Targeted surveillance for influenza A(H7N9) identified 24 cases of infection with this virus in Guangzhou, China, during April 1, 2013–March 7, 2014. The spectrum of illness ranged from severe pneumonia to asymptomatic infection. Epidemiologic findings for 2 family clusters of infection highlight the importance of rigorous close contact monitoring.
During February–May 2013, the initial outbreak of human infection with avian influenza A(H7N9) virus in China resulted in 133 cases (1). Influenza A(H7N9) virus reemerged in southern China in October 2013 and had caused 85 laboratory-confirmed cases of infection in Guangdong Province as of March 7, 2014. In response to the outbreak, targeted surveillance programs were established in April 2013 in Guangzhou, the provincial capital of Guangdong Province. Here, we report results of this surveillance program through early 2014.
The Study
Since 2004, all clinical facilities in Guangzhou (population 13 million in 2013) have been required by the China National Health and Family Planning Commission to report any patient who meets the criteria of having pneumonia of unknown etiology (PUE): fever (>38°C), radiologic characteristics consistent with pneumonia, low-normal leukocyte count or low lymphocyte count in early-stage disease, and no alternative etiology (2). Upper or lower respiratory samples from these patients are collected for identification of possible causative pathogens. In response to the influenza A(H7N9) outbreak, PUE surveillance was enhanced in April 2013 by implementing laboratory testing specific for influenza A(H7N9) virus (3). Specimens are initially screened for influenza A and B viruses by real-time reverse transcription PCR (rRT-PCR); samples positive for influenza A are then subtyped as H1N1, H3N2, H5N1, or H7N9.
Surveillance for influenza-like illness (ILI) was initially conducted in 4 sentinel hospitals in Guangzhou and expanded to 19 hospitals in November 2013. Each hospital collects 10–20 convenience throat swab specimens weekly from ILI patients visiting the hospitals within 3 days of illness onset. The same laboratory screening protocols were adopted as for PUE surveillance.
Surveillance for influenza A(H7N9) virus was established in 24 live poultry markets (LPMs) in April 2013 and expanded to 42 LPMs in November 2013, covering all 12 districts in Guangzhou. From each LPM, 10–30 environmental samples are collected biweekly and tested by rRT-PCR. When human influenza A(H7N9) infection is confirmed, additional environmental sampling from epidemiologically linked LPMs is immediately launched to trace the possible source of infection. All poultry workers linked to influenza A(H7N9) virus–contaminated LPMs (i.e., LPMs with >1 virus-positive environmental samples identified) are placed under medical observation for 7 days. Throat swab specimens are collected within 24 hours for detection of influenza A(H7N9) infection and second swab specimens are collected if symptoms appear.
Close contacts of influenza A(H7N9) case-patients are defined as any family member who shares residence, social contacts who visit, and health care workers who provide medical services without effective personal protection (4) during the period from 1 day before illness onset to isolation (5). All close contacts are monitored for 7 days for any symptoms. Pared serum samples, tested by hemagglutinin inhibition assay (6), and throat swab specimens are collected to detect possible secondary cases.
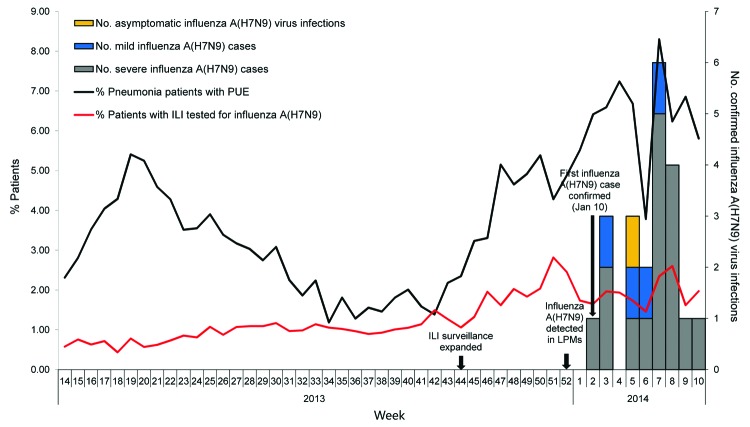
From April 1, 2013, through March 7, 2014, a total of 47,937 patients with pneumonia were reported in Guangzhou (Table 1). Of these, 1,923 (4.0%) met PUE criteria, and respiratory specimens were collected and tested. An influenza A(H7N9) case in Guangzhou was confirmed on January 10, 2014 (Figure 1); since then, an additional 15 patients with PUE were confirmed as influenza A(H7N9) case-patients. All were adults; 11 (69%) were >60 years of age. Recent poultry exposure history was available for 14 (88%) patients (Table 2).
