Abstract
Hyponatremia, the most frequent electrolyte disorder, is caused predominantly by the syndrome of inappropriate antidiuresis (SIAD). A comprehensive characterization of SIAD subtypes, defined by type of osmotic dysregulation, is lacking, but may aid in predicting therapeutic success. Here, we analyzed serial measurements of serum osmolality and serum sodium, plasma arginine vasopressin (AVP), and plasma copeptin concentrations from 50 patients with hyponatremia who underwent hypertonic saline infusion. A close correlation between copeptin concentrations and serum osmolality existed in 68 healthy controls, with a mean osmotic threshold±SD of 282±4 mOsM/kg H2O. Furthermore, saline-induced changes in copeptin concentrations correlated with changes in AVP concentrations in controls and patients. With use of copeptin concentration as a surrogate measure of AVP concentration, patients with SIAD could be grouped according to osmoregulatory defect: Ten percent of patients had grossly elevated copeptin concentrations independent of serum osmolality (type A); 14% had copeptin concentrations that increased linearly with rising serum osmolality but had abnormally low osmotic thresholds (type B); 44% had normal copeptin concentrations independent of osmolality (type C), and 12% had suppressed copeptin concentrations independent of osmolality (type D). A novel SIAD subtype discovered in 20% of patients was characterized by a linear decrease in copeptin concentrations with increasing serum osmolality (type E or “barostat reset”). In conclusion, a partial or complete loss of AVP osmoregulation occurs in patients with SIAD. Although the mechanisms underlying osmoregulatory defects in individual patients are presumably diverse, we hypothesize that treatment responses and patient outcomes will vary according to SIAD subtype.
Hyponatremia is the most common electrolyte disorder, associated with significant morbidity and mortality.1–3 Most hyponatremic states are characterized by inappropriately elevated plasma levels of arginine vasopressin (AVP), with the syndrome of inappropriate antidiuresis (SIAD) as the most frequent underlying pathophysiologic abnormality. The diagnosis of SIAD is based on criteria defined by Schwartz and Bartter in 1957, including hypotonic hyponatremia, euvolemia, inappropriately high urine osmolality in relation to serum hypo-osmolality, increased renal sodium excretion on a normal salt and water intake, and absence of renal disease or other nonosmotic (i.e., “appropriate”) stimuli of AVP release.4 Zerbe et al. were the first to suggest distinct types of osmotic dysregulation in SIAD based on the AVP response to hypertonic saline: a type with erratic fluctuations of high plasma AVP levels that are nonresponsive to osmotic stimulation (type A); another type, with a preserved linear correlation between AVP and serum osmolality but an abnormally low osmotic threshold (“reset osmostat” or type B); type C, with constant and nonsuppressible AVP release in the hypo-osmotic range; and type D, with undetectable AVP levels.5
Unfortunately, this subtype description was reported only as individual case examples; it has never been validated using quantitative criteria. A better understanding of the different forms of SIAD may influence future treatment strategies because response to therapy (e.g., with vaptans or fluid restriction) varies widely in SIAD.6,7
Using the hypertonic saline infusion test, the present study aimed to establish quantitatively defined SIAD subtypes based on serial measurements of copeptin, a stable and easily measurable surrogate for AVP release.
Results
Baseline Characteristics of Patients with SIAD and Healthy Controls
In total, 50 patients with SIAD (30 women) were included. Eighty-two percent of patients had a malignancy-associated SIAD (41% solid tumors, 59% hematologic disorders), 6% had HIV-associated SIAD, 4% were drug-induced, 4% were infection-associated, and another 4% were considered idiopathic. Baseline serum sodium levels ranged between 111 and 129 mmol/L, and serum osmolality ranged between 231 and 271 mOsM/kg H2O. Both individual and median baseline serum sodium and osmolality concentrations were lower in the SIAD group than in healthy controls: 126 (interquartile range [IQR], 123, 128) versus 136 (IQR, 135, 138) mmol/L and 260 (IQR, 256, 260) versus 283 (IQR, 280 285) mOsM/kg H2O (both P<0.001) (Table 1). No differences were found between the SIAD and control group regarding plasma renin, serum aldosterone, creatinine, BP, and heart rate (all P>0.05) (Table 1). Moreover, isotonic saline infusion, performed in all but two patients (patients 11 and 37; see Supplemental Table 1), caused no relevant increase in serum sodium concentration (defined as ≥5 mmol/L in 24 hours) in the patient group.
Table 1.
Baseline characteristics of the healthy control and SIAD patient groups
| Characteristic | SIAD Group (n=50) | Control Group (n=10) |
|---|---|---|
| Age (yr) | 59.8±12.3 | 37.8±14.1a |
| Serum sodium (mmol/L) | 125±4 | 137±2 b |
| Serum osmolality (mOsM/kg H2O) | 259±8 | 283±4b |
| Urine osmolality (mOsM/kg H2O) | 509 (391, 691) | 570 (456, 718) |
| Urine sodium (mmol/L) | 95 (67,130) | 83 (37, 126) |
| Serum uric acid (mg/dl) | 3.4±0.8 | 3.6±0.6 |
| Plasma copeptin (pmol/L) | 8.4 (4.2, 36) | 3.4 (2.9, 6.9)a |
| Plasma AVP (pmol/L) | 2.8 (1.5, 4.8) | 1.3 (0.8, 2.6)b |
| Mean arterial pressure (mmHg) | 88 (79, 92) | 81 (74, 87) |
| Heart rate (beats/min) | 72 (66, 82) | 72 (63, 85) |
Data are expressed as mean±SD or median (IQR).
