As opposed to unfed ticks, transmission of R. rickettsii occurred in <10 minutes of attachment by adult fed ticks.
Feeding by A. aureolatum and Transmission of R. rickettsii
Keywords: Rickettsia rickettsii, Amblyomma aureolatum, Rocky Mountain spotted fever, vectorborne, ticks, tickborne, parasite, bacteria, Brazil
Abstract
Rocky Mountain spotted fever is endemic to the São Paulo metropolitan area, Brazil, where the etiologic agent, Rickettsia rickettsii, is transmitted to humans by adult Amblyomma aureolatum ticks. We determined the minimal feeding period required by A. aureolatum nymphs and adults to transmit R. rickettsii to guinea pigs. Unfed nymphs and unfed adult ticks had to be attached to the host for >10 hours to transmit R. rickettsii. In contrast, fed ticks needed a minimum of 10 minutes of attachment to transmit R. rickettsii to hosts. Most confirmed infections of Rocky Mountain spotted fever in humans in the São Paulo metropolitan area have been associated with contact with domestic dogs, the main host of A. aureolatum adult ticks. The typical expectation that transmission of tickborne bacteria to humans as well as to dogs requires ≥2 hours of tick attachment may discourage persons from immediately removing them and result in transmission of this lethal bacterium.
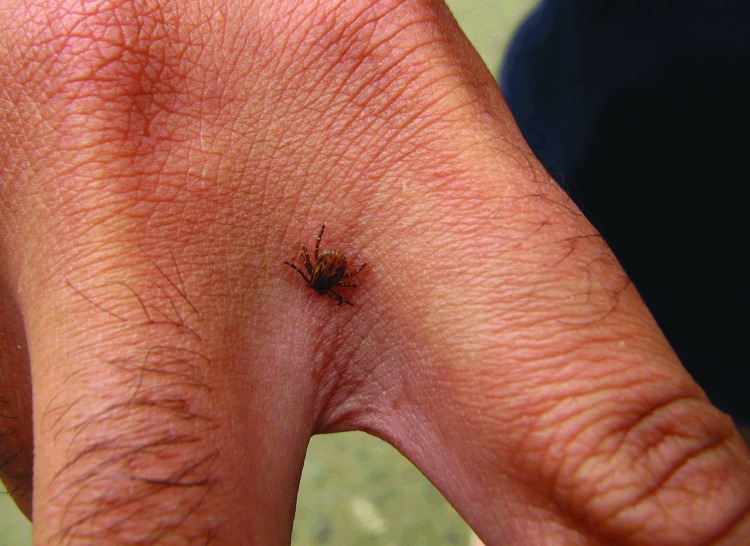
The tickborne bacterium Rickettsia rickettsii is the etiologic agent of the deadliest known rickettsiosis, Rocky Mountain spotted fever (RMSF). RMSF is referred to as Brazilian spotted fever in Brazil, where case-fatality rates are 20%–40% (1,2). The known distribution of R. rickettsii is restricted to the Americas, where different tick species have been implicated as vectors. The ticks Dermacentor andersoni and D. variabilis are the main vectors in the United States, and ticks of the Amblyomma cajennense complex are the most common vectors in Central and South America (3,4). The tick Rhipicephalus sanguineus has also been implicated as a vector for R. rickettsii in a few areas of Mexico and the state of Arizona in the United States (5,6). In the state of São Paulo, southeastern Brazil, there are 2 distinct epidemiologic scenarios of RMSF. Although A. cajennense is the identified vector in the countryside of the state of São Paulo (1,4), the tick A. aureolatum is the main vector in the metropolitan area of the city of São Paulo (7). A recent study on experimental infection of A. aureolatum with R. rickettsii demonstrated that the agent was preserved between life stages (transstadial maintenance) and by transovarial transmission in 100% of the A. aureolatum ticks for several consecutive generations; in addition, larvae, nymphs, and adults transmitted R. rickettsii to susceptible guinea pigs (8). Figure 1 illustrates an A. aureolatum adult tick.
Figure 1.
An adult male Amblyomma aureolatum tick attached to the hand of a person who became infested while in direct contact with a naturally infested dog in the metropolitan area of São Paulo, Brazil.
The life cycle of ticks in the hard tick family, Ixodidae, is characterized by a short parasitic phase and a long nonparasitic or free-living phase. The former consists of few days or weeks for the feeding of each of the ticks at the larval, nymphal, and adult stages; the free-living phase varies from several months to years, encompassing the off-host developmental stages (egg laying and incubation, molting), and the host-seeking period of unfed ticks (9). Unfed ticks are known for their capacity to survive extremely long fasting periods of months to years until they find a suitable host on which to start a new parasitic phase (9). During the fasting period, metabolic activity of salivary glands, midgut, reproductive organs, the excretory system, and circulation system of the tick are at much lower levels than they are during feeding periods (9).
