Abstract
A pathogenic role of p53 in AKI was suggested a decade ago but remains controversial. Indeed, recent work indicates that inhibition of p53 protects against ischemic AKI in rats but exacerbates AKI in mice. One intriguing possibility is that p53 has cell type-specific roles in AKI. To determine the role of tubular p53, we generated two conditional gene knockout mouse models, in which p53 is specifically ablated from proximal tubules or other tubular segments, including distal tubules, loops of Henle, and medullary collecting ducts. Proximal tubule p53 knockout (PT-p53-KO) mice were resistant to ischemic and cisplatin nephrotoxic AKI, which was indicated by the analysis of renal function, histology, apoptosis, and inflammation. However, other tubular p53 knockout (OT-p53-KO) mice were sensitive to AKI. Mechanistically, AKI associated with the upregulation of several known p53 target genes, including Bax, p53-upregulated modulator of apoptosis-α, p21, and Siva, and this association was attenuated in PT-p53-KO mice. In global expression analysis, ischemic AKI induced 371 genes in wild-type kidney cortical tissues, but the induction of 31 of these genes was abrogated in PT-p53-KO tissues. These 31 genes included regulators of cell death, metabolism, signal transduction, oxidative stress, and mitochondria. These results suggest that p53 in proximal tubular cells promotes AKI, whereas p53 in other tubular cells does not.
AKI, often resulting from ischemic, nephrotoxic, and septic insults, is a devastating clinical condition that is associated with unacceptably high rates of mortality and morbidity.1,2 Moreover, AKI is frequently associated with and may contribute to the development of CKD.3–6 The pathogenesis of AKI is multifactorial, involving multiple cell types, cellular processes, and molecular mediators and regulators.7–9 Among them, p53 seems to play a pathologic role.10,11 In 2003, Dagher and colleagues12 showed the first evidence for the involvement of p53 in a rat model of ischemic AKI. We and others established a critical role of p53 and associated DNA damage response in cisplatin-induced AKI using both cell culture and animal models including global p53 knockout mice.13–16 p53 was also implicated in kidney injury induced by folic acid, aristolochic acid, and glycerol injection.17–19 As a result, inhibition of p53 may offer an effective therapy for AKI,12,16,20 and small interfering RNA (siRNA) targeting p53 has been tested for AKI in clinical trials (http://clinicaltrials.gov/ct2/results?term=I5NP&Search=Search). In AKI, p53 was shown to regulate several genes involved in cell death and survival, including Bcl-2–associated x (Bax), p53 upregulated modulator of apoptosis (PUMA)-α, CD27 binding protein (Siva), and p21.12,21–23 However, a global analysis of p53-regulated genes in AKI is not available.
Despite these findings, recent studies suggest that the pathologic role of p53 in AKI is far more complicated and that its inhibition may not always be beneficial. In this regard, the pharmacological p53 inhibitor pifithrin-α, when given after initial injury in AKI, promoted renal fibrosis in rats.24 Even more surprisingly, pifithrin-α and global p53 deletion exacerbated ischemic AKI in mice.25 Although this study indicates that the action of p53 is animal species-dependent, mechanistically, it is puzzling how p53 may be injurious to AKI in rats but protective in mice. One explanation is that AKI in rats depends largely on renal tubular injury, whereas AKI in mice depends more on inflammation and inflammatory damage. This possibility is based on the assumption that p53 in different cell/tissue types may have distinct or opposite roles in the pathogenesis of AKI: whereas leukocyte p53 is anti-inflammatory and thus, renoprotective, tubular p53 is a critical trigger and/or mediator of AKI. The anti-inflammatory function of leukocyte p53 was recently suggested by the experiments using chimeric mouse models.25 However, the pathogenic role of tubular p53 has yet to be established by using kidney tubule-specific p53 knockout models.
In the present study, we established two conditional knockout mouse models, in which p53 was specifically ablated from proximal tubules or other tubular segments. Knockout of p53 from proximal tubules but not other tubules protected against ischemic and cisplatin nephrotoxic AKI. AKI-associated upregulation of several known p53 target genes was shown to be attenuated in proximal tubule p53 knockout (PT-p53-KO) kidney tissues. Additional global gene expression analysis showed the induction of 371 genes by ischemic AKI in wild-type kidneys, of which the induction of 31 genes was abrogated in PT-p53-KO tissues. These 31 genes included regulators of cell death, metabolism, signal transduction, oxidative stress, and mitochondrial carriers. Together, the results suggest that p53 in proximal tubules contributes critically to AKI by regulating multiple genes involved in kidney tissue injury, remodeling, and repair.
Results
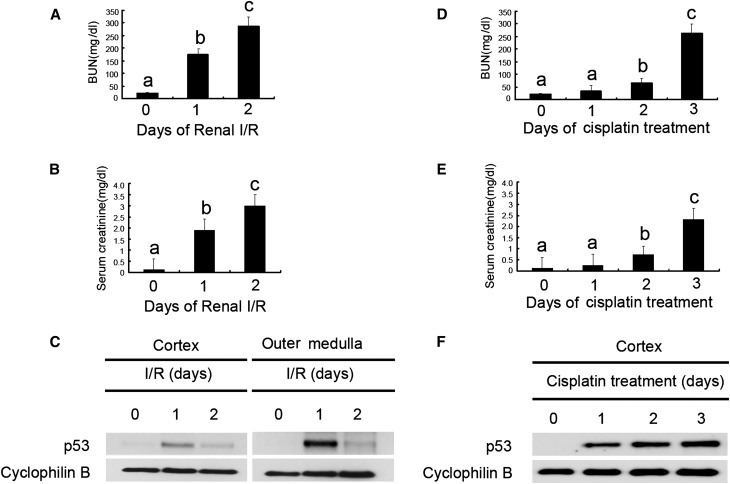
We first verified p53 expression in kidney tissues during AKI. Bilateral renal ischemia-reperfusion induced AKI in C57/Bl6 mice as indicated by marked increases in BUN and serum creatinine (Figure 1, A and B); p53 expression was very low in sham control (day 0) but induced by ischemic AKI in renal cortex and outer medulla (Figure 1C), and p53 induction seemed significantly higher in outer medulla than renal cortex. Temporally, p53 induction peaked at day 1 of reperfusion and then decreased by day 2. In cisplatin nephrotoxic AKI, p53 was induced in kidneys gradually from day 1 to day 3 and accompanied by increases in BUN and serum creatinine (Figure 1, D–F). These data, confirming previous studies,12–16 indicate the induction of p53 in AKI.
Figure 1.
p53 is induced in ischemic and cisplatin nephrotoxic AKI in mice. Male C57BL/6 mice were (A–C) subjected to 28 minutes of bilateral renal ischemia followed by 0–2 days of reperfusion (n=20) or (D–F) injected with 30 mg/kg cisplatin (n=28) for 0–3 days of examination. In A, B, D, and E, blood samples were collected at indicated time points to measure BUN and serum creatinine. Data were expressed as means±SDs; the bars with different superscripts (a–c) in each panel were significantly different (P<0.05). In C and F, kidneys were harvested to extract cortical and outer medulla tissues at indicated time points for immunoblot analysis of p53 and cyclophilin B (loading control). I/R, ischemia/reperfusion.
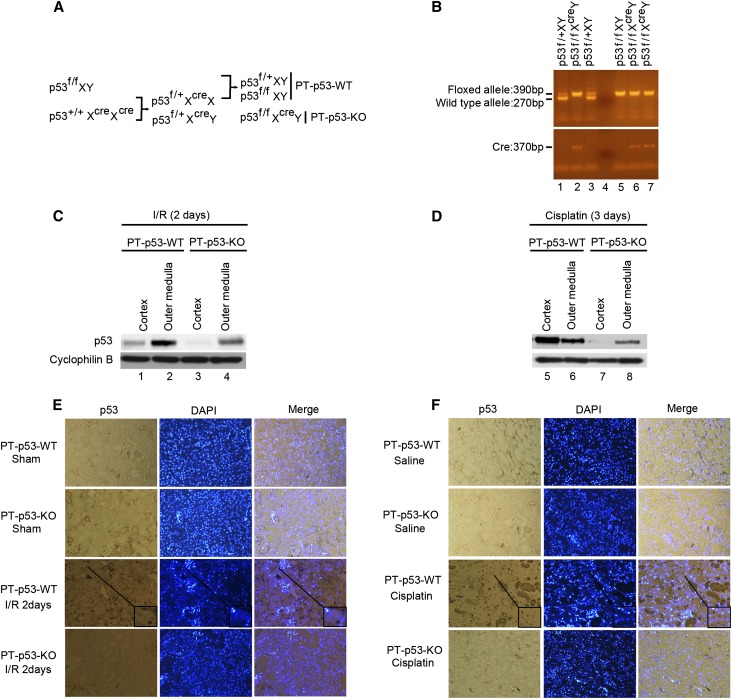
To determine the pathologic role of tubular p53, we initially established a conditional knockout mouse model, in which p53 was deleted specifically from kidney proximal tubules. The breeding protocol is shown in Figure 2A. Briefly, male mice bearing the floxed p53 alleles (p53f/f XY) were crossed with female phosphoenolpyruvate carboxykinase-cAMP-response element (PEPCK-Cre) transgenic mice (p53+/+XcreXcre) that express Cre recombinase under the control of a modified PEPCK promoter, which directs Cre expression predominantly in kidney proximal tubular cells.26 After the first round of breeding, heterozygous female progenies (p53f/+XcreX) were selected to further breeding with p53f/fXY males to generate PT-p53-KO (p53f/fXcreY) and proximal tubule p53 wild-type (PT-p53-WT) littermate mice for testing.
Figure 2.
Creation and characterization of the PT-p53-KO mouse model. (A) Breeding protocol for generating PT-p53-KO mice. After confirming genotype, male littermate mice at 8–10 weeks of age were used for experiments. (B) Representative gel images of PCR-based genotyping. Genomic DNA was extracted from tail biopsy and amplified to detect wild-type and floxed alleles of p53 and PEPCK-Cre allele as indicated. (C and D) Kidney cortex and outer medulla were collected from PT-p53-KO and PT-p53-WT littermate mice after I/R injury or cisplatin injection for immunoblot analysis of p53 and cyclophilin B. (E and F) Immunohistochemical staining of p53 in kidney cortical tissues of wild-type and PT-p53-KO mice after I/R or cisplatin injury. The selected areas are shown as insets at a higher magnification. Original magnification, ×200. DAPI, 4′,6-diamidino-2-phenylindole; I/R, ischemia/reperfusion.
To verify the genotypes, three sets of PCR were conducted for each mouse. The genotype of PT-p53-KO mice was indicated by (1) amplification of the 390-bp fragment of the floxed allele, (2) lack of amplification of the 270-bp fragment of the wild-type allele, and (3) amplification of the 370-bp fragment of the Cre gene (Figure 2B, lanes 2, 6, and 7). The absence of the Cre gene ensured the genotype of wild-type (PT-p53-WT) mice (Figure 2B, lanes 1, 3, and 5). At the protein level, because p53 in control kidney tissues was very low (Figure 1), we compared p53 expression in PT-p53-KO and wild-type tissues after AKI. After ischemic AKI, wild-type mice showed p53 expression in both cortical and outer medullary tissues (Figure 2C, lanes 1 and 2) that was suppressed in PT-p53-KO tissues (Figure 2C, lanes 3 and 4). Similarly, p53 expression during cisplatin-induced AKI was significantly lower in PT-p53-KO kidney tissues than wild-type tissues (Figure 2D, lanes 7 and 8 versus 5 and 6). In immunohistochemistry (Figure 2, E and F), p53 was induced during AKI in the nuclei of wide-spread tubular cells (Figure 2, E, inset and F, inset) and the cytoplasm of a subset of tubules in wild-type kidney tissues, and again, p53 expression was largely attenuated in PT-p53-KO tissues, verifying tubular p53 ablation in this conditional model.
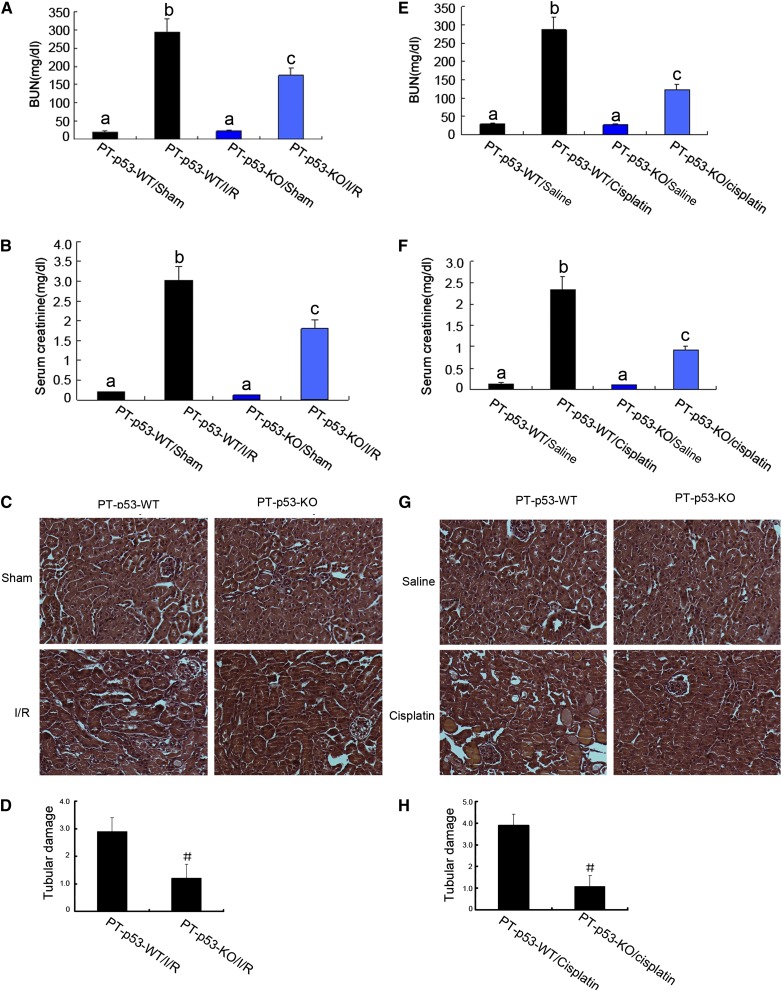
Without AKI treatment, these mice showed similarly low levels of BUN and serum creatinine, indicating normal renal function. Renal ischemia-reperfusion induced severe renal failure in wild-type mice, raising BUN and serum creatinine levels to 295 and 3.02 mg/dl, respectively (Figure 3, A and B). In contrast, after the same treatment, PT-p53-KO littermate mice had 175 mg/dl BUN and 1.8 mg/dl serum creatinine (significantly lower than the levels of the wild type). Similarly, at day 3 of cisplatin treatment, wild-type mice developed severe renal failure, with 287 mg/dl BUN and 2.34 mg/dl serum creatinine, whereas PT-p53-KO mice had 122 mg/dl BUN and 0.92 mg/dl serum creatinine (Figure 3, C and D). Histologic analysis confirmed that both cisplatin and ischemia-reperfusion induced severe kidney tissue damage in PT-p53-WT mice, which was significantly ameliorated in PT-p53-KO mice (Figure 3, C and G). In wild-type mice, the tubular damage scores were 2.9 and 3.9 after ischemic and cisplatin AKI, respectively, whereas the scores were markedly decreased to 1.2 and 1.0 after ischemic and cisplatin AKI, respectively, for PT-p53-KO tissues (Figure 3, D and H).
Figure 3.
Ischemic and cisplatin-induced AKI is attenuated in PT-p53-KO mice. (A–D) Wild-type and PT-p53-KO littermate mice were subjected to 28 minutes of bilateral renal ischemia followed by 2 days of reperfusion or sham operation as control. (E–H) Wild-type and PT-p53-KO littermate mice were injected with 30 mg/kg cisplatin or saline as control for 3 days. (A, B, E, and F) Blood samples were collected for measurements of BUN and serum creatinine levels. Data were expressed as means±SDs (n=8); the bars with different superscripts (a–c) were significantly different from each other (P<0.05). In C and G, kidney cortical tissues were stained with hematoxylin-eosin to show histology. Original magnification, ×200. In D and H, tubular damage in I/R and cisplatin-treated cortical tissues was semiquantified as pathologic scores. Data were expressed as means±SDs (n=8). #P<0.05, significantly different from PT-p53-WT group. I/R, ischemia/reperfusion.
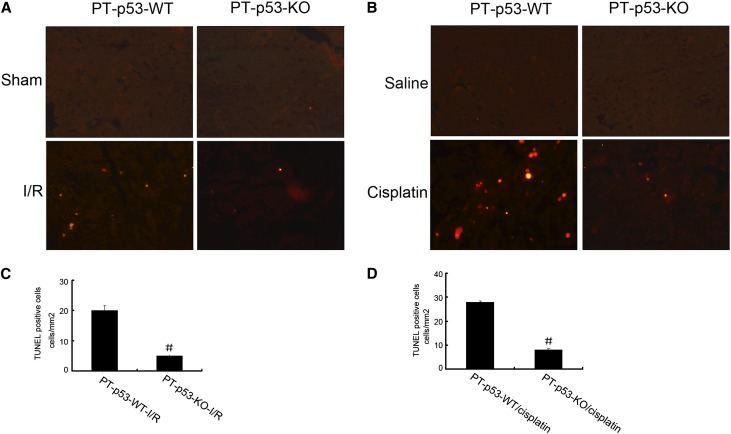
Apoptosis contributes significantly to the pathogenesis of AKI,27 and p53 is known as a trigger of apoptosis under cell stress.28 We, therefore, analyzed apoptosis in kidney cortical tissues by terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay. Representative TUNEL staining is presented in Figure 4, A and B. No apoptosis was detected in the kidney tissues of sham-operated or saline-injected mice. However, after renal ischemia-reperfusion and cisplatin treatment, significant apoptosis was induced in kidney cortical tissues in wild-type mice. Importantly, much less apoptosis was induced in PT-p53-KO mice. This observation was further verified by counting apoptotic cells in cortical and outer medulla regions from independent experiments (Figure 4, C and D). We further examined the infiltration of inflammatory cells in ischemic AKI. As shown in Supplemental Figure 1, renal ischemia-reperfusion induced the infiltration of leukocytes, including neutrophils and macrophages, into wild-type kidney tissues, which was suppressed in PT-p53-KO tissues. The decrease of inflammation in PT-p53 KO mice correlated with lower apoptosis, supporting the inference that tubular apoptosis may be a driving factor for inflammation.29
Figure 4.
AKI-associated renal apoptosis is suppressed in PT-p53-KO mice. Wild-type and PT-p53-KO littermate mice were subjected to 28 minutes of bilateral renal ischemia followed by 2 days of reperfusion or injected with 30 mg/kg cisplatin for 3 days. Control mice were sham-operated or injected with saline. Kidney cortical tissues were examined by TUNEL assay to reveal apoptosis. (A and B) Representative images of TUNEL staining. Original magnification, ×200. (C and D) Quantification of TUNEL-positive cells in the tissues from each condition. Data were expressed as means±SDs (n=8); #P<0.05, significantly different from the PT-p53-WT group. I/R, ischemia/reperfusion.