Table 1. Investigation of weekly reported number of patients with pneumonia, PUE, and ILI and confirmed cases of influenza A(H7N9) virus infection, Guangzhou, China, April 1, 2013–March 7, 2014*.
| Year, wk | PUE surveillance |
ILI surveillance |
|||||
|---|---|---|---|---|---|---|---|
| No. patients with pneumonia | No. (%) patients with PUE | No. confirmed influenza A(H7N9) infections | No. patients with ILI | No. (%) samples tested | No. confirmed influenza A(H7N9) infections | ||
| 2013 | |||||||
| 14 | 951 | 22 (2.3) | 0 | 8,089 | 47 (0.6) | 0 | |
| 15 | 996 | 28 (2.8) | 0 | 8,555 | 65 (0.8) | 0 | |
| 16 | 1,021 | 36 (3.5) | 0 | 8,698 | 55 (0.6) | 0 | |
| 17 | 1,087 | 44 (4.1) | 0 | 8,759 | 63 (0.7) | 0 | |
| 18 | 1,118 | 48 (4.3) | 0 | 9,852 | 43 (0.4) | 0 | |
| 19 | 1,146 | 62 (5.4) | 0 | 8,682 | 68 (0.8) | 0 | |
| 20 | 1,238 | 65 (5.4) | 0 | 9,621 | 55 (0.6) | 0 | |
| 21 | 1,197 | 55 (4.6) | 0 | 10,248 | 64 (0.6) | 0 | |
| 22 | 1,121 | 48 (4.3) | 0 | 11,264 | 83 (0.7) | 0 | |
| 23 | 1,166 | 41 (3.5) | 0 | 9,546 | 82 (0.9) | 0 | |
| 24 | 1,041 | 37 (3.6) | 0 | 9,962 | 81 (0.8) | 0 | |
| 25 | 1,075 | 42 (3.9) | 0 | 8,910 | 96 (1.1) | 0 | |
| 26 | 1,032 | 35 (3.4) | 0 | 7,735 | 68 (0.9) | 0 | |
| 27 | 976 | 31 (3.2) | 0 | 7,431 | 80 (1.1) | 0 | |
| 28 | 922 | 28 (3.0) | 0 | 7,567 | 83 (1.1) | 0 | |
| 29 | 945 | 26 (2.8) | 0 | 7,306 | 80 (1.1) | 0 | |
| 30 | 908 | 28 (3.1) | 0 | 6,998 | 82 (1.2) | 0 | |
| 31 | 887 | 20 (2.3) | 0 | 7,824 | 76 (1.0) | 0 | |
| 32 | 911 | 17 (1.9) | 0 | 7,484 | 74 (1.0) | 0 | |
| 33 | 848 | 19 (2.2) | 0 | 7,176 | 82 (1.1) | 0 | |
| 34 | 925 | 11 (1.2) | 0 | 8,018 | 85 (1.1) | 0 | |
| 35 | 883 | 16 (1.8) | 0 | 8,186 | 84 (1.0) | 0 | |
| 36 | 856 | 11 (1.3) | 0 | 8,768 | 85 (1.0) | 0 | |
| 37 | 833 | 13 (1.6) | 0 | 9,549 | 86 (0.9) | 0 | |
| 38 | 821 | 12 (1.5) | 0 | 8,788 | 82 (0.9) | 0 | |
| 39 | 773 | 14 (1.8) | 0 | 7,217 | 73 (1.0) | 0 | |
| 40 | 844 | 17 (2.0) | 0 | 6,448 | 68 (1.1) | 0 | |
| 41 | 755 | 12 (1.6) | 0 | 5,513 | 63 (1.1) | 0 | |
| 42 | 721 | 10 (1.4) | 0 | 5,284 | 79 (1.5) | 0 | |
| 43 | 733 | 16 (2.2) | 0 | 5,746 | 73 (1.3) | 0 | |
| 44 | 766 | 18 (2.4) | 0 | 6,599 | 70 (1.1) | 0 | |
| 45 | 803 | 26 (3.2) | 0 | 5,655 | 75 (1.3) | 0 | |
