Abstract
Spleen tyrosine kinase (SYK) has an important role in immunoreceptor signaling, and SYK inhibition has accordingly attenuated immune-mediated injury in several in vivo models. However, the effect of SYK inhibition on autoantibody production remains unclear, and SYK inhibition has not been studied in an autoimmune model of renal disease. We, therefore, studied the effect of SYK inhibition in experimental autoimmune GN, a rodent model of antiglomerular basement membrane disease. We show glomerular SYK expression and activation by immunohistochemistry in both experimental and clinical disease, and we show that treatment with fostamatinib, a small molecule kinase inhibitor selective for SYK, completely prevents the induction of experimental autoimmune GN. In established experimental disease, introduction of fostamatinib treatment led to cessation of autoantibody production, reversal of renal injury, preservation of biochemical renal function, and complete protection from lung hemorrhage. B cell ELISpot and flow cytometric analysis suggest that short-term fostamatinib treatment inhibits the generation and activity of antigen-specific B cells without affecting overall B-cell survival. Additionally, fostamatinib inhibited proinflammatory cytokine production by nephritic glomeruli ex vivo and cultured bone marrow-derived macrophages in vitro, suggesting additional therapeutic effects independent of effects on autoantibody production that are likely related to inhibited Fc receptor signaling within macrophages in diseased glomeruli. Given these encouraging results in an in vivo model that is highly applicable to human disease, we believe clinical studies targeting SYK in GN are now warranted.
Spleen tyrosine kinase (SYK) is a nonreceptor tyrosine kinase that has a well characterized role in the intracellular signaling cascade for classic immunoreceptors, such as activatory Fc receptors (FcRs) and the B-cell receptor.1 SYK is critical for mediating FcR-induced responses in a variety of cell types, including myeloid cells,2,3 dendritic cells,4 and mast cells.5 In B cells, SYK-mediated B-cell receptor signaling is necessary for cell maturation and survival, and SYK-deficient cells developmentally arrest at the pro–B-cell stage.6,7
SYK has, therefore, emerged as a potential therapeutic target in autoimmune and allergic disease. Genetic disruption of SYK expression using small interfering RNA, antisense oligonucleotides, or inducible deletion has been shown to attenuate responses in animal models of airway hyper-responsiveness and asthma.8,9 A number of small molecule inhibitors directed against SYK are also in development. One such agent—fostamatinib—has progressed to late-phase clinical trials, where it has shown biologic activity in patients with rheumatoid arthritis (RA).10,11
We have previously reported that SYK inhibition with fostamatinib—the orally bioavailable prodrug of active moiety R406—is remarkably effective in reducing injury in rat nephrotoxic nephritis (NTN), even when treatment was delayed until disease was well established.12 It has similarly shown efficacy in models of autoimmune disease, including murine lupus,13,14 collagen-induced arthritis (CIA),15,16 and spontaneous diabetes in nonobese diabetic (NOD) mice.17
Although inhibition of antibody-dependent FcR-mediated responses has been shown to contribute to the reduction in injury seen in these models, the specific impact of SYK inhibition on autoantibody production remains unclear. No effect on circulating autoantibody levels was observed in CIA or murine lupus. Conversely, in NTN, there was a significant reduction in autologous rat anti-rabbit antibody titer in animals pretreated with fostamatinib. In NOD mice, treatment resulted in a reduction in antiglutamate decarboxylase antibodies but not anti-insulin antibodies. These conflicting results are of particular interest given that the role of SYK in antibody production in mature B cells and plasma cells is not defined, because constitutively SYK-deficient B cells arrest at the pro-B cell stage. Several factors may account for these discrepancies, such as timing and duration of SYK inhibitor exposure and potential differences in response to auto- or alloantigens. Notably, while modeling autoimmune diseases, all of the reported nonspontaneous models rely on immunization with alloantigen or passive transfer of antibody that acts as a planted alloantigen in target tissue; therefore, their translation to clinical autoimmunity is limited.
To address these issues, we have studied the effects of SYK inhibition in experimental autoimmune GN (EAG). This rodent model closely recapitulates the immunobiology and pathology of Goodpasture’s (or antiglomerular basement membrane [anti-GBM]) disease. In our laboratory, it is induced by immunizing susceptible rat strains with a well defined recombinant rat protein (noncollagenous domain of the α3-chain of type IV collagen [α3(IV)NC1]),18,19 the universal Goodpasture autoantigen that is germane to human disease.20 Both the model and clinical disease are critically dependent on the development of autoantibodies directed against this autoantigen, and both manifest features of crescentic GN and alveolar hemorrhage.21,22 EAG, therefore, can be regarded as a genuine model of autoimmunity, and because it is characterized by the ongoing production of a directly pathogenic, disease-relevant autoantibody, it more accurately reproduces clinical disease than our previous studies in NTN, and in particular, it allows for study of pathogenic humoral responses in addition to renal and lung end organ damage.
Here, we show the presence of the target protein, SYK, in both experimental and clinical anti-GBM disease. We show that SYK inhibition prevents the induction of autoimmunity in this model. In established EAG, we report, for the first time, significant attenuation of pathogenic humoral autoimmune responses along with reversal of end organ damage. Taken together, these data provide a clear rationale for targeting SYK in autoimmune glomerular disease.
Results
SYK Is Expressed and Activated in Experimental and Clinical Anti-GBM Disease
In normal rat kidney (Figure 1A), staining for total SYK (T-SYK) was often positive in distal tubular epithelial cells but consistently negative in glomeruli. Staining for phosphorylated SYK (P-SYK) was negative in both glomeruli and tubular epithelial cells, suggesting that SYK may be expressed but not activated in the latter. In nephritic glomeruli from animals 18 days after induction of EAG (Figure 1B), the same pattern of tubular staining was observed. Within diseased glomeruli, staining for both T-SYK and P-SYK was positive and localized to areas of endocapillary and extracapillary proliferation. Double staining confirmed strong colocalization of P-SYK to ED-1–positive macrophages (Figure 1G).
Figure 1.