P<0.05 for comparison of the respective SIAD subgroup with the healthy control group (Mann–Whitney U test).
P<0.01 for comparison of the respective SIAD subgroup with the healthy control group (Mann–Whitney U test).
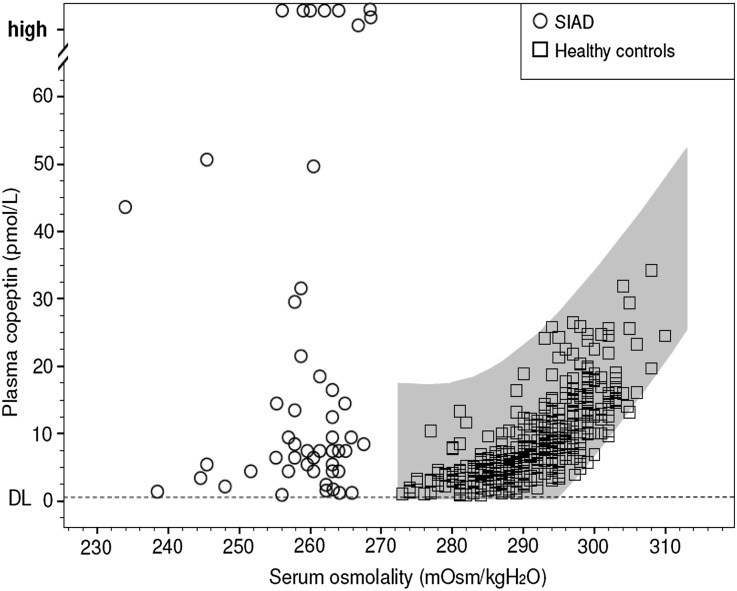
Baseline copeptin and AVP levels were higher in patients with SIAD (median, 8.4 [IQR, 4.4, 32.4] and 2.8 [IQR, 1.5, 4.7] pmol/L; P<0.001) compared with the control group (median, 3.4 [IQR, 2.9, 6.9] L and 1.3 [IQR, 0.8, 2.6] pmol/L; P<0.01). Other than in healthy controls, baseline copeptin concentrations varied substantially among patients with SIAD: In the hypo-osmotic range (<280 mOsM/kg H2O), baseline hormone levels ranged from 0.9 to 251 pmol/L (Figure 1). In six patients (12%), baseline copeptin levels were appropriately suppressed to <2 pmol/L, whereas supraphysiologically high baseline levels (>38.0 pmol/L) were found in 15 patients (30%). A majority of patients (58%) had plasma copeptin concentrations within the normal physiologic range (2–38 pmol/L); levels were inappropriately high only in relation to the corresponding hypo-osmotic state. The patients’ individual baseline characteristics, causes of SIAD, and specific treatments are described in detail in Supplemental Table 1.
Figure 1.
Physiological plasma copeptin response to hypertonic saline-induced osmotic stimulation. The gray area illustrates the area of normality describing the relationship between plasma copeptin and serum osmolality based on 384 data pairs of 68 healthy controls that were osmotically stimulated using a hypertonic saline infusion. The regression curves were constructed with 95% CIs for the relation between serum osmolality and plasma copeptin levels with a cubic regression yielding the best fit. The detection limit (DL) of copeptin determination was 0.4 pmol/L. In contrast, the data points in the hypo-osmotic range illustrate the relationship between baseline plasma copeptin and serum osmolality levels in 50 patients with clinical features of SIAD (n=50).
Hypertonic Saline Infusion Test: Correlation of Plasma Copeptin and AVP in Healthy Controls and Patients with SIAD
The physiologic osmotic response of copeptin to hypertonic saline infusion was characterized by averaging the individual slopes of the 68 controls, and a slope of 0.74 pmol/L and mOsM/kg H2O was considered the normal mean linear response (95% confidence interval [95% CI], 0.66 to 0.83; 99% confidence interval, 0.63 to 0.86). The physiologic osmotic threshold for copeptin release was defined by the calculated mean x intercept with its 95% CI: 282±4.3 mOsM/kg H2O (95% CI, 281to 285 mOsM/kg H2O; range, 274–288 mOsM/kg H2O). Accordingly, the expected close linear correlation was confirmed between saline-induced rise in serum sodium (Δ11.2±1.5 mmol/L) and osmolality concentrations (Δ16.7±2.2 mOsM/kg H2O) and resulting elevation in plasma copeptin levels (from 4.1 [IQR, 2.9, 4.7] to 15.2 [IQR, 12.7, 20.6] pmol/L; both r=0.83; P<0.001). The saline-induced rise in plasma copeptin and AVP levels correlated closely (r=0.67; P<0.001), while no significant correlation was found between plasma copeptin and AVP concentrations at baseline (r=0.43; P=0.22).
In patients with SIAD, changes in copeptin levels in response to osmotic stimulation (from 8.4 [IQR, 4.3, 32.4] to 10.5 [IQR, 5.2, 22.1] pmol/L; median Δ−0.01 [IQR, −4.8, 6.2] pmol/L) did not correlate with corresponding rises in serum sodium (Δ12 [IQR, 11, 14] mmol/L; r=−0.22) or osmolality levels (Δ30 [IQR, 27, 34] mOsM/kg H2O; r=0.43). Accordingly, no correlation was found between sodium or osmolality increase and plasma AVP levels (2.8 [IQR, 1.5, 4.7] to 2.5 [IQR, 1.6, 5.8] pmol/L [r=0.03]; Δ0.04 [IQR, −1.2, 1.5] pmol/L [r=0.54]; all P>0.3). Strong correlations were found between saline-induced changes in absolute levels of plasma copeptin and AVP (r=0.90; P<0.0001), as well between their mean linear slopes (r=0.85; P<0.0001).