| 46 | 846 | 28 (3.3) | 0 | 4,234 | 83 (2.0) | 0 | |
| 47 | 815 | 42 (5.2) | 0 | 5,054 | 82 (1.6) | 0 | |
| 48 | 859 | 40 (4.7) | 0 | 4,683 | 95 (2.0) | 0 | |
| 49 | 935 | 46 (4.9) | 0 | 5,493 | 101 (1.8) | 0 | |
| 50 | 947 | 51 (5.4) | 0 | 5,488 | 112 (2.0) | 0 | |
| 51 | 1,004 | 43 (4.3) | 0 | 4,151 | 117 (2.8) | 0 | |
| 52 |
1,066 |
52 (4.9) |
0 |
|
4,840 |
119 (2.5) |
0 |
| 2014 | |||||||
| 01 | 1,124 | 62 (5.5) | 0 | 6,497 | 113 (1.7) | 0 | |
| 02 | 1,091 | 70 (6.4) | 1 | 7,313 | 121 (1.7) | 0 | |
| 03 | 1,198 | 79 (6.6) | 3 | 6,401 | 126 (2.0) | 0 | |
| 04 | 1,257 | 91 (7.2) | 0 | 6,089 | 118 (1.9) | 0 | |
| 05 | 1,286 | 86 (6.7) | 1 | 6,048 | 105 (1.7) | 1 | |
| 06 | 845 | 32 (3.8) | 1 | 4,656 | 68 (1.5) | 1 | |
| 07 | 1,036 | 86 (8.3) | 5 | 4,172 | 98 (2.4) | 1 | |
| 08 | 1,122 | 70 (6.2) | 4 | 4,340 | 113 (2.6) | 0 | |
| 09 | 1,123 | 77 (6.9) | 1 | 6,850 | 111 (1.6) | 0 | |
| 10 | 1,084 | 63 (5.8) | 1 | 5,925 | 117 (2.0) | 0 | |
| Total | 47,934 | 1923 (4.0) | 16 | 349,712 | 4,149 (1.2) | 3 | |
*Real-time reverse transcription PCR testing for influenza A(H7N9) virus was implemented in April 1, 2013 (2013 week 14). Samples were collected from all patients with PUE and were laboratory tested. PUE, pneumonia of unknown etiology; ILI, influenza-like illness.
Figure 1.
Weekly number of confirmed influenza A(H7N9) cases detected by real-time reverse transcription PCR, percentage of pneumonia patients with pneumonia of unknown etiology (PUE), and percentage of patients with influenza-like illness (ILI) tested for influenza A(H7N9), Guangzhou, China, April 1, 2013–March 7, 2014. For PUE and ILI surveillance in Guangzhou, laboratory testing for influenza A(H7N9) virus using real-time reverse transcription PCR was implemented in week 14 of 2013 (April 1, 2013). ILI surveillance was expanded to 19 sentinel hospitals in week 44 (November 2013), according to the requirements of the public health authority of Guangdong Province after 4 confirmed influenza A(H7N9) cases were reported in Guangdong.
Table 2. Demographic, epidemiologic, and clinical characteristics of patients with severe and mild influenza A(H7N9) cases, Guangzhou, China, April 1, 2013–March 7, 2014*.
