Abstract
Macroalbuminuria, defined as urine albumin excretion rate (AER)≥300 mg/d, has long been considered a stage of irreversible kidney damage that leads reliably to GFR loss. We examined the long-term renal outcomes of persons with type 1 diabetes who developed incident macroalbuminuria during the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study. One hundred fifty-nine participants developed incident macroalbuminuria and were subsequently followed for a median duration of 9 years (maximum of 25 years). At the time of macroalbuminuria diagnosis, mean (SD) age was 37 (9) years, mean (SD) duration of diabetes was 17 (5) years, median AER was 524 mg/d, and mean (SD) eGFR was 108 (20) ml/min per 1.73 m2. Ten years after macroalbuminuria diagnosis, the cumulative incidence of a sustained reduction in AER to <300 mg/d was 52%, mostly but not entirely under treatment with renin-angiotensin system inhibitors. The cumulative incidence of impaired GFR (sustained eGFR<60 ml/min per 1.73 m2) 10 years after macroalbuminuria diagnosis was 32%, including 16% who developed ESRD. Lower hemoglobin A1c and BP and regression to AER<300 mg/d were associated with reduced risk of developing impaired GFR. In conclusion, people with type 1 diabetes who develop macroalbuminuria are at high risk of progressive kidney disease. However, through at least 10 years of follow-up, AER could often be controlled, and GFR frequently remained in the normal range.
Macroalbuminuria, defined as urine albumin excretion rate (AER)≥300 mg/d, has long been considered a stage of irreversible kidney damage that leads reliably to GFR loss.1 In early published type 1 diabetes cohorts, macroalbuminuria was associated with a 15-year cumulative incidence of ESRD as high as 75%.2,3 However, contemporary long-term renal outcomes of macroalbuminuria have not been fully characterized.
The Diabetes Control and Complications Trial (DCCT) and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, present a valuable opportunity to examine macroalbuminuria and its long-term clinical outcomes. In DCCT/EDIC, the onset of macroalbuminuria can be defined with confidence using frequent longitudinal measurements of AER, participants have been followed for up to 25 years after the diagnosis of macroalbuminuria, and outcomes were meticulously recorded using standardized methods. Previous work in this cohort has shown that most cases of impaired GFR are preceded by macroalbuminuria,4 which is associated with a 50-fold higher risk of developing impaired GFR (eGFR<60 ml/min per 1.73 m2).5 Here, we extend these studies by comprehensively evaluating the long-term renal outcomes of incident macroalbuminuria in the DCCT/EDIC cohort and examining the risk factors for its progression to impaired GFR.
Results
Incidence of Macroalbuminuria
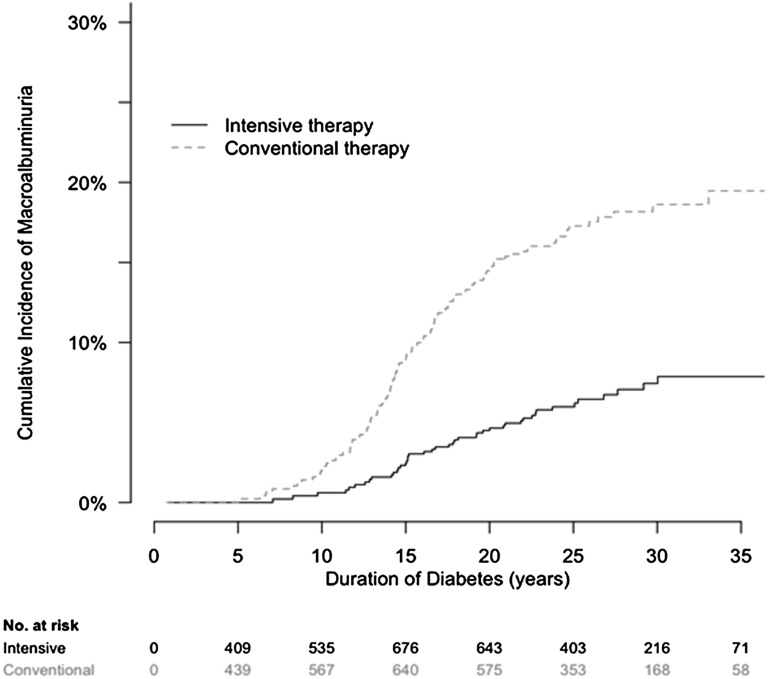
In the DCCT/EDIC population, the development of macroalbuminuria was rare before 10 years of diabetes duration and increased steadily thereafter (Figure 1). Macroalbuminuria developed less frequently among participants assigned to intensive therapy compared with conventional therapy during the DCCT. The cumulative incidence of macroalbuminuria at 25 years after diabetes onset was 6% in participants assigned to intensive therapy and 17% in participants assigned to conventional therapy.
Figure 1.
Cumulative incidence of macroalbuminuria by treatment assignment in the DCCT/EDIC study by duration of type 1 diabetes. The cumulative incidence of macroalbuminuria, defined as AER≥300 mg/d, is shown according to the group to which participants had been randomly assigned in the DCCT.
Clinical Characteristics at Macroalbuminuria Diagnosis
Of 159 participants who developed incident macroalbuminuria, 68% were men, and 72% had been assigned to conventional diabetes therapy (Table 1). At the time of macroalbuminuria diagnosis, mean age was 37 years; mean duration of diabetes was 17 years, 55% had hypertension, and 31% were taking renin-angiotensin-aldosterone system (RAAS) inhibitors. Median AER was 524 mg/24 h; mean eGFR was 108 ml/min per 1.73 m2, and four participants had an eGFR<60 ml/min per 1.73 m2.
Table 1.
Characteristics of participants in the DCCT/EDIC study at the time that incident macroalbuminuria was diagnosed
| Variable | Mean (SD) or N (%) | |
|---|---|---|
| All Macroalbuminuria | Persistent Macroalbuminuria | |
| Number | 159 | 123 |
| Age (yr) | 37 (9) | 36 (9) |
| Women | 51 (32%) | 37 (30%) |
| Caucasian race | 146 (92%) | 114 (93%) |
| Duration of diabetes (yr) | 17 (5) | 17 (5) |
| DCCT cohort | ||
| Primary prevention | 64 (40%) | 45 (37%) |
| Secondary prevention | 95 (60%) | 78 (63%) |
| DCCT treatment assignment | ||
| Intensive therapy | 44 (28%) | 35 (28%) |
| Conventional therapy | 115 (72%) | 88 (72%) |
| Time of diagnosis of macroalbuminuria | ||
| During DCCT | 52 (33%) | 45 (37%) |
| During EDIC study | 107 (67%) | 78 (63%) |
| Active smoking | 45 (29%) | 39 (33%) |
| Current RAAS inhibitor use | 49 (31%) | 35 (29%) |
| Lipid-lowering medication use | 18 (12%) | 11 (9%) |
| Body mass index (kg/m2) | 26.9 (4.3) | 26.6 (4.0) |
| SBP (mmHg) | 133 (17) | 132 (17) |
| DBP (mmHg) | 82 (10) | 83 (10) |
| Hypertension (SBP>140 or DBP>90 or use of antihypertensive medications) | 84 (55%) | 63 (54%) |
| Hemoglobin A1c (%) | 9.3 (1.6) | 9.4 (1.6) |
| AER (mg/24 h) | 524 (383–796) | 549 (393–842) |
| eGFR (ml/min per 1.73 m2) | 108 (20) | 109 (21) |
| Total cholesterol (mg/dl) | 203 (45) | 206 (47) |
| HDL cholesterol (mg/dl) | 52 (15) | 52 (14) |
| Triglycerides (mg/dl) | 120 (77) | 123 (79) |
| LDL cholesterol (mg/dl) | 126 (35) | 128 (37) |
Data are mean (SD) or number (%), except for albumin excretion ratio, which is median (interquartile range). SBP, systolic BP; DBP, diastolic BP.
Albuminuria Outcomes after Macroalbuminuria Diagnosis
After macroalbuminuria diagnosis, 61 participants regressed to sustained AER<300 mg/d over a median follow-up of 4 years (maximum of 22 years). The cumulative incidence of sustained AER<300 mg/d 10 years after macroalbuminuria diagnosis was 52% (Table 2). The prevalence of RAAS inhibitor use in people with AER<300 mg/d 10 years after macroalbuminuria diagnosis was 80%. After macroalbuminuria diagnosis, 14 participants regressed to sustained AER<30 mg/d over a median follow-up of 8 years (maximum of 22 years). The cumulative incidence of sustained AER<30 mg/d 10 years after macroalbuminuria diagnosis was 13% (Table 2). Two patients were treated with RAAS inhibitors at or before the time that they developed AER<300 mg/d, eight patients were treated with RAAS inhibitors after AER had dropped to <300 mg/d, and four patients were not treated with RAAS inhibitors. Of 14 people who regressed to normoalbuminuria, all but 2 people maintained eGFR≥60 ml/min per 1.73 m2 for the duration of follow-up.
Table 2.
Renal outcomes among 159 participants who developed incident macroalbuminuria during the DCCT/EDIC study
| Outcomes | Number at Risk | Number of Cases | Follow-Up (yr after Macroalbuminuria Onset) | Incidence Rate (per 100 person-yr) | 10-yr Cumulative Incidence (%) | |
|---|---|---|---|---|---|---|
| Median | Maximum | |||||
| Sustained microalbuminuria | 130a | 61 | 4 | 22 | 8.2 | 52 |
| Sustained normoalbuminuria | 130a | 14 | 8 | 22 | 1.3 | 13 |
| Nephrotic-range albuminuria | 159 | 40 | 6 | 24 | 3.0 | 27 |
| Sustained eGFR<60 ml/min per 1.73 m2 | 159 | 51 | 7 | 24 | 4.0 | 32 |
| eGFR<45 ml/min per 1.73 m2 | 159 | 46 | 8 | 25 | 3.1 | 26 |
| eGFR<30 ml/min per 1.73 m2 | 159 | 33 | 8 | 25 | 2.1 | 22 |
| ESRD | 159 | 22 | 9 | 25 | 1.4 | 16 |
Sustained microalbuminuria was defined as urine AER<300 mg/d on two successive occasions. Sustained normoalbuminuria was defined as urine AER<30 mg/d on two successive occasions. Nephrotic-range albuminuria was defined as AER≥3000 mg/d. Participants who developed ESRD were considered to have met each eGFR outcome (<60, <45, and <30 ml/min per 1.73 m2).
Twenty-nine of one hundred fifty-nine participants with macroalbuminuria were not at risk for sustained microalbuminuria or normoalbuminuria, because they did not have two AER measurements after macroalbuminuria diagnosis.
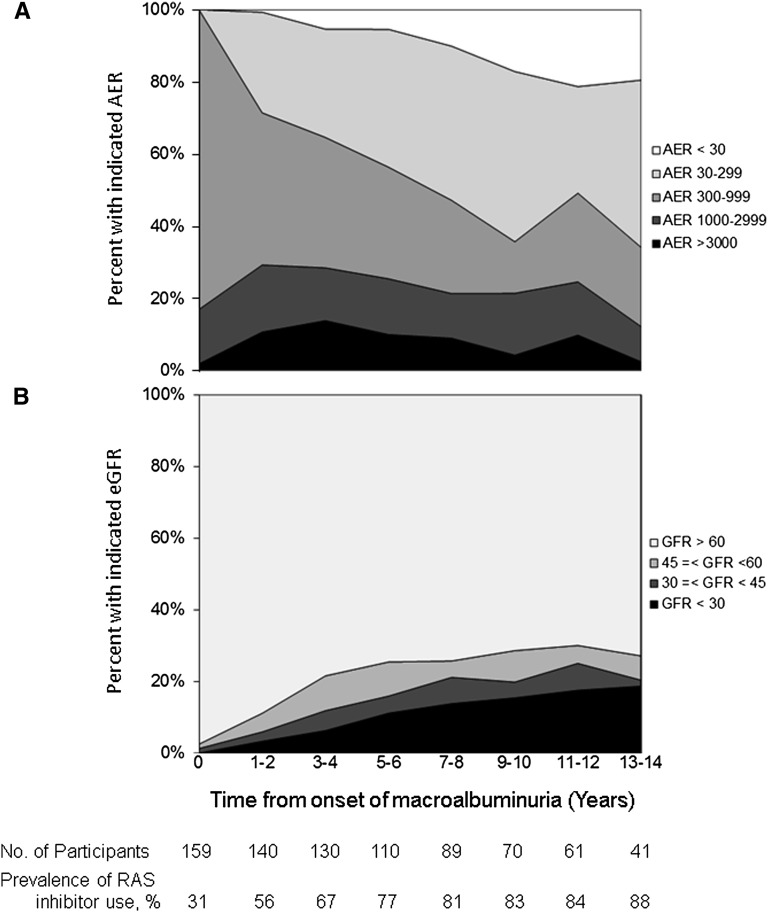
The point prevalