Abstract
Substantial evidence indicates the importance of elevated cAMP in polycystic kidney disease (PKD). Accumulation of cAMP in cystic tissues may be, in part, caused by enhanced adenylyl cyclase activity, but inhibition of cAMP degradation by phosphodiesterases (PDE) likely has an important role, because cAMP is inactivated much faster than it is synthesized. PDE1 is the only PDE family activated by Ca2+, which is reduced in PKD cells. To assess the contribution of the PDE1A subfamily to renal cyst formation, we examined the expression and function of PDE1A in zebrafish. We identified two splice isoforms with alternative starts corresponding to human PDE1A1 and PDE1A4. Expression of the two isoforms varied in embryos and adult tissues, and both isoforms hydrolyzed cAMP with Ca2+/calmodulin dependence. Depletion of PDE1A in zebrafish embryos using splice- and translation-blocking morpholinos (MOs) caused pronephric cysts, hydrocephalus, and body curvature. Human PDE1A RNA and the PKA inhibitors, H89 and Rp-cAMPS, partially rescued phenotypes of pde1a morphants. Additionally, MO depletion of PDE1A aggravated phenotypes in pkd2 morphants, causing more severe body curvature, and human PDE1A RNA partially rescued pkd2 morphant phenotypes, pronephric cysts, hydrocephalus, and body curvature. Together, these data indicate the integral role of PDE1A and cAMP signaling in renal development and cystogenesis, imply that PDE1A activity is altered downstream of polycystin-2, and suggest that PDE1A is a viable drug target for PKD.
Autosomal dominant polycystic kidney disease (PKD) is characterized by the development and enlargement of kidney cysts, which progress and interfere with renal function. It affects from 1 in 400 to 1 in 1000 people, and it is the most common genetic cause of renal failure and the fourth leading cause of kidney failure overall, accounting for approximately 5%–10% of patients receiving dialysis or kidney transplants.1–4 Most cases have mutations in the PKD1 gene, encoding Polycystin-1 (PC1), or the PKD2 gene, encoding Polycystin-2 (PC2). PC1 and PC2 comprise a subfamily of transient receptor potential channels. They form a complex with Ca2+ channel activity located at multiple subcellular locations, including primary cilia.
Among the most consistently described changes associated with PKD is increased cAMP levels, which contribute to cystogenesis by multiple mechanisms. These findings have led to the identification of vasopressin V2 and somatostatin receptors as possible therapeutic targets because of their modulation of cAMP synthesis by adenylyl cyclase. Resulting preclinical and clinical trials have indicated the promise of this approach.2,5–9 A recent clinical trial found that the V2 receptor antagonist, Tolvaptan, slowed disease progression over 3 years, which was indicated by a 50% reduction in total kidney volume increase and a 30% slower decline in kidney function indicated by serum creatinine.2 Lowering cAMP levels by additional means may prove even more effective.
A powerful additional approach to lowering cAMP levels is through cAMP hydrolysis by phosphodiesterases (PDEs). As therapeutic targets, PDEs have several advantages.10–12 The capacity for hydrolysis of cyclic nucleotides by PDEs far exceeds the maximum rate of synthesis by adenylyl cyclases. Additionally, the complexity of the PDE superfamily (11 families, 21 genes, and 100 isoforms) provides an opportunity for cell-specific or even subcellular compartment-specific regulation of cAMP levels and control of cystogenesis with relative safety.
Of all the PDE families, only one (PDE1) is regulated by Ca2+.10–12 It is highly expressed in the kidney and likely inhibited in response to decreased Ca2+ associated with PC1/PC2 mutations.13 The PDE1 family is, therefore, a logical candidate affecting renal cystogenesis. In mammals, the PDE1 family has three subfamilies: PDE1A, PDE1B, and PDE1C.
The ease of monitoring cystic phenotypes in zebrafish makes it an ideal model to dissect the function of individual PDE superfamily members. Experimental approaches are straightforward for either down- or upregulation of specific transcripts through antisense morpholinos (MOs) or exogenous RNA expression, respectively. Additionally, several zebrafish resources are available that facilitate these studies, including validated genetic models of PKD and transgenic lines with fluorescence highlighting kidney anatomy and morphology.
We have focused here on PDE1A, showing that depletion was associated with cystogenic phenotypes and that overexpression partially rescued phenotypes from PC2 depletion. These data validate PDE1A as a target for therapeutic intervention for PKD.
