Abstract
Previous studies report a higher risk of cancer in patients with ESRD, but the impact of less severe CKD on risk of cancer is uncertain. Our objective was to evaluate the association between level of kidney function and subsequent cancer risk. We performed a retrospective cohort study of 1,190,538 adults who were receiving care within a health care delivery system, had a measurement of kidney function obtained between 2000 and 2008, and had no prior cancer. We examined the association between level of eGFR and the risk of incident cancer; the primary outcome was renal cancer, and secondary outcomes were any cancer and specific cancers (urothelial, prostate, breast, lung, and colorectal). During 6,000,420 person-years of follow-up, we identified 76,809 incident cancers in 72,875 subjects. After adjustment for time-updated confounders, lower eGFR (in milliliters per minute per 1.73 m2) was associated with an increased risk of renal cancer (adjusted hazard ratio [HR], 1.39; 95% confidence interval [95% CI], 1.22 to 1.58 for eGFR=45–59; HR, 1.81; 95% CI, 1.51 to 2.17 for eGFR=30–44; HR, 2.28; 95% CI, 1.78 to 2.92 for eGFR<30). We also observed an increased risk of urothelial cancer at eGFR<30 but no significant associations between eGFR and prostate, breast, lung, colorectal, or any cancer overall. In conclusion, reduced eGFR is associated with an independently higher risk of renal and urothelial cancer but not other cancer types.
CKD and cancer are both major and growing public health problems nationally and internationally.1,2 The incidence of CKD continues to rise, with an estimated 11.5% of the United States population having reduced eGFR and/or proteinuria and approximately 13.5 million Americans with stage 3 or higher CKD.1,3 Concurrently, >1.5 million new cancer diagnoses and approximately 577,000 cancer deaths are projected nationally in 2012.2 Although cancer incidence has decreased slightly since 2000, it remains one of the leading causes of morbidity and mortality.
The link between CKD and the risk of developing cancer has not been well delineated. Although multiple studies have observed higher risks of cancer in persons with ESRD requiring dialysis or renal transplantation,4–11 whether less severe kidney disease is associated with cancer remains poorly understood.12–15 Previous studies are limited by modest sample sizes, restricted diversity, and their ability to adequately control for confounders. Determining whether there is a robust association between the presence and severity of CKD with subsequent cancer risk and specifically examining if level of kidney function is differentially associated with specific cancer type could have important public health implications for screening and early detection of cancer in patients with CKD.
To address this knowledge gap, we evaluated the association between level of kidney function and the risk of incident cancer and cancer type within a large, diverse, community-based population linked to a regional cancer registry. We hypothesized that there would be an independent increased risk of kidney cancer with lower GFR levels but no significant relationship between renal function and other types of malignancies.
Results
We identified 1,190,538 adults ages 40 years and older with known kidney function and no history of cancer, dialysis, or renal transplantation. Median follow-up for the cohort was 5.3 years (interquartile range=2.6–7.6 years), with a total of 6,000,420 person-years of follow-up. During follow-up, the median number of outpatient serum creatinine measurements per subject was four (interquartile range=2–9).
At cohort entry, patients with lower GFR were more likely to be older, be persons of color, have lower socioeconomic status, have a higher burden of comorbidity, and be current or former smokers (Table 1). The frequency of documented urine dipstick proteinuria was substantially higher at lower entry GFR levels along with the receipt of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aldosterone receptor antagonists, α-blockers, β-blockers, calcium channel blocker, statins, other lipid-lowering agents, and 5-α reductase inhibitors. Approximately 34% of the cohort had an abnormal dipstick hematuria evaluation and 30% obtained abdominal imaging during follow-up. However, the median number of ambulatory health care visits during the year before cohort entry was similar across all GFR categories (Table 1).
Table 1.
Baseline characteristics stratified by level of kidney function at entry among 1,190,538 adults ages 40 years and older with no previously known cancer, dialysis, or renal transplantation
| Characteristic | Overall Cohort | eGFR (ml/min per 1.73 m2) | ||||
|---|---|---|---|---|---|---|
| 90–150 | 60–89 | 45–59 | 30–44 | <30 | ||
| N | 1,190,538 | 309,927 | 704,437 | 131,889 | 34,669 | 9616 |
| Age, yr | ||||||
| Median age (interquartile range) | 55 (48–65) | 49 (44–54) | 56 (49–64) | 69 (60–77) | 76 (68–83) | 76 (67–83) |
| Age category, %a | ||||||
| 40–49 | 33.34 | 56.27 | 29.98 | 7.47 | 2.88 | 5.10 |
| 50–59 | 30.84 | 31.09 | 34.65 | 17.39 | 8.38 | 9.65 |
| 60–69 | 18.95 | 9.56 | 21.56 | 27.61 | 17.45 | 17.42 |
| 70–79 | 11.93 | 2.89 | 10.70 | 31.65 | 37.04 | 31.99 |
| ≥80 | 4.93 | 0.19 | 3.11 | 15.87 | 34.25 | 35.85 |
| Sex, % | ||||||
| Men | 47.04 | 35.50 | 50.95 | 53.03 | 47.85 | 47.54 |
| Women | 52.96 | 64.50 | 49.05 | 46.97 | 52.15 | 52.46 |
| Race, % | ||||||
| White | 58.21 | 45.25 | 60.31 | 71.94 | 75.61 | 71.42 |
| Black | 6.82 | 9.24 | 5.93 | 5.60 | 6.97 | 10.64 |
| Native American | 1.51 | 1.24 | 1.46 | 2.11 | 2.51 | 2.44 |
| Asian | 11.00 | 14.83 | 9.96 | 8.26 | 8.64 | 10.43 |
| Other, multiple, or unknown race | 22.45 | 29.45 | 22.35 | 12.10 | 6.26 | 5.06 |
| Socioeconomic status, % | ||||||
| Low annual household income | 10.04 | 10.89 | 9.21 | 11.09 | 13.87 | 15.72 |
| Low educational attainment | 19.41 | 24.10 | 17.59 | 17.55 | 20.39 | 24.27 |
| Medical history, % | ||||||
| Heart failure | 1.77 | 0.42 | 1.02 | 4.53 | 12.96 | 21.58 |
| Hypertension | 31.40 | 21.78 | 29.61 | 50.68 | 68.92 | 73.11 |
| Diabetes | 10.27 | 11.11 | 8.60 | 12.92 | 20.84 | 30.82 |
| Coronary heart disease | 2.44 | 1.04 | 2.09 | 5.14 | 9.07 | 11.57 |
| Chronic lung disease | 24.05 | 23.45 | 23.28 | 26.77 | 31.99 | 33.55 |
| Chronic liver disease | 0.37 | 0.47 | 0.30 | 0.36 | 0.61 | 0.88 |
| Proteinuria | 6.21 | 5.15 | 4.94 | 9.44 | 19.89 | 39.57 |
| Hematuria | ||||||
| Negative | 9.33 | 9.58 | 9.91 | 7.19 | 5.15 | 3.48 |
| Abnormal | 6.85 | 7.32 | 6.58 | 6.84 | 7.26 | 10.46 |
| Missing | 83.81 | 83.10 | 83.51 | 85.97 | 87.59 | 86.05 |
| Body mass index categoryb | ||||||
| <25 | 24.48 | 26.00 | 24.33 | 22.79 | 21.92 | 18.87 |
| 25–29 | 25.06 | 24.34 | 26.04 | 24.10 | 18.37 | 13.87 |
| 30–39 | 34.04 | 35.03 | 34.60 | 32.10 | 25.37 | 18.22 |
| ≥40 | 3.30 | 4.01 | 3.20 | 2.62 | 2.07 | 1.72 |
| Missing | 13.12 | 10.63 | 11.83 | 18.39 | 32.28 | 47.32 |
| Smoking statusb | ||||||
| Never | 72.63 | 71.45 | 73.72 | 72.66 | 65.11 | 57.17 |
| Current | 13.78 | 16.91 | 13.28 | 10.03 | 10.59 | 12.10 |
| Former | 11.48 | 9.96 | 11.07 | 14.43 | 19.22 | 22.30 |
| Unknown | 2.11 | 1.68 | 1.92 | 2.88 | 5.08 | 8.43 |
| Baseline medication use, % | ||||||
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker | 20.92 | 15.34 | 19.04 | 33.35 | 50.82 | 59.58 |
| Aldosterone receptor blocker | 6.83 | 5.87 | 6.32 | 9.25 | 13.75 | 17.02 |
| α-Blocker | 6.30 | 2.55 | 5.70 | 13.29 | 19.82 | 26.98 |
| β-Blocker | 22.36 | 16.35 | 21.04 | 34.92 | 47.04 | 51.52 |
| Calcium channel blocker | 14.98 | 10.56 | 13.49 | 24.83 | 37.84 | 49.14 |
| Statin | 10.49 | 6.01 | 10.22 | 18.12 | 22.78 | 25.45 |
| Nonstatin lipid-lowering drug | 8.68 | 7.07 | 8.26 | 12.12 | 15.97 | 18.77 |
| 5-α Reductase inhibitor | 0.37 | 0.15 | 0.38 | 0.62 | 0.81 | 0.82 |
| Health care use during 12 months before cohort entry | ||||||
| Median (interquartile range) ambulatory visits | 2 (1–5) | 2 (1–5) | 2 (1–5) | 2 (1–5) | 2 (1–5) | 2 (0–4) |
| Median (interquartile range) hospitalizations | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Abdominal imaging use | ||||||
| Computed tomography | 2.10 | 2.04 | 1.89 | 2.66 | 3.76 | 5.05 |
| Magnetic resonance imaging | 0.03 | 0.02 | 0.02 | 0.03 | 0.09 | 0.63 |
| Ultrasound | 2.92 | 2.96 | 2.49 | 3.35 | 6.34 | 14.75 |
Column percent unless otherwise specified.