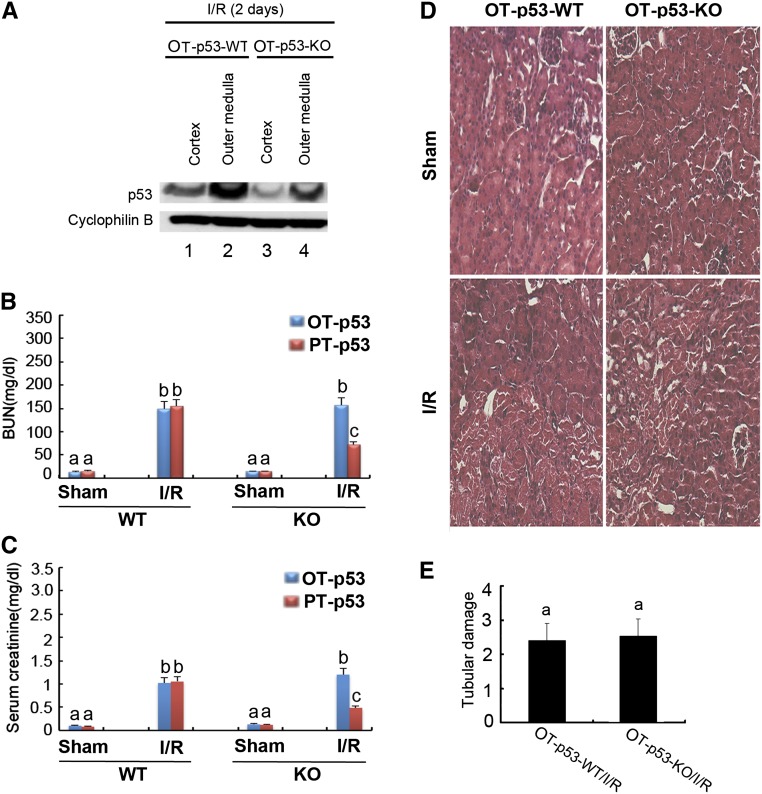
To determine if p53 in renal tubular segments other than proximal tubules contributes significantly to AKI, we established the other tubular p53 knockout (OT-p53-KO) model by crossing p53-floxed mice with kidney-specific cadherin (Ksp-Cre) mice. Ksp promoter drives Cre expression (and therefore, floxed gene deletion) in distal tubules, loops of Henle, and medullary collecting ducts with significantly fewer effects on proximal tubules (http://jaxmice.jax.org/strain/012237.html).30 As shown in Figure 5A, p53 expression during ischemic AKI seemed lower in OT-p53-KO kidney tissues than wild-type tissues (Figure 5A, lanes 3 and 4 versus 1 and 2). However, ischemic AKI shown by BUN, serum creatinine, and tubular damage was not significantly ameliorated in OT-p53-KO mice (Figure 5, B–E). It was noticed that 28 minutes of renal ischemia-reperfusion induced moderate AKI in OT-p53-WT mice (Figure 5) but severe AKI in PT-p53-WT mice (Figure 3). For comparison, we subjected the PT-p53 mice to 23 minutes of ischemia-reperfusion and showed that PT-p53-KO mice were protected in this model of moderate AKI (Figure 5, B and C, Supplemental Figure 2). Together, these results suggest that p53 in proximal tubules (and not p53 in other tubular segments) plays a critical pathogenic role in ischemic AKI.
Figure 5.
Ischemic AKI is diminished in PT-p53-KO mice, but not in OT-p53-KO mice. OT-p53-KO and wild-type littermate mice were subjected to 28 minutes of bilateral renal ischemia, whereas PT-p53-KO and wild-type littermate mice were subjected to 23 minutes of bilateral renal ischemia. Samples were collected after 2 days of reperfusion. (A) p53 expression in kidney tissues of OT-p53-KO and wild-type littermate mice. (B and C) BUN and serum creatinine, respectively. Data were expressed as means±SDs; bars with different superscripts (a–c) in each panel were significantly different (P<0.05). (D) Representative histology of cortical tissues from OT-p53-KO and wild-type littermate mice after hematoxylin-eosin staining. Original magnification, ×200. (E) Tubular damage score in renal cortical tissues from OT-p53-KO and wild-type littermate mice. I/R, ischemia/reperfusion.
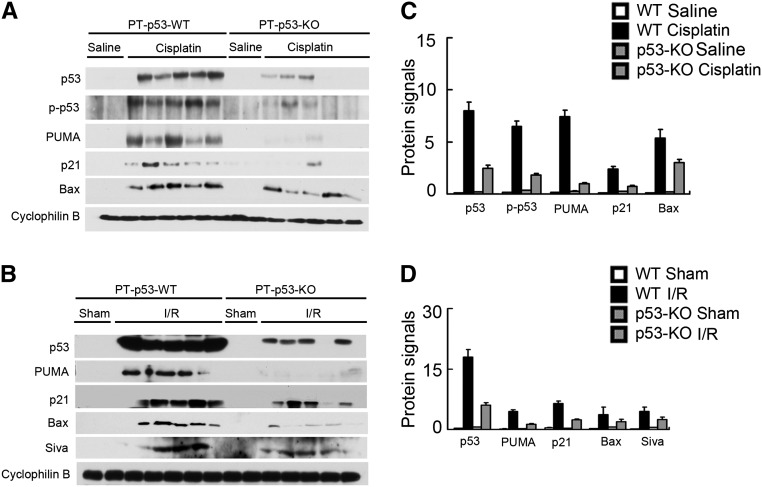
p53 regulates cell death and survival through both transcription-dependent and -independent mechanisms.29 Interestingly, several apoptotic genes subjected to p53 regulation have been reported to be upregulated in AKI. For example, PUMA-α is induced by cisplatin in kidney tissues,21,31,32 whereas Bax and Siva are induced in ischemic AKI.12,23 In addition, p21, a p53 target gene involved in cell cycle arrest and cytoprotection, is induced markedly in various AKI models.22,33,34 We, therefore, analyzed the expression of these genes to determine their dependence on proximal tubular p53. As shown in Figure 6, both p53 and its serine-15 phosphorylated form were induced by cisplatin in kidney cortical tissues in PT-p53-WT mice. Concomitantly, Bax, PUMA-α, and p21 were induced. In PT-p53-KO mice, the induction of p53 as well as Bax, PUMA-α, and p21 was suppressed (Figure 6A). In ischemic AKI, Bax, PUMA-α, Siva, and p21 were markedly induced along with p53 in wild-type kidney tissues, and the inductive response was largely diminished in PT-p53-KO tissues (Figure 6B). The dependence of Bax, PUMA-α, Siva, and p21 induction on proximal tubule p53 was further substantiated by densitometric analysis of the immunoblots (Figure 6, C and D). Together, these data suggest that p53 in proximal tubules is responsible for the induction of key cell death regulatory genes during AKI.
Figure 6.