SYK is expressed and activated in experimental and clinical anti-GBM disease. Immunostaining for T-SYK and P-SYK in (A) normal rat kidney shows tubular SYK expression without activation (phosphorylation) and no evidence of glomerular expression, (B) nephritic rat kidney shows a similar pattern of tubular staining accompanied by both SYK expression and activation within inflamed glomeruli, (C) thin basement membrane (TBM) disease shows no glomerular SYK expression, and (D) anti-GBM disease shows SYK expression and activation localized to areas of crescent formation and in occasional endocapillary cells (thin arrows). (E and F) T-SYK and P-SYK expression and activation in lymphoid follicles in rat spleen tissue and human lymph node (LN). (G) Double immunostaining for ED-1 (blue) and P-SYK (brown) shows significant colocalization of P-SYK to infiltrating macrophages (black arrows), the predominant infiltrating leukocyte in this model, along with a small number of ED-1–negative cells (white arrow) that express P-SYK. A–F are T-SYK N-19 (Santa Cruz Biotechnology), rat P-SYK Tyr323 (Abcam, Inc.), and human P-SYK Tyr535/536 (Cell Signaling Technology) immunoperoxidase stains with hematoxylin counterstain. Original magnification, ×200. G is rat ED-1 (Serotec) immunophosphatase stain and rat P-SYK Tyr 323 immunoperoxidase stain without counterstain. Original magnification, ×400.
In renal tissue from patients with benign, nonproliferative glomerular pathology (thin basement membrane lesion), a pattern of distal tubular expression for T-SYK was observed, which was comparable with that seen in rat tissue, and glomerular staining for both T-SYK and P-SYK was negative (Figure 1C). In biopsies from patients with anti-GBM disease, there was strong staining for both T-SYK and P-SYK that localized predominantly to areas of crescent formation within abnormal glomeruli (Figure 1D) and occasionally, proliferating cells within the glomerular tuft.
We also noted that immunostaining of both rat and human lymphoid tissue (Figure 1, E and F, respectively) for T-SYK and P-SYK was strongly positive and centered on follicles and marginal zone areas, consistent with the well characterized role for SYK in the generation of adaptive immune responses.
SYK Inhibition with Fostamatinib Prevents the Induction of Autoimmunity
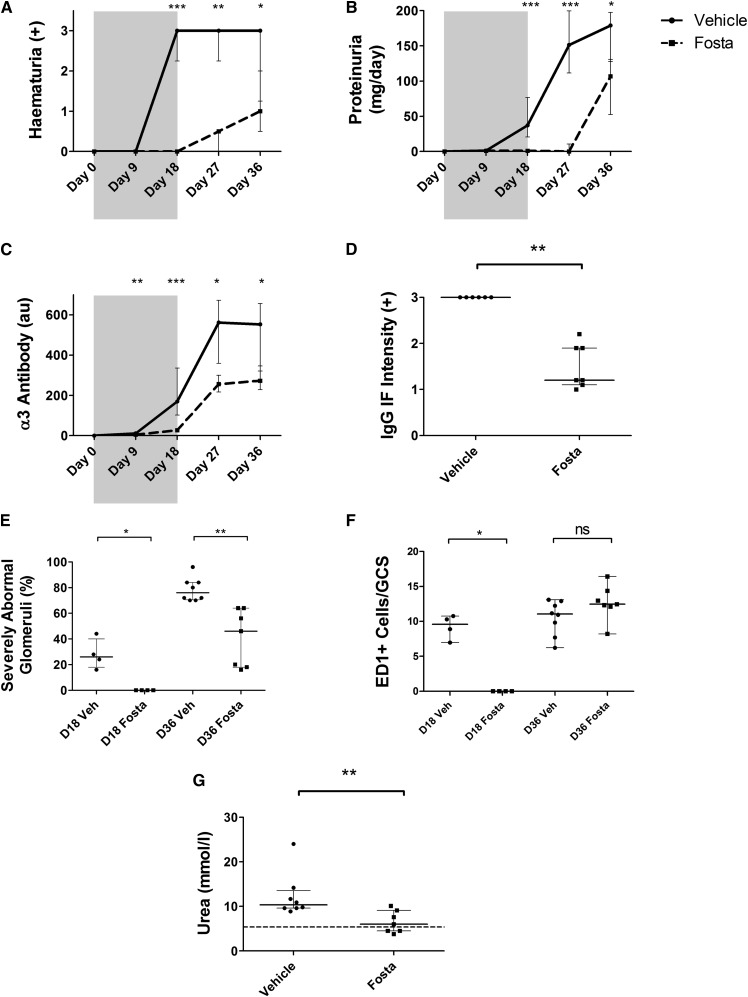
To establish if SYK has a role in the pathogenesis of autoimmunity in EAG, we examined the effect of SYK inhibition using fostamatinib in a preventive in vivo study. Wistar Kyoto rats (n=8/group) were treated with either vehicle or fostamatinib by oral gavage from 1 hour before immunization with α3(IV)NC1 to day 18 after disease induction. This time point was chosen, because previous studies have shown reproducible disease in all animals by day 18. From day 18 on, treatment was discontinued, and animals were monitored for another 18-day treatment-free period to observe any progression of disease after treatment withdrawal. A dose of 40 mg/kg two times daily was chosen based on our previous dose–response study in NTN that showed maximal biologic effect without toxicity, and it is consistent with the dose used in murine lupus studies.13
During the treatment period, fostamatinib-treated rats were completely protected from hematuria (100% reduction compared with vehicle controls; P<0.001) (Figure 2A) and proteinuria (97% reduction; P<0.001) (Figure 2B). Circulating autoantibodies to the GBM were virtually undetectable in treated animals (84% reduction; P<0.001) (Figure 2C). Fostamatinib-treated animals had entirely normal renal histology at day 18, whereas vehicle-treated animals had severe pathology (i.e., >50% of the glomerular tuft affected by necrosis or crescent formation) affecting 26% of glomeruli (100% reduction; P=0.02) (Figure 2E). Similarly, fostamatinib treatment completely prevented glomerular macrophage infiltration at day 18 (100% reduction; P=0.02) (Figure 2F). These observations suggest that SYK activity is an absolute requirement for the induction of autoimmunity in this model.
Figure 2.
SYK inhibition prevents induction of autoimmunity in experimental anti-GBM disease. (A) Hematuria, (B) proteinuria, and (C) circulating anti-GBM antibody levels in fostamatinib- (Fosta; dashed plots) and vehicle-treated (solid plots) animals during the 18-day treatment period (shaded) and 18-day withdrawal period (unshaded) showing complete protection from these key autoimmune phenotypes during fostamatinib exposure. Protection was sustained after treatment withdrawal until day 36. (D) In keeping with reduced circulating antibody levels, fostamatinib treatment resulted in less deposited anti-GBM antibody as detected by direct immunofluorescence (IF) at day 36. (E) Quantification of glomerular pathology in fostamatinib- and vehicle-treated (Veh) animals at the end of the 18-day treatment period (D18) shows full protection from nephritis with fostamatinib treatment; at the end of the 18-day withdrawal period (D36), glomerular injury was still reduced in the treated group. (F) Fostamatinib treatment completely prevented macrophage infiltration until day 18. After treatment withdrawal, macrophage infiltration proceeded and by day 36, was comparable with untreated animals at day 18. (G) Fostamatinib-treated animals had significantly lower levels of serum urea at day 36; the dashed line represents the upper reference limit for serum urea in healthy rats (5.4 mmol/L). Animals: n=8/group. Data are reported as median per group±interquartile range. GCS, glomerular cross-section; ns, not significant. *P<0.05; **P<0.01; ***P<0.001.
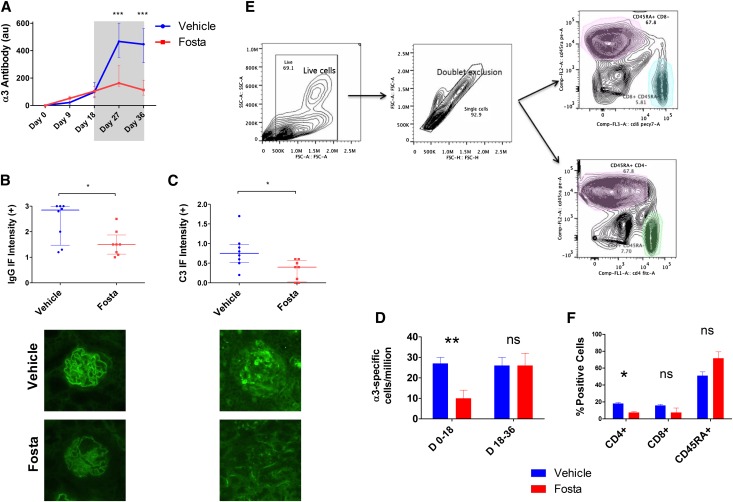
When treatment was withdrawn at day 18, the treated group developed typical features of disease, although with sustained protection compared with controls at day 36. Fostamatinib-treated animals had preserved biochemical renal function as reflected by normal serum urea levels (73% increase in the vehicle-treated group; P<0.01) (Figure 2G), and there remained significant differences in hematuria (66% reduction in fostamatinib group; P=0.03), proteinuria (40% reduction; P=0.01), and severe glomerular pathology (38% reduction; P=0.001) (Figure 2E, Supplemental Figure 1A). These indices of disease severity approximated the indices in untreated animals at day 18, suggesting that disinhibition of SYK by treatment withdrawal at day 18 allowed the natural history of disease to be restored. It is notable, however, that anti-GBM antibody levels in the fostamatinib-treated animals reached a lower plateau level than vehicle controls (51% reduction; P=0.02) (Figure 2C), suggesting possible immunomodulatory effects after exposure to SYK inhibition, which was previously suggested in murine lupus.14 In keeping with lower levels of circulating autoantibodies, there was less deposited antibody detected in the glomeruli of fostamatinib-treated animals at day 36 (60% reduction; P=0.002) (Figure 2D).
SYK Inhibition Is an Effective Treatment in Established EAG
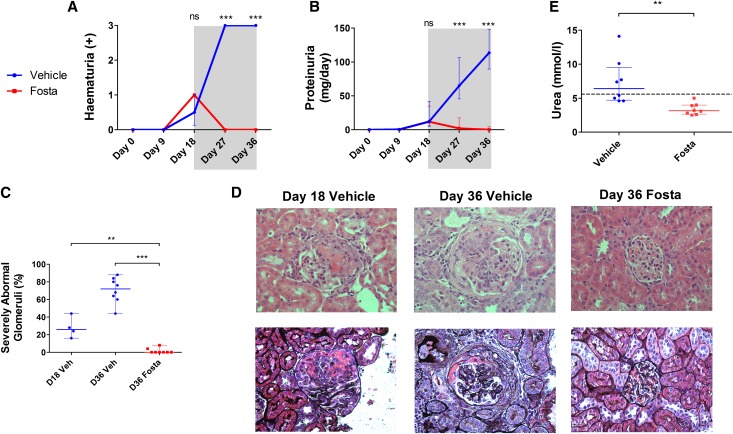
In a second in vivo study, we examined the effects of SYK inhibition in established EAG to more accurately reflect the potential effect of treatment in clinical practice. Rats were treated with either fostamatinib (40 mg/kg) or vehicle by two times daily oral gavage from day 18 to day 36 and then assessed for disease severity.
At day 18, all animals had comparable degrees of hematuria (Figure 3A) and proteinuria (Figure 3B). Histologic assessment in vehicle-treated control animals at this time point confirmed the presence of severe segmental necrotizing injury and crescent formation in approximately 26% of glomeruli. Disruption of the GBM was confirmed by Jones methenamine silver stain, and crescents were acute in nature, being characterized by extravasation of fibrin and cellular proliferation (Figure 3, C and D).
Figure 3.
SYK inhibition is an effective treatment for established experimental anti-GBM disease. (A) Hematuria and (B) proteinuria in fostamatinib- (Fosta; red plots) and vehicle-treated (blue plots) animals during the 18-day treatment-free period (unshaded) and 18-day treatment period (shaded) showing complete resolution of urinary abnormalities after treatment initiation. At day 36, there was 100% reduction in hematuria and proteinuria in fostamatinib-treated animals. (C) Glomerular pathology in untreated animals at day 18 before initiation of treatment (repeated from Figure 2E for comparison) and fostamatinib- and vehicle-treated animals after 18 days of treatment at day 36, showing significant reversal of severe glomerular abnormalities with fostamatinib. (D) Representative photomicrographs of hematoxylin and eosin-stained sections (upper panel) and Jones methenamine silver-stained sections (lower panel) showing typical glomerular pathology in vehicle- and fostamatinib-treated animals at days 18 and 36. Notably, silver stain confirmed disruption of the GBM and extracapillary crescent formation at day 18 in untreated animals. Original magnification, ×400. (E) Fostamatinib-treated animals had lower levels of serum urea at day 36; dashed line represents the upper reference limit for serum urea in healthy rats (5.4 mmol/L). Animals: n=8/group. Data are reported as median per group±interquartile range. **P<0.01; ***P<0.001.