Hypertonic Saline Infusion Test: Copeptin Osmoregulation in Patients with SIAD
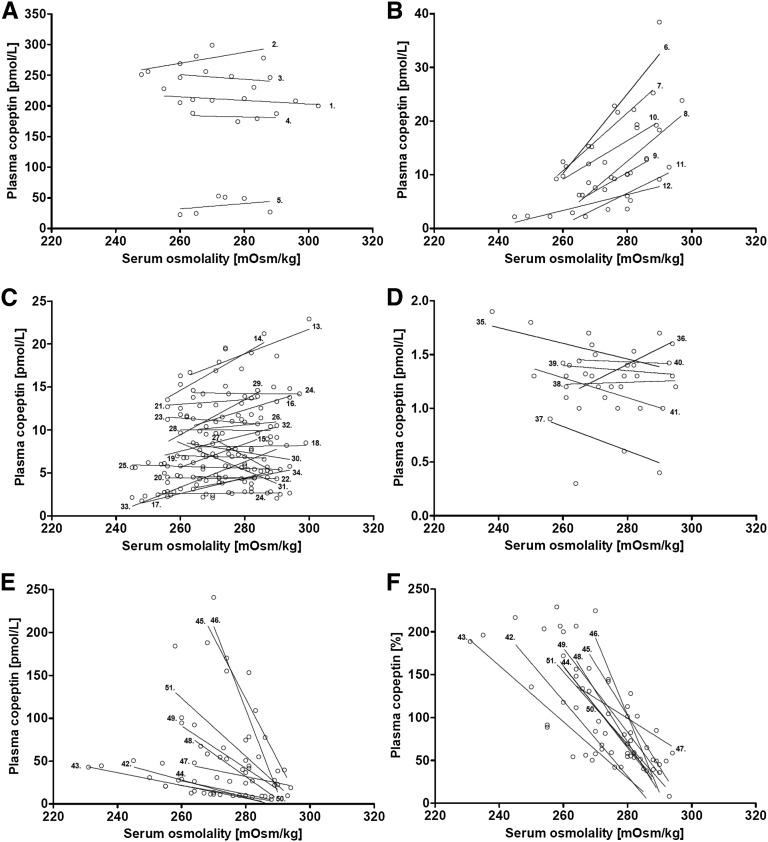
Consistent with the broad range in copeptin baseline levels in the SIAD group (Figure 1), copeptin response to hypertonic saline infusion also varied widely. Three predefined criteria, considered to best catch abnormal behavior of osmotically stimulated copeptin, were used for individual patient characterization: (1) the slope of copeptin concentration (see Concise Methods), (2) the osmotic threshold of copeptin release, and (3) the maximum absolute plasma copeptin concentration. According to these three parameters, all patients were subclassified into one of five overall distinct groups of osmoregulatory defect (types A–E) (Table 2).
Table 2.
Summary of SIAD subtype definition in response to 3% NaCl infusion
| SIAD Subtype | Definition |
|---|---|
| A | Plasma copeptin consistently >38 pmol/L with a copeptin slope <95% CI for that of healthy persons (i.e., <0.66 pmol/L/mOsM/kg H2O) |
| B | Any plasma copeptin concentration with a positive copeptin slope >0.25 pmol/L/mOsM/kg H2O, but a low osmotic threshold <95% CI for that of healthy persons (i.e., <281 mOsM/kg H2O) |
| C | Plasma copeptin concentration between 2 and 38 pmol/L with a copeptin slope between −0.25 and 0.25 pmol/L/mOsM/kg H2O |
| D | Plasma copeptin concentration consistently <2 pmol/L regardless of the copeptin slope |
| E | Any plasma copeptin concentration with a copeptin slope <−0.25 pmol/L/mOsM/kg H2O |
Patients with a consistently supraphysiologically high copeptin release, exceeding the highest hormone value measured under stimulating conditions in the control group (38 pmol/L), and without relation to osmotic stimulation (i.e., copeptin slope less than the 95% CI for that of healthy persons; Figure 2A), were defined as having a type A defect.
Figure 2.
Individual patient response to saline-induced osmotic stimulation of all 50 patients with SIADH. Graphic characterization of the different subtypes of SIAD (A–E) illustrates all plasma copeptin data points of all individuals (n=50) during hypertonic saline infusion. Please note that the y axis varies according to variation in absolute plasma copeptin release. (F) Relative plasma copeptin response to osmotic stimulation, in percentages, when defining the mean copeptin concentration of each patient as 100%.
Patients showing a linear rise in copeptin levels in response to increasing serum osmolality (i.e., positive slope above 0.25 pmol/L/mOsM/kg H2O), despite an abnormally low osmotic threshold (i.e., below the lowest individual x axis intercept measured in healthy persons; Figure 2B), were allocated to type B defect.
Patients with copeptin levels constantly within the normal physiologic range (i.e., between 2 and 38 pmol/L), being nonsuppressible in the hypo-osmotic range, and not further stimulated in response to hypertonic saline infusion (i.e., copeptin slope between −0.25 and 0.25 pmol/L/mOsM/kg H2O; Figure 2C), were categorized as having type C defect.