Closest to baseline body mass index.
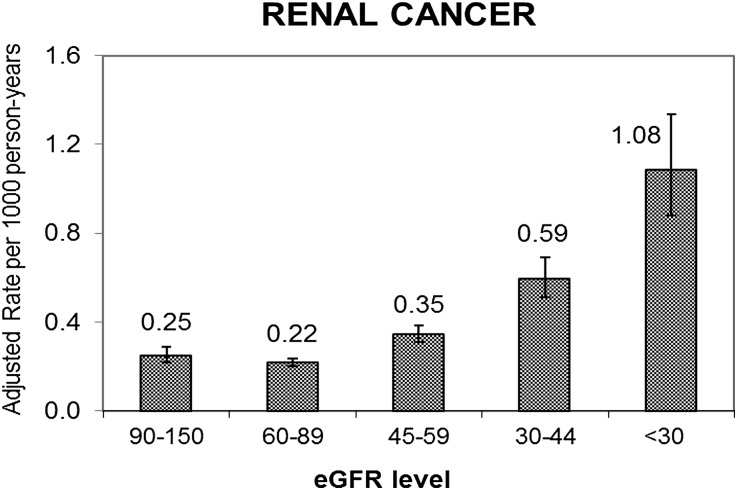
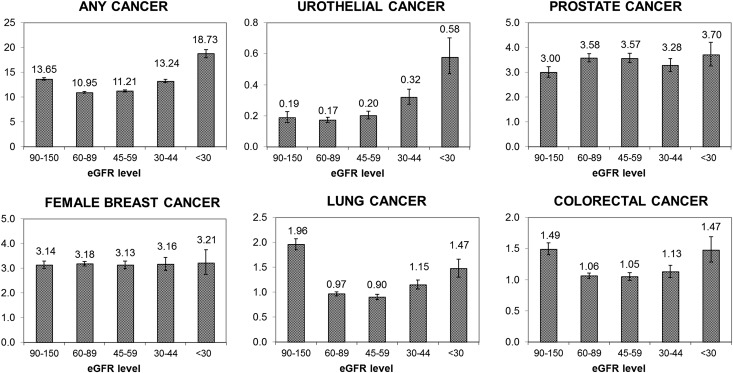
During follow-up, 81,545 subjects died, 4389 subjects initiated chronic dialysis, 1436 subjects received a renal transplant, and 296,734 subjects disenrolled from the health plan. During follow-up, in total, 76,809 incident cancers were documented among 72,875 subjects (38,744 men and 34,131 women). The age–sex-adjusted rates of incident renal cancer increased with lower GFR level, ranging from 0.22 per 1000 person-years for GFR=60–89 ml/min per 1.73 m2 to 1.08 per 1000 person-years for GFR<30 ml/min per 1.73 m2 (Figure 1). The age–sex-adjusted incidence rates of any cancer as well as urothelial cancer were also significantly higher at lower GFR levels (Figure 2). For breast, lung, colorectal, and prostate cancer types, there was no apparent stepwise progression observed like with renal or urothelial cancer types (Figure 2).
Figure 1.
Age–sex-adjusted crude rate per 1000 person-years of incident renal cancer by level of eGFR among 1,190,538 adults. Subjects were 40 years and older with known renal function between 2000 and 2008.
Figure 2.
Age–sex-adjusted crude rate per 1000 person-years of any incident cancer and specific cancers (urothelial, prostate, breast, lung, and colorectal) by level of eGFR among 1,190,538 adults. Subjects were 40 years and older with known renal function between 2000 and 2008.