| Characteristic | Severe cases, n = 16 | Mild cases, n = 4† |
|---|---|---|
| Sex ratio, M:F | 11:5 | 1:3 |
| Age, y, median (range) |
66 (29–83) |
5 (4–17) |
| Age group, y | ||
| 0–14 | 0 | 3 (75) |
| 15–59 | 5 (31) | 1 (25) |
|
>60 |
11 (69) |
0 |
| Type of residence | ||
| Urban | 12 (75) | 4 (100) |
| Rural |
4 (25) |
0 |
| Occupation | ||
| Retired | 11 (69) | 0 |
| Housewife | 2 (13) | 0 |
| Farmer | 2 (13) | 0 |
| Tofu vendor in retail wet market | 1 (6) | 0 |
| Kindergarten student | 0 | 3 (75) |
| Primary or high school student |
0 |
1 (25) |
| History of poultry exposure‡ | ||
| Direct contact | 6§ (38) | 0 |
| Indirect contact | 9¶ (56) | 1# (25) |
| Unknown |
3 (19) |
3 (75) |
| Underlying medical conditions** |
12 (75) |
0 |
| Preliminary diagnosis at the first hospital visit | ||
| Influenza | 0 | 3 (75) |
| Upper respiratory tract infection | 6 (38) | 1 (25) |
| Pneumonia |
10†† (63) |
0 |
| Required hospitalization | 16 (100) | 1 (25) |
| Illness progressed to pneumonia | 16 (100) | 0 |
| Received oseltamivir treatment | 15 (94) | 4 (100) |
| Admitted to intensive care unit | 14 (88) | 0 |
| No. hospitals visited, median (IQR) |
3 (1–4) |
1 (1–2) |
| Time from illness onset to first medical care, median (IQR) | 5 d (1–12 d) | 6h (3–24h) |
| For patients in >60 y age group |
7 d (2–12 d) |
|
| Time from illness onset to antiviral therapy, median (IQR) | 7 d (3–12 d) | 1 d (6 h–4 d) |
| Time from illness onset to laboratory confirmation, median (IQR) | 8 d (7–13 d) | 3 d (2–5 d) |
| Length of illness, median (IQR) |
24 d (11–32 d) |
7 d (6–8 d) |
| Outcome | ||
| Recovered and discharged | 4 (25) | 4 (100) |
| Still in hospital‡‡ | 1 (6) | 0 |
| Died | 11 (69) | 0 |
*Values are no. (%) patients except as indicated. LPM, live poultry market; IQR, interquartile range. †Includes the confirmed mild case detected from follow-up of close contacts. ‡Direct contact: bought poultry, slaughtered poultry, handled poultry meat, raised backyard poultry. Indirect contact: visited LPMs. §Four case-patients raised backyard chickens, 3 slaughtered live chickens, and 1 handled chicken meat. ¶Five case-patients visited LPMs daily; 4 visited LPMs several times. #Case-patient’s father managed a live poultry stall in a contaminated LPM; case-patient visited the stall several times. **Diabetes, hypertension, uremia, chronic obstructive pulmonary disease, and coronary heart disease. ††Nine case-patients were >60 y of age. ‡‡As of April 7, 2013.
During the same period (April 1, 2013–March 7, 2014), a total of 4,149 throat swab specimens were collected from 349,712 ILI patients (Table 1); 3 (0.1%) specimens were positive for influenza A(H7N9) virus. All 3 patients were young urban residents who had mild upper respiratory symptoms (Table 2). As a safety measure, these patients were isolated and treated with oseltamivir. All 3 patients recovered quickly (within 5–7 days) and were discharged after test results for throat swab samples were negative for 2 successive days.
During April–October 2013, 3 of 3,355 environmental samples collected from 24 LPMs were positive for influenza A(H7N9) virus, all on May 16. In contrast, of the 5,220 samples collected from 48 LPMs during November 2013 through March 7, 2014, a total of 141 (2.70%) samples were positive (Technical Appendix). A total of 375 poultry workers from 24 influenza A(H7N9) virus–contaminated LPMs were recruited and monitored, and 381 throat swab specimens were collected; repeat specimens were collected from 6 workers who showed symptoms. Asymptomatic influenza A(H7N9) virus infection was detected in 1 worker who managed a live poultry stall and had daily direct contact with live poultry. Two environmental samples collected from his stall on January 27, 2014, and a throat swab sample collected from the worker on January 28 were positive for influenza A(H7N9) virus. The worker was isolated, but test results for 3 consecutive throat swab specimens collected on January 30 and 31 and February 6 were negative, and in the absence of any symptoms or abnormal chest radiograph findings, he was discharged.