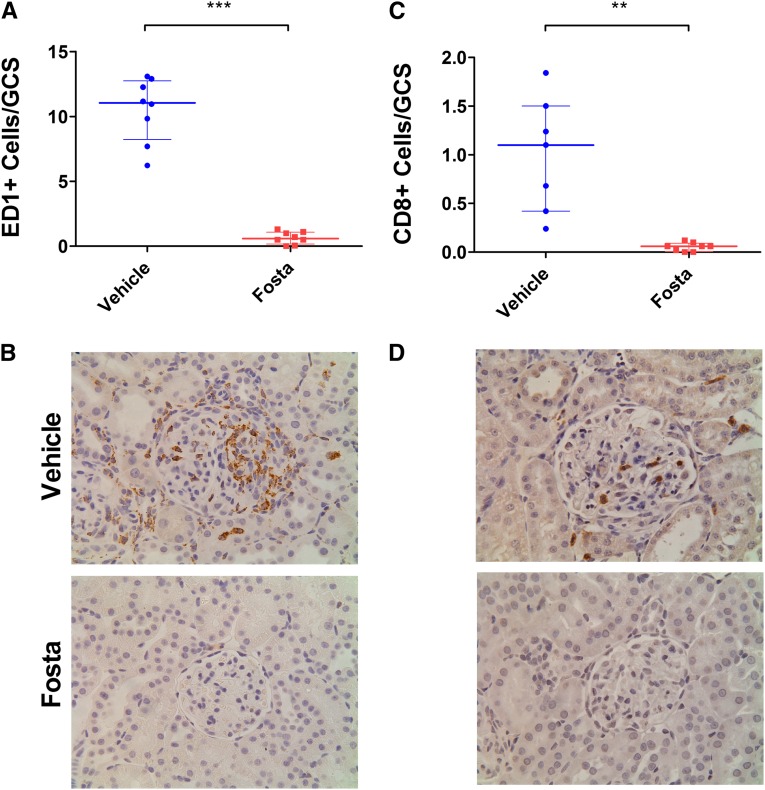
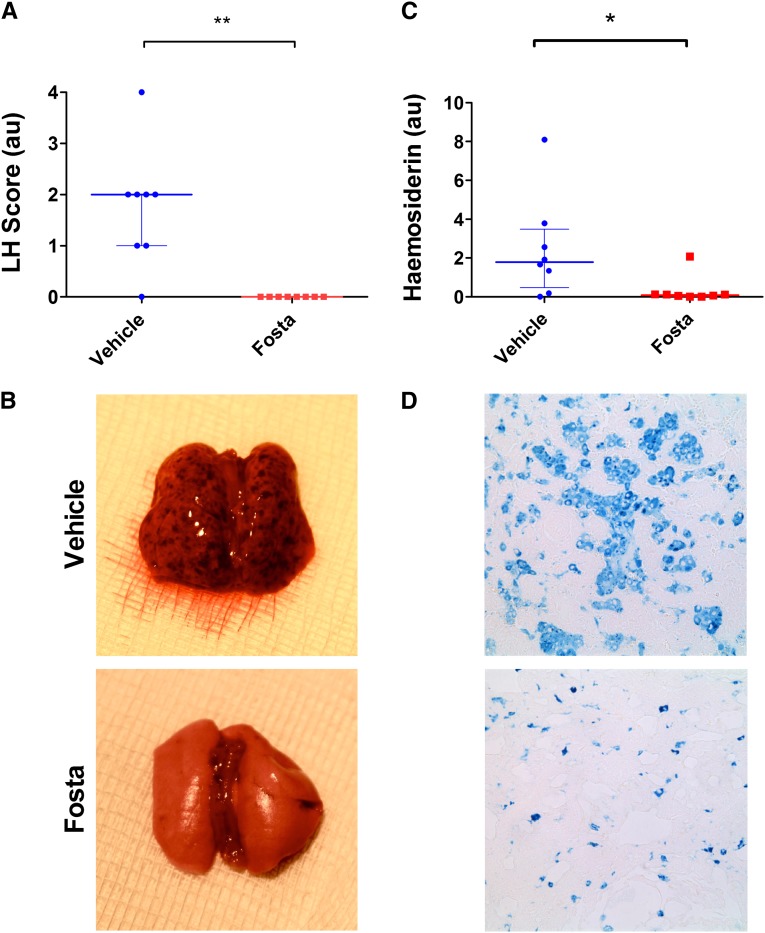
After the introduction of fostamatinib treatment at day 18, there was a rapid and complete resolution of urinary abnormalities that was sustained until day 36, whereas vehicle-treated animals had marked progression of disease (100% reduction of both hematuria and proteinuria at day 36; P<0.001 and P<0.001, respectively). Examination of renal histology (Figure 3, C and D) at day 36 confirmed severe pathology (>50% of the glomerular tuft affected by necrosis or crescent formation) affecting 76% of glomeruli in vehicle-treated animals, whereas fostamatinib-treated animals had essentially normal glomerular histology (100% reduction; P=0.001) (Supplemental Figure 1B). Compared with histology in untreated animals at day 18, this finding implied that SYK inhibitor treatment led to reversal of necrosis and crescent formation (100% reduction; P<0.01). In keeping with the normal histology, fostamatinib-treated animals had preserved levels of serum urea (103% increase in vehicle group; P=0.002) (Figure 3E). Fostamatinib-treated animals had minimal evidence of ED-1– or CD8-positive cell infiltration into glomeruli (95% reduction in both; P<0.001 and P=0.001, respectively) (Figure 4). SYK inhibition also resulted in complete protection from pulmonary hemorrhage (Figure 5), a late feature of disease in this model that develops after day 18, which was assessed by macroscopic inspection (100% reduction in fostamatinib group; P=0.001) and histologic quantification of hemosiderin-laden cells (95% reduction; P=0.03).
Figure 4.
SYK inhibition abrogates glomerular inflammatory cell infiltration in experimental anti-GBM disease. (A) Glomerular macrophages were identified using anti–ED-1 mAb and quantified per GCS. Fostamatinib-treated animals had 95% reduction in glomerular macrophage infiltration at day 36. (B) Representative photomicrographs showing ED-1 staining in vehicle- and fostamatinib-treated animals at day 36. (C) Fostamatinib-treated animals also had 95% reduction in glomerular CD8+ cells at day 36. (D) Representative CD8 staining for vehicle- and fostamatinib-treated animals at day 36. Original magnification for all images with hematoxylin counterstain, ×400. Animals: n=8/group. Data are reported as median per group±interquartile range. **P<0.01; ***P<0.001.
Figure 5.
SYK inhibition protects from the development of lung hemorrhage (LH) in experimental anti-GBM disease. (A) Lung injury was assessed by macroscopic inspection of the lung surfaces at the point of cull on day 36 and scored on the basis of the number of visible petechiae. Fostamatinib-treated animals were completely protected from developing macroscopic lung hemorrhage. B shows representative lung appearances for vehicle- and fostamatinib-treated animals. (C) Lung sections were also stained for hemosiderin with Perls’ Prussian blue (without counterstain), and the number of hemosiderin-positive cells were quantified using automated image analysis. Fostamatinib-treated animals had minimal evidence of hemosiderin deposition. (D) Photomicrographs show representative Perls’ stain in fostamatinib- and vehicle-treated groups. Original magnification, ×400. Animals: n=8/group. Data are reported as median per group±interquartile range. *P<0.05; **P<0.01.