Patients with appropriately suppressed plasma copeptin levels in the hypo-osmotic range (<2 pmol/L), showing an appropriate or inappropriate copeptin increase in response to saline infusion in the normo-osmotic range (i.e., positive slope within or less than the 95% CI for healthy persons; Figure 2D), were categorized as having type D defect. The threshold level of <2 pmol/L for copeptin suppression derives from previous data demonstrating that patients with neurogenic diabetes insipidus and complete AVP deficiency reveal copeptin levels constantly <2 pmol/L.8
Finally, patients showing a linear decline in plasma copeptin response to increasing serum osmolality (i.e., a negative copeptin slope below −0.25 pmol/L/mOsM/kg H2O; Figure 2, E and F) were classified as having a type E defect. A careful clinical and biochemical re-evaluation of patient records before hypertonic saline infusion revealed no evidence of baseline hypovolemia in this group (Supplemental Table 2).
The individual copeptin-osmolality slopes capturing the full information during osmotic stimulation are given in Figure 2, A–F, and their hemodynamic responses to saline infusion are illustrated in Supplemental Table 2.
The subtype classification was unaffected by differences in sodium concentrations before the hypertonic saline test, the rate of osmotic response to hypertonic saline, or resulting variances in the test duration (in our sample, 90–150 minutes). Upon comparing the copeptin-osmolality slopes using all data points and reducing them to only the first and last data pair, no difference became apparent (all P values for subgroups >0.9).
DNA Sequencing of Six Patients with SIAD of Type D Defect
In all patients with a type D defect, DNA was sequenced for gain-of-function mutations of the AVPR2 gene as previously described.9 No activating mutation (R137C, R137L, or F229V) was found for the AVPR2 gene, thus probably excluding constitutive AVPR2 activity.
Discussion
Our investigation substantially extends the database on different subtypes in SIAD by taking advantage of a now-available stable surrogate for circulating AVP. As hypothesized, in healthy controls, we found a close relationship between copeptin and osmolality with a significant increase in copeptin levels above an osmotic threshold of 282±4.3 mOsM/kg H2O. This threshold is in excellent agreement with previously reported results for AVP.10,11 Furthermore, the close correlation between changes in AVP and copeptin release strongly endorses the value of copeptin as a surrogate of AVP. These findings in healthy persons were used to quantify osmotic dysregulation in SIAD based on abnormalities in osmotic threshold, osmotic gain, and maximum copeptin concentration.
As expected, in almost all patients with SIAD, neither copeptin nor AVP was appropriately suppressed for the given hypo-osmolality, and no relationship was seen between serum osmolality and copeptin or AVP levels when looking at the entire patient group. Furthermore, median plasma copeptin and AVP levels were clearly higher at baseline in patients with SIAD than in controls. This observation confirms nonsuppression of AVP despite low serum osmolality as the key mechanism for hyponatremia in SIAD.4
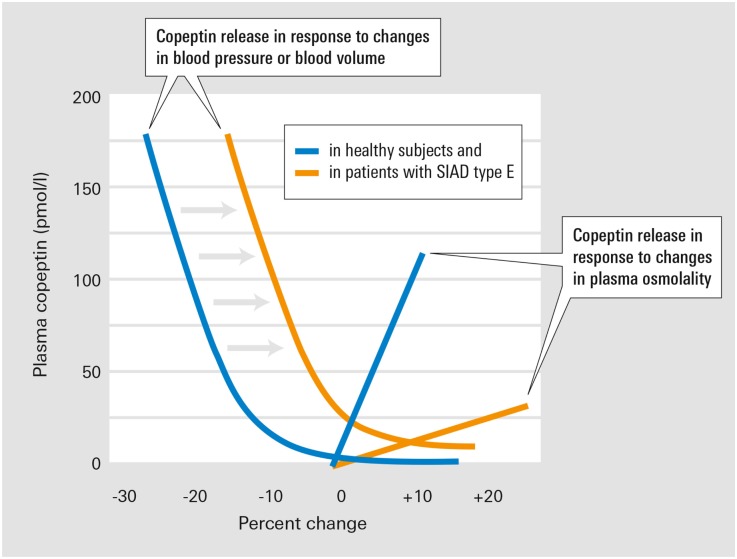
An important finding of our investigation is the discovery of a novel osmoregulatory defect, type E SIAD, characterized by a decline in plasma copeptin levels with increasing saline-stimulated serum osmolality. Kamoi et al. observed a similar pattern in two patients with untreated primary adrenal failure.12 They speculated that this response was related to nausea, which ameliorated under hypertonic saline infusion. However, nausea was not present in our patients and adrenal insufficiency was excluded. The pathophysiologic basis of this new subtype is not fully clear. Baseline hypovolemia could not be identified (Supplemental Table 2). Although mild volume depletion is sometimes difficult to detect,13,14 it is normally insufficient to generate a median rise in baseline copeptin levels to about 100 pmol/L (Supplemental Table 1). Rather, a substantial decrease in blood volume is considered necessary to nonosmotically affect AVP secretion.15 Mild changes in blood volume still may indirectly influence AVP regulation by altering the sensitivity of AVP response to osmotic stimuli. However, the expected response to elevating serum osmolality in a hypovolemic state is a gain in the osmoregulatory response with an enhancement rather than a decline in AVP/copeptin secretion,16,17 as observed in type E SIAD. Because our patients appeared euvolemic, reduced nonosmotic inhibition is one likely mechanism in type E SIAD. Such impaired inhibition may activate AVP release despite euvolemia and a serum osmolality below the osmotic set-point, consistent with a barostat reset. Figure 3 illustrates this hypothesized mechanism in patients with type E SIAD.