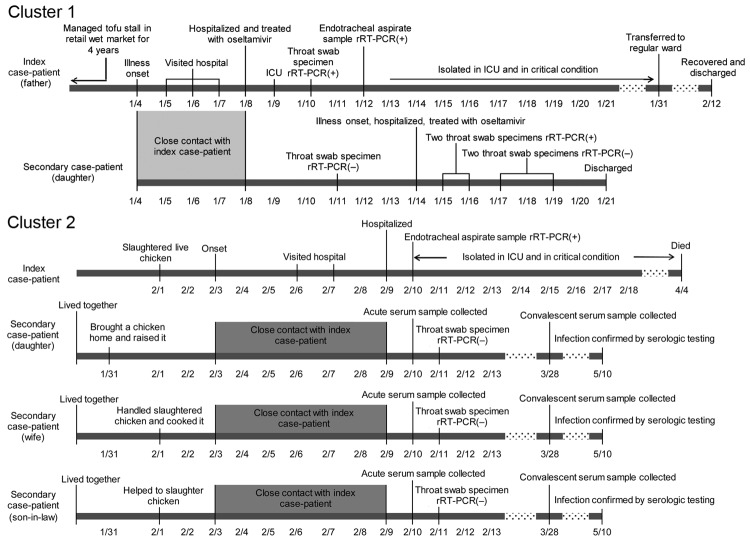
A total of 361 pairs of serum samples and 411 throat swab specimens were collected from 384 close contacts of influenza A(H7N9) case-patients; 2 family clusters were detected. In family cluster 1 (Figure 2), influenza A(H7N9) infection was laboratory confirmed in the index case-patient on January 10 and in 1 close contact (his daughter) on January 14 by positive test results on 2 throat swab specimens. The daughter showed mild respiratory symptoms and recovered quickly. She had no known history of poultry exposure before illness onset but had close, prolonged, and unprotected contact with her sick father. In family cluster 2, the index case-patient slaughtered a live chicken on February 1, became ill on February 3, and had influenza A(H7N9) infection laboratory confirmed on February 10. Three asymptomatic close family contacts of this patient had influenza A(H7N9) infection confirmed by a 4-fold rise in HI titer, although test results on throat swab specimens were negative. All 3 of these contacts had been involved in buying, slaughtering, or handling chickens and had close and unprotected contact with the index case-patient before he was isolated (Figure 2).
Figure 2.
Timeline of illness for 2 family clusters of persons with confirmed influenza A(H7N9) virus infection, Guangzhou, China, 2014. ICU, intensive care unit; rRT-PCR, real-time reverse transcription PCR.
Conclusions
Human infection with influenza A(H7N9) virus has been characterized by severe illness, in particular, rapidly progressive pneumonia and acute respiratory distress syndrome (7). However, the 21 case-patients with laboratory-confirmed influenza A(H7N9) that we identified in Guangzhou showed a wider spectrum of illness, ranging from severe pneumonia to mild ILI to asymptomatic infection. Clinical signs and symptoms differed notably across age groups; all mild cases occurred in those <20 years of age, whereas most severe cases occurred in older patients, similar to findings from previous studies (8,9). The age variances may be attributed to more frequent poultry exposure, more co-existing chronic diseases, or delayed medical admission and antiviral treatment among older patients.
Evidence shows the potential for influenza A(H7N9) virus transmission from person to person (10,11). In particular, epidemiologic findings of the father-daughter cluster indicate that person-to-person transmission may occur among family members after prolonged and intimate contact, consistent with findings in several other family clusters (8,12,13). However, no widespread mild influenza A(H7N9) infection was detected through ILI surveillance, which indicates that the likelihood of community-level transmission is low.
Subclinical influenza A(H7N9) virus infections of poultry workers have been identified by serologic testing (14). However, the possibility of cross-reactivity with other antigenically similar viruses cannot be ruled out. Using rRT-PCR, our surveillance identified a poultry worker with asymptomatic influenza A(H7N9) virus infection, providing further evidence for an occupational risk for asymptomatic infection.
Our study is limited by potential underreporting and by the increased use of PUE and ILI surveillance during the study period compared with previous periods. However, our results show that targeted surveillance during a period of elevated disease activity improved identification of mild or asymptomatic infections.
Geographic distribution of confirmed influenza A(H7N9) cases and live poultry markets sampled in Guangzhou, China.
Acknowledgments
We thank staff members of 12 district Center for Disease Control and Prevention offices in Guangzhou for their assistance in the field investigation and data collection.
This work was supported by grants from the National Science and Technology Major Projects of China (no. 2012ZX100004213-005) and Science and Technology Program of Guangzhou, China (no. 201102A213222).
Biography
Dr Chen is an epidemiologist at the Department of Infectious Disease Control and Prevention, Guangzhou Center for Disease Control and Prevention, Guangdong, China. His research interests include surveillance of infectious diseases especially for influenza, prevention and control strategies for emerging infectious diseases, and preparedness for pandemic influenza.