SYK Inhibition Suppresses Autoantibody Production in Established Disease
The introduction of SYK inhibitor treatment at day 18 caused circulating autoantibody levels to plateau at a time when levels were rising rapidly in the vehicle-treated group (Figure 6A). Because the half-life of these autoantibodies exceeds 2–3 weeks, this finding suggests that there was no ongoing antibody production after the introduction of treatment, implying significant effects of SYK inhibition on mature, antibody-secreting B cells or plasma cells. At the end of the treatment period, there was a 75% reduction in circulating antibody levels (P<0.001) with a concomitant decrease in the amount of deposited antibody (37% reduction; P=0.04) (Figure 6B). There was also a decrease in deposited complement component C3 (47% reduction; P=0.02) (Figure 6C) in keeping with reduced activation of the classic complement pathway.
Figure 6.
SYK inhibition terminates pathogenic autoantibody production in experimental anti-GBM disease. (A) Circulating anti-GBM antibody levels in fostamatinib- (Fosta; red plots) and vehicle-treated (blue) animals during the 18-day treatment-free period (unshaded) and the 18-day treatment period (shaded) show cessation of autoantibody production after introduction of fostamatinib treatment. (B) At day 36, fostamatinib-treated animals had less deposited anti-GBM antibody, which was detected by direct IF. Lower panel photomicrographs show representative linear IF for deposited anti-GBM antibodies in fostamatinib- and vehicle-treated animals at day 36. Original magnification, ×400. (C) Fostamatinib-treated animals also had less complement C3 deposition within glomeruli, suggesting reduced activation of the classic complement pathway secondary to decreased deposition of antibody. Lower panel photomicrographs show representative IF for C3 in glomeruli of vehicle- and fostamatinib-treated rats. (D) α3-Specific splenic B cells were enumerated by B-cell ELISpot assay. Fostamatinib treatment from day 0 to day 18 inhibited the generation of α3-specific splenic B cells (measured at day 18). Fostamatinib treatment from day 18 to day 36 did not affect the overall number of α3-specific splenic B cells at day 36. Replicate results (×8) from one of three representative biologic replicated experiments are shown. (E) Gating strategy for flow cytometric analysis of splenocyte subsets. (F) Fostamatinib treatment for 18 days did not significantly affect the survival of CD45RA- or CD8-positive splenocytes, although there was a reduction in CD4-positive cells. Animals: n=8/group, except flow cytometric analysis (n=4/group). Data are reported as median per group±interquartile range. *P<0.05; **P<0.01; ***P<0.001.
SYK Inhibition Reduces the Generation and Activity of Antigen-Specific Autoantibody-Producing Splenocytes In Vivo
Because we observed significant effects of SYK inhibition on humoral immune responses in both in vivo studies, we went on to enumerate antigen-specific splenic B cells using B-cell ELISpot assays. We found that fostamatinib treatment from day 0 to day 18 after disease induction reduced the generation of antigen-specific cells by 63% (P=0.002) (Figure 6C). However, in animals treated from day 18 to day 36, after disease was established, there was no significant difference in the number of antigen-specific splenic B cells at the end of the treatment period (P=1.00). This finding supports our observation that the introduction of fostamatinib treatment at day 18 had a direct effect on antibody production by mature antigen-specific cells, because there was no ongoing antibody production during this period, despite equal numbers of antigen-specific cells being present in the spleen.
We found only modest changes in the overall proportion of CD8-, CD4-, and CD45RA-positive splenocyte subsets after 18 days of treatment with fostamatinib (Figure 6, D and E). The maintenance of the CD45RA-positive population, in particular, suggests that B-cell survival was not adversely affected by a short period of treatment and implies a direct effect of SYK inhibition on autoantibody production.
SYK Inhibition Has Additional Effects in EAG Independent of Effects on Autoantibody Production
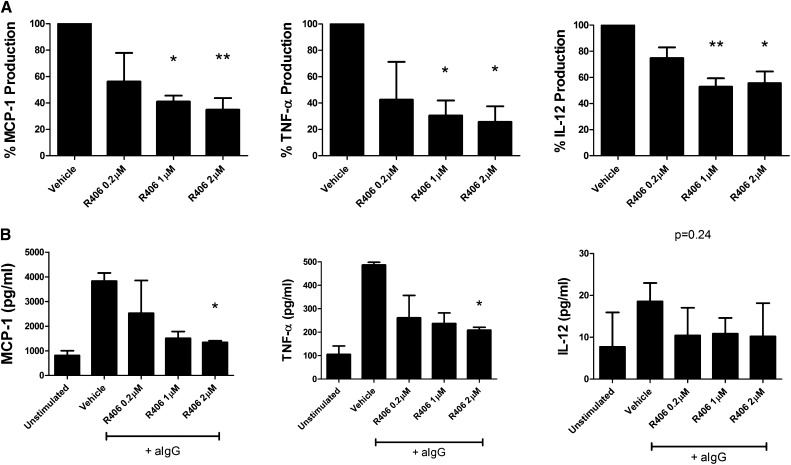
Based on our previous studies in NTN and our immunohistochemical findings, we hypothesized that, in addition to inhibiting the production of autoantibodies, fostamatinib may prevent their downstream function through inhibition of FcR signaling. To investigate a role for SYK independent of autoantibody production in EAG, we isolated nephritic glomeruli from untreated animals 28 days after disease induction to examine the effects of SYK inhibition in diseased tissue dissociated from the systemic humoral response. When these nephritic glomeruli were incubated with R406, the active metabolite of fostamatinib, there was a dose-dependent reduction in the spontaneous production of a number of proinflammatory cytokines (Figure 7A), including monocyte chemoattractant protein-1 (MCP-1), TNF-α, and IL-12, each of which has been implicated in the pathogenesis of experimental GN.23–25 In addition, we studied the effects of R406 on cytokine production by primary bone marrow-derived macrophages (BMDMs) in vitro after stimulation by crossligation of FcRs using heat-aggregated IgG.26 We observed a dose-dependent reduction in proinflammatory cytokine production (Figure 7B), similar to that seen in glomerular preparations.
Figure 7.
SYK inhibition has additional effects in EAG independent of effects on autoantibody production. (A) Incubation with R406, the active metabolite of fostamatinib, reduced spontaneous proinflammatory cytokine production by nephritic glomeruli ex vivo in a dose-dependent manner. Results from four biologic replicate experiments are shown, with data from each experiment normalized to vehicle group measurements. (B) R406 inhibited the production of proinflammatory cytokines by primary BMDMs in vitro after stimulation with heat-aggregated IgG (aIgG). Replicate results (×3) from one of at least two biologic replicate experiments are shown. Data are reported as median per group±interquartile range. *P<0.05; **P<0.01.