Figure 3.
Working hypothesis on osmotic and nonosmotic dysregulation underlying the SIADH subtype E. In healthy persons, minor decreases in BP or blood volume induce small increases in copeptin release. In patients with SIAD type E, the altered baroreceptor signaling mimics volume depletion despite normovolemia, thus shifting the copeptin response to the right (gray shaded arrows). Modified from ref.31 with permission.
Hemodynamic influences on AVP regulation are mediated at least in part by neural pathways originating from stretch-sensitive baroreceptors in the cardiac atria, aorta, and carotid sinus. Afferent nerve fibers from these receptors ascend to the nuclei of the solitary tract in the brainstem18 and project to the hypothalamic paraventricular and supraoptic nuclei.19 Impairment of the afferent baroreceptor signaling (e.g., via tumor infiltration or compression or other neuronal damage) may result in inappropriate nonosmotic stimulation of AVP release causing hypo-osmotic hyponatremia.20 Thus, volume expansion due to hypertonic saline infusion may compensate for the reduced sensitivity of baroreceptor-activated pathways, resulting in suppression of AVP release. Furthermore, an attenuated gain of the osmoregulatory response may be contributing to this defect. This would explain the overriding importance of the nonosmotic AVP regulation via volume expansion during hypertonic saline infusion. Theoretically, an alternative explanation of type E could be a reversed osmotic response from stimulation to inhibition. However, such reversal would imply positive feedback in the hypo-osmotic and negative feedback in the hyperosmotic range, promoting both rapidly decompensating hypo- and hyperosmolality.
Importantly, our study also revealed major deviations from the original report5 as to both subtype prevalence and phenotype: On the basis of our quantitative classification, 10% of patients (n=5) were assigned to type A SIAD, characterized by grossly elevated baseline copeptin concentrations unresponsive to osmotic regulation. Erratic and markedly elevated AVP fluctuations had previously been reported to characterize the osmoregulatory defect of type A.5 However, with the exception of patient 5, such fluctuations remained very moderate in our series, indicating a predominantly continuous release of AVP. The high hormone levels above the physiologic range suggest an ectopic tumor–associated AVP secretion. In fact, lung cancer has been the predominant cause of SIAD in our patient series (Table 1), and bronchogenic carcinoma is still the best-characterized entity of ectopic AVP production in SIAD.21,22
Regarding type B SIAD, our findings substantially differ from the previous subtype concept of SIAD.5 In contrast to the 33% prevalence reported by Zerbe et al.,5 we detected only one patient (patient 6, who had acute myeloid leukemia as the underlying disease) with a clear shift of the osmotic threshold to hypo-osmolar levels, but a maintained osmotic gain within the normal range. All other patients with a lowered osmotic threshold and an increase of copeptin with rising osmolality (patients 7–12; Supplemental Table 1) demonstrated a subnormal osmotic gain with an attenuated slope also in the normo-osmotic range. These observations resemble those from Smith et al.,10 who found in six of eight patients with SIAD an attenuated AVP response to hypertonic saline. Therefore, the incidence of true reset osmostat appears to be lower than hitherto reported.5,23 Most patients, rather, seem to present a combination of an abnormally lowered osmotic set-point for the initiation of AVP release, with blunted responsivity to an osmotic challenge, probably with a variable contribution of both elements. Hence, the reset osmostat may in part be considered as a less severe variant of the type C defect (found in 44% of patients), where responsivity to osmotic challenges is completely lost. Copeptin release in this subtype was stable at levels within the normal physiologic range but was not suppressed by hypotonicity or stimulated in response to osmotic stimulation; thus, it deviates from the previously described type C.5 Obviously, this defect could also result from an ectopic source of AVP release with a lower secretory activity. Thus, in many cases type A and type C may differ only on a quantitative level, while type B and type C may differ with regard to the activity of the magnocellular neurosecretory cells. Thus, we presume that distinct pathologic conditions may manifest in the same SIAD subtype by a common final pathway.
The central nervous mechanisms underlying the shift in osmotic response in type B or the blunted osmotic gain in type C defect remain elusive. In principle, it might be caused by changes in the magnocellular neurosecretory cells themselves. Previous studies have shown that pathologic conditions, such as epilepsy, axonal injury, and neuropathy, can cause a depolarizing shift in the γ-aminobutyric acid (GABA)–ergic response in neurons, reducing the magnitude of GABA-ergic inhibition in these cells and in some cases even producing GABA-ergic excitation.24 Intracellular clamp recording experiments could be used to determine whether a state of prolonged hypo-osmotic stress can also induce such an inhibitory-to-excitatory switch in GABA-ergic transmission.