Results
Identification of Zebrafish pde1a Isoforms, Expression Patterns, and Catalytic Activity
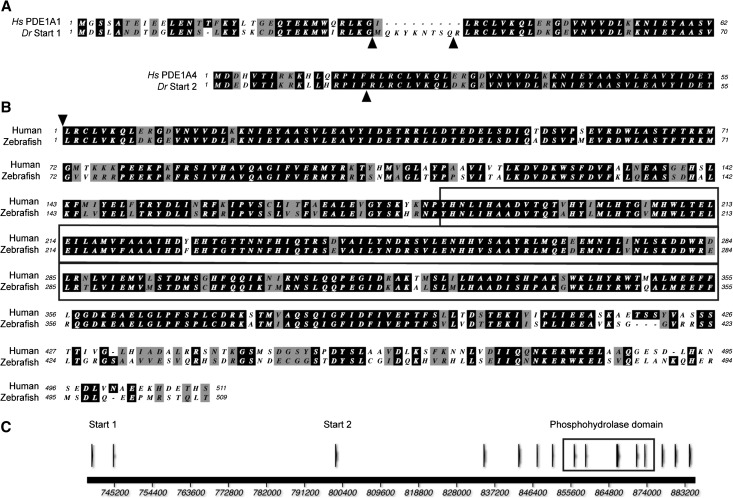
Tblastn searches of the zebrafish genome with human and mouse isoforms of PDE1A identified two isoforms with alternative starts constituting a single gene on chromosome 9, and full-length sequences of the two isoforms were amplified by RT-PCR. The transcript from the upstream start (start 1) was predicted to encode a protein with 78% similarity overall to its closest human ortholog, PDE1A2 (determined by protein–protein BLAST). The protein encoded by the downstream start of pde1a (start 2) had 79% similarity overall to its closest human ortholog, PDE1A4 (determined by protein–protein BLAST). By ClustalW alignment, the start 1 isoform had the greatest similarity to human PDE1A1, and the start 2 isoform had the greatest similarity to PDE1A4 (Figure 1A). The zebrafish and human isoforms have highly conserved catalytic domains, with 98% similarity, but they apparently differ at the C terminus. We identified an additional 73 amino acids in the zebrafish sequence after the region corresponding to the human stop codon.
Figure 1.
Alignment and overview of zebrafish PDE1A shows substantial similarity to human PDE1A. (A) Protein encoded by exons 1a/2a/3a of zebrafish PDE1A start 1 isoform and 1b/2b of zebrafish PDE1A start 2 isoform each aligned with the closest human ortholog. Arrowheads show the zebrafish exon boundaries. (B) Alignment of zebrafish PDE1A with human PDE1A1 starting with the protein encoded by human exon 2. Additional 73 amino acids of C-terminal zebrafish sequence is not shown. (C) Overview of genomic structure showing alternative starts and the conserved domain of the phosphohydrolases. Boxed region indicates the conserved domain of the metal-dependent phosphohydrolases. Hs PDE1A1 is translated from Genbank accession number NM005019.3, and Hs PDE1A4 is translated from Genbank accession number NM_001258313. Hs, Homo sapiens; Dr, Danio rerio.
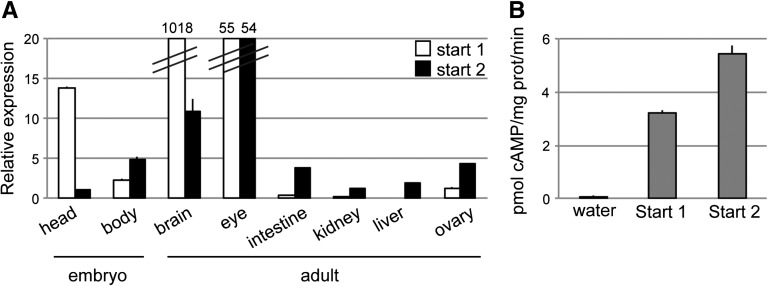
Quantitative PCR (qPCR) showed that relative expression of the two isoforms varied in embryo and adult tissues. In particular, the start 1 pde1a isoform was expressed at a level 14 times that of the start 2 isoform in the head of 2-day postfertilization (2-dpf) embryos (Figure 2A). Among the adult tissues tested, the start 1 isoform was expressed at a higher level than the start 2 isoform only in brain, where its expression was 100 times that of start 2.
Figure 2.
Zebrafish pde1a isoform expression varies among tissues and both isoforms have cAMP catalytic activity. (A) qPCR showing relative expression levels with expression set at one for the start 2 isoform in 2-dpf embryo head. Data are shown as means±SEMs of triplicate samples using tissue pooled from 50 embryos or 3–10 adults. (B) Enzyme assays on lysates from Xenopus oocytes injected with water or zebrafish pde1a RNA. Ca2+/calmodulin-dependent cAMP hydrolysis is shown expressed as mean±SEM (n=3 tubes containing 10 oocytes each).
To examine the cAMP hydrolytic activity of zebrafish PDE1A, RNA was made from either the start 1 or the start 2 isoform and injected into Xenopus oocytes, and oocyte lysates were tested for cAMP hydrolytic activity. Lysates of oocytes injected with either isoform had Ca2+/calmodulin-dependent cAMP catalytic activity, whereas lysates of water-injected oocytes did not (Figure 2B).