Spencer and Parker (10) postulated that virulence of R. rickettsii in tick vectors is linked directly to the physiologic state of the tick. In fasting ticks, virulent R. rickettsii lose their pathogenicity and virulence for guinea pigs; however, incubation of infected fasting ticks at elevated temperature (37°C) for 24 to 48 h or allowing them to feed for >48 h induces R. rickettsii to revert to a virulent state (reactivation). This reactivation process, or restoration of virulence, is accompanied by a series of changes in the surface structure of R. rickettsii, demonstrated by an ultrastructure study of the bacterium in D. andersoni ticks (11). In addition, a recent study demonstrated that the expression of some R. rickettsii genes is modulated by the physiologic state of the host, such as a fasting or feeding A. aureolatum tick (12); however, specific genes responsible for rickettsial reactivation remain unknown.
Earlier studies by Ricketts (13) and Moore (14) reported that adult D. andersoni ticks usually required a 10-hour feeding period to transmit R. rickettsii to vertebrate hosts, although a minimal period of 1 hour and 45 minutes was demonstrated for ticks that had previously fed on another host. Spencer and Parker (10) reported that this period would be >48 hours for unfed D. andersoni ticks. In Brazil, Magalhães (15) reported that R. rickettsii–infected A. cajennense adult ticks required 36 hours of feeding to transmit the agent to guinea pigs. The current literature, including medical textbooks, guidelines, and reviews on RMSF (16,17), has repeatedly advised that an infected tick requires a minimum feeding period varying from 2 to 10 hours to transmit R. rickettsii to humans. On the basis of this information, gathered from the above-mentioned earlier studies during the first half of the 20th century, it is widely recommended that adult persons entering wooded or grassy areas should inspect themselves and their children frequently for ticks and remove the parasites before they could efficiently transmit R. rickettsii (17).
Herein, we determined the minimal feeding period required by nymphs and adult male A. aureolatum ticks to transmit R. rickettsii to guinea pigs, since no such data have been reported for A. aureolatum. Male ticks were tested instead of adult female ticks because male Amblyomma ticks are highly motile on hosts, constantly seeking attached females (9). In addition, male Amblyomma ticks typically outnumber female ticks on hosts because male ticks can stay on hosts for a much longer period (18,19). Therefore, adult male A. aureolatum ticks, hereafter referred to as adult ticks, would be more likely to transmit R. rickettsii to humans.
Materials and Methods
We collected 4 engorged female A. aureolatum ticks from dogs in São Bernardo do Campo, São Paulo metropolitan area, and brought them to the laboratory of the University of São Paulo, where we placed them in an incubator at 24°C and 95%–100% relative humidity for egg laying. We indirectly found the female offspring to be free of Rickettsia infection by testing the collected female ticks after oviposition by PCR, targeting a 401-bp fragment, the rickettsial gltA gene, as previously described (20). For acquisition feeding, the first generation larval progeny were allowed to feed on 5 R. rickettsii–infected guinea pigs previously inoculated with R. rickettsii strain Taiaçu, as described (8,20). This rickettsial strain had been isolated from an A. aureolatum tick from an RMSF–endemic area in the São Paulo metropolitan area (21). Recovered engorged larvae molted to nymphs; using the PCR method referenced above, we found that 10 nymphs that comprised a random sample were infected by R. rickettsii. Previous studies have shown that this acquisition protocol usually results in the infection of 100% of A. aureolatum ticks, which are capable of sustaining the rickettsial infection by transstadial maintenance and transovarial transmission (8,20).
For determination of the minimal feeding period required by an A. aureolatum unfed nymph to transmit R. rickettsii to a vertebrate host, we used 32 guinea pigs (nos. 1–32). Each guinea pig was infested by 10 A. aureolatum unfed nymphs, which were placed within a cotton sleeve glued to the shaved back of the animal, as described (20). Each of the 32 guinea pigs had a specific period in which the nymphs were allowed to feed; however, for each feeding period, we used 2 or 4 guinea pigs to replicate a given feeding period. For example, on guinea pigs 3 and 4 (Table 1), nymphs were allowed to feed for 4 hours. In this case, when the first nymph was seen attached to the skin of each animal, we started counting the feeding period. Four hours after the attachment of the first nymph, all 10 nymphs were manually removed from the guinea pig and stored frozen at −80°C until further analysis. The same procedure was used for the remaining guinea pigs, with variation of 2- to 48-hour feeding periods (Table 1). On guinea pigs 31 and 32, unfed nymphs were allowed to feed until they detached naturally as engorged nymphs, which varied from 4 to 7 days. Additional guinea pigs were infested by infected nymphs that were left to molt into adults to obtain unfed adults to be used in the following infestations.