After adjustment for age, sex, race, socioeconomic status, comorbidities, proteinuria, hematuria, body mass index, smoking status, imaging use, health care use, and specific prescription medications, compared with GFR=60–89 ml/min per 1.73 m2, there was an increased rate of any incident renal cancer, ranging from a 39% increased rate for GFR=45–59 ml/min per 1.73 m2 to a more than 2-fold increased rate for GFR<30 ml/min per 1.73 m2 (Table 2). This increased rate was of greater magnitude for clear cell renal cancer compared with nonclear cell renal cancer (Table 2). Compared with GFR=60–89 ml/min per 1.73 m2, we also found that GFR<30 ml/min per 1.73 m2 was associated with a 48% increased adjusted rate of urothelial cancer (Table 3). However, after adjustment for potential confounders, compared with GFR=60–89 ml/min per 1.73 m2, GFR levels below 60 ml/min per 1.73 m2 were not significantly associated with prostate, colorectal, lung, breast, or any cancer (Table 3). Of note, GFR=90–150 ml/min per 1.73 m2 was associated with a reduced adjusted rate of prostate cancer and higher adjusted rates of lung and colorectal cancer compared with GFR=60–89 ml/min per 1.73 m2 (Table 3).
Table 2.
Multivariable association between measures of kidney function and risk of incident renal cancer (overall and by type)
| eGFR (ml/min per 1.73 m2) | Cancer Cases Number and Adjusted HR (95% CI)a | |||||
|---|---|---|---|---|---|---|
| Any Renal Cell Carcinomab | Clear Cell Renal Carcinomac | Nonclear Cell Renal Carcinomac | ||||
| N | HR (95% CI) | N | HR (95% CI) | N | HR (95% CI) | |
| 90–150 | 241 | 1.08 (0.93 to 1.26) | 116 | 1.12 (0.9 to 1.39) | 40 | 1.37 (0.93 to 2.00) |
| 60–89 | 826 | Reference | 383 | Reference | 117 | Reference |
| 45–59 | 395 | 1.39 (1.22 to 1.58) | 165 | 1.29 (1.06 to 1.58) | 52 | 1.24 (0.87 to 1.76) |
| 30–44 | 196 | 1.81 (1.51 to 2.17) | 80 | 1.71 (1.29 to 2.26) | 25 | 1.55 (0.96 to 2.49) |
| <30 | 92 | 2.28 (1.78 to 2.92) | 28 | 1.68 (1.09 to 2.58) | 6 | 0.92 (0.38 to 2.21) |
HR, hazard ratio; 95% CI, 95% confidence interval.
Final models adjusted for age, sex, race, income, education, smoking, body mass index, prior health care use, proteinuria, hematuria, imaging use, specific comorbidities, and prescription drug use as listed in Table 1.
List of histology codes: 80003, 80103, 80503, 81403, 82113, 82553, 82603, 83103, 83123, 83163, 83173, 83183, 83193, 83203, and 83233.
Histology codes: clear cell carcinomas, 83103; nonclear cell carcinomas, 80503, 82603, 83163, 83173, 83183, 83193, and 83203.
Table 3.
Multivariable association between measures of kidney function and risk of incident cancer (overall and by cancer type)
| eGFR (ml/min per 1.73 m2) | Cancer Cases Number and Adjusted HR (95% CI)a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Cancer | Urothelialb | Prostate | Breast | Lung | Colorectal | |||||||
| N | HR (95% CI) | N | HR (95% CI) | N | HR (95% CI) | N | HR (95% CI) | N | HR (95% CI) | N | HR (95% CI) | |
| 90–150 | 10,271 | 1.04 (1.01 to 1.06) | 114 | 0.95 (0.77 to 1.17) | 1014 | 0.76 (0.71 to 0.81) | 2100 | 0.98 (0.92 to 1.03) | 1414 | 1.32 (1.22 to 1.43) | 1066 | 1.18 (1.09 to 1.28) |
| 60–89 | 39,105 | Reference | 727 | Reference | 9001 | Reference | 5966 | Reference | 4258 | Reference | 3728 | Reference |
| 45–59 | 15,151 | 1.00 (0.98 to 1.02) | 439 | 1.12 (0.99 to 1.27) | 3422 | 1.02 (0.98 to 1.06) | 1733 | 0.96 (0.91 to 1.02) | 1849 | 0.96 (0.90 to 1.02) | 1578 | 0.98 (0.91 to 1.04) |
| 30–44 | 6116 | 1.05 (1.01 to 1.08) | 267 | 1.42 (1.21 to 1.67) | 887 | 0.98 (0.91 to 1.06) | 617 | 0.92 (0.84 to 1.01) | 865 | 1.01 (0.92 to 1.1) | 653 | 1.02 (0.92 to 1.12) |
| <30 | 2218 | 1.05 (0.99 to 1.11) | 126 | 1.48 (1.19 to 1.85) | 259 | 1.02 (0.89 to 1.18) | 161 | 0.85 (0.71 to 1.01) | 285 | 0.80 (0.68 to 0.93) | 223 | 1.05 (0.89 to 1.24) |
HR, hazard ratio; 95% CI, 95% confidence interval.