PDE1A Depletion Caused Pronephric Cysts and Hydrocephalus
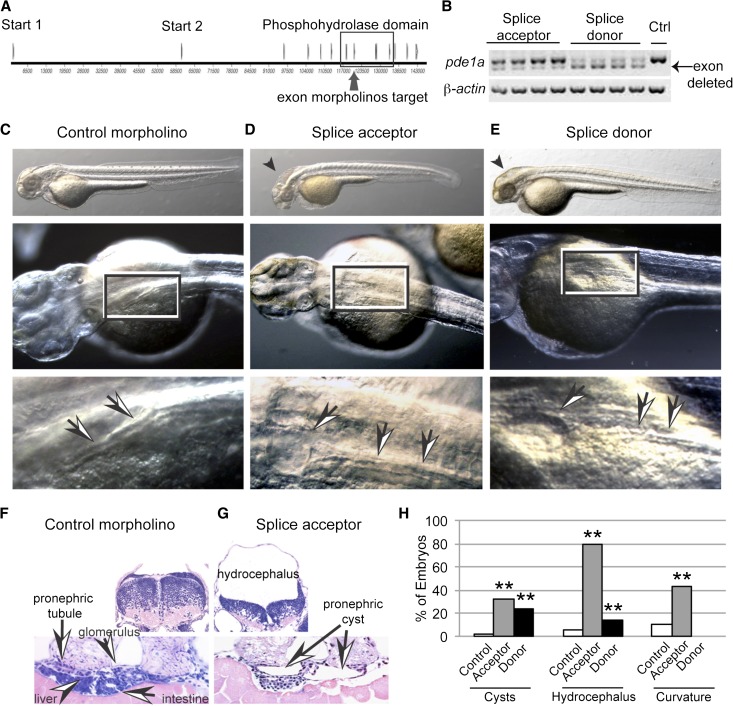
To determine the role of PDE1A during renal development, splice- and translation-blocking MOs were developed. Splice-blocking MOs targeted each end of exon 7, the second exon encoding the conserved phosphohydrolase domain including the critical conserved HD motif required for metal binding and hydrolytic activity (Figure 3A). The efficacy of both of these splice-blocking MOs was shown by RT-PCR and sequencing of individual embryos, showing an in-frame deletion of exon 7 (Figure 3B). Injections of either MO (10 ng) caused significant development of pronephric cysts and hydrocephalus compared with the control MO (10 ng) (Figure 3, C–H), and some embryos showed apparent tubule dilation. Sectioned splice acceptor morphants showed compression of the brain underlying hydrocephalus and compression of the epithelium lining pronephric cysts (Figure 3, F and G). The relatively milder apparent effect of the acceptor MO on splicing contrasts with its more potent effect on phenotypes, suggesting that the altered transcript may have a disproportionately strong effect on translation. Additionally, it is possible that unanticipated splice forms resulted that were not detected.
Figure 3.
Two splice-blocking MOs targeting pde1a caused pronephric cysts in 2-dpf embryos. (A) Map of exons, including the exon containing the conserved HD motif targeted at each end by splice acceptor and splice donor MOs. (B) RT-PCR and sequencing of four individual embryos (one embryo per lane) showed that both MOs caused an in-frame deletion of exon 7. Ctrl, control. (C–E) In embryos, 10 ng pde1a MO caused pronephric cysts and hydrocephalus compared with embryos injected with 10 ng control MO. Arrows and boxes show tubules and cysts, and arrowheads show hydrocephalus. (F and G) JB4 sections with hematoxylin and eosin staining showing hydrocephalus and pronephric cysts. (H) Quantification shows penetrance of phenotypes. **P<0.005 by chi-squared test versus control MO (n≥192 embryos in four or more batches of 20–69 embryos each; data shown are the total percents of embryos).
Translation-blocking MOs targeting either start 1 or start 2 caused pronephric cysts, and some embryos showed apparent tubule dilation (Figure 4). Cystogenesis resulting from the start 1 MO was dose-dependent, with a greater effect on cystogenesis at the maximum dose compared with start 2. Additionally, the start 1 MO caused hydrocephalus and curvature, whereas the start 2 MO did not. Both dose dependence and the more potent effects overall in start 1 morphants are consistent with higher expression of this isoform during embryogenesis (Figure 2). In particular, the lack of hydrocephalus in start 2 morphants is consistent with low expression of this isoform in the head at 2 dpf.
Figure 4.
Two translation-blocking MOs targeting either start 1 or start 2 of pde1a caused pronephric cysts in 2-dpf embryos. (A) Representative phenotypes of 5 and 10 ng control and pde1a translation-blocking morphants. MO targeting start 1 caused pronephric cysts, hydrocephalus, and curvature, whereas MO targeting start 2 caused pronephric cysts without hydrocephalus or curvature. Arrows show cysts and tubules, and the arrowhead shows hydrocephalus. Scale bar, 100 μm. (B) Quantification shows dose responses and greater maximal penetrance of start 1 MO compared with start 2 MO. *P<0.05; **P<0.005 by chi-squared test (n≥173 embryos in three or more batches of 29–62 embryos each; data shown are the total percents of embryos).
To test whether depletion of PDE1A affects cilia, we compared cilia beating in 2-dpf pde1a morphants (5 ng each start 1 and start 2 MOs) containing pronephric cysts with controls. The average beat frequency measured in undilated regions of the tubule in pde1a morphants was the same as in controls (32±1 versus 30±1 Hz in pde1a versus control MO; P=0.24 by two-tailed t test; n≥9 fish in three batches), and in these regions in both cases, cilia were bundled and beat in a coordinated fashion. In contrast, cilia in dilated regions of the tubule of pde1a morphants were not bundled, and instead, beat individually in an uncoordinated fashion (Supplemental Movie 1).