Table 1. Fever, seroconversion to Rickettsia rickettsii antigens, and ear and/or scrotal lesions in guinea pigs exposed to R. rickettsii-infected Amblyomma aureolatum unfed nymphs through different feeding periods, Brazil.
| Guinea pig no. | Tick feeding period, h* | Fever† | R. rickettsii antibody titers‡ | Ear and/or scrotal lesions§ |
|---|---|---|---|---|
| 1 | 2 | No | <1:64 | No |
| 2 | 2 | No | <1:64 | No |
| 3 | 4 | No | <1:64 | No |
| 4 | 4 | No | <1:64 | No |
| 5 | 6 | No | <1:64 | No |
| 6 | 6 | No | <1:64 | No |
| 7 | 8 | No | <1:64 | No |
| 8 | 8 | No | <1:64 | No |
| 9 | 8 | No | <1:64 | No |
| 10 | 8 | No | <1:64 | No |
| 11 | 10 | No | <1:64 | No |
| 12 | 10 | No | <1:64 | No |
| 13 | 12 | Yes | ¶ | Yes |
| 14 | 12 | No | <1:64 | No |
| 15 | 12 | No | <1:64 | No |
| 16 | 12 | No | <1:64 | No |
| 17 | 14 | Yes | 2,048 | Yes |
| 18 | 14 | No | 256 | No |
| 19 | 16 | Yes | 512 | No |
| 20 | 16 | Yes | 512 | No |
| 21 | 18 | Yes | 8,192 | Yes |
| 22 | 18 | Yes | 256 | No |
| 23 | 24 | Yes | 4,096 | Yes |
| 24 | 24 | Yes | 8,192 | Yes |
| 25 | 24 | Yes | 4,096 | Yes |
| 26 | 24 | Yes | 512 | No |
| 27 | 36 | Yes | 16,384 | Yes |
| 28 | 36 | Yes | 8,192 | Yes |
| 29 | 48 | Yes | 4,096 | Yes |
| 30 | 48 | Yes | 8,192 | Yes |
| 31 | >96h | Yes | 16,384 | Yes |
| 32 | >96h | Yes | 16,384 | Yes |
*Number of hours that infected nymphs were allowed to feed on each guinea pig before ticks were manually removed from host. †Rrectal temperature >39.5°C during 21 d after tick infestation. ‡Anti-R. rickettsii IgG endpoint titers determined 21 d after tick infestation. §Ear or scrotal lesions (edema, necrosis) during the febrile period within 21 d after tick infestation. ¶Guinea pig died during the febrile period, before the 21st d after tick infestation; its lung was PCR-positive for Rickettsia spp.
To determine the minimal feeding period of A. aureolatum unfed adult ticks required to enable transmission of R. rickettsii to a vertebrate host, we used 24 guinea pigs (nos. 33–56). Each guinea pig was infested by 1 A. aureolatum unfed adult tick, as described for nymphs. Each of the 24 guinea pigs was assigned a specific feeding period in which the adult tick was allowed to feed. For example, on guinea pigs 39 and 40 (Table 2), adult ticks (1 per guinea pig) were allowed to feed for 8 hours. In this case, when the single adult tick was seen attached to the skin of each animal, we started counting the feeding period. Eight hours after attachment of the adult tick, it was manually removed from the guinea pig, and stored frozen at −80°C until further analysis. The same procedure was adopted for the remaining guinea pigs, except for the period in which the adult ticks were allowed to feed, which varied from 2 to 48 hours (Table 2). Unfed adult ticks were allowed to feed on 2 guinea pigs (nos. 55 and 56) for 7 days (168 hours), to simulate a feeding period that would last at least 7 days under natural conditions.
Table 2. Fever, seroconversion to Rickettsia rickettsii antigens, and ear and/or scrotal lesions in guinea pigs that were exposed to R. rickettsii–infected Amblyomma aureolatum unfed adult male ticks, Brazil.
| Guinea pig no. | Tick feeding period, h* | Fever† | Anti-R. rickettsii antibody titers‡ | Ear and/or scrotal lesions§ |
|---|---|---|---|---|
| 33 | 2 | No | <1:64 | No |
| 34 | 2 | No | <1:64 | No |
| 35 | 4 | No | <1:64 | No |
| 36 | 4 | No | <1:64 | No |
| 37 | 6 | No | <1:64 | No |
| 38 | 6 | No | <1:64 | No |
| 39 | 8 | No | <1:64 | No |
| 40 | 8 | No | <1:64 | No |
| 41 | 10 | No | <1:64 | No |
| 42 | 10 | No | <1:64 | No |
| 43 | 12 | Yes | 4,096 | Yes |
| 44 | 12 | Yes | 256 | No |
| 45 | 16 | Yes | 2,048 | Yes |
| 46 | 16 | Yes | 1,024 | Yes |
| 47 | 20 | Yes | 512 | Yes |
| 48 | 20 | Yes | 2,048 | Yes |
| 49 | 24 | Yes | 2,048 | Yes |
| 50 | 24 | Yes | ¶ | Yes |
| 51 | 36 | Yes | 2,048 | Yes |
| 52 | 36 | Yes | 4,096 | Yes |
| 53 | 48 | Yes | ¶ | Yes |
| 54 | 48 | Yes | 8,192 | Yes |
| 55 | 168 | Yes | 16,384 | Yes |
| 56 | 168 | Yes | 16,384 | Yes |
*Number of hours that an infected male adult tick was allowed to feed on each guinea pig before the tick was manually removed from the host. †Rectal temperature >39.5°C during 21 days after tick infestation. ‡Anti-R. rickettsii IgG endpoint titers determined 21 days after tick infestation. §Occurrence of ear or scrotal lesions (edema, necrosis) during the febrile period within 21 days after tick infestation. ¶Guinea pig died during the febrile period, before the 21st day after tick infestation; its lung tissue was PCR positive for Rickettsia spp.
To determine the minimal feeding period required by a previously fed A. aureolatum adult tick to transmit R. rickettsii to a vertebrate host, we first allowed adult male ticks to feed with adult female ticks for 48 hours on the shaved back of tick-naïve domestic rabbits (Oryctolagus cuniculus), as described (8). Then, the fed ticks were removed from the rabbits and immediately used to infest 34 guinea pigs (nos. 57–90), as described above, except that the period in which the adult ticks were allowed to feed varied from 1 minute to 168 hours (Table 3).
Table 3. Fever, seroconversion to Rickettsia rickettsii antigens, and ear and/or scrotal lesions in guinea pigs that were infested by previously fed R. rickettsii–infected Amblyomma aureolatum adult male ticks through different feeding periods, Brazil.
| Guinea pig number | Tick feeding period* | Fever† | Anti-R. rickettsii antibody titers‡ | Ear and/or scrotal lesions§ |
|---|---|---|---|---|
| 57 | 1 min | No | <1:64 | No |
| 58 | 1 min | No | <1:64 | No |
| 59 | 3 min | No | <1:64 | No |
| 60 | 3 min | No | <1:64 | No |
| 61 | 5 min | No | <1:64 | No |
| 62 | 5 min | No | <1:64 | No |
| 63 | 10 min | No | <1:64 | No |
| 64 | 10 min | Yes | 1,024 | No |
| 65 | 20 min | Yes | 1,024 | No |
| 66 | 20 min | Yes | 512 | No |
| 67 | 40 min | Yes | 1,024 | No |
| 68 | 40 min | Yes | 4,096 | Yes |
| 69 | 1 h | Yes | 4,096 | Yes |
| 70 | 1 h | Yes | 8,192 | Yes |
| 71 | 2 h | Yes | ¶ | Yes |
| 72 | 2 h | Yes | 512 | No |
| 73 | 4 h | Yes | ¶ | Yes |
| 74 | 4 h | Yes | 16,384 | Yes |
| 75 | 6 h | Yes | ¶ | Yes |
| 76 | 6 h | Yes | ¶ | Yes |
| 77 | 8 h | Yes | ¶ | Yes |
| 78 | 8 h | Yes | ¶ | Yes |
| 79 | 12 h | Yes | ¶ | Yes |
| 80 | 12 h | Yes | ¶ | Yes |
| 81 | 18 h | Yes | ¶ | Yes |
| 82 | 18 h | Yes | 8,192 | Yes |
| 83 | 24 h | Yes | 8,192 | Yes |
| 84 | 24 h | Yes | 16,384 | No |
| 85 | 36 h | Yes | ¶ | Yes |
| 86 | 36 h | Yes | ¶ | Yes |
| 87 | 48 h | Yes | ¶ | Yes |
| 88 | 48 h | Yes | ¶ | Yes |
| 89 | 168 h | Yes | 16,384 | Yes |
| 90 | 168 h | Yes | ¶ | Yes |
*Number minutes or hours that an infected adult male tick was allowed to feed on each guinea pig before the tick was manually removed from the host. All ticks had previously fed on rabbits for 48 h.
†Rectal temperature >39.5°C during 21 days after tick infestation.
‡ Anti-R. rickettsii IgG endpoint titers determined at 21 days after tick infestation.
§ Ear or scrotal lesions (edema, necrosis) during the febrile period within 21 days after tick infestation.
¶Guinea pig died during the febrile period, before day 21 after tick infestation; its lung was PCR positive for Rickettsia spp.
Every guinea pig or rabbit used in this study was tick naive; these animals were provided by a private laboratory that raised the animals under proper sanitary conditions. The rectal temperatures of guinea pigs and rabbits were measured daily from the day of infestation through 21 days afterward. These animals were considered febrile if rectal temperature reached values >39.5°C (guinea pigs) or >40°C (rabbits). All animals were tested for seroconversion to R. rickettsii antigens. For this purpose, we collected blood samples at 0 and 21 days postinfestation and tested for anti–R. rickettsii reactive antibodies by immunofluorescence assay, as described (8,22). Animals were considered seronegative if their serum was not reactive at the 1:64 dilution. Some infested guinea pigs that died before day 21 postinfestation were not tested by immunofluorescence assay because a second blood sample was not obtained; however, we submitted a fragment of their lung tissue to DNA extraction using the DNeasy Tissue Kit (QIAGEN, Chatsworth, CA, USA) and tested the samples by the same PCR protocol referenced above. Clinical alterations, such as ear or scrotal necrosis, were noted when observed. We tested all nymphal and adult ticks that were manually removed from the infested guinea pigs individually by the same PCR protocol referenced above.