Important evidence for altered central osmoregulation in SIAD comes from the observation that not only is AVP release inappropriately modified but the thirst threshold is lowered as well.10 In six patients (12%; patients 35–40), we found a compromised urinary diluting capacity despite suppressed copeptin levels. This suggests an AVP-independent mechanism of antidiuresis and resembles the osmoregulatory defect of type D with a prevalence similar to the former description.5 However, our patients differed from the previous case report in that plasma copeptin levels remained suppressed, even when serum osmolality increased into the normal or hyperosmotic range (Figure 2D). Thus, in contrast to the initial report, our patients also featured a clear pathology of hormone release above the normal osmotic threshold. The pathomechanism of type D SIAD remains unclear after constitutive AVP2R activity could be excluded. Still, this type of defect is particularly interesting because it raises the question of whether affected patients are capable of responding to treatment with AVPR2-blocking agents.6,25 Intriguingly, one patient in this group (patient 37) was successfully treated with tolvaptan, resulting in sustained normonatremia. Thus, it is conceivable that individual patients with type D SIAD become hypersensitive to the antidiuretic effects of AVP,26 possibly as a result of upregulated AVP2R expression.27
Although not systematically addressed in this study, treatment efficacy may vary depending on the underlying type of osmoregulatory defect. For example, patients with a type C defect are expected to respond better to a competitive V2R antagonist than are patients with a type A defect. The latter probably require a much higher drug dose to reach the same therapeutic effect on sodium increase. In a reset osmostat defect, the rise in plasma copeptin when serum sodium exceeds the new osmotic threshold (Figure 2B) may overcome V2R blockade, thereby possibly limiting the efficacy of vaptans. Some patients with type D antidiuresis may be independent of AVP action, thereby limiting the use of vaptans. In contrast, patients with a type E defect are expected to respond well to V2R antagonism, while fluid restriction is expected to show a rather limited effect on sodium concentration.
Our study does have some limitations. Owing to the demanding protocol and the often only transient manifestation of SIAD, hypertonic saline testing was performed only once in all but three patients. In these patients (patients 1, 37, and 42), however, the respective phenotype was reproducible. Furthermore, for safety reasons the saline-induced rise in serum sodium levels needed to be limited to a moderate extent and to patients in a stable clinical condition. Thus, the prevalence of the different subtypes of SIAD does not necessarily apply to critical care patients. In addition, the number of patients with high normal or elevated serum osmolality levels is relatively small, limiting the database for the response to osmolality levels above the physiologic osmotic threshold. However, in SIAD types C, D, and E, copeptin concentrations at the highest measured serum osmolality were not higher than the average copeptin concentration below the normal osmotic threshold, indicating that impaired osmoregulation persists in the normal range. Moreover, future studies still need to clarify the pathomechanism of type E SIAD. Separating volume effects from osmotic effects by inducing volume expansion without a corresponding rise in plasma osmolality should enable validation of the hypothesis of an altered baroreceptor signaling mimicking volume depletion despite normovolemia in type E defect.
A sample size of only 50 patients with SIAD may seem small. However, in comparison to previously published data addressing subtype analysis of SIAD,5,9 our series is quite large. Additional strengths of this work include the multicentric acquisition of data from controls, supporting the homogeneity and generalizability of the hypertonic saline test results as a basis for the phenotyping of the abnormalities in SIAD.
In conclusion, our data emphasize the heterogeneity of SIAD, and the copeptin-based classification system of SIAD provides important novel information as to different osmoregulatory defects.
Our findings support the concept of ectopic AVP secretion and AVP-independent mechanisms in subsets of patients with SIAD. Profoundly impaired endogenous osmoregulation is evident in most patients with type B, type C, and type D defects and seems to extend well into the normo-osmotic range. In addition, we describe a novel SIAD subtype (type E or barostat reset), which may be related to impaired nonosmotic inhibitory pathways, presumably in combination with altered osmoregulation. This definition of distinct subtypes may serve as a starting point to assess the heterogeneous treatment response in SIAD.
Concise Methods
Study Population
From March 2008 to March 2010, all hyponatremic patients presenting at the Medical Department of the University Hospital of Würzburg were screened for study participation. Eligibility criteria included the diagnosis of SIAD (persistent for at least 1 week before screening), serum sodium concentration ≤130 mmol/L, and age>18 years. Patients with symptomatic hyponatremia, acute infectious disease, vomiting and nausea, or critical illness (including patients in the intensive care unit) were excluded.
The diagnosis of SIAD was based on the following criteria: (1) serum osmolality<275 mOsM/kg; (2) urine osmolality>200 mOsM/kg; (3) clinical euvolemia (confirmed by isotonic saline challenge test if necessary); (4) urine sodium concentration>30 mmol/L; and (5) normal renal, adrenal, and thyroid function. In case of diagnostic uncertainty, euvolemia was ascertained by the response to an infusion of isotonic saline. Adrenal integrity was confirmed by a standard Synacthen test (250 μg 1–24 adrenocorticotropic hormone [ACTH] intravenously) in patients with baseline serum cortisol ≤5 µg/dl.
Sixty-eight nonsmoking, normal-weight healthy persons underwent the same (n=40) or a very similar (n=28) hypertonic saline infusion protocol at four different sites (Würzburg, Germany; London, United Kingdom; Basel, Switzerland; Biel-Bienne, Switzerland). The study was approved by the Ethics Committee of the University of Würzburg (21/08) and preregistered at ClinicalTrials.gov (NCT01341665). Written informed consent was obtained from all participants prior to participation.