Rescue of PDE1A Depletion Phenotypes with PDE1A RNA or Protein Kinase A Inhibitors
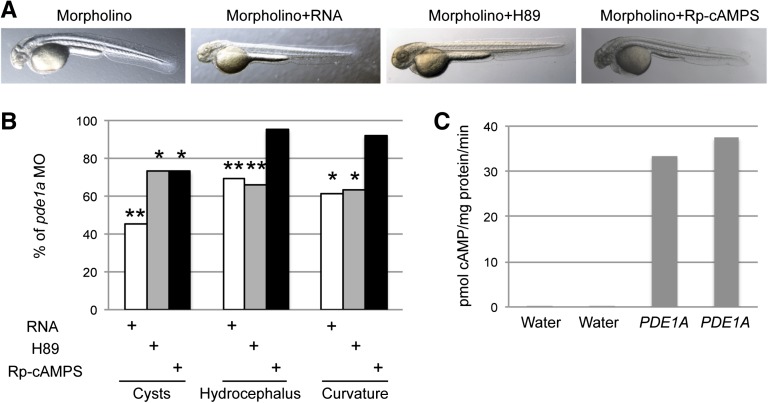
Coinjection of human PDE1A RNA into one-cell embryos with pde1a start 1 and start 2 MOs significantly decreased the percent of embryos with pronephric cysts, hydrocephalus, and curvature (Figure 5, A and B). This rescue was most effective for cystogenesis (55% lower) but also decreased hydrocephalus by approximately 30% and body curvature by approximately 40%. The same PDE1A RNA injected into Xenopus oocytes increased PDE1 activity compared with water-injected oocytes (Figure 5C). The protein kinase A (PKA) inhibitors H89 and Rp-cAMPS were tested by adding them to the water when embryos reached 50% epiboly (approximately 6 hours postfertilization) (Figure 5, A and B). Data shown were obtained using 1–5 μM H89, but similar levels of rescue were seen in preliminary experiments using 1–25 μM H89. Similar to PDE1A RNA, H89 partially rescued the phenotypes, decreasing cystogenesis by approximately 30%, hydrocephalus by 35%, and curvature by approximately 40%. Similar to H89, Rp-cAMPS decreased cystogenesis by approximately 30%, although it did not rescue hydrocephalus or curvature.
Figure 5.
Human PDE1A RNA and PKA inhibitors rescued pde1a morphant phenotypes. (A) Two-dpf embryos injected with 5 ng start 1 MO+5 ng start 2 MO were coinjected with human PDE1A1 RNA (125 pg) or incubated with PKA inhibitors H89 (1–5 μM) or Rp-cAMPS (1 μM). (B) Quantification of phenotypes shows partial rescue of pronephric cysts, hydrocephalus, and body curvature by treatment with PDE1A1 RNA, H89, and Rp-cAMPS. *P<0.05; **P<0.005 by chi-squared test versus MO alone (n≥218 embryos in five or more batches of 22–71 embryos each; data shown are the total incidence of phenotypes in treated pde1a morphants as percentages of phenotypes in untreated pde1a morphants). (C) Enzyme assays on lysates from Xenopus oocytes injected with water or PDE1A1 RNA confirmed that the RNA used for rescue expressed PDE1A1 and induced PDE1 activity.
Functional Interaction of PDE1A and PC2
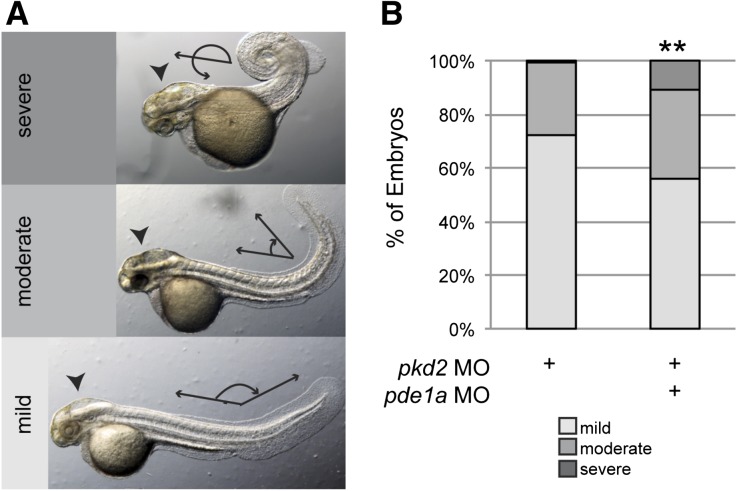
Interaction of PDE1A and PC2 was tested using both loss- and gain-of-function studies. In loss-of-function studies, coinjection of the pde1a start 1 MO (5 ng) with the pkd2 MO (2 ng) aggravated pkd2 morphant phenotypes, causing more severe body curvature than embryos coinjected with the same amounts of pkd2 MO (2 ng) plus control MO (5 ng) (Figure 6). In both cases, 100% of embryos showed curvature, which was categorized as mild (body/tail angle≥90°), moderate (<90°), or severe (crossing the body axis) as previously described.14 Phenotypes from the pkd2 MO were similar to those described previously for this MO and other pkd2 MOs and mutants.15–18 Embryos injected with the pkd2 MO and pde1a MO had a significantly more severe distribution of body curvature phenotypes with less mild curvature and more moderate and severe curvature than embryos injected with the same amount of pkd2 MO and control MO. The interaction of pkd2 MO and pde1a start 1 MO was synergistic, because the pde1a start 1 MO increased the severity of dorsal curvature when coinjected with the pkd2 MO, but the pde1a MO alone did not induce dorsal curvature, even when injected at 10 ng (Figure 4A). Additionally, embryos coinjected with pde1a MO seemed to have larger hydrocephalus (Figure 6B) and pronephric cysts.
Figure 6.
PDE1A depletion aggravated phenotypes from PC2 depletion. (A) Phenotypes of 2-dpf morphants were classified as having body curvature that was mild (body/tail angle≥90°), moderate (<90°), or severe (crossing the body axis). (B) Quantification of incidence of body curvature phenotypes. Embryos coinjected with 2 ng pkd2 and 5 ng pde1a start 1 MO had a significantly different distribution of body curvature phenotypes, with more severe body curvature overall than embryos injected with 2 ng pkd2 and 5 ng control MO, as well as increased hydrocephalus (arrowheads in A) and larger pronephric cysts (not shown). **P<0.005 by chi-squared test (n≥114 embryos in three batches of 29–55 embryos each; data shown are the total percents of embryos).
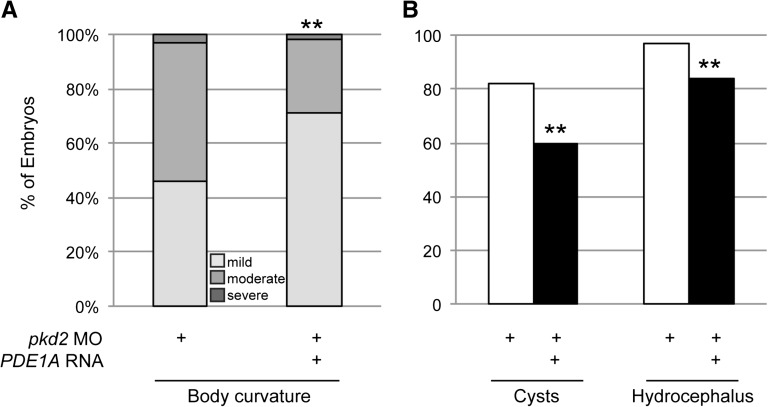
In rescue studies, coinjection of the same PDE1A RNA that rescued pde1a morphants and induced PDE1 activity (Figure 5, B and C) partially rescued phenotypes resulting from the pkd2 MO (Figure 7). Body curvature was classified in 3-dpf morphants as mild, moderate, or severe as described above, and 100% of pkd2 morphants showed curvature. However, coinjection of PDE1A RNA significantly changed the distribution of body curvature phenotypes, such that the incidence of mild curvature increased and the incidence of moderate body curvature decreased compared with pkd2 MO alone. Additionally, the PDE1A RNA reduced the incidence of cysts and hydrocephalus by approximately 24% and 15%, respectively.
Figure 7.
PDE1A RNA rescued phenotypes from PC2 depletion. (A) Extent of body curvature in 3-dpf morphants was classified as in Figure 6. Coinjection of human PDE1A RNA (125 pg) with pkd2 MO (2 ng) significantly increased the percent of embryos with mild body curvature and decreased the percent of embryos with moderate body curvature compared with pkd2 MO alone. (B) Human PDE1A RNA (125 pg) coinjected with pkd2 MO (5 ng) partially rescued cysts and hydrocephalus at 2 dpf. **P<0.005 by chi-squared test (n≥88 embryos in three batches of 27–51 embryos each; data shown are the total percents of embryos).
Discussion
We report here the identification, expression, and requirement of two zebrafish PDE1A isoforms for preventing renal cysts and associated phenotypes. Both PDE1A isoforms have a high level of conservation with mammalian orthologs, particularly in the conserved phosphohydrolase domain. Depletion of either isoform caused renal cystogenesis, which was rescued by human PDE1A RNA. In addition, PDE1A depletion aggravated and PDE1A overexpression rescued pkd2 morphant phenotypes, indicating a functional interaction between PDE1A and PC2. To our knowledge, this work is the first demonstration of an interaction between a PDE and PC2 in zebrafish, indicative of a role for PDE1A downstream of PC2 leading to renal cystogenesis.
The specificity of the pde1a morphant phenotypes is supported by the similar effects of four distinct MOs, particularly regarding induction of pronephric cysts, and the rescue of pde1a morphant phenotypes by PDE1A RNA. In depletion studies, loss of PDE1A is expected to cause elevated cAMP levels and PKA activity. Rescue studies using the PKA inhibitors H89 and Rp-cAMPS tested the contribution of PKA signaling to PDE1A depletion phenotypes. Rescue was only partial but significant, indicating the contribution of PKA signaling to the induced cystic phenotypes. Additional cAMP effectors (i.e., exchange proteins activated by cAMP) may contribute to cystogenesis.19 Importantly, PDE1A hydrolyzes cGMP as well as cAMP, and cGMP is elevated in mouse and rat PKD models.10–13 Therefore, it is also possible that PDE1A depletion causes elevated cGMP, which contributes directly to the phenotypes reported here. As with PDE1A depletion, the rescue of PC2 depletion by PDE1A RNA was partial, suggesting that PC2 acts through cAMP as well as other mechanisms to cause renal cystogenesis, hydrocephalus, and body curvature. This finding is not surprising, because PC2 regulates intracellular Ca2+, which has many cellular effects, including activation of the Ca2+/calmodulin-dependent protein kinase type II that also acts downstream of PC2 in the development of the zebrafish pronephros.20
The two isoforms of pde1a identified here in zebrafish correspond to at least two of five human isoforms. Alignment of five human PDE1A isoforms listed by the National Center for Biotechnology Information (NCBI) shows that there are four distinct N termini, one of which varies by only four additional N-terminal amino acids from two others. Therefore, there are three substantially distinct N termini in humans, two of which have clear zebrafish orthologs. Human PDE1A also has two distinct C termini, neither of which is similar to the zebrafish pde1a C terminus. The poorly conserved C terminus of zebrafish pde1a, after the conserved phosphohydrolase domain, adds a substantial polypeptide after the stop codon in human PDE1A isoforms, but its functional significance is unclear.
The second exon in the start 1 isoform encodes 9 amino acids not present in human PDE1A but present in human PDE1C. Nevertheless, the sequence reported here is most similar to human PDE1A by protein–protein BLAST. Additionally, by ClustalW, it has greater identity to PDE1A than PDE1C (using the closest isoforms PDE1A1 and PDE1C1) overall (66% versus 60%) and in the hydrolase domain (89% versus 79%). Finally, we amplified a distinct partial zebrafish pde1c predicted to encode 314 N-terminal amino acids, including the start ATG (C.R. Sussman, unpublished data). The pde1c transcript was 95% similar to human PDE1C and mapped most closely to loci on chromosome 24 and an unmapped chromosome (i.e., distinct from zebrafish pde1a on chromosome 9). These data are consistent with inclusion of exon 2 in an ancestral pde1, which was subsequently deleted in human pde1a.
The functional significance of the two different zebrafish pde1a starts is not clear. We observed pronephric cystogenesis from depletion of either isoform but hydrocephalus and body curvature only from depletion of the start 1 isoform. Isoform differences in causing hydrocephalus and body curvature are consistent with the relative expression levels of their transcripts in the head versus body of 2-dpf embryos and the brain in the adult. Similar to their human orthologs, both isoforms had Ca2+/calmoduli-dependent cAMP hydrolytic activity.10–12 It remains unknown whether there are additional differences in the biochemistry or regulation of the two isoforms.
By showing that depletion of PDE1A alone induces renal cysts in zebrafish, our data indicate that cAMP is a primary determinant of cystogenesis in an immature kidney with vigorous epithelial cell proliferation, similar to that shown in metanephric organ cultures treated with 8-Br-cAMP.21,22 Studies on metanephric mesenchyme in explant culture have additionally shown that PKA activation disrupts tubulogenesis and induces cystic dilations.23 Together, these studies suggest that a highly proliferative background alters the cellular response to cAMP, predisposing to cystogenesis. Additional studies are needed to test the contributions of proliferation and fluid transport to cystogenesis in these settings.
The effects of PDE1A and PC2 depletion were synergistic, implying that the proteins act in a common pathway. For example, body curvature was more severe in pde1a and pkd2 double morphants compared with pkd2 single morphants, and pde1a MO alone caused no dorsal curvature, even at high doses (10 ng). Moreover, PDE1A RNA partially rescued pronephric cysts, hydrocephalus, and body curvature resulting from PC2 depletion. Although the details of the mechanism responsible for this rescue are unknown, these data imply that increased cAMP is involved in causing these phenotypes of PC2 depletion. Zebrafish body curvature in pkd1a/b or pkd2 morphants involves changes in collagen expression, which is regulated in a complex relationship with cAMP levels.14,24
Similarly, high-speed video microscopy showed that pde1a MO did not affect cilia organization or beat frequency, except in tubule regions that were dilated, suggesting these effects were secondary effects of dilation. These observations are consistent with an effect downstream of cilia and PC2 and similar to other studies showing that PC2 depletion does not affect cilia.15,17
Together with previous studies, these data support a model for renal cystogenesis that is similar to mammalian PKD (i.e., disruption of cilia or PC2 function affects Ca2+ signaling, PDE1A activity, cAMP levels, and PKA activity).15,17,18,25 These data establish zebrafish as a model for studies of PDE and cAMP signaling in PKD and show the central role of cAMP signaling in pronephric development and cystogenesis in an intact vertebrate. Interestingly, another recent study showed that MO depletion of PDE6D, one of several genes mutated in Joubert syndrome, also causes pronephric cysts.26
The large size of the PDE family makes the rapid dissection in zebrafish an attractive way to explore the significance of family members. Although it is not clear whether PDE1A will prove to be among the more potent PDEs regulating cystogenesis, it is worth noting that depletion of PDE1A alone was sufficient to induce cysts. That is, another PDE family member did not compensate for its depletion, suggesting that PDE1A has a unique function in kidney maintenance and/or development and prevention of cystogenesis. These data support the hypothesis that different PDEs have unique effects on cystogenesis and may provide relatively specific targets for PKD therapy.
Concise Methods
Zebrafish
Zebrafish were maintained and raised as described.27 All animal work adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Mayo Clinic Institutional Animal Care and Use Committee. Embryos were developed at 28.5°C with 0.003% 1-phenyl-2-thiourea (Sigma-Aldrich) added at 24 hours postfertilization to prevent pigmentation for facilitating cyst visualization. Embryos were anesthetized using 0.02% tricaine (Aquatic Habitats) before RNA extraction or observation by microscopy. Fish were obtained from Segrest Farms (Gibsonton, FL).
Pde1a Cloning, PCR, and Catalytic Activity
Zebrafish pde1a genomic contigs were identified in a tblastn search with human PDE1A. Putative exons were used to design primers, and zebrafish pde1a was amplified by RT-PCR and sequenced. cDNA template was synthesized using SuperScript III (Invitrogen) from total RNA isolated using TRIzol (Invitrogen). Primers used for PCR are shown in Table 1. PCR products were purified (Qiagen) and sequenced.
Table 1.