Results
All PCRs performed on the DNA of nymphal and adult ticks that fed on guinea pigs for different periods resulted in amplicons compatible with R. rickettsii, indicating that all 90 guinea pigs in this study were exposed to R. rickettsii–infected ticks. Among guinea pigs exposed to R. rickettsii–infected unfed nymphs, animals remained afebrile and seronegative when nymphs fed for ≤10 hours (Table 1). When nymphs fed for 12 hours on 4 guinea pigs, 3 of these animals (nos. 14–16) remained seronegative and afebrile, but the fourth animal (no. 13) became febrile and died on the second week, when ear and scrotal necrosis were evident. Its lung tissue sample was PCR–positive for rickettsiae. All guinea pigs on which nymphs fed for 14 to ≥96 hours seroconverted to R. rickettsii, and fever developed in all but 1 (no. 18). Of 16 these animals, 5 did not show ear or scrotal lesions.
Among the 24 guinea pigs exposed to R. rickettsii–infected unfed adult ticks, 10 animals remained afebrile and seronegative when ticks fed for <10 hours (Table 2). Fever developed in the 14 guinea pigs on which the male ticks fed for 12–168 hours. Seroconversion to R. rickettsii was demonstrated in 12 of the 14 febrile guinea pigs. Two guinea pigs, nos. 50 and 53, died before 21 days; their lung tissue specimens were PCR–positive for rickettsiae. Thirteen of these febrile animals showed ear and scrotal lesions.
Of the 2 rabbits on which adult A. aureolatum ticks fed for 48 hours, 1 became febrile at day 5 and the other at day 7 postinfestation; ear necrosis developed in both rabbits, and blood samples seroconverted to R. rickettsii with endpoint titers of 8,192 or 16,384. When exposed to the R. rickettsii–infected adult ticks that had fed for 48 hours on rabbits, guinea pigs remained afebrile and seronegative when the ticks fed for ≤5 minutes (Table 3). Of 2 guinea pigs on which adult ticks fed for 10 minutes (nos. 63 and 64), no. 63 remained seronegative and afebrile, but no. 64 became febrile and seroconverted. Fever developed in all 26 guinea pigs on which fed adult ticks fed for 20 minutes to 168 hours; of these, 21 had ear or scrotal lesions, or both. Thirteen animals seroconverted to R. rickettsii, and 14 died during the febrile period; their lungs were positive for Rickettsia spp. by PCR.
The infection with R. rickettsii in guinea pigs was confirmed by seroconversion (nonfatal cases) or by PCR on lung tissue (fatal cases). Fever onset was registered between 5 and 9 days (mean 6.8) postinfestation with nymphs, between 4 and 8 days (mean 5.6) postinfestation with infected unfed adult ticks, and between 4 and 11 days (mean 6.7) postinfestation with prefed adult ticks. Among the 17 guinea pigs that became infected by R. rickettsii after being exposed to unfed nymphs, only 1 died of spotted fever (6% fatality rate). Among the 14 guinea pigs that became infected after being exposed to unfed adult ticks, 2 (14% fatality rate) died of spotted fever. When guinea pigs were exposed to infected ticks that had previously fed on rabbits (prefed adult ticks), the fatality rate rose to 52% (14/27).
Discussion
This work showed that unfed nymphs and unfed adult male ticks of A. aureolatum needed to be attached for >10 hours on the host, to successfully transmit a virulent strain of R. rickettsii. In contrast, fed adults needed only up to 10 minutes of attachment for transmission of R. rickettsii to the host. The >10-hour feeding period observed for unfed ticks is similar to the 10-hour period previously reported for D. andersoni ticks in 2 earlier studies (13,14); albeit much lower than the periods previously reported for D. andersoni (>48 hours) in another study (10) and for A. cajennense ticks (36 hours) in Brazil (15). Regarding fed ticks, the 10-minuteperiod herein observed for A. aureolatum ticks is much shorter than the 1 hour and 45 minutes previously reported for prefed D. andersoni ticks (14). It is possible that different tick species require different feeding periods for effective inoculation of R. rickettsii into the host; however, it is clear that prefed ticks require much shorter periods than unfed ticks. This difference should be related to the reactivation phenomenon; i.e., R. rickettsii was in a nonvirulent state in unfed nymphal and adult A. aureolatum ticks and in its virulent state (reactivated) in the prefed adult ticks used to infest guinea pigs.
