Abstract
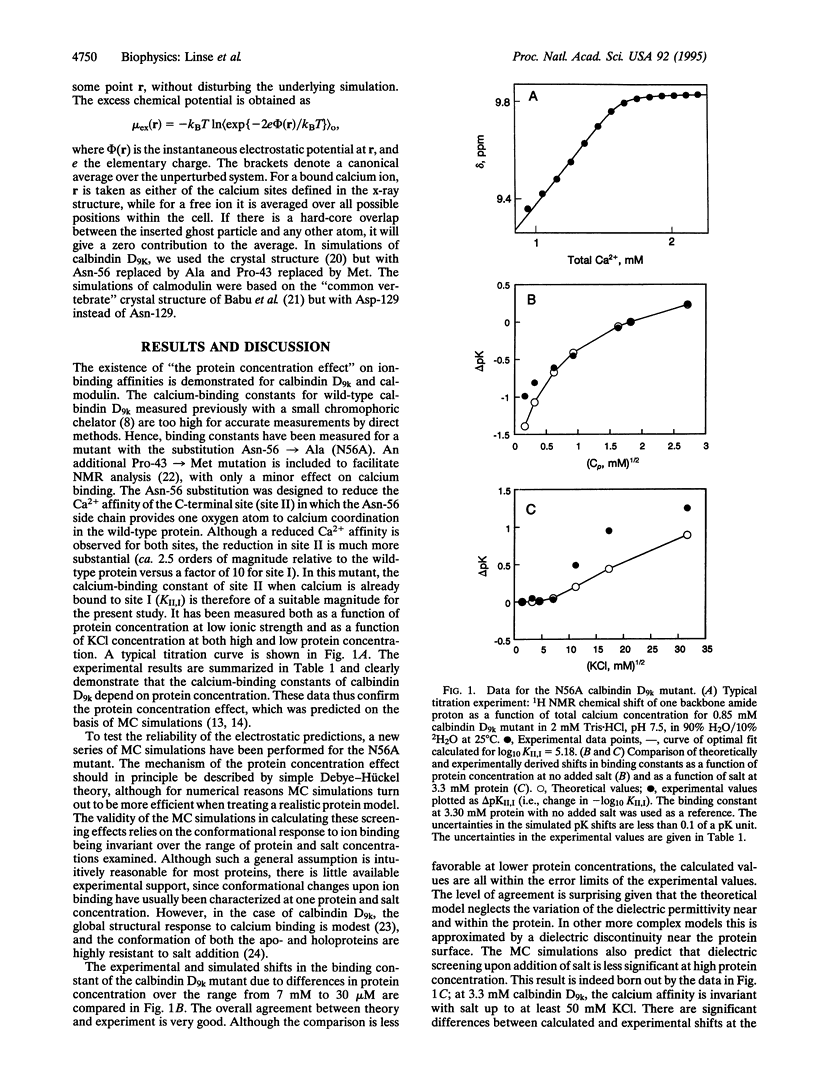
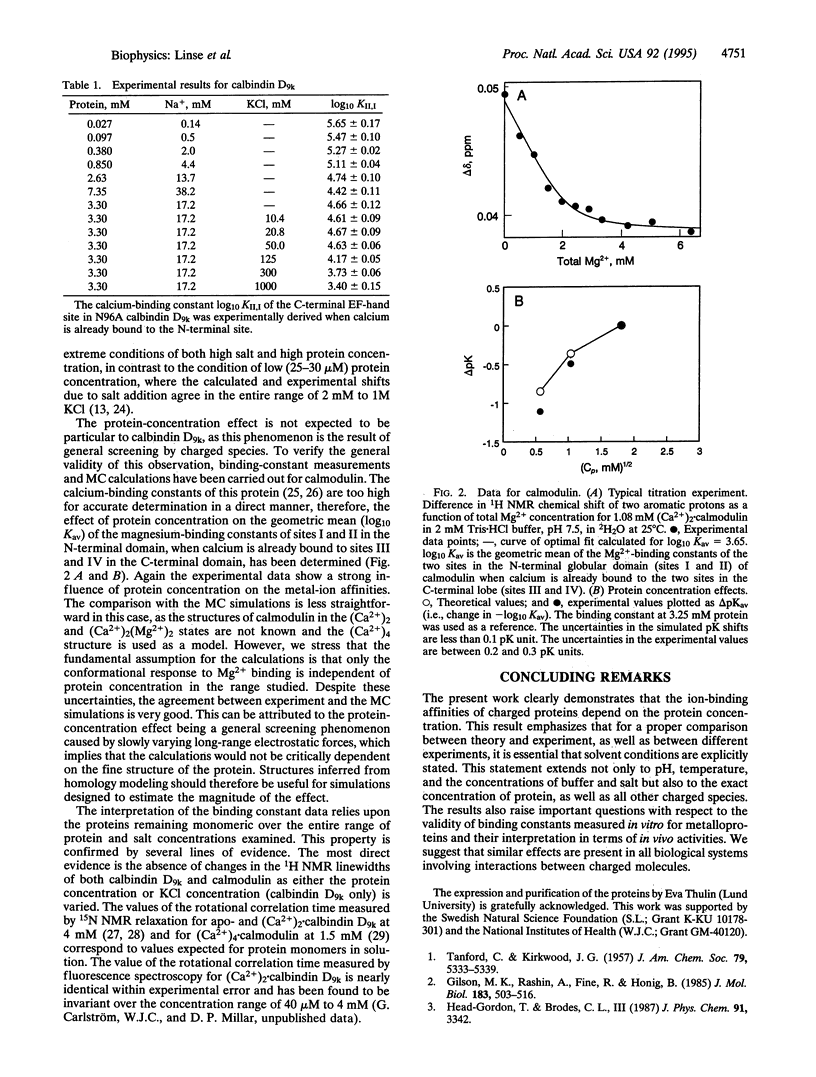
The concentration of protein in a solution has been found to have a significant effect on ion binding affinity. It is well known that an increase in ionic strength of the solvent medium by addition of salt modulates the ion-binding affinity of a charged protein due to electrostatic screening. In recent Monte Carlo simulations, a similar screening has been detected to arise from an increase in the concentration of the protein itself. Experimental results are presented here that verify the theoretical predictions; high concentrations of the negatively charged proteins calbindin D9k and calmodulin are found to reduce their affinity for divalent cations. The Ca(2+)-binding constant of the C-terminal site in the Asn-56 --> Ala mutant of calbindin D9k has been measured at seven different protein concentrations ranging from 27 microM to 7.35 mM by using 1H NMR. A 94% reduction in affinity is observed when going from the lowest to the highest protein concentration. For calmodulin, we have measured the average Mg(2+)-binding constant of sites I and II at 0.325, 1.08, and 3.25 mM protein and find a 13-fold difference between the two extremes. Monte Carlo calculations have been performed for the two cases described above to provide a direct comparison of the experimental and simulated effects of protein concentration on metal ion affinities. The overall agreement between theory and experiment is good. The results have important implications for all biological systems involving interactions between charged species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akke M., Skelton N. J., Kördel J., Palmer A. G., 3rd, Chazin W. J. Effects of ion binding on the backbone dynamics of calbindin D9k determined by 15N NMR relaxation. Biochemistry. 1993 Sep 21;32(37):9832–9844. doi: 10.1021/bi00088a039. [DOI] [PubMed] [Google Scholar]
- Babu Y. S., Bugg C. E., Cook W. J. Structure of calmodulin refined at 2.2 A resolution. J Mol Biol. 1988 Nov 5;204(1):191–204. doi: 10.1016/0022-2836(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Barbato G., Ikura M., Kay L. E., Pastor R. W., Bax A. Backbone dynamics of calmodulin studied by 15N relaxation using inverse detected two-dimensional NMR spectroscopy: the central helix is flexible. Biochemistry. 1992 Jun 16;31(23):5269–5278. doi: 10.1021/bi00138a005. [DOI] [PubMed] [Google Scholar]