PCR primers
| Target | Exons | Primer Identification | Forward | Reverse |
|---|---|---|---|---|
| pde1a | ||||
| start 1 | 1, 15 | 376F, 355Ra | 5′-GCATAACTGCGTATCCCTCTGC | 5′-CCGTTCCAGGGCAGCGTTTCT |
| start 1 | 1, 15 | 374F, 411Ra | 5′-AACAGGACACTCTGGGAAACGC | 5′-CTGAGCTGTGCTCCTCCGGG |
| start 2 | 1, 14 | 360F, 355Ra | 5′-AGTTTAGCGTTTGGTGTTTGCC | 5′-CCGTTCCAGGGCAGCGTTTCT |
| start 1 | 1, 3 | 498F, 499Rb | 5′-AATGACACGGACGGACTGG | 5′-CCTTTATCCAGCTGCTTCACC |
| start 2 | 1, 2 | 502F, 503Rb | 5′-CTGAAGCCTGGACTTGTGC | 5′-ACAGCCTCCAAAACTGATGC |
| Common | 6, 10c | 380F, 381Rd | 5′-TCAAGCTCCAGGAGGCGAGC | 5′-AGGCTGCTGCAGAGAGTTCCTCA |
| β-actin | 50F, 150Rd | 5′-TCAGTGCACGCTGAGAAGAT | 5′-AGGAAGGAAGGCTGGAAGAG | |
| elfa | 496F, 497Rb | 5′-CTTCTCAGGCTGACTGTGC | 5′-CCGCTAGCATTACCCTCC |
For qPCR, tissues from 50 zebrafish embryos or 3–10 adults were pooled, total RNA was extracted using RNeasy (Qiagen), and first-strand cDNA was synthesized using SuperScript III (Invitrogen). qPCR was done using an ABI 7900HT (Applied Biosystems) with SYBR GreenER qPCR Supermix (Invitrogen). Primers used are shown in Table 1. Fold differences in expression were calculated from ΔΔCt, normalized using elfa, which was previously determined to be one of the most stably expressed genes across tissues in zebrafish.28 Expression levels are shown relative to the expression of the start 2 isoform in 2-dpf embryo head. qPCR was performed in triplicate on cDNA obtained from pooled embryo or adult tissues. Data are shown as means±SEMs. Products were confirmed by sequencing.
Full-length start 1 and start 2 pde1a isoforms were amplified from adult zebrafish brain (start 1) or eye (start 2) and subcloned into pGEMHE expression vector containing Xenopus β-globin 5′- and 3′-untranslated region for increased expression and stability (provided by Michael F. Romero, Mayo Clinic, Rochester, MN) using ligation-independent cloning.29 The construct was confirmed by sequencing and used for transcription of capped and tailed RNA (Ambion).
For measurement of PDE1 activity, PDE1A1 RNA (25 ng) was injected into Xenopus oocytes, and PDE1 activity was determined as described previously.13,30 Oocytes were frozen 4 days after injection and then lysed in homogenization buffer with protease inhibitors (Roche). PDE activities were measured using cAMP with 3H-cAMP included as a tracer for quantification. Hydrolyzed nucleotide was separated on ion exchange resin (FabGennix) and quantified by liquid scintillation counting.
MO and RNA Rescue Studies
MO and/or cRNA was injected at the one- to four-cell stage using capillary pipettes (World Precision Instruments) pulled on a Sutter pipette puller and a nitrogen-driven injector (Femtojet; Eppendorf). MOs were obtained from Gene Tools, and MO targeting pde1a had sequences, which are shown in Table 2. Translation-blocking pkd2 MO is the hi4166 oligo 1.17,18 Control MO was the standard control for Gene Tools with no target in zebrafish, and it has the sequence 5′-CCTCTTACCTCAGTTACAATTTATA.
Table 2.
Pde1a MOs used
| MO | Sequence |
|---|---|
| Splice acceptor | 5′-TGCTGCGGAAGAGCGAATCAAATAA |
| Splice donor | 5′-CATTGAAGATGATCACAAACCTGGT |
| Start 1 translation blocking | 5′-CCAGAGAATCCATGGTATGCGCGAA |
| Start 2 translation blocking | 5′-AGAGCACAAGTCCAGGCTTCAGTCA |
Human PDE1A1 clone (NCBI BC022480; IMAGE 4814064) was obtained from Open Biosystems and subcloned into pGEMHE expression vector as described above. The construct was confirmed by sequencing and used for transcription of capped and tailed RNA (Ambion), which was injected into zebrafish embryos for rescue studies or Xenopus oocytes for cAMP hydrolysis assay as described above.
Pharmacologic Inhibitors
H89 or Rp-cAMPS (Sigma-Aldrich) was diluted to final concentration in embryo water, added to embryos at 50% epiboly, and replaced daily.
Analysis of the Embryonic Phenotype
Embryos were examined at 2 or 3 dpf, as indicated, for pronephric cysts, hydrocephalus, and body curvature using a Zeiss Lumar stereo fluorescence microscope and Axiovision software. Cysts were defined as being larger than otolith crystals (15±1.0 μm, mean±SEM, n=15).
Cilia beating was examined in 2-dpf embryos, anesthetized in tricaine, embedded in 3% methylcellulose, and examined on coverslips. Cilia beating was recorded using high-speed video microscopy on a Zeiss Axiovert with a Zeiss AxioCam HSr camera and Axiovision software. Images were recorded at 80 frames/s and replayed at 6 frames/s for measurement of beat frequency. At least two measurements were made on each fish to obtain an average beat frequency for each fish. Beat frequencies from 9 to 10 fish were averaged to obtain the average and SEM for each treatment.