Induction of p53 target genes in AKI is suppressed in PT-p53-KO mice. Wild-type and PT-p53-KO littermate mice were subjected to 28 minutes of bilateral renal ischemia followed by 2 days of reperfusion or injected with 30 mg/kg cisplatin for 3 days. Control mice were subjected to sham operation or injected with saline. The lysate of kidney cortex and outer medulla was collected for immunoblot analysis of p53, p-p53 (ser15), p21, PUMA-α, Bax, Siva, and Cyclophilin B by using specific antibodies. (A and B) Representative immunoblots. (C and D) Densitometry of immunoblot signals. For densitometric analysis, the protein signal of the wild-type control group (n=5) was arbitrarily set as one, and the signals of other conditions were normalized (n=8). I/R, ischemia/reperfusion.
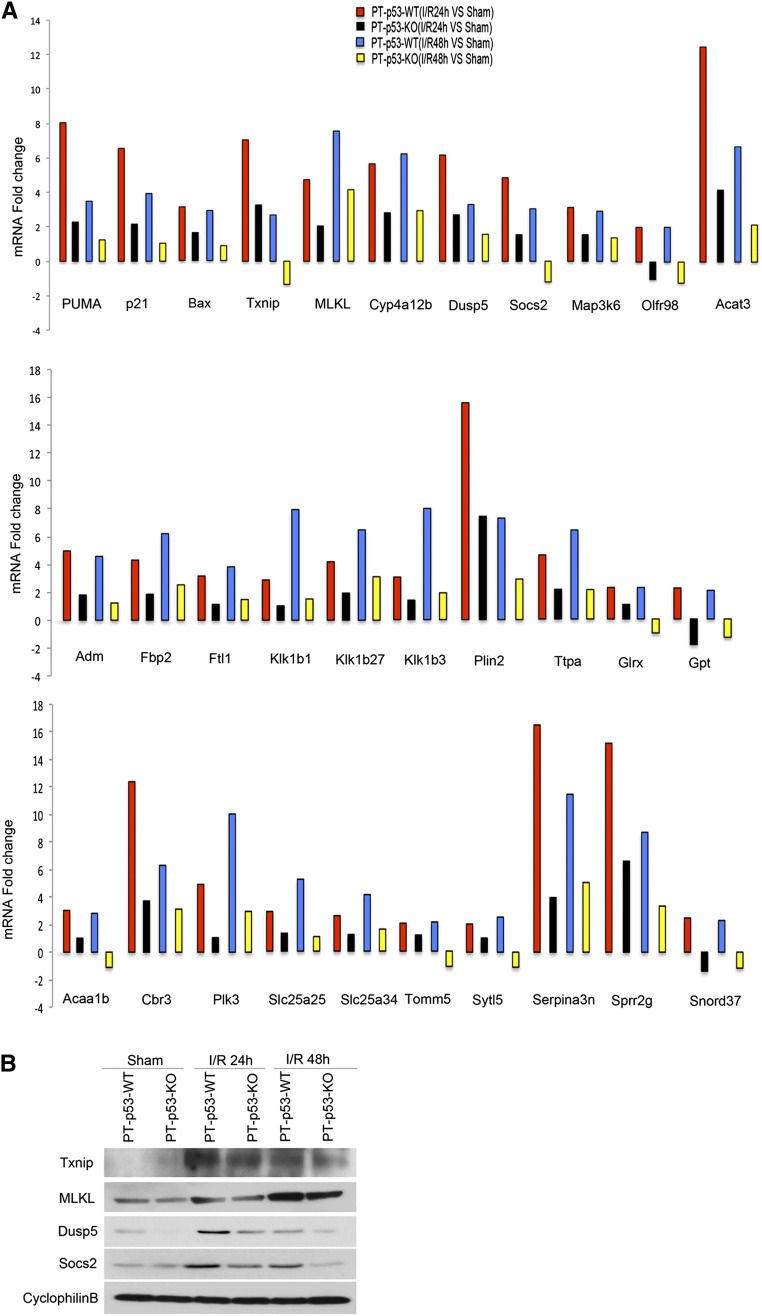
To gain a comprehensive understanding of p53-regulated gene expression in AKI, we conducted a global gene expression analysis. To this end, RNA samples were isolated from kidney cortex, amplified, and hybridized to a cDNA microarray containing 26,515 genes. In wild-type mice, renal ischemia followed by 24 and 48 hours of reperfusion led to the induction of 769 and 800 genes, respectively, of which 341 genes were induced at both time points (Supplemental Tables 1–3). Compared with wild type, the expression of 204 and 257 genes at 24 and 48 hours of reperfusion, respectively, was suppressed in PT-p53-KO kidneys (Supplemental Tables 4 and 5). In these genes, 83 were suppressed at both reperfusion time points (Supplemental Table 6). Notably, 31 of 83 suppressed genes were among 371 genes that were induced by both 24 and 48 hours of reperfusion in wild-type kidneys (Figure 7A). These 31 genes included the regulators of cell death, signal transduction, metabolism, oxidative stress, and mitochondrial carriers (Table 1). Seven of thirty-one genes have been reported to be induced by ischemia-reperfusion in kidneys, brain, heart, or lung. Interestingly, the analysis with the JASPAR CORE database (http://jaspar.genereg.net/) showed that 7 of 31 genes contain p53 binding sites in their promoters (Table 2). By immunoblot analysis, we further confirmed the induction of thioredoxin-interacting protein (Txnip), mixed lineage kinase domain–like protein (MLKL), dual specificity phosphatase 5 (DUSP5), and suppressors of cytokine signaling 2 (SOCS2) during renal ischemia-reperfusion in wild-type kidneys, which was suppressed in PT-p53-KO tissues (Figure 7B).
Figure 7.
The induction of 31 genes during ischemic AKI is suppressed in PT-p53-KO mice. Wild-type (n=4) and PT-p53-KO (n=4) littermate mice were subjected to 28 minutes of bilateral renal ischemia followed by 24 or 48 hours of reperfusion or sham operation as control. (A) Total RNA samples isolated from renal cortical tissues were analyzed by a cDNA microarray containing 26,515 genes. The amount of the mRNA of each gene from the I/R group was divided by the amount of sham control to calculate the fold change. (B) The lysate of renal cortical tissues was examined by immunoblot analysis of Txnip, MLKL, DUSP5, SOCS2, and cyclophilin B. I/R, ischemia/reperfusion.
Table 1.
Thirty-one genes induced by p53 during renal ischemia-reperfusion
| Cell Death/Survival | Signal Transduction | Metabolic Processes | Mitochondrial Carrier | Oxidative Stress | Others |
|---|---|---|---|---|---|
| Baxa,b | Dusp5b | Acat3 | Slc25a25 | Cyp4a12b | Plk3 |
| PUMAa,b | Socs2a,b | Acaa1b | Slc25a34 | Txnipb | SYTL5 |
| p21a,b | Map3k6 | Adm | Tomm5 | Serpina3na | |
| Txnipb | Olfr98a | Cbr3 | Sprr2g | ||
| MLKL | Fbp2 | Snord37 | |||
| Ftl1 | |||||
| Glrxb | |||||
| Gpta | |||||
| Klk1b1 | |||||
| Klk1b27 | |||||
| Klk1b3 | |||||
| Plin2 | |||||
| Ttpa |
Genes with p53 binding sites in their promoters.