Final models adjusted for age, sex, race, income, education, smoking, body mass index, prior health care use, proteinuria, specific comorbidities, and prescription drug use as listed in Table 1. Urothelial cancer final model is additionally adjusted for hematuria.
Urothelial cancers included transitional cell carcinoma (histology codes 81203 and 81303).
Discussion
Within a contemporary, community-based sample of more than 1 million adults with known kidney function, we found that lower level of GFR was independently associated with a higher risk of incident diagnosed renal cancer, even after adjustment for a broad set of potential confounders and relevant medication exposures. Subjects with GFR<30 ml/min per 1.73 m2 had a more than 2-fold increased adjusted rate of incident renal cancer compared with those subjects who had GFR=60–89 ml/min per 1.73 m2. We also found an association between GFR and incident urothelial cancer, although the magnitude of this association was less pronounced than observed with renal cancer. In contrast, there was no significant association between lower level of GFR and all combined cancers or prostate, breast, lung, or colorectal cancer after accounting for potential confounding factors.
Previous studies have shown an increased overall risk of cancer in persons with ESRD requiring RRT,4–11 but the impact of less severe kidney dysfunction and cancer risk has been less certain.12–15 In contrast to our findings, two prior studies found an association between renal function and overall cancer risk,13,14 whereas another study found no association between CKD and cancer risk in diabetic patients.15 When focusing on specific cancer types, three studies found that lower kidney function was associated with higher risks of renal and urothelial cancer and support our findings.12–14 In a cohort of approximately 3600 participants, men had a significantly increased risk of cancer with lower GFR; stage 3 CKD was an independent risk factor for cancer, and this risk increased in a linear fashion with lower baseline eGFR.14 The relation between kidney function and cancer risk was site-specific, with the most robust association seen with urinary tract (excluding prostate) and lung cancers.14 Higher albumin-to-creatinine ratio has also been reported to be associated with a higher incidence of cancer (especially bladder and renal cancer) in adjusted analyses.13 A separate study has also observed increased renal and urothelial cancer-specific mortality among patients with CKD.16 Among a much larger, diverse population-based sample, we did not find GFR to be an independent predictor of overall cancer risk or specific cancers, such as prostate, breast, lung, or colorectal, after rigorously adjusting for potential confounders and excluding any incident cancers diagnosed within the first 2 years of follow-up and all GFR measurements within 3 months before a cancer diagnosis. Our findings reveal that the association of CKD and cancer risk is site-specific for renal and urothelial cancers and does not seem to be associated with an individual’s overall cancer risk.
Several possible biologic mechanisms may help to explain the association between level of kidney function and renal or urothelial cancers. Kidney dysfunction results in a state of chronic inflammation and oxidative stress,17,18 and such an inflammatory microenvironment may play a role in cancer development.19 Severe CKD may additionally create a relative state of immunodeficiency,20 which could influence the development of cancer. Kidney transplant patients with medically induced immunodeficiency are known to have higher rates of cancer, and some, but not all, of that increased risk is associated with viral-induced malignancies.11 Certain medical therapies21–26 used in patients with CKD have been suggested to increase cancer risk, but adjustment for differential longitudinal use of these medications did not materially affect the association between level of GFR and renal or urothelial cancer. These mechanisms and others deserve additional study to better define the link between kidney function and site-specific cancer risk. The stronger association of GFR and clear cell type renal cancer compared with nonclear cell renal cancer may further elucidate the biologic underpinnings of the association of CKD and renal cancer.