Hypertonic Saline Infusion Test
Before testing, all participants had refrained from caffeine, nicotine, and alcohol for 24 hours and were fasted overnight. Patients were not under therapeutic fluid restriction before saline infusion, but had ad libitum access to nonalcoholic beverages until start of the infusion. Study participants were kept in a recumbent position for at least 1 hour before and throughout the test infusion. In the SIAD group and in most controls (n=40), hypertonic saline infusion (3%, 513 mOsM/L) was started between 8 am and 9 am at a rate of 0.1 ml/kg per minute. Blood samples for serum osmolality, sodium, plasma AVP, and copeptin levels were taken every 30 minutes, and BP, heart rate, and serum sodium at blood gas analysis (ABL800 FLEX; Drott Medical Technique GmbH, Vienna, Austria) were monitored at 15-minute intervals. Volume parameters were measured before and after the infusion, including serum creatinine, uric acid, urea, hematocrit, plasma renin, and aldosterone levels.
The saline infusion was stopped once serum sodium concentration had increased by ≥10 mmol/L. In patients with baseline serum sodium levels<120 mmol/L, efforts were made to increase serum sodium to levels between >120 and 125 mmol/L before the test. After the infusion, serum sodium levels were monitored for another 36 hours to prevent overly rapid correction.
The remaining 28 healthy persons underwent a slightly different infusion protocol: eight male volunteers received 2% saline infusion, 1 ml/kg per hour, from 8 pm to 8 am, followed by 5% saline infusion, 200 ml/hr, from 8 am to 1 pm Blood samples were taken at baseline as well as 12, 16, and 17 hours after start of the infusion.28 General linear models for repeated measurements confirmed a high degree of comparability of saline infusion–induced changes of osmolality and copeptin by the different protocols; hence, all controls were included in the analyses described below.
Clinical and Biochemical Assessment
Before hypertonic saline infusion, each patient underwent a comprehensive physical examination and an orthostatic challenge with measurements of BP and heart rate after 30 minutes in the supine position and after 1 minute standing in the upright position. Baseline biochemical evaluation (serum sodium, potassium, creatinine, urea, uric acid, hematocrit, albumin, glucose, triglycerides, osmolality, cortisol, aldosterone, thyroid-stimulating hormone, plasma ACTH, and renin measurement) and urine analyses (sodium, creatinine, uric acid, urea, potassium, osmolality) were performed in all patients and controls.
Routine clinical chemistry parameters were determined by automated analyzers in the Central Laboratory of the respective university hospital. Osmolality was measured by determination of freezing-point depression. Cortisol, ACTH, and thyroid-stimulating hormone levels were analyzed by established immunoassays (IMMULITE 2000; Siemens Medical Solution Diagnostic GmbH, Bad Nauheim, Germany). Aldosterone and renin measurements were performed by commercially available radioimmunoassays (Diagnostic Products Corp., Los Angeles, CA; Cis-Bio Intl., Marcoule, France). Copeptin was analyzed in a single batch with a commercial sandwich immunoluminometric assay (LUMItest CT-proAVP; B.R.A.H.M.S. AG, Hennigsdorf/Berlin, Germany) as previously described. Plasma AVP concentration was determined by RIA as described previously29 with a lower limit of detection of 0.4 pmol/L. Changes in blood volume were determined from the changes in hematocrit using the formula BV2/BV1=Hct1/Hct2, assuming no changes in circulating erythrocyte volume.30
Statistical Analyses
Characteristics of study participants are presented as mean±SD, median (IQR), and frequencies, as appropriate. Correlations are expressed by Pearson correlation coefficient (r) after log-normalization. Continuous variables among different groups were compared by the Kruskal–Wallis test. To discern different osmotic patterns, we inspected all graphs visually. All healthy controls exhibited a positive slope distinctly larger than 0.5 pmol/L/mOsM/kg H2O. We therefore empirically defined a slope between −0.25 and 0.25 pmol/L/mOsM/kg H2O as a neutral slope, a slope>0.25 pmol/L/mOsM/kg H2O as a positive slope, and a slope<−0.25 pmol/L/mOsM/kg H2O as a negative slope. The area of normality for plasma copeptin in relation to osmolality was calculated by using all 384 data points from the 68 healthy controls taken during the hypertonic saline infusion. Regression curves with 95% CIs were fitted for the individual regression analyses based upon all available data pairs that had been obtained at baseline and during test infusion. Cubic regression yielded the best fit (R2=0.57; Figure 1), but was only slightly better than the fit from linear regression (R2=0.53). SPSS software, version 21, was used for statistical analyses (SPSS Inc., Chicago, IL). All tests were two sided. A P value <0.05 was considered to represent a statistically significant difference.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank all patients and investigators who participated in this study, the interdisciplinary center of clinical research at the University of Würzburg, the German Research Society (DFG) for research grants and funding (W.K.F.), and the Swiss National Foundation (M.C.C., PP00P3-123346). This work has also been supported by grants from the Bundesministerium für Bildung und Forschung (BMBF01 EO1004). Finally, we thank Thermo Scientific Biomarkers Clinical Diagnostics for providing the reagents for measurement of copeptin.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080895/-/DCSupplemental.