Genes that have been reported to be induced in ischemia-reperfusion of kidney, brain, heart, or liver.
Table 2.
p53 binding sites in the promoter of seven induced genes
| Genes | p53 Binding Sites |
|---|---|
| PUMA | Site 1 AGGGGGTGCCCGGGCATGT |
| PUMA | Site 2 ACAGACATGCCCGGGCAGCC |
| p21 | Site 1 AGGAACATGTCTTGACATGT |
| p21 | Site 2 CTGAACATGTCAAGACATGT |
| Bax | Site 1 GCAGGCCCGGGCTTG |
| Bax | Site 2 ACAAGCCCGGGCCTG |
| Socs2 | Site 1 GCATCCCCAGGCATCTCCTT |
| Olfr98 | Site 1 CTGGGCATGTCTGGGAAGGT |
| Olfr98 | Site 2 CCAGACATGCCCAGAGGTGT |
| Gpt | Site 1 AGGTGCATGCTCGGGCTTGC |
| Gpt | Site 2 AGGTGCATGCTCGGGCTTGC |
| Serpina3n | Site 1 AAGCATATGCCCTGACATGT |
| Serpina3n | Site 2 GGAAACATGTCAGGGCATAT |
Discussion
A pathologic role of p53 in AKI has been supported by several lines of evidence. p53 is upregulated and activated in a variety of AKI models accompanied by the induction of downstream target genes.12–23 p53 inhibitors and siRNA afford protection against ischemic and cisplatin nephrotoxic AKI in rats.12,16,20 In addition, genetic and pharmacologic inhibition of p53 is protective during cisplatin AKI in mice.13–16 Based on these findings, recent clinical trials have tested the therapeutic effect of p53 siRNA in human patients (http://clinicaltrials.gov/ct2/results?term=I5NP&Search=Search). However, recent studies have suggested a much more complex role of p53 in AKI. Specifically, it was shown that, although systemic inhibition of p53 protects against ischemic AKI in rats,12 it enhances AKI in mice.25 One intriguing possibility is that AKI in rats depends largely on p53-mediated renal tubular injury, whereas AKI in mice depends more on inflammation and inflammatory damage that is suppressible by p53 in leukocytes. The anti-inflammatory function of leukocyte p53 was recently suggested by using chimeric mouse models.25 Our study has now shown the injurious role of proximal tubular p53 in AKI. Together, these studies indicate that p53 in different cell/tissue types plays distinguished roles in the pathogenesis of AKI. Moreover, by global gene expression analysis, we have identified several classes of genes that are upregulated through p53 in ischemic AKI, gaining new insights into the mechanism of p53 regulation in the disease condition.
This study has established two renal tubular p53 knockout mouse models. One model was generated by crossing PEPCK-Cre mice, and the other was generated by crossing Ksp-Cre with p53-floxed mice. PEPCK-Cre results in the deletion of floxed genes in the majority (70%–80%) of proximal tubules without deletion in other tubular segments.26 In contrast, Ksp-Cre leads to floxed gene deletion mainly in collecting ducts, loops of Henle, and distal tubules but very weak or limited deletion in proximal tubules.30 Using these two models, our study determined the effects of specific p53 deletion from proximal tubules or other tubules on AKI. Considering the pleiotropic role of p53 in cell cycle, differentiation, and apoptosis, one may expect significant effects of tubular p53 ablation on kidney development, histology, and function. However, these effects were not observed in either PT-p53-KO or OT-p53-KO mice. This result is not surprising, because global p53 knockout does not result in notable abnormalities in kidneys, although some newborn pups were reported to have an aberrant renal phenotype.35 It is possible that p53 knockout delays kidney development, but at adult age, the kidneys have completed the development process and gained normal histology and function. Regardless, PT-p53-KO mice were shown to be resistant to AKI. Of note, p53 ablation from proximal tubules in this model was obvious but incomplete (Figure 2, C and D), an observation that is consistent with the 70%–80% recombination efficiency of the PEPCK-Cre model.26 The notable renoprotection shown in this model further supports a role of proximal tubular p53 in AKI. In contrast, OT-p53-KO mice were as sensitive to AKI as wild-type animals, suggesting that p53 in other tubular segments does not seem to have a critical role in AKI. However, these results do not rule out the involvement of other tubular segments in the pathogenesis of AKI. Other tubules, such as distal tubules and thick ascending limbs, may contribute to AKI through p53-independent mechanisms.
p53, although known as a tumor suppressor, responds rapidly to cellular stress in both cancer and normal cells. Depending on the severity of the stress, p53 may result in cell cycle arrest and/or cell death under various pathophysiologic conditions. Mechanistically, p53 is a key regulator of gene expression in the nucleus. Nonetheless, p53 may also participate in cell regulation through transcription-independent mechanisms in the cytoplasm.29,36 In a rat model of ischemic AKI, p53 induction was detected mainly in the cytoplasm of renal tubular cells, with limited signals in the nucleus.12 In our study using mouse models, p53 was induced in the nuclei of widespread tubular cells, although some cytoplasmic staining was revealed in a subset of tubular cells. The subcellular localization difference in p53 observed in these two studies was most likely caused by the variations in the animal models. Notably, p53-related gene expression was shown in both studies, supporting the significance of transcriptional regulation by p53 in AKI.
Previous studies showed the induction of several p53-regulated genes in AKI.12,21–23 Consistently, we confirmed the induction of Bax, PUMA-α, Siva, and p21 during ischemic and cisplatin AKI. Importantly, the inductive response was largely diminished in PT-p53-KO mice, indicating that proximal tubular p53 is the key to the expression of these genes. Interestingly, although Bax, PUMA-α, and Siva are known to be proapoptotic, p21 arrests the cell cycle and has a critical cytoprotective role in kidneys.27,37 The paradoxical gene induction by p53 in AKI remains puzzling. It is plausible that, in response to stress, the cells initially activate defensive or cytoprotective mechanisms, including p21 induction through p53; however, as the insult continues and/or intensifies, prodeath gene expression is triggered. Of note, both p53-dependent and -independent mechanisms contribute to p21 induction in AKI.33
To further elucidate the p53-mediated gene expression in AKI, we conducted a global gene expression analysis using a cDNA microarray containing 26,515 genes. In wild-type mice, renal ischemia-reperfusion for 24–48 hours led to the consistent induction of 341 genes. The number of genes identified in our microarray analysis is, therefore, much larger than the number in a previous report,38 in which only 91 genes were shown to be upregulated in ischemic AKI. The larger number of genes identified in our analysis was likely because of the larger coverage (26,515 versus 9000 genes) of the cDNA microarray used in our study. In addition, the previous study examined the effect of unilateral renal ischemia-reperfusion in Swiss–Webster mice, whereas bilateral ischemia-reperfusion in C57/Bl6 mice was analyzed in the current study. Despite these differences, 14 genes were shown to be induced in both studies.