Our study has several notable strengths. We evaluated approximately 1.2 million adults with known renal function and no prior known cancer, representing the largest study to date exploring the relation between CKD and cancer risk. Our study focused on patients across a broad spectrum of kidney function and excluded patients with known ESRD. We ascertained longitudinal measures of GFR based on the recommended Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation3,27 and statistically adjusted for a large set of potential time-updated confounders as well as a comprehensive list of medications that potentially impact cancer risk. Another key strength of this study was our ability to minimize the impact of detection bias by additional adjustment for hematuria and receipt of abdominal imaging. None of the previous studies have been able to adjust for these important confounding factors. Importantly, in a subgroup analysis not shown here, we found an association between eGFR and renal cancer among those subjects with nonlocalized cancer who did not have abdominal imaging; arguably, this group is the least susceptible to detection bias, adding to the evidence supporting an etiologic role of reduced kidney function in renal carcinogenesis. Additionally, we comprehensively ascertained the occurrence of cancer through use of a regional cancer registry linked to Surveillance, Epidemiology, and End Results (SEER).28
This study had certain limitations. We cannot completely exclude the impact of residual confounding. For example, information on family history of cancer, severity of comorbid conditions, and drug dosage was unavailable. However, we controlled for the presence or absence of major confounders and longitudinal exposure to the most common relevant medications that may impact cancer risk, and the strong increased risk of renal cancer (and to a lesser degree, urothelial cancer) argues against missing a major confounder. Data were also unavailable for selected over-the-counter analgesics and toxin exposure. Phenacetin, an analgesic that has been off the market for more than three decades, has been closely linked to both urothelial and renal cancer,29,30 but it is very unlikely to have been used by our cohort members in our contemporary study period. However, we recognize that recent data raise some concern about the association of renal cancer and nonaspirin, nonsteroidal anti-inflammatory drug use.31 Any of the serum creatinine-based GFR estimating equations may provide imperfect measurements of GFR, introducing the potential for misclassification of kidney function level. We sought to minimize this misclassification by using longitudinal, time-updated measurements of GFR calculated by the CKD-EPI equation3,27 and isotope dilution mass spectrometry–traceable serum creatinine measures, with a median of four outpatient, nonemergency department serum creatinine measurements per subject. Given that patients with worse kidney function may be more closely followed clinically and possibly, more likely to have incident cancer detected, we adjusted for prior medical resource use, which did not materially affect the relations between level of kidney function and incident renal or urothelial cancer. We also limited potential effect cause by excluding any incident cancers diagnosed during the first 2 years of follow-up as well as any GFR measures performed within 3 months before an incident cancer diagnosis. Finally, although our study sample was large and diverse, results may not be fully generalizable to other populations and practice settings.
In conclusion, we found that lower GFR level was an independent risk factor for incident renal and urothelial cancer but not prostate, colorectal, lung, breast, or all cancers combined. If GFR is associated with an increased risk of renal and urothelial cancer, then it could have implications for directing cancer screening efforts in select populations. Currently, there are no evidence-based cancer screening recommendations tailored for patients with CKD. Additional studies are needed to elucidate the reasons for this association and delineate the potential use of targeted cancer screening in patients with CKD.
Concise Methods
Study Sample
We identified all persons with measured serum creatinine concentration who are receiving care within Kaiser Permanente Northern California, a large integrated health care delivery system providing comprehensive care to >3.2 million persons in the San Francisco and greater Bay area. The Kaiser Permanente Northern California population is highly representative of the surrounding local and statewide population, except for slightly lower representation at the extremes of age and incomes.32 The analytic sample included members who were ages 40 years and older, had one or more outpatient, nonemergency department serum creatinine measurements between January 2000 and December 2008, and had ≥12 months of continuous health plan membership before cohort entry. Patients were excluded if they had unknown sex or preexisting cancer diagnosis (except for localized, nonmelanoma skin cancer) identified in the Kaiser Permanente Cancer Registry,28 which also links to the SEER cancer registry.33 We also excluded patients with a documented history of hemodialysis, peritoneal dialysis, or renal transplantation before entry based on a comprehensive health plan ESRD treatment registry.34
The Institutional Review Board of the Kaiser Foundation Research Institute approved this study. Waiver of informed consent was obtained because of the nature of the research.
Measurement of Renal Function
The primary predictor of interest was level of kidney function as determined by eGFR at baseline and during follow-up. GFR was calculated using all available outpatient, nonemergency department serum creatinine concentration values derived from isotope dilution mass spectrometry–traceable assays using the CKD-EPI equation and incorporated as time-varying values in our analyses. The CKD-EPI equation has been shown to be more accurate than the Modification of Diet in Renal Disease study estimating equation, especially when estimating higher levels of GFR.3,27 GFR values (in units of milliliters per minute per 1.73 m2) were categorized according to the modified National Kidney Foundation classification system for CKD: ≥90, 60–89, 45–59, 30–44, 15–29 and <15 ml/min per 1.73 m2 not requiring dialysis. Based on available sample size, we combined the latter two groups into a single category (<30 ml/min per 1.73 m2).
Follow-Up and Outcomes
Follow-up occurred through December 31, 2008, which was the latest date in which complete vital status data were available at the time of analysis. Patients were censored at the end of follow-up, initiation of dialysis, date of renal transplantation, disenrollment from the health plan, or death. Death was identified from health plan administrative data (including proxy reporting), California death certificate data, and Social Security Administrative vital status files.35,36
The primary outcome of interest was occurrence of incident renal cancer during the study period identified from the Kaiser Permanente Cancer Registry. Renal cancer histology codes used in our dataset were in compliance with the World Health Organization International Classification of Diseases for Oncology international standard for primary site and histology (December 2009; www.seer.cancer.gov/icd-o-3/index.html). Specific histology codes used are listed in Table 2. In secondary analyses, we evaluated the association of renal function with any incident cancer diagnosis (excluding nonmelanoma skin cancers) and other specific cancer types, including urothelial, prostate, breast, lung, and colorectal. Specific urothelial histology codes used are listed in Table 3.