Footnotes
Suggested citation for this article: Chen Z, Liu H, Lu J, Luo L, Li K, Liu Y, et al. Asymptomatic, mild, and severe influenza A(H7N9) virus infection in humans, Guangzhou, China. Emerg Infect Dis [Internet]. 2014 Sep [date cited]. http://dx.doi.org/10.3201/eid2009.140424
These first authors contributed equally to this article.
These senior authors contributed equally to this article.
References
- 1.World Health Organization. Risk assessment: human infections with avian influenza A(H7N9) virus [cited 2014 Jan 21]. http://www.who.int/influenza/human_animal_interface/RiskAssessment_H7N9_21Jan14.pdf?ua=1
- 2.Xiang N, Havers F, Chen T, Song Y, Tu W, Li L, et al. Use of national pneumonia surveillance to describe influenza A(H7N9) virus epidemiology, China, 2004–2013. Emerg Infect Dis. 2013;19:1784–90 . 10.3201/eid1911.130865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Real-time RT-PCR protocol for the detection of avian influenza A(H7N9) virus. Beijing: WHO Collaborating Center for Reference and Research on Influenza at the Chinese National Influenza Center [cited 2013 Apr 15]. http://www.who.int/influenza/gisrs_laboratory/cnic_realtime_rt_pcr_protocol_a_h7n9.pdf
- 4.China National Health and Family Planning Commission. Technical guidelines for prevention and control of nosocomial infections of avian influenza A(H7N9) (2013. edition) [in Chinese]. Beijing: Chinese Center for Disease Control and Prevention; 2013 [cited 2014 Apr 3]. http://www.moh.gov.cn/ewebeditor/uploadfile/2013/04/20130403184606651.doc
- 5.China National Health and Family Planning Commission. Chinese guideline for prevention and control for human infection with A(H7N9) avian influenza (2014 edition) [in Chinese]. Beijing: Chinese Center for Disease Control and Prevention; 2014. [cited 2014 Jan 29]. http://www.moh.gov.cn/jkj/s3577/201401/8c1828375a7949cd85454a76bb84f23a.shtml
- 6.World Health Organization. Serological detection of avian influenza A(H7N9) virus infections by modified horse red blood cells haemagglutination-inhibition assay. Beijing: WHO Collaborating Center for Reference and Research on Influenza at the Chinese National Influenza Center; 2013. [cited 2013 Dec 20]. http://www.who.int/influenza/gisrs_laboratory/cnic_serological_diagnosis_hai_a_h7n9_20131220.pdf 10.1056/NEJMoa1304617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368:2277–85 . 10.1056/NEJMoa1305584 [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014;370:520–32 . 10.1056/NEJMoa1304617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowling BJ, Freeman G, Wong J, Wu P, Liao Q, Lau E, et al. Preliminary inferences on the age-specific seriousness of human disease caused by avian influenza A(H7N9) infections in China, March to April 2013. Euro Surveill. 2013;18:20475 . [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Wang D, Gao R, Zhao B, Song J, Qi X, et al. Biological features of novel avian influenza A (H7N9) virus. Nature. 2013;499:500–3 . 10.1038/nature12379 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science. 2013;341:410–4 . 10.1126/science.1240532 [DOI] [PubMed] [Google Scholar]
- 12.Qi X, Qian YH, Bao CJ, Guo XL, Cui LB, Tang FY, et al. Probable person to person transmission of novel avian influenza A (H7N9) virus in Eastern China, 2013: epidemiological investigation. BMJ. 2013;347:f4752 . 10.1136/bmj.f4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T, Bi Z, Wang X, Li Z, Ding S, Bi Z, et al. One family cluster of avian influenza A(H7N9) virus infection in Shandong, China. BMC Infect Dis. 2014;14:98 . 10.1186/1471-2334-14-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang S, Chen Y, Cui D, Yao H, Lou J, Huo Z, et al. Avian-origin influenza A(H7N9) infection in influenza A(H7N9)–affected areas of China: a serological study. J Infect Dis. 2014;209:265–9 . 10.1093/infdis/jit430 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Geographic distribution of confirmed influenza A(H7N9) cases and live poultry markets sampled in Guangzhou, China.