In PT-p53-KO mice, ischemic AKI was also associated with significant changes in gene expression. Notably, compared with wild-type tissues, 83 genes were suppressed in PT-p53-KO kidneys during ischemic AKI, suggesting that these genes are subjected to p53 regulation in proximal tubules in AKI. Interestingly, in these 83 genes, 31 genes were among 371 genes that were induced by renal ischemia-reperfusion in wild-type kidneys (Figure 7A). The analysis suggests that these 31 genes are induced during ischemic AKI in a p53-dependent manner. These 31 genes include important regulators of cell death, oxidative stress, metabolism, mitochondrial carriers, and others (Table 1). Seven of thirty-one genes have been reported to be induced by ischemia-reperfusion in kidneys or other organs. Moreover, 7 of 31 genes contain p53 binding sites in their promoters (Table 2), suggesting a direct transcriptional regulation of these genes by p53 in AKI. Immunoblot analysis further confirmed the induction of several interesting genes (i.e., MLKL, DUSP5, Socs2, and Txnip) during renal ischemia-reperfusion in wild-type mice, which was suppressed in PT-p53-KO mice (Figure 7B). MLKL is a newly identified key regulator of necrosis downstream of the receptor interacting protein kinase 3.39,40 p53-Dependent induction of MLKL suggests that, in addition to apoptosis, p53 may also be involved in tubular cell necrosis in AKI. In support of this possibility, PT-p53-KO mice showed less necrotic damage in renal tubules after ischemic or cisplatin AKI than wild-type mice (Figure 3). SOCS2 is a suppressor of cytokine signaling that blocks the Janus kinase/signal transducer and activation of the transcription (JAK/STAT) pathway, which has been implicated in ischemic injury in kidneys.41 DUSP5 is an inducible nuclear phosphatase that functions as both an inactivator of and a nuclear anchor for extracellular signal-regulated kinase in mammalian cells. p53-mediated induction of DUSP5 may, therefore, block extracellular signal-regulated kinase signaling and its role in ischemic AKI.42 Txnip is a modulator of cellular redox state that contributes to cell apoptosis.43 In mice, knockout of Txnip impairs mitochondrial function but protects myocardium from ischemia-reperfusion injury.44 Thus, the pathologic role of p53 in AKI may not be limited to the regulation of tubular cell death/survival; rather, it may be extended to the regulation of metabolism, oxidative stress, and mitochondria. It is important to recognize that the differential gene expression shown in PT-p53-KO and wild-type kidney tissues after AKI may also be secondary to the lower injury associated with the PT-p53-KO model. Nonetheless, identification of these genes lays a foundation for additional investigation to distinguish the genes that are directly regulated by p53 from those genes that are secondary to the effect of p53 ablation-related protection.
In conclusion, this study has established two conditional knockout mouse models, in which p53 is specifically deleted from either proximal tubules or other tubular segments. By using these models, the study has shown a critical pathogenic role of proximal tubular p53 (but not other tubular p53) in AKI. Global gene expression analysis has further identified 31 genes induced in ischemic AKI directly or indirectly through p53, which may regulate cell death, metabolism, oxidative stress, and mitochondrial activity. Additional investigation in these directions will generate significant new insights into p53 regulation of kidney injury, tissue remodeling, and repair.
Concise Methods
Reagents and Antibodies
Chemicals, including the 3-hydroxy-4′-nitro-2-naphthanilide chloroacetate (specific esterase) kit for leukocyte staining, were purchased from Sigma-Aldrich. Antibodies included anti-cyclophilin B, anti-Txnip, anti-SOCS2, anti-MLKL, antineutrophil, and antimacrophage from Abcam, Inc.; anti-PUMA, anti-p53, and antiphospho-p53 (ser15) from Cell Signaling Technology; and anti-p21, anti-Bax, and anti-DUSP5 from Santa Cruz. All secondary antibodies were from Thermo Fisher Scientific.
Animals
Ksp-Cre and p53flox/flox mouse lines were obtained from The Jackson Laboratory. PEPCK-Cre mice were provided by Volker Haase (Vanderbilt University School of Medicine, Nashville, TN). p53flox/flox mice were crossed with PEPCK-Cre mice to produce PT-p53-KO (p53flox/floxXcreY) and PT-p53-WT littermate mice, which is depicted in the breeding protocol in Figure 1A. Similarly, p53flox/flox mice were crossed with Ksp-Cre mice to produce OT-p53-KO mice. The mice were housed in the animal facility of the Charlie Norwood Veterans Affairs Medical Center under a 12-hour light/dark pattern with free access to food and water. All animal experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the Charlie Norwood Veterans Affairs Medical Center.
AKI Models
Ischemic and cisplatin nephrotoxic AKI was induced in male mice of 8–10 weeks of age as described recently.45–47 For cisplatin injury, mice were intraperitoneally injected with a single dose of cisplatin at 30 mg/kg. For ischemic AKI, renal pedicles were exposed by flank incisions for the indicated duration of bilateral clamping followed by release for reperfusion. During ischemic AKI, the body temperatures of the mice were controlled at approximately 36.5°C. Sham control mice underwent the same operation without renal pedicle clamping.
Microarray
Total RNA was isolated from kidney cortical tissues for reverse transcription using the Ambion WT Expression Kit (Life Technologies). The synthesized cDNAs were fragmented and biotin-labeled using the GeneChip WT Terminal Labeling Kit (Affymetrix). The labeled cDNAs were then hybridized onto the Affymetrix Mouse Gene 2.0ST Array according to the manufacturer’s protocol. After 16 hours of hybridization, the arrays were washed and stained using Affymetrix GeneChip Fluidics Station 450 Systems. The stained arrays were scanned with an Affymetrix GeneChip Scanner 3000.
Renal Function, Histology, and TUNEL Assay
BUN and serum creatinine were measured using commercial kits from Stanbio Laboratory to indicate renal function.45–47 Renal histology was subjected to a blind examination after hematoxylin and eosin staining. Tissue damage was scored according to the percentage of damaged tubules: 0, no damage; 1, less than 25% damage; 2, 25%–50% damage; 3, 50%–75% damage; 4, more than 75% damage. The criteria of tubular damage included the loss of brush border, tubular dilation, cast formation, and cell lysis. TUNEL assay was conducted using the In Situ Cell Death Detection Kit from Roche Applied Science. For quantification, 10–20 fields were randomly selected from each tissue section to count the TUNEL-positive cells per millimeter2.