- Chazin W. J., Kördel J., Drakenberg T., Thulin E., Brodin P., Grundström T., Forsén S. Proline isomerism leads to multiple folded conformations of calbindin D9k: direct evidence from two-dimensional 1H NMR spectroscopy. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2195–2198. doi: 10.1073/pnas.86.7.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getzoff E. D., Cabelli D. E., Fisher C. L., Parge H. E., Viezzoli M. S., Banci L., Hallewell R. A. Faster superoxide dismutase mutants designed by enhancing electrostatic guidance. Nature. 1992 Jul 23;358(6384):347–351. doi: 10.1038/358347a0. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Rashin A., Fine R., Honig B. On the calculation of electrostatic interactions in proteins. J Mol Biol. 1985 Aug 5;184(3):503–516. doi: 10.1016/0022-2836(85)90297-9. [DOI] [PubMed] [Google Scholar]
- Johansson C., Brodin P., Grundström T., Thulin E., Forsén S., Drakenberg T. Biophysical studies of engineered mutant proteins based on calbindin D9k modified in the pseudo EF-hand. Eur J Biochem. 1990 Jan 26;187(2):455–460. doi: 10.1111/j.1432-1033.1990.tb15325.x. [DOI] [PubMed] [Google Scholar]
- Kesvatera T., Jönsson B., Thulin E., Linse S. Binding of Ca2+ to calbindin D9k: structural stability and function at high salt concentration. Biochemistry. 1994 Nov 29;33(47):14170–14176. doi: 10.1021/bi00251a028. [DOI] [PubMed] [Google Scholar]
- Kördel J., Skelton N. J., Akke M., Palmer A. G., 3rd, Chazin W. J. Backbone dynamics of calcium-loaded calbindin D9k studied by two-dimensional proton-detected 15N NMR spectroscopy. Biochemistry. 1992 May 26;31(20):4856–4866. doi: 10.1021/bi00135a017. [DOI] [PubMed] [Google Scholar]
- Linse S., Brodin P., Johansson C., Thulin E., Grundström T., Forsén S. The role of protein surface charges in ion binding. Nature. 1988 Oct 13;335(6191):651–652. doi: 10.1038/335651a0. [DOI] [PubMed] [Google Scholar]
- Linse S., Helmersson A., Forsén S. Calcium binding to calmodulin and its globular domains. J Biol Chem. 1991 May 5;266(13):8050–8054. [PubMed] [Google Scholar]
- Linse S., Johansson C., Brodin P., Grundström T., Drakenberg T., Forsén S. Electrostatic contributions to the binding of Ca2+ in calbindin D9k. Biochemistry. 1991 Jan 8;30(1):154–162. doi: 10.1021/bi00215a023. [DOI] [PubMed] [Google Scholar]
- Long J. E., Durham B., Okamura M., Millett F. Role of specific lysine residues in binding cytochrome c2 to the Rhodobacter sphaeroides reaction center in optimal orientation for rapid electron transfer. Biochemistry. 1989 Aug 22;28(17):6970–6974. doi: 10.1021/bi00443a029. [DOI] [PubMed] [Google Scholar]