Covariates
Information on age, sex, and race was obtained from health plan databases. Socioeconomic characteristics were assigned using US Census-block data.32,34 Subjects living in a Census-block area where median annual household income was less than $35,000 were classified as low income. Low educational attainment was also categorized according to US Census-block data and defined as those individuals living in an area where more than 25% of residents over age 25 years have less than a 12th grade education level.
The presence of proteinuria and hematuria was based on the urine dipstick results found in health plan laboratory databases. As previously described,34 proteinuria was defined as a urine dipstick protein result of 1+ or greater (approximately 30 mg/dl or greater) in the absence of possible urinary tract infection (i.e., concomitant positive urine nitrite or esterase).
We identified baseline and time-updated comorbidities within our cohort by methods previously described using health plan databases for inpatient and ambulatory diagnoses, laboratory results, and prescription medications.34,37–40 Coexisting illnesses of interest included diabetes mellitus, hypertension, coronary heart disease, heart failure, and chronic lung or liver disease. Body mass index category41 and tobacco history were ascertained from ambulatory visit databases. Measures of health care use before cohort entry included the number of ambulatory visits and hospitalizations during the 12 months before index date. We identified the total number of radiology studies (computed tomography, magnetic resonance imaging, and ultrasound of the abdomen) obtained during follow-up. Longitudinal medication use was ascertained from ambulatory pharmacy records for common medications potentially associated with cancer risk using previously described algorithms.37,42 These medications included oral antihypertensive agents21,22 (β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, α-blockers, calcium channel blockers, and aldosterone receptor antagonists), nonstatin lipid-lowering agents, statins,24,25 and 5-α reductase inhibitors.23,26
Analytic Approach
Analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC). Baseline characteristics were compared across GFR categories using ANOVAs for continuous variables and chi-squared tests for categorical variables. We calculated rates (per 1000 person-years) of incident cancer by GFR category, overall, and specific cancer type. We conducted extended Cox regression models to examine the independent association between measures of kidney function and risk of any incident cancer and specific cancer type after adjustment for potential confounders. Final models were adjusted for age, sex, race, socioeconomic status, smoking status, body mass index, health care use, proteinuria, comorbid conditions (heart failure, hypertension, diabetes mellitus, coronary heart disease, chronic lung disease, and chronic liver disease), and longitudinal exposure to targeted prescription drugs. The final urothelial cancer model was additionally adjusted for hematuria, whereas the final model for renal cell cancer was also adjusted for hematuria and receipt of abdominal imaging (Table 1). To further evaluate any potential association of kidney function and renal cell cancer, we fit the above-described model for conventional clear cell renal cancer and nonclear cell renal cancer. We separately examined associations using baseline and time-updated measures of kidney function. For all models, we used a sandwich estimate of the variance–covariance matrix to obtain SEMs accommodating the clustering of observations on subjects.43 Because of concern for potential effect cause (or reverse causation) for the association between GFR and risk of cancer, we excluded any incident cancers that occurred during the first 2 years of follow-up and any GFR measures performed within 3 months before an incident cancer diagnosis during follow-up.
Disclosures
None.