Embryos were sectioned in JB4 (Electron Microscopy Sciences) and stained with hematoxylin and eosin as described.31 Sections were photographed on a Zeiss Axiovert with Axiovision software.
Statistical Analyses
Experiments were repeated in at least three unique clutches of at least 20 embryos each. Phenotypes were scored as present or absent. The total percent of embryos with phenotypes across batches is shown in graphs, and statistics were calculated using chi-squared tests. Cilia beat frequency was analyzed using a two-tailed t test.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Michael F. Romero, Heather Holmes, and Taku Hirata for help with oocyte injections and Xiaofang Wang for help with PDE1 activity assays.
This work was supported by National Institutes of Health Pilot and Feasibility Grant DK090728 (to C.R.S.), Grant DK059597 (to P.C.H.), DK44863 (to V.E.T.), and DK090728 (to V.E.T.), Mayo Clinic Fulk Career Development Grant (to C.R.S.), and the Zell Family Foundation.
These results have been partially reported in abstract form (Sussman et al., J Am Soc Nephrol 23: 1A, 2012; Sussman et al., FASEB J 27: 910–916, 2013; Sussman et al., J Am Soc Nephrol 24: 394A, 2013).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013040421/-/DCSupplemental.
References
- 1.Harris PC, Torres VE: Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 3.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477–1485, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Rees S, Kittikulsuth W, Roos K, Strait KA, Van Hoek A, Kohan DE: Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 25: 232–237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76: 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattone VH, 2nd, Wang X, Harris PC, Torres VE: Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH, 2nd: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Gattone V, 2nd, Harris PC, Torres VE: Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16: 846–851, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Lugnier C: Cyclic nucleotide phosphodiesterase (PDE) superfamily: A new target for the development of specific therapeutic agents. Pharmacol Ther 109: 366–398, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Bender AT, Beavo JA: Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Conti M, Beavo J: Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481–511, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Ward CJ, Harris PC, Torres VE: Cyclic nucleotide signaling in polycystic kidney disease. Kidney Int 77: 129–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangos S, Lam PY, Zhao A, Liu Y, Mudumana S, Vasilyev A, Liu A, Drummond IA: The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis Model Mech 3: 354–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA: Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol 17: 2706–2718, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisgrove BW, Snarr BS, Emrazian A, Yost HJ: Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer’s vesicle are required for specification of the zebrafish left-right axis. Dev Biol 287: 274–288, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Schottenfeld J, Sullivan-Brown J, Burdine RD: Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development 134: 1605–1615, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N: A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF: The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD). Hepatology 49: 160–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothschild SC, Francescatto L, Drummond IA, Tombes RM: CaMK-II is a PKD2 target that promotes pronephric kidney development and stabilizes cilia. Development 138: 3387–3397, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP: Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl(-) Co-transporter-dependent cystic dilation. J Am Soc Nephrol 17: 3424–3437, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Mahajan N, Kaur T, Puri V, Singla SK, Jha V, Puri S: Calcium ameliorates renal cyst growth in metanephric organ culture: A morphological study. J Environ Pathol Toxicol Oncol 31: 285–293, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Gallegos TF, Kouznetsova V, Kudlicka K, Sweeney DE, Bush KT, Willert K, Farquhar MG, Nigam SK: A protein kinase A and Wnt-dependent network regulating an intermediate stage in epithelial tubulogenesis during kidney development. Dev Biol 364: 11–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Aso M, Fernandez P, Mediero A, Chan ES, Cronstein BN: Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. Faseb J 28: 802–812, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, Lipschutz JH: The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet 7: e1001361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas S, Wright KJ, Le Corre S, Micalizzi A, Romani M, Abhyankar A, Saada J, Perrault I, Amiel J, Litzler J, Filhol E, Elkhartoufi N, Kwong M, Casanova JL, Boddaert N, Baehr W, Lyonnet S, Munnich A, Burglen L, Chassaing N, Encha-Ravazi F, Vekemans M, Gleeson JG, Valente EM, Jackson PK, Drummond IA, Saunier S, Attie-Bitach T: A homozygous PDE6D mutation in Joubert syndrome impairs targeting of farnesylated INPP5E protein to the primary cilium. Hum Mutat 35: 137–146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westerfield M: The Zebrafish Book, Eugene, OR, University of Oregon Press, 2000 [Google Scholar]
- 28.McCurley AT, Callard GV: Characterization of housekeeping genes in zebrafish: Male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol Biol 9: 102, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Wen A, Shen B, Lu J, Huang Y, Chang Y: FastCloning: A highly simplified, purification-free, sequence- and ligation-independent PCR cloning method. BMC Biotechnol 11: 92, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sussman CR, Zhao J, Plata C, Lu J, Daly C, Angle N, DiPiero J, Drummond IA, Liang JO, Boron WF, Romero MF, Chang MH: Cloning, localization, and functional expression of the electrogenic Na+ bicarbonate cotransporter (NBCe1) from zebrafish. Am J Physiol Cell Physiol 297: C865–C875, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan-Brown J, Bisher ME, Burdine RD: Embedding, serial sectioning and staining of zebrafish embryos using JB-4 resin. Nat Protoc 6: 46–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.