- Martin S. R., Linse S., Johansson C., Bayley P. M., Forsén S. Protein surface charges and Ca2+ binding to individual sites in calbindin D9k: stopped-flow studies. Biochemistry. 1990 May 1;29(17):4188–4193. doi: 10.1021/bi00469a023. [DOI] [PubMed] [Google Scholar]
- Sines J. J., Allison S. A., McCammon J. A. Point charge distributions and electrostatic steering in enzyme/substrate encounter: Brownian dynamics of modified copper/zinc superoxide dismutases. Biochemistry. 1990 Oct 9;29(40):9403–9412. doi: 10.1021/bi00492a014. [DOI] [PubMed] [Google Scholar]
- Skelton N. J., Kördel J., Akke M., Forsén S., Chazin W. J. Signal transduction versus buffering activity in Ca(2+)-binding proteins. Nat Struct Biol. 1994 Apr;1(4):239–245. doi: 10.1038/nsb0494-239. [DOI] [PubMed] [Google Scholar]
- Svensson B., Jönsson B., Thulin E., Woodward C. E. Binding of Ca2+ to calmodulin and its tryptic fragments: theory and experiment. Biochemistry. 1993 Mar 23;32(11):2828–2834. doi: 10.1021/bi00062a014. [DOI] [PubMed] [Google Scholar]
- Svensson B., Jönsson B., Woodward C. E., Linse S. Ion-binding properties of calbindin D9k: a Monte Carlo simulation study. Biochemistry. 1991 May 28;30(21):5209–5217. doi: 10.1021/bi00235a014. [DOI] [PubMed] [Google Scholar]
- Svensson B., Jönsson B., Woodward C. Electrostatic contributions to the binding of Ca2+ in calbindin mutants. A Monte Carlo study. Biophys Chem. 1990 Oct;38(1-2):179–183. doi: 10.1016/0301-4622(90)80053-a. [DOI] [PubMed] [Google Scholar]
- Szebenyi D. M., Moffat K. The refined structure of vitamin D-dependent calcium-binding protein from bovine intestine. Molecular details, ion binding, and implications for the structure of other calcium-binding proteins. J Biol Chem. 1986 Jul 5;261(19):8761–8777. [PubMed] [Google Scholar]
- Waltersson Y., Linse S., Brodin P., Grundström T. Mutational effects on the cooperativity of Ca2+ binding in calmodulin. Biochemistry. 1993 Aug 10;32(31):7866–7871. doi: 10.1021/bi00082a005. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Lukas T. J., Craig T. A., Wilson E., King M. M., Kwiatkowski A. P., Watterson D. M. Computational and site-specific mutagenesis analyses of the asymmetric charge distribution on calmodulin. Proteins. 1989;6(1):70–85. doi: 10.1002/prot.340060107. [DOI] [PubMed] [Google Scholar]