Acknowledgments
This work was supported by National Institutes of Health Urologic Oncology Training Grant T32-CA82088 (to W.T.L.), a Huntsman Cancer Institute Cancer Control and Population Sciences FY13 Pilot Project Award (to W.T.L.), and National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health, Department of Health and Human Services Grant U01-DK060902 (to A.S.G.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “CKD and Risk of Renal Cell Carcinoma: A Causal Association?,” on pages 2147–2148.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A: Cancer statistics, 2012. CA Cancer J Clin 62: 10–29, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cengiz K: Increased incidence of neoplasia in chronic renal failure (20-year experience). Int Urol Nephrol 33: 121–126, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Holley JL: Screening, diagnosis, and treatment of cancer in long-term dialysis patients. Clin J Am Soc Nephrol 2: 604–610, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Iseki K, Osawa A, Fukiyama K: Evidence for increased cancer deaths in chronic dialysis patients. Am J Kidney Dis 22: 308–313, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney AP, Briggs D, McCredie M, Boyle P: Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet 354: 93–99, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Matas AJ, Simmons RL, Kjellstrand CM, Buselmeier TJ, Najarian JS: Increased incidence of malignancy during chronic renal failure. Lancet 1: 883–886, 1975 [DOI] [PubMed] [Google Scholar]
- 9.Port FK, Ragheb NE, Schwartz AG, Hawthorne VM: Neoplasms in dialysis patients: A population-based study. Am J Kidney Dis 14: 119–123, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Stewart JH, Buccianti G, Agodoa L, Gellert R, McCredie MR, Lowenfels AB, Disney AP, Wolfe RA, Boyle P, Maisonneuve P: Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: Analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 14: 197–207, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE: Cancer incidence before and after kidney transplantation. JAMA 296: 2823–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Christensson A, Savage C, Sjoberg DD, Cronin AM, Frank O'Brien M, Lowrance W, Nilsson PM, Vickers AJ, Russo P, Lilja H: Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years. Int J Cancer 133: 1452–1458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jørgensen L, Heuch I, Jenssen T, Jacobsen BK: Association of albuminuria and cancer incidence. J Am Soc Nephrol 19: 992–998, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong G, Hayen A, Chapman JR, Webster AC, Wang JJ, Mitchell P, Craig JC: Association of CKD and cancer risk in older people. J Am Soc Nephrol 20: 1341–1350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong G, Zoungas S, Lo S, Chalmers J, Cass A, Neal B, Woodward M, Perkovic V, Glasziou P, Williams B, Howard K, Chapman JR, Craig JC: The risk of cancer in people with diabetes and chronic kidney disease. Nephrol Dial Transplant 27: 3337–3344, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Weng PH, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC: Cancer-specific mortality in chronic kidney disease: Longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol 6: 1121–1128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J: The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 140: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 107: 87–92, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Coussens LM, Werb Z: Inflammation and cancer. Nature 420: 860–867, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giacchino F, Alloatti S, Quarello F, Bosticardo GM, Giraudo G, Piccoli G: The immunological state in chronic renal insufficiency. Int J Artif Organs 5: 237–242, 1982 [PubMed] [Google Scholar]
- 21.Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C, Messerli FH: Antihypertensive drugs and risk of cancer: Network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol 12: 65–82, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Pahor M, Guralnik JM, Ferrucci L, Corti MC, Salive ME, Cerhan JR, Wallace RB, Havlik RJ: Calcium-channel blockade and incidence of cancer in aged populations. Lancet 348: 493–497, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS, REDUCE Study Group : Effect of dutasteride on the risk of prostate cancer. N Engl J Med 362: 1192–1202, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Choueiri TK, Cho E: Statin use and the risk of renal cell carcinoma in 2 prospective US cohorts. Cancer 118: 797–803, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor ML, Wells BJ, Smolak MJ: Statins and cancer: A meta-analysis of case-control studies. Eur J Cancer Prev 17: 259–268, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA, Jr.: The influence of finasteride on the development of prostate cancer. N Engl J Med 349: 215–224, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen C-P, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS, Chronic Kidney Disease Prognosis Consortium : Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 307: 1941–1951, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fireman BH, Fehrenbacher L, Gruskin EP, Ray GT: Cost of care for patients in cancer clinical trials. J Natl Cancer Inst 92: 136–142, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Dubach UC, Rosner B, Stürmer T: An epidemiologic study of abuse of analgesic drugs. Effects of phenacetin and salicylate on mortality and cardiovascular morbidity (1968 to 1987). N Engl J Med 324: 155–160, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Lornoy W, Becaus S, de Vleeschouwer M, Morelle V, Fonteyne E, Thienpoint L, Mestdagh J: Renal cell carcinoma, a new complication of analgesic nephropathy. Lancet 1: 1271–1272, 1986 [DOI] [PubMed] [Google Scholar]
- 31.Cho E, Curhan G, Hankinson SE, Kantoff P, Atkins MB, Stampfer M, Choueiri TK: Prospective evaluation of analgesic use and risk of renal cell cancer. Arch Intern Med 171: 1487–1493, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krieger N: Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health 82: 703–710, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF: Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care 40[Suppl]: IV-3–IV-18, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Arellano MG, Petersen GR, Petitti DB, Smith RE: The California Automated Mortality Linkage System (CAMLIS). Am J Public Health 74: 1324–1330, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman TB, Brown AN: Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc 4: 233–237, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH: Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA 296: 2105–2111, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV: Ethnic disparities in diabetic complications in an insured population. JAMA 287: 2519–2527, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Selby JV, Ray GT, Zhang D, Colby CJ: Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care 20: 1396–1402, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS: Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 362: 2155–2165, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ: Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363: 2211–2219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R: Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Intern Med 168: 2415–2421, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Therneau TM, Grambsch PM: Modeling Survival Data: Extending the Cox Model, New York, Springer-Verlag, 2000 [Google Scholar]