Immunohistochemistry and Immunoblot Analyses
For immunohistochemistry, kidney tissues were fixed with 4% paraformaldehyde and paraffin-embedded to collect tissue sections, which were then deparaffinized and incubated with 0.1 M sodium citrate (pH 6.0) at 65°C for antigen retrieval. After the incubation with blocking buffers, tissue sections were exposed sequentially to the primary antibody, the biotinylated secondary antibody, and the Tyramide Signal Amplification Biotin System (PerkinElmer). The signals were developed with the VECTASTAIN ABC Standard Kit and DAB Peroxidase Substrate Kit (Vector Laboratories) following the protocols of the manufacturer. Cell nuclei were counterstained with Hoechst 33342. For immunoblot analysis, tissue lysate from kidney cortex and outer medulla was extracted for SDS–polyacrylamide electrophoresis, blotting, and antibody exposure by standard procedures.
Statistical Analyses
Qualitative data, including immunoblots and tissue histology images, are representatives of at least three experiments. Quantitative data are expressed as means±SDs. Statistical analysis was conducted using the GraphPad Prism software. Multiple groups were compared with ANOVA followed by Tukey’s post-tests. Statistical differences between two groups were determined by two-tailed unpaired or paired t tests. P<0.05 was considered significantly different.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Volker Haase at Vanderbilt University School of Medicine for the PEPCK-Cre mouse line.
The study was supported, in part, by National Natural Science Foundation of China Grants 81370791 and 81100507, National Basic Research Program of China 973 Program 2012CB517600, and the National Institutes of Health and Department of Veterans Administration of the United States.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080902/-/DCSupplemental.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY: Yes, AKI truly leads to CKD. J Am Soc Nephrol 23: 967–969, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK: Acute kidney injury: A springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla LS, Kimmel PL: Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int 82: 516–524, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Okusa MD, Chertow GM, Portilla D, Acute Kidney Injury Advisory Group of the American Society of Nephrology : The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol 4: 520–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Sharfuddin AA, Molitoris BA: Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Dagher PC: Apoptosis in ischemic renal injury: Roles of GTP depletion and p53. Kidney Int 66: 506–509, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Jiang M, Dong Z: Regulation and pathological role of p53 in cisplatin nephrotoxicity. J Pharmacol Exp Ther 327: 300–307, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC: P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: Protective role of a p53 inhibitor. J Am Soc Nephrol 14: 128–138, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Cummings BS, Schnellmann RG: Cisplatin-induced renal cell apoptosis: Caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther 302: 8–17, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Jiang M, Yi X, Hsu S, Wang CY, Dong Z: Role of p53 in cisplatin-induced tubular cell apoptosis: Dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287: F1140–F1147, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Pabla N, Huang S, Mi QS, Daniel R, Dong Z: ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 283: 6572–6583, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z: Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293: F1282–F1291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y: Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int 82: 537–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Fu P, Huang XR, Liu F, Lai KN, Lan HY: Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J Am Soc Nephrol 21: 31–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homsi E, Mota da Silva S, Jr., Machado de Brito S, Bouçada Inácio Peixoto E, Butori Lopes de Faria J, Janino P: p53-Mediated oxidative stress and tubular injury in rats with glycerol-induced acute kidney injury. Am J Nephrol 33: 49–59, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E: siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 20: 1754–1764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z: Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4056–4066, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Megyesi J, Safirstein RL, Price PM: Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest 101: 777–782, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singaravelu K, Padanilam BJ: p53 target Siva regulates apoptosis in ischemic kidneys. Am J Physiol Renal Physiol 300: F1130–F1141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dagher PC, Mai EM, Hato T, Lee SY, Anderson MD, Karozos SC, Mang HE, Knipe NL, Plotkin Z, Sutton TA: The p53 inhibitor pifithrin-α can stimulate fibrosis in a rat model of ischemic acute kidney injury. Am J Physiol Renal Physiol 302: F284–F291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton TA, Hato T, Mai E, Yoshimoto M, Kuehl S, Anderson M, Mang H, Plotkin Z, Chan RJ, Dagher PC: p53 is renoprotective after ischemic kidney injury by reducing inflammation. J Am Soc Nephrol 24: 113–124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Havasi A, Borkan SC: Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chipuk JE, Green DR: Dissecting p53-dependent apoptosis. Cell Death Differ 13: 994–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Daemen MA, van ’t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA: Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest 104: 541–549, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P: A minimal Ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1824–1836, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Vousden KH, Prives C: Blinded by the light: The growing complexity of p53. Cell 137: 413–431, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Han X, Yue J, Chesney RW: Functional TauT protects against acute kidney injury. J Am Soc Nephrol 20: 1323–1332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuruya K, Yotsueda H, Ikeda H, Taniguchi M, Masutani K, Hayashida H, Hirakata H, Iida M: Involvement of p53-transactivated Puma in cisplatin-induced renal tubular cell death. Life Sci 83: 550–556, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Megyesi J, Udvarhelyi N, Safirstein RL, Price PM: The p53-independent activation of transcription of p21 WAF1/CIP1/SDI1 after acute renal failure. Am J Physiol 271: F1211–F1216, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Yu F, Megyesi J, Safirstein RL, Price PM: Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol 289: F514–F520, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Saifudeen Z, Dipp S, El-Dahr SS: A role for p53 in terminal epithelial cell differentiation. J Clin Invest 109: 1021–1030, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vousden KH, Lane DP: p53 in health and disease. Nat Rev Mol Cell Biol 8: 275–283, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Price PM, Megyesi J, Safirstein RL: Cell cycle regulation: Repair and regeneration in acute renal failure. Semin Nephrol 23: 449–459, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P: Differential gene expression following early renal ischemia/reperfusion. Kidney Int 63: 1714–1724, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X: Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148: 213–227, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Jiang H, Chen S, Du F, Wang X: The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148: 228–243, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Yang N, Luo M, Li R, Huang Y, Zhang R, Wu Q, Wang F, Li Y, Yu X: Blockage of JAK/STAT signalling attenuates renal ischaemia-reperfusion injury in rat. Nephrol Dial Transplant 23: 91–100, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG: Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. J Pharmacol Exp Ther 325: 732–740, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Lu J, Holmgren A: Thioredoxin system in cell death progression. Antioxid Redox Signal 17: 1738–1747, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Brooks C, Wei Q, Cho SG, Dong Z: Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z: Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z: Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest 121: 2709–2722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.