References
- 1.Upadhyay A, Jaber BL, Madias NE: Incidence and prevalence of hyponatremia. Am J Med 119[Suppl 1]: S30–S35, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Waikar SS, Mount DB, Curhan GC: Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 122: 857–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein L, O’Connor CM, Leimberger JD, Gattis-Stough W, Piña IL, Felker GM, Adams KF, Jr, Califf RM, Gheorghiade M, OPTIME-CHF Investigators : Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 111: 2454–2460, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz WB, Bennett W, Curelop S, Bartter FC: A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med 23: 529–542, 1957 [DOI] [PubMed] [Google Scholar]
- 5.Zerbe R, Stropes L, Robertson G: Vasopressin function in the syndrome of inappropriate antidiuresis. Annu Rev Med 31: 315–327, 1980 [DOI] [PubMed] [Google Scholar]
- 6.Soupart A, Gross P, Legros JJ, Alföldi S, Annane D, Heshmati HM, Decaux G: Successful long-term treatment of hyponatremia in syndrome of inappropriate antidiuretic hormone secretion with satavaptan (SR121463B), an orally active nonpeptide vasopressin V2-receptor antagonist. Clin J Am Soc Nephrol 1: 1154–1160, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Robertson GL: Vaptans for the treatment of hyponatremia. Nat Rev Endocrinol 7: 151–161, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Fenske W, Quinkler M, Lorenz D, Zopf K, Haagen U, Papassotiriou J, Pfeiffer AF, Fassnacht M, Störk S, Allolio B: Copeptin in the differential diagnosis of the polydipsia-polyuria syndrome—revisiting the direct and indirect water deprivation tests. J Clin Endocrinol Metab 96: 1506–1515, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Decaux G, Vandergheynst F, Bouko Y, Parma J, Vassart G, Vilain C: Nephrogenic syndrome of inappropriate antidiuresis in adults: High phenotypic variability in men and women from a large pedigree. J Am Soc Nephrol 18: 606–612, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Smith D, Moore K, Tormey W, Baylis PH, Thompson CJ: Downward resetting of the osmotic threshold for thirst in patients with SIADH. Am J Physiol Endocrinol Metab 287: E1019–E1023, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Robertson GL, Mahr EA, Athar S, Sinha T: Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest 52: 2340–2352, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamoi K, Tamura T, Tanaka K, Ishibashi M, Yamaji T: Hyponatremia and osmoregulation of thirst and vasopressin secretion in patients with adrenal insufficiency. J Clin Endocrinol Metab 77: 1584–1588, 1993 [DOI] [PubMed] [Google Scholar]
- 13.McGee S, Abernethy WB, 3rd, Simel DL: The rational clinical examination. Is this patient hypovolemic? JAMA 281: 1022–1029, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Fenske W, Störk S, Koschker AC, Blechschmidt A, Lorenz D, Wortmann S, Allolio B: Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab 93: 2991–2997, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Verbalis JG: Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab 17: 471–503, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Robertson GL, Athar S: The interaction of blood osmolality and blood volume in regulating plasma vasopressin in man. J Clin Endocrinol Metab 42: 613–620, 1976 [DOI] [PubMed] [Google Scholar]
- 17.Dunn FL, Brennan TJ, Nelson AE, Robertson GL: The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest 52: 3212–3219, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andresen MC, Doyle MW, Jin YH, Bailey TW: Cellular mechanisms of baroreceptor integration at the nucleus tractus solitarius. Ann N Y Acad Sci 940: 132–141, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Renaud LP: CNS pathways mediating cardiovascular regulation of vasopressin. Clin Exp Pharmacol Physiol 23: 157–160, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Blessing WW, Sved AF, Reis DJ: Destruction of noradrenergic neurons in rabbit brainstem elevates plasma vasopressin, causing hypertension. Science 217: 661–663, 1982 [DOI] [PubMed] [Google Scholar]
- 21.Amatruda TT, Jr, Mulrow PJ, Gallagher JC, Sawyer WH: Carcinoma of the Lung with Inappropriate Antidiuresis. Demonstration of Antidiuretic-Hormone-Like Activity in Tumor Extract. N Engl J Med 269: 544–549, 1963 [DOI] [PubMed] [Google Scholar]
- 22.George JM, Capen CC, Phillips AS: Biosynthesis of vasopressin in vitro and ultrastructure of a bronchogenic carcinoma. Patient with the syndrome of inappropriate secretion of antidiuretic hormone. J Clin Invest 51: 141–148, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson GL, Aycinena P, Zerbe RL: Neurogenic disorders of osmoregulation. Am J Med 72: 339–353, 1982 [DOI] [PubMed] [Google Scholar]
- 24.De Koninck Y: Altered chloride homeostasis in neurological disorders: A new target. Curr Opin Pharmacol 7: 93–99, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Babey M, Kopp P, Robertson GL: Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat Rev Endocrinol 7: 701–714, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Berliner RW, Davidson DG: Production of hypertonic urine in the absence of pituitary antidiuretic hormone. J Clin Invest 36: 1416–1427, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block LH, Furrer J, Locher RA, Siegenthaler W, Vetter W: Changes in tissue sensitivity to vasopressin in hereditary hypothalamic diabetes insipidus. Klin Wochenschr 59: 831–836, 1981 [DOI] [PubMed] [Google Scholar]
- 28.Szinnai G, Morgenthaler NG, Berneis K, Struck J, Müller B, Keller U, Christ-Crain M: Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab 92: 3973–3978, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Fenske W, Störk S, Blechschmidt A, Maier SG, Morgenthaler NG, Allolio B: Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab 94: 123–129, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Kamoi K, Toyama M, Takagi M, Koizumi T, Niishiyama K, Takahashi K, Sasaki H, Muto T: Osmoregulation of vasopressin secretion in patients with the syndrome of inappropriate antidiuresis associated with central nervous system disorders. Endocr J 46: 269–277, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Robertson G: Posterior Pituitary. In: Endocrinology and Metabolism, 3rd Ed., edited by Baxter JD, Felig F, Frohman LA, New York, McGraw-Hill, 1995, pp 385–432 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.