Discussion
Here, we report several important observations that suggest that SYK warrants clinical investigation as a therapeutic target in the autoimmune glomerulonephritides. These observations include the first description of SYK expression in experimental and clinical anti-GBM disease, the novel finding that SYK inhibition has a significant impact on the production of pathogenic autoantibody in addition to its known effects on antibody-dependent FcR-mediated responses, and it is accompanied by reversal of crescentic GN and complete protection from alveolar hemorrhage, the life-threatening manifestations of anti-GBM disease, in a highly accurate preclinical autoimmune model.
SYK inhibition using fostamatinib or other small molecule inhibitors has been studied in a number of in vivo models of immune-mediated injury. However, confirmation of SYK expression by immunohistochemistry in these models is limited. T-SYK expression has been described in the skin lesions of MRL/lpr lupus-prone mice14 and the synovium of CIA rats,16 although the identification of SYK in its activated, phosphorylated state is limited to the alloimmune heterologous phase of rat nephrotoxic nephritis.27 This study, therefore, is the first report of P-SYK detection by immunohistochemistry in an autoimmune model of renal injury, and the observation that P-SYK localized to areas of pathology in diseased glomeruli robustly implicates SYK in the pathogenesis of EAG. P-SYK colocalized strongly (although not exclusively) to ED-1–positive macrophages, the predominant infiltrating leukocytes in the model. Macrophages are well recognized mediators of glomerular injury in clinical glomerulonephritides,28 and this observation supports potential targeting of SYK in clinical disease. To this end, we have previously reported both T-SYK and P-SYK expression in IgA nephropathy,23 and P-SYK detection is also described in postinfectious GN.27 The identification of P-SYK in anti-GBM disease, the most severe form of GN, suggests that SYK may contribute to the pathogenesis of a range of proliferative glomerulonephritides and that they may be responsive to SYK inhibitor therapy.
In addition to SYK expression in diseased end organ renal tissue, we observed strong staining for both T-SYK and P-SYK in the lymphoid tissue of both rats and humans, consistent with a well characterized role for SYK in the generation of adaptive immune responses. In both in vivo studies, we observed significant effects of SYK inhibition on the induction and progression of autoimmunity. In particular, we found considerable attenuation of humoral responses after the introduction of treatment in the second study. This finding is in contrast to previous reports in murine lupus, CIA, and spontaneous diabetes in NOD mice, where no clear-cut effects on anti–double-stranded DNA, anticollagen, or anti-insulin antibody levels, respectively, were observed. However, the exposure period in these studies was not optimal for studying humoral responses, because treatment was initiated after maximal autoantibody responses were established, and it did not continue beyond the lifetime of these preexisting antibodies. Previous studies in mice have suggested that prolonged exposure to fostamatinib (>1–3 months) is associated with a decline in total B-cell number and altered proportions of certain B-cell subpopulations.13,17 Our data, however, suggest that overall B-cell survival was not affected by short-term fostamatinib treatment in rats and that the effect on autoantibody production was because of a direct inhibitory effect on mature antibody-producing B cells and plasma cells. Because constitutively SYK-deficient B cells arrest at the pro-B cell stage, it has only been possible to study the role of SYK in mature cells with the advent of specific small molecule inhibitors (and potentially, conditional genetic techniques29), and our novel observation suggests that SYK inhibition may prevent the production of pathogenic autoantibody, even after aberrant clonal responses have been established. Additional analysis of the effects of fostamatinib treatment on B-lymphocyte subsets or B-cell function in vitro, however, is limited by the paucity of validated B-cell markers in the rat.
In addition to preventing the production of pathogenic autoantibodies, SYK inhibition seemed to have the potentially therapeutic second effect of inhibiting their downstream effector functions. Spontaneous proinflammatory cytokine production by nephritic glomeruli was inhibited by incubation with R406, the active metabolite of fostamatinib, independent of its effects on systemic humoral immunity. We observed a similar pattern of attenuated cytokine production by primary BMDMs after FcR ligation, suggesting that the effect in glomeruli was mediated, at least in part, by inhibition of antibody-dependent, FcR-mediated responses in macrophages. Notably, the effect on IL-12 production by BMDM was less dramatic than that seen in whole glomerular preparations in keeping with previous reports that IL-12 production by intrinsic renal cells is important in the pathogenesis of GN30 and that resident renal cells may also respond to SYK inhibition.23
The combined effect of SYK inhibition on antibody production and antibody-mediated effector functions was a striking reversal of severe glomerular pathology in EAG, confirming our previous observations in NTN, where we observed an approximately 20% reduction in glomerular crescent formation when treatment was initiated in severe diseases with greater than 90% established glomerular crescents. We also observed complete protection from alveolar hemorrhage both by macroscopic assessment and as a reduction in hemosiderin-laden cells, a characteristic finding in the broncho-alveolar lavage fluid of patients with lung hemorrhage,31 a life-threatening phenomenon that may accompany certain forms of GN.
Although our data suggest an important effect of SYK inhibition on B cells and macrophages in this model, our study has not addressed the role of other cell types that express SYK and that have been implicated in the pathogenesis of GN, such as neutrophils,32 mast cells,33 and dendritic cells.34 Systemic exposure to a small molecule inhibitor, such as fostamatinib, limits the extent to which the effect in individual cells can be delineated, and only future work using genetic approaches will allow complete dissection of the potential cellular mechanisms involved in vivo. Strengths of our study, however, include the use of a genuine autoimmune model using a well characterized autoantigen that is relevant to human disease along with a selective SYK inhibitor that has already shown biologic activity and an acceptable toxicity profile in several clinical studies.10,11,35,36 Although fostamatinib did not show sufficient efficacy in a phase III study program to support regulatory submissions in rheumatoid arthritis, we believe that the striking findings in this experimental model suggest that clinical studies in GN are now desirable.
Concise Methods
A detailed description of all methods is provided in Supplemental Material.
Study Approval
Human renal tissue samples were obtained from patients under local ethics committee approval (04/Q0406/25 Hammersmith and Queen Charlotte’s and Chelsea Hospitals Research Ethics Committee). Lymph node samples were provided by the Imperial College Healthcare National Health Service Trust Tissue Bank. Animal studies were carried out with local ethics committee approval in accordance with the United Kingdom Animals (Scientific Procedures Act) 1986.