Adult A. aureolatum ticks feed chiefly on Carnivora species (mostly domestic dogs), but immature ticks (larvae, nymphs) generally feed on passerine birds and a few rodent species (7,23). Humans have reported being attacked only by adult ticks, and usually by a single tick (24), because the population density of A. aureolatum ticks is usually low (18). In southeastern Brazil, the distribution of A. aureolatum populations is restricted to Atlantic rainforest fragments where optimal conditions of high humidity and cool temperatures prevail throughout the year (7,18). Therefore, infestations occur typically on domestic dogs that are reared unrestrained, with access to Atlantic rainforest fragments (18,25). However, to our knowledge, A. aureolatum–human infestation acquired in the forest has not been studied and documented. In fact, in an Atlantic rainforest reserve in the state of São Paulo, 4 Amblyomma tick species (including A. aureolatum) were collected in wild animal trails during a 4-year period (26), when A. aureolatum was the only 1 of the 4 tick species that was not reported to have attached to researchers during their field activities in the forest (27). Thus, we hypothesize that many of the RMSF-confirmed cases in the São Paulo metropolitan area were transmitted by A. aureolatum ticks that had fed on domestic dogs. In this case, the domestic dog would have become infested in the forest and brought an infected tick indoors, where it came into direct contact with humans (Figure 2). This statement is corroborated by a study that reported that 69% of the RMSF cases in the São Paulo metropolitan area occurred in children and women, who usually did not enter the forest (habitat of A. aureolatum) as frequently as did adult men (28). In addition, 93% of the cases in this area have been associated with direct contact with dogs (29).
Figure 2.
A typical area where infection with the Rickettsia rickettsii bacterium occurs, manifested as Rocky Mountain spotted fever, in the metropolitan area of São Paulo, Brazil. Humans have constructed their homes in the Atlantic rainforest fragment (habitat of the Amblyomma aureolatum tick, a vector of R. rickettsii), where many dogs are unrestrained. Dogs frequently enter the forest, become infested by adult A.aureolatum ticks, and bring them into homes, allowing the direct transfer of feeding ticks from dogs to humans.
In this study, the fatality rate for guinea pigs exposed to prefed adult ticks (52%) was much higher than the rate for guinea pigs exposed to unfed ticks (14%). A recent study reported that the fatality rate for patients with RMSF in a region of the São Paulo metropolitan area (transmission by A. aureolatum ticks) was 62.5% during 2003–2010, which was substantially higher than the 33.3% fatality rate observed in a region of the countryside of the state of São Paulo (transmission by A. cajennense ticks) during a similar period (29). Similarly to the situation with the guinea pigs in this study, this marked difference among RMSF case-patients could be related to the reactivation state of R. rickettsii in the tick vector, since we postulated above that infestation by fed ticks would predominate in the metropolitan area of São Paulo. In the countryside, acquisition of R. rickettsii infection could be predominantly related to infestations by unfed A. cajennense ticks acquired directly in the field, since such infestations are commonly reported in this area (4,30,31).
According to results of this study, a fed A. aureolatum tick could transmit R. rickettsii to a human in as few as 10 minutes of parasitism. Because this route of transmission seems to be common in the metropolitan area of São Paulo, health authorities must be aware that current textbooks and guidelines that indicate that an infected tick takes 2 to 10 hours to transmit R. rickettsii to humans (16,17) do not apply to the São Paulo metropolitan area.
In the eastern United States, R. rickettsii is transmitted to humans typically by the D. variabilis tick in the adult stage, commonly known as the American dog tick, which feeds chiefly on domestic dogs (17). Similarly to the circumstances in the São Paulo metropolitan area, most of the RMSF cases in the eastern United States have occurred in children and women (32,33), and infections in canines have been associated repeatedly with an increased risk for disease in owners (34). Because numerous reports of infected humans were associated with tick-infested dogs or tick removal within 4 weeks of disease onset, researchers have proposed that many of these cases were a result of direct contact with rickettsiae from tick body fluids during tick removal (34,35). Although this postulated mechanism cannot be discarded (including in the São Paulo metropolitan area), the current literature has considered that an attached tick needs several to many hours of attachment for a successful inoculation of rickettsiae into human skin. Once it is forcibly removed from a host, a partially fed tick loses its discriminatory senses and strives to feed wherever possible on any available vertebrate animal (36). Thus, it is reasonable to consider that tick removal habits in RMSF-endemic areas could have implications for the transmission of R. rickettsii, not only caused by potential direct contact with tick fluids, but also, as shown in this study, because detached ticks could readily attach to humans and inoculate them with rickettsiae within few minutes.
Acknowledgments
We thank the staff of Laboratório Biovet, Brazil, for providing naive guinea pigs and rabbits.
This work was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and Coordenadoria de Apoio a Pesquisa e Desenvolvimento.
This study was based on Mr Saraiva’s Master of Science dissertation, Feeding Period Required by Amblyomma aureolatum Ticks for Transmission of Rickettsia rickettsii to Vertebrate Hosts, presented in 2012 at the University of São Paulo.
This study was approved by the Ethics Committee on Animal Research for the Faculty of Veterinary Medicine of the University of São Paulo.
Biography
Mr Saraiva is a researcher of wildlife ecology at Bicho do Mato Research Institute, Brazil. His research interests focus on the ecology of ticks, tickborne diseases, and wildlife.
Footnotes
Suggested citation for this article: Saraiva DG, Soares HS, Soares JF, Labruna MB. Feeding period required by Amblyomma aureolatum ticks for transmission of Rickettsia rickettsii to vertebrate hosts. Emerg Infect Dis. 2014 Sep [date cited]. http://dx.doi.org/10.3201/eid2009.140189
References
- 1.Labruna MB. Ecology of rickettsia in South America. Ann N Y Acad Sci. 2009;1166:156–66. 10.1111/j.1749-6632.2009.04516.x [DOI] [PubMed] [Google Scholar]
- 2.de Sá Del Fiol FS, Junqueira FM, Rocha MCP, Toledo MI, Filho SB. Febre maculosa no Brasil. Rev Panam Salud Publica. 2010;27:461–6. 10.1590/S1020-49892010000600008 [DOI] [PubMed] [Google Scholar]
- 3.de Rodaniche EC. Natural infection of the tick Amblyomma cajennense with Rickettsia rickettsii in Panama. Am J Trop Med Hyg. 1953;2:696–9 . [DOI] [PubMed] [Google Scholar]
- 4.Krawczak FS, Nieri-Bastos FA, Nunes FP, Soares JF, Moraes-Filho J, Labruna MB. Rickettsial infection in Amblyomma cajennense ticks and capybaras (Hydrochoerus hydrochaeris) in a Brazilian spotted fever-endemic area. Parasit Vectors. 2014;7:7. [DOI] [PMC free article] [PubMed]
- 5.Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–94. 10.1056/NEJMoa050043 [DOI] [PubMed] [Google Scholar]
- 6.Eremeeva ME, Zambrano ML, Anaya L, Beati L, Karpathy SE, Santos-Silva MM, et al. Rickettsia rickettsii in Rhipicephalus ticks, Mexicali, Mexico. J Med Entomol. 2011;48:418–21. 10.1603/ME10181 [DOI] [PubMed] [Google Scholar]
- 7.Ogrzewalska M, Saraiva DG, Moraes-Filho J, Martins TF, Costa FB, Pinter A, et al. Epidemiology of Brazilian spotted fever in the Atlantic Forest, state of São Paulo, Brazil. Parasitology. 2012;139:1283–300. 10.1017/S0031182012000546 [DOI] [PubMed] [Google Scholar]
- 8.Labruna MB, Ogrzewalska M, Soares JF, Martins TF, Soares HS, Moraes-Filho J, et al. Experimental infection of Amblyomma aureolatum ticks with Rickettsia rickettsii. Emerg Infect Dis. 2011;17:829–34. 10.3201/eid1705.101524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonenshine DE. Biology of ticks. Vol. 1. New York: Oxford, 1991. [Google Scholar]
- 10.Spencer RR, Parker RR. Rocky Mountain spotted fever: infectivity of fasting and recently fed ticks. Public Health Rep. 1923;38:333–81. 10.2307/457666719314866 [DOI] [Google Scholar]
- 11.Hayes SF, Burgdorfer W. Reactivation of Rickettsia rickettsii in Dermacentor andersoni ticks: an ultrastructural analysis. Infect Immun. 1982;37:779–85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galletti MF, Fujita A, Nishiyama MY Jr, Malossi CD, Pinter A, Soares JF, et al. Natural blood feeding and temperature shift modulate the global transcriptional profile of Rickettsia rickettsii infecting its tick vector. PLoS ONE. 2013;8:e77388. 10.1371/journal.pone.0077388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricketts HT. Some aspects of Rocky Mountain spotted fever as shown by recent investigations. Medical Record. 1909;76:843–55. [DOI] [PubMed] [Google Scholar]
- 14.Moore JJ. Time relationships of the wood tick in the transmission of Rocky Mountain spotted fever. J Infect Dis. 1911;8:339–47 and. 10.1093/infdis/8.3.339 [DOI] [Google Scholar]
- 15.Magalhães O. Contribuição ao conhecimento das doenças do grupo tifo exantematico. Rio de Janeiro: Instituto Oswaldo Cruz; 1952. [Google Scholar]
- 16.Burgdorfer W. Ecological and epidemiological considerations of Rocky Mountain spotted fever and scrub typhus. In: Walker DH, editor. Biology of rickettsial diseases. Vol. 1. Boca Raton (FL): CRC Inc.; 1988. p. 33–50. [Google Scholar]
- 17.Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27 . [PubMed] [Google Scholar]
- 18.Pinter A, Dias RA, Gennari SM, Labruna MB. Study of the seasonal dynamics, life cycle, and host specificity of Amblyomma aureolatum (Acari: Ixodidae). J Med Entomol. 2004;41:324–32. 10.1603/0022-2585-41.3.324 [DOI] [PubMed] [Google Scholar]
- 19.Labruna MB, Kasai N, Ferreira F, Faccini JL, Gennari SM. Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Vet Parasitol. 2002;105:65–77. 10.1016/S0304-4017(01)00649-5 [DOI] [PubMed] [Google Scholar]
- 20.Labruna MB, Ogrzewalska M, Martins TF, Pinter A, Horta MC. Comparative susceptibility of larval stages of Amblyomma aureolatum, Amblyomma cajennense, and Rhipicephalus sanguineus to infection by Rickettsia rickettsii. J Med Entomol. 2008;45:1156–9. 10.1603/0022-2585(2008)45[1156:CSOLSO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 21.Pinter A, Labruna MB. Isolation of Rickettsia rickettsii and Rickettsia bellii in cell culture from the tick Amblyomma aureolatum in Brazil. Ann N Y Acad Sci. 2006;1078:523–9. 10.1196/annals.1374.103 [DOI] [PubMed] [Google Scholar]
- 22.Horta MC, Moraes-Filho J, Casagrande RA, Saito TB, Rosa SC, Ogrzewalska M, et al. Experimental infection of opossums Didelphis aurita by Rickettsia rickettsii and evaluation of the transmission of the infection to ticks Amblyomma cajennense. Vector Borne Zoonotic Dis. 2009;9:109–18. 10.1089/vbz.2008.0114 [DOI] [PubMed] [Google Scholar]
- 23.Guglielmone AA, Estrada-Peña A, Mangold AJ, Barros-Battesti DM, Labruna MB, Martins JR, et al. Amblyomma aureolatum (Pallas, 1772) and Amblyomma ovale Kock, 1844: hosts, distribution and 16S rDNA sequences. Vet Parasitol. 2003;113:273–88. 10.1016/S0304-4017(03)00083-9 [DOI] [PubMed] [Google Scholar]
- 24.Guglielmone AA, Beati L, Barros-Battesti DM, Labruna MB, Nava S, Venzal JM, et al. Ticks (Ixodidae) on humans in South America. Exp Appl Acarol. 2006;40:83–100. 10.1007/s10493-006-9027-0 [DOI] [PubMed] [Google Scholar]
- 25.Moraes-Filho J, Pinter A, Pacheco RC, Gutmann TB, Barbosa SO, Gonzáles MA, et al. New epidemiological data on Brazilian spotted fever in an endemic area of the state of São Paulo, Brazil. Vector Borne Zoonotic Dis. 2009;9:73–8. 10.1089/vbz.2007.0227 [DOI] [PubMed] [Google Scholar]
- 26.Szabó MP, Labruna MB, Garcia MV, Pinter A, Castagnolli KC, Pacheco RC, et al. Ecological aspects of the free-living ticks (Acari: Ixodidae) on animal trails within Atlantic rainforest in south-eastern Brazil. Ann Trop Med Parasitol. 2009;103:57–72. 10.1179/136485909X384956 [DOI] [PubMed] [Google Scholar]
- 27.Szabó MP, Labruna MB, Castagnolli KC, Garcia MV, Pinter A, Veronez VA, et al. Ticks (Acari: Ixodidae) parasitizing humans in an Atlantic rainforest reserve of Southeastern Brazil with notes on host suitability. Exp Appl Acarol. 2006;39:339–46. 10.1007/s10493-006-9013-6 [DOI] [PubMed] [Google Scholar]
- 28.Fialho A. Tifo exantematico de São Paulo. Revista Médico-Cirúrgica do Brasil. 1932;40:183–205. [Google Scholar]
- 29.Angerami RN, Câmara M, Pacola MR, Rezende RC, Duarte RM, Nascimento EM, et al. Features of Brazilian spotted fever in two different endemic areas in Brazil. Ticks Tick Borne Dis. 2012;3:346–8. [DOI] [PubMed]
- 30.Sangioni LA, Horta MC, Vianna MCB, Gennari SM, Soares RM, Galvão MAM, et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis. 2005;11:265–70. 10.3201/eid1102.040656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brites-Neto J, Nieri-Bastos FA, Brasil J, Duarte KMR, Martins TF, Veríssimo CJ, et al. Environmental infestation and rickettsial infection in ticks in a Brazilian spotted fever-endemic area. Rev Bras Parasitol Vet. 2013;22:367–72. 10.1590/S1984-29612013000300008 [DOI] [PubMed] [Google Scholar]
- 32.Topping NH. The epidemiology of Rocky Mountain spotted fever. N Y State J Med. 1947;47:1585–7 . [PubMed] [Google Scholar]
- 33.Hoogstraal H. Ticks in relation to humans diseases caused by Rickettsia species. Annu Rev Entomol. 1967;12:377–420. 10.1146/annurev.en.12.010167.002113 [DOI] [PubMed] [Google Scholar]
- 34.Paddock CD, Brenner O, Vaid C, Boyd DB, Berg JM, Joseph RJ, et al. Short report: concurrent Rocky Mountain spotted fever in a dog and its owner. Am J Trop Med Hyg. 2002;66:197–9 . [DOI] [PubMed] [Google Scholar]
- 35.Greene CE. Rocky Mountain spotted fever. J Am Vet Med Assoc. 1987;191:666–71 . [PubMed] [Google Scholar]
- 36.Hoogstraal H, Aeschlimann A. Tick-host specificity. Bull Soc Entomol Suisse. 1982;55:5–32. [Google Scholar]