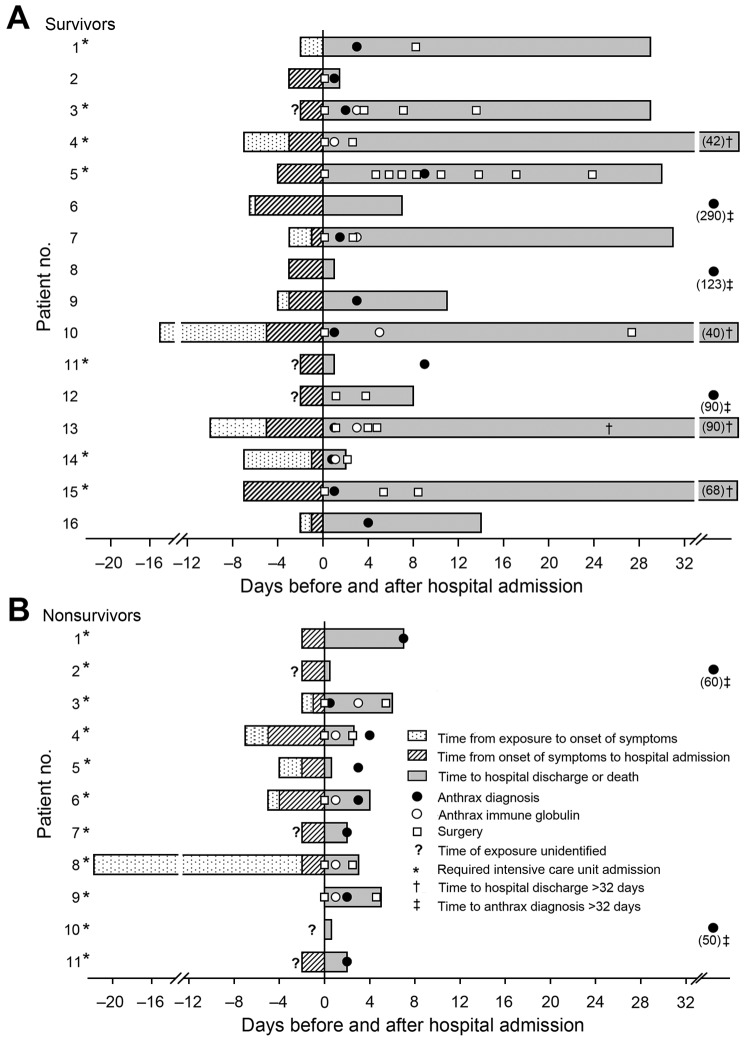
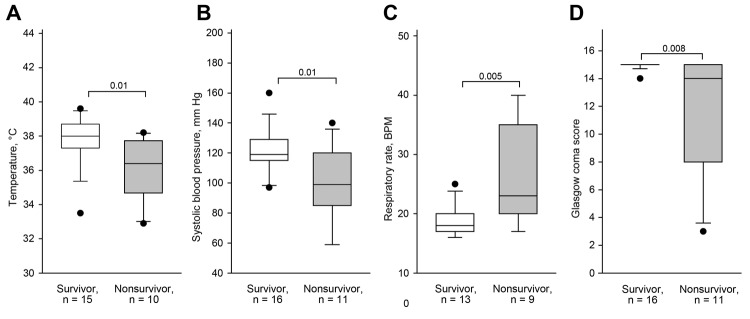
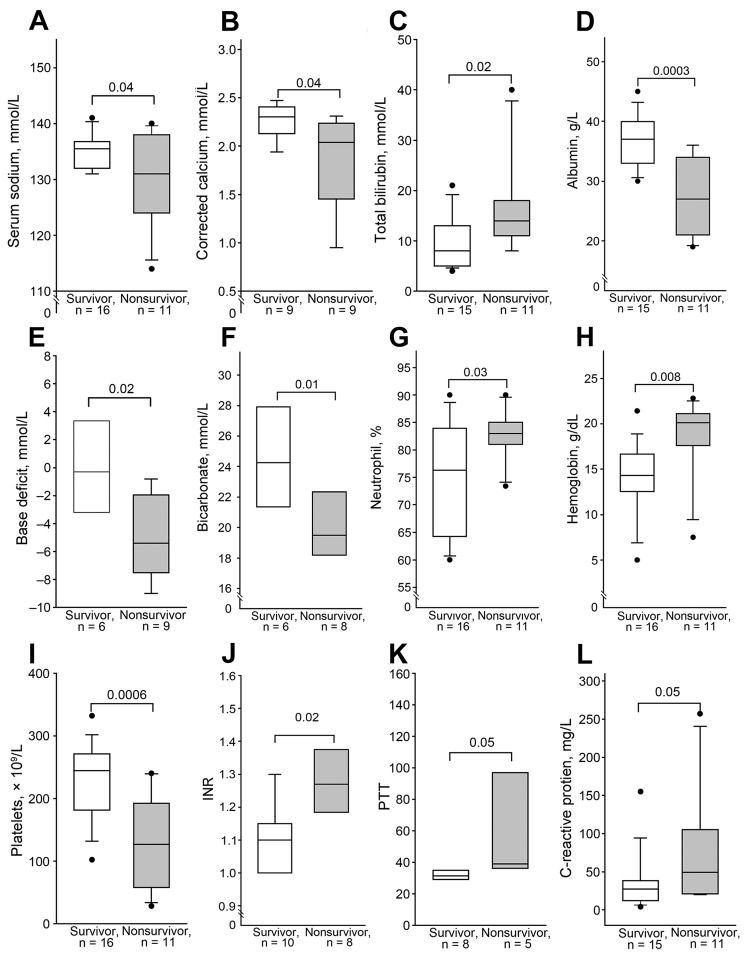
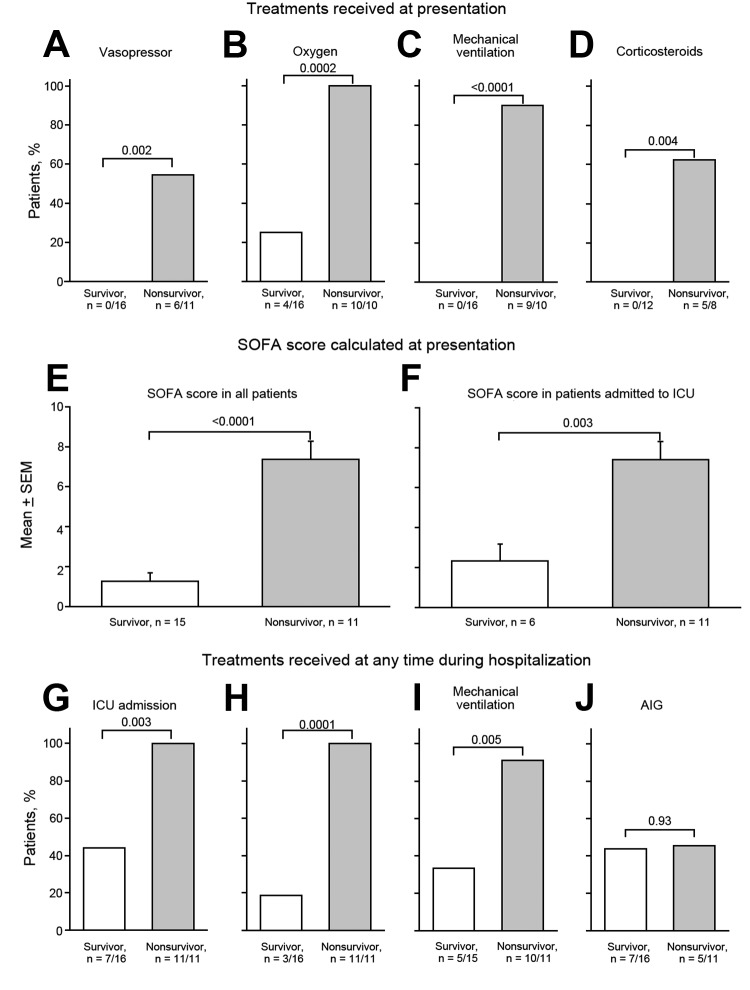
Malcolm Booth
Malcolm Booth
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Lindsay Donaldson
Lindsay Donaldson
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Xizhong Cui
Xizhong Cui
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Junfeng Sun
Junfeng Sun
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Stephen Cole
Stephen Cole
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Susan Dailsey
Susan Dailsey
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Andrew Hart
Andrew Hart
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Neil Johns
Neil Johns
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Paul McConnell
Paul McConnell
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Tina McLennan
Tina McLennan
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Benjamin Parcell
Benjamin Parcell
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Henry Robb
Henry Robb
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Benjamin Shippey
Benjamin Shippey
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Malcolm Sim
Malcolm Sim
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Charles Wallis
Charles Wallis
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,
Peter Q Eichacker
Peter Q Eichacker
1Glasgow Royal Infirmary, Glasgow, Scotland, UK (M. Booth, L. Donaldson, A. Hart);
2National Institutes of Health, Bethesda, Maryland, USA (X. Cui, J. Sun, P.Q. Eichacker);
3Ninewells Hospital, Dundee, Scotland, UK (S. Cole, B. Parcell);
4Victoria Infirmary, Glasgow (S. Dailsey);
5Queen Margaret and Victoria Hospitals, Dumfermline, Scotland, UK (N. Johns, B. Shippey);
6Crosshouse Hospital, Kilmarnock, Scotland, UK (P. McConnell);
7Hairmyres Hospital, East Kilbride, Scotland, UK (T. McLennan);
8Forth Valley Royal Hospital, Larbert, Scotland, UK (H. Robb);
9Western Infirmary, Glasgow (M. Sim);
10Western General Hospital, Edinburgh, Scotland, UK (C. Wallis)
1,2,3,4,5,6,7,8,9,10,✉