Immunohistochemistry for SYK
All immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissues using the following primary antibodies: T-SYK N-19 (Santa Cruz Biotechnology), human P-SYK Tyr525/526 (Cell Signaling Technology), and rodent P-SYK Try323 (Abcam, Inc.) developed using the Envision+System (DakoCytomation). For double staining, sections were first stained for P-SYK as described, then incubated with primary antibody to ED-1 (Serotec), and developed using the Vector Blue ALP Substrate Kit III (Vector Laboratories).
SYK Inhibitors
Fostamatinib and its prodrug, R406, were provided by Rigel Pharmaceuticals and AstraZeneca. The details of these molecules have been reported previously.13,15
Experimental Autoimmune GN
Disease was induced by immunizing 6-week-old female Wistar Kyoto rats (n=8/group) with 100 μg α3(IV)NC1 emulsified in complete Freund’s adjuvant as previously described.18 To obtain comparative histology at day 18, an additional two groups of n=4 were used. Fostamatinib (40 mg/kg) or vehicle was administered by two times per day oral gavage.
Assessment of Renal Disease
Hematuria was quantified by dipstick analysis (Multistix 8 SG; Siemens Healthcare), and proteinuria was quantified by sulphosalicylic acid method.37 Kidney sections stained with periodic acid–Schiff, hematoxylin and eosin, and Jones methenamine silver stain were assessed for glomerular injury; 50 consecutive glomeruli were graded as normal, abnormal (<50% tuft affected by necrosis or crescent formation), or severely abnormal (>50% tuft affected) by a blinded observer, and results were expressed as the mean proportion for each animal. Macrophages and CD8+ cells were immunostained using mAbs to ED-1 and CD8 (Serotec), respectively, and the number of positive cells per cross-section counted in 20–50 consecutive glomeruli by a blinded observer, with results expressed as the mean for each animal.
Assessment of Lung Injury
Lung surfaces were inspected at the time of cull, and severity of lung hemorrhage was graded as zero (normal), one (<10 petechaie), two (>10 petechaie), three (>20 petechaie), or four (massive hemorrhage). Lung sections were stained with Perls’ Prussian blue without counterstain and quantified by a blinded observer using automated image analysis software, with results expressed in arbitrary units.
Assessment of Autoantibody Response
Circulating α3(IV)NC1 antibodies were assayed in serum by ELISA. Deposited antibodies and complement C3 were detected on frozen kidney sections using an FITC-labeled anti-rat IgG (Sigma-Aldrich) and an FITC-labeled anti-rat C3 antibody (Nordic MUbio), respectively. Fluorescence intensity was graded (0–3+) in 20 consecutive glomeruli by a blinded observer, and the results were expressed as mean per animal.
B-Cell ELISpots
Splenocytes were obtained from rats after treatment either from day 0 to day 18 or from day 18 to day 36 (n=3/group). Cells were suspended in cell culture medium and incubated (500,000 cells/well) in ELISpot plates (Multiscreen HTS 96-well filter plates; EMD Millipore) previously coated with α3(IV)NC1 (50 μg/ml) in sterile PBS. Cells were incubated for 48 hours without moving, and then, plates were washed and developed using a biotin-streptavidin–based secondary system. Spots were counted using an ELISpot platereader and dedicated software (ELISpot 4.0; Autoimmun Diagnostika). Replicate results from one of three pairs of animals are reported in the manuscript.
Flow Cytometry
Analysis was performed on splenocytes obtained from animals after treatment from day 0 to day 18 after disease induction (n=4). Cells were stained with the antibodies CD45RA-PE (OX-33; BD Biosciences), CD8-PECy7 (OX8; eBiosciences), and CD4-FITC (W3/25; eBiosciences) and run on a BD Accuri C6 flow cytometer. Analysis was performed on FlowJo X software (Treestar).
Ex Vivo Glomeruli and BMDMs
Glomeruli were extracted from untreated animals 28 days after induction of EAG by differential sieving of whole-kidney tissue and cultured in full culture media with 0.1% DMSO (vehicle) and 0.2, 1, or 2 µM R406 for 48 hours. MCP-1, TNF-α, and IL-12 levels in the media were quantified by ELISA or cytometric bead array (OptEIA Rat MCP-1 ELISA Set and Cytometric Bead Array Rat TNF-α Flex Set; BD Biosciences; Rat IL-12+p40 ELISA Kit; Invitrogen) used according to the manufacturers’ instructions. Primary BMDMs were grown in L929-conditioned culture media to day 7. Cell culture supernatants were collected 24 hours after stimulation with heat-aggregated rat IgG26 (250 μg/ml; Sigma-Aldrich) and/or incubation with R406/vehicle in serum-free conditions, and cytokine levels were quantified as above.
Statistical Analyses
Statistical analysis was conducted using GraphPad Prism 5.0 (GraphPad Software Inc.). All data are reported as median per group±interquartile range unless otherwise stated. Comparison between groups was by Mann–Whitney U test and Kruskal–Wallis test with Dunn’s multiple comparison.
Disclosures
E.S.M. is an employee of and owns stocks/stock options for Rigel Pharmaceuticals. C.D.P. has received a research project grant from GlaxoSmithKline and has a consultancy agreement with Genzyme. F.W.K.T. has received research project grants from AstraZeneca Limited and has a consultancy agreement with Rigel Pharmaceuticals.
Supplementary Material
Acknowledgments
S.P.M. received a Clinical Research Training Fellowship funded by United Kingdom Medical Research Council Grant G0901997/1. F.W.K.T. is supported by the Diamond Fund from Imperial College Healthcare Charity.
Components of this work have been presented in abstract form at the American Society of Nephrology Renal Week, October 30–November 4, 2012, in San Diego, CA, and the 16th International Vasculitis and ANCA Workshop, April 14–17, 2013, in Paris, France.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013090978/-/DCSupplemental.
References
- 1.Mócsai A, Ruland J, Tybulewicz VL: The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat Rev Immunol 10: 387–402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL: A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med 186: 1027–1039, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T: The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol Cell Biol 18: 4209–4220, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedlik C, Orbach D, Veron P, Schweighoffer E, Colucci F, Gamberale R, Ioan-Facsinay A, Verbeek S, Ricciardi-Castagnoli P, Bonnerot C, Tybulewicz VL, Di Santo J, Amigorena S: A critical role for Syk protein tyrosine kinase in Fc receptor-mediated antigen presentation and induction of dendritic cell maturation. J Immunol 170: 846–852, 2003 [DOI] [PubMed] [Google Scholar]
- 5.de Castro RO: Regulation and function of syk tyrosine kinase in mast cell signaling and beyond. J Signal Transduct 2011: 507291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T: Syk tyrosine kinase required for mouse viability and B-cell development. Nature 378: 303–306, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL: Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature 378: 298–302, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Wex E, Bouyssou T, Duechs MJ, Erb KJ, Gantner F, Sanderson MP, Schnapp A, Stierstorfer BE, Wollin L: Induced Syk deletion leads to suppressed allergic responses but has no effect on neutrophil or monocyte migration in vivo. Eur J Immunol 41: 3208–3218, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Ruzza P, Biondi B, Calderan A: Therapeutic prospect of Syk inhibitors. Expert Opin Ther Pat 19: 1361–1376, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Weinblatt ME, Kavanaugh A, Burgos-Vargas R, Dikranian AH, Medrano-Ramirez G, Morales-Torres JL, Murphy FT, Musser TK, Straniero N, Vicente-Gonzales AV, Grossbard E: Treatment of rheumatoid arthritis with a Syk kinase inhibitor: A twelve-week, randomized, placebo-controlled trial. Arthritis Rheum 58: 3309–3318, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Weinblatt ME, Kavanaugh A, Genovese MC, Musser TK, Grossbard EB, Magilavy DB: An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N Engl J Med 363: 1303–1312, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Smith J, McDaid JP, Bhangal G, Chawanasuntorapoj R, Masuda ES, Cook HT, Pusey CD, Tam FW: A spleen tyrosine kinase inhibitor reduces the severity of established glomerulonephritis. J Am Soc Nephrol 21: 231–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahjat FR, Pine PR, Reitsma A, Cassafer G, Baluom M, Grillo S, Chang B, Zhao FF, Payan DG, Grossbard EB, Daikh DI: An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum 58: 1433–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Deng GM, Liu L, Bahjat FR, Pine PR, Tsokos GC: Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum 62: 2086–2092, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, Qu K, Herlaar E, Lau A, Young C, Wong BR, Lovell S, Sun T, Park G, Argade A, Jurcevic S, Pine P, Singh R, Grossbard EB, Payan DG, Masuda ES: R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther 319: 998–1008, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Pine PR, Chang B, Schoettler N, Banquerigo ML, Wang S, Lau A, Zhao F, Grossbard EB, Payan DG, Brahn E: Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clin Immunol 124: 244–257, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Colonna L, Catalano G, Chew C, D’Agati V, Thomas JW, Wong FS, Schmitz J, Masuda ES, Reizis B, Tarakhovsky A, Clynes R: Therapeutic targeting of Syk in autoimmune diabetes. J Immunol 185: 1532–1543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan JJ, Reynolds J, Norgan VA, Pusey CD: Expression and characterization of recombinant rat alpha 3(IV)NC1 and its use in induction of experimental autoimmune glomerulonephritis. Nephrol Dial Transplant 16: 253–261, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Reynolds J, Abbott DS, Karegli J, Evans DJ, Pusey CD: Mucosal tolerance induced by an immunodominant peptide from rat alpha3(IV)NC1 in established experimental autoimmune glomerulonephritis. Am J Pathol 174: 2202–2210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, Wilson CB, Hudson BG: Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 363: 343–354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds J, Moss J, Duda MA, Smith J, Karkar AM, Macherla V, Shore I, Evans DJ, Woodrow DF, Pusey CD: The evolution of crescentic nephritis and alveolar haemorrhage following induction of autoimmunity to glomerular basement membrane in an experimental model of Goodpasture’s disease. J Pathol 200: 118–129, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Pusey CD: Anti-glomerular basement membrane disease. Kidney Int 64: 1535–1550, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kim MJ, McDaid JP, McAdoo SP, Barratt J, Molyneux K, Masuda ES, Pusey CD, Tam FW: Spleen tyrosine kinase is important in the production of proinflammatory cytokines and cell proliferation in human mesangial cells following stimulation with IgA1 isolated from IgA nephropathy patients. J Immunol 189: 3751–3758, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Khan SB, Cook HT, Bhangal G, Smith J, Tam FW, Pusey CD: Antibody blockade of TNF-alpha reduces inflammation and scarring in experimental crescentic glomerulonephritis. Kidney Int 67: 1812–1820, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kitching AR, Tipping PG, Holdsworth SR: IL-12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur J Immunol 29: 1–10, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Smith J, Lai PC, Behmoaras J, Roufosse C, Bhangal G, McDaid JP, Aitman T, Tam FW, Pusey CD, Cook HT: Genes expressed by both mesangial cells and bone marrow-derived cells underlie genetic susceptibility to crescentic glomerulonephritis in the rat. J Am Soc Nephrol 18: 1816–1823, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ryan J, Ma FY, Kanellis J, Delgado M, Blease K, Nikolic-Paterson DJ: Spleen tyrosine kinase promotes acute neutrophil-mediated glomerular injury via activation of JNK and p38 MAPK in rat nephrotoxic serum nephritis. Lab Invest 91: 1727–1738, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Duffield JS: Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30: 234–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozaki N, Suzuki S, Ishida M, Harada Y, Tanaka K, Sato Y, Kono T, Kubo M, Kitamura D, Encinas J, Hara H, Yoshida H: Syk-dependent signaling pathways in neutrophils and macrophages are indispensable in the pathogenesis of anti-collagen antibody-induced arthritis. Int Immunol 24: 539–550, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Timoshanko JR, Kitching AR, Holdsworth SR, Tipping PG: Interleukin-12 from intrinsic cells is an effector of renal injury in crescentic glomerulonephritis. J Am Soc Nephrol 12: 464–471, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Colby TV, Fukuoka J, Ewaskow SP, Helmers R, Leslie KO: Pathologic approach to pulmonary hemorrhage. Ann Diagn Pathol 5: 309–319, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Mayadas TN, Rosetti F, Ernandez T, Sethi S: Neutrophils: Game changers in glomerulonephritis? Trends Mol Med 16: 368–378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holdsworth SR, Summers SA: Role of mast cells in progressive renal diseases. J Am Soc Nephrol 19: 2254–2261, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Hochheiser K, Engel DR, Hammerich L, Heymann F, Knolle PA, Panzer U, Kurts C: Kidney dendritic cells become pathogenic during crescentic glomerulonephritis with proteinuria. J Am Soc Nephrol 22: 306–316, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB: Of mice and men: An open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood 113: 3154–3160, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe AM, Levy R, Shipp MA: Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115: 2578–2585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheryanna A, Bhangal G, McDaid J, Smith J, Manning A, Foxwell BM, Feldmann M, Cook HT, Pusey CD, Tam FW: Inhibition of p38 mitogen-activated protein kinase is effective in the treatment of experimental crescentic glomerulonephritis and suppresses monocyte chemoattractant protein-1 but not IL-1beta or IL-6. J Am Soc Nephrol 18: 1167–1179, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.