haematol. 2010;150:102–7. doi: 10.1111/j.1365-2141.2010.08195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed SG, Bukar AA, Jolayemi B. Hematological indices of sickle cell anaemia patients with pulmonary tuberculosis in northern Nigeria. Mediterr J Hemat Infect Dis. 2010;2:e2010014. doi: 10.4084/MJHID.2010.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 7.De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary hypertension associated with sickle cell disease: Clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2008;83:19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–30. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 9.Buchowski MS, Swift LL, Akohoue SA, Shankar SM, Flakoll PJ, Abumrad N. Defects in postabsorptive plasma homeostasis of fatty acids in sickle cell disease. JPEN J Parenter Enteral Nutr. 2007;31:263–8. doi: 10.1177/0148607107031004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haghpanah S, Davani M, Samadi B, Ashrafi A, Karimi M. Serum lipid profiles in patients with beta-thalassemia major and intermedia in southern Iran. J Res Med Sci. 2010;15:150–4. [PMC free article] [PubMed] [Google Scholar]
- 11.Zorca S, Freeman L, Hildesheim M, Allen D, Remaley AT, Taylor JG, 6th, et al. Lipid levels in sickle-cell disease associated with haemolytic severity, vascular dysfunction and pulmonary hypertension. Br J Haematol. 2010;149:436–45. doi: 10.1111/j.1365-2141.2010.08109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shores J, Peterson J, VanderJagt D, Glew RH. Reduced cholesterol levels in African-American adults with sickle cell disease. J Natl Med Assoc. 2003;95:813–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Shalev H, Kapelushnik J, Moser A, Knobler H, Tamary H. Hypocholesterolemia in chronic anemias with increased erythropoietic activity. Am J Hematol. 2007;82:199–202. doi: 10.1002/ajh.20804. [DOI] [PubMed] [Google Scholar]
- 14.Akinlade KS, Atere AD, Olaniyi JA, Rahamon SK, Adewale CO. Serum copeptin and cortisol do not accurately predict sickle cell anaemia vaso-occlusive crisis as C-reactive protein. PLoS One. 2013;8:e77913. doi: 10.1371/journal.pone.0077913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2. Cambridge: Cambridge University Press; 2000. PCV and red cell indices; pp. 309–313. [Google Scholar]
- 16.Frieldwald WT, Levy RI, Fredricson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of prepreparative ultra centrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Westerman MP. Hypocholesterolaemia and anaemia. Br J Haematol. 1975;31:87–94. doi: 10.1111/j.1365-2141.1975.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 18.Ngogang J, Mouray H, Lebreton de Vonne T, Raisonnier A. Erythrocyte and plasma cholesterol exchange in sickle cell anemia. Clin Chim Acta. 1989;179:295–304. doi: 10.1016/0009-8981(89)90092-2. [DOI] [PubMed] [Google Scholar]
- 19.Olaniyi JA, Akinlade KS, Atere AD, Arinola OG. Plasma homocysteine, methyl-malonic acid, vitamin B12 and folate levels in adult nigerian sickle cell anaemia patients. Br J Med Med Res. 2013;4:1327–34. [Google Scholar]
- 20.Nouraie M, Lee JS, Zhang Y, Kanias T, Zhao X, Xiong Z, et al. Walk-PHASST Investigators and Patients. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica. 2013;98:464–72. doi: 10.3324/haematol.2012.068965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boizel R, Benhamou PY, Lardy B, Laporte F, Foulon T, Halimi S. Ratio of triglycerides to HDL cholesterol is an indicator of LDL particle size in patients with type 2 diabetes and normal HDL cholesterol levels. Diabetes Care. 2000;23:1679–85. doi: 10.2337/diacare.23.11.1679. [DOI] [PubMed] [Google Scholar]
- 22.Belcher JD, Marker PH, Geiger P, Girotti AW, Steinberg MH, Hebbel RP, et al. Low density lipoprotein susceptibility to oxidation and cytotoxicity to endothelium in sickle cell anemia. J Lab Clin Med. 1999;133:605–12. doi: 10.1016/s0022-2143(99)90191-9. [DOI] [PubMed] [Google Scholar]
- 23.Ren H, Ghebremeskel K, Okpala I, Ugochukwu CC, Crawford M, Ibegbulam O. Abnormality of erythrocyte membrane n-3 long chain polyunsaturated fatty acids in sickle cell haemoglobin C (HbSC) disease is not as remarkable as in sickle cell anaemia (HbSS) Prostaglandins Leukot Essent Fatty Acids. 2006;74:1–6. doi: 10.1016/j.plefa.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki J, Waterman MR, Buchanan GR, Cottam GL. Plasma and erythrocyte lipids in sickle cell anaemia. Clin Lab Haematol. 1983;5:35–44. doi: 10.1111/j.1365-2257.1983.tb00494.x. [DOI] [PubMed] [Google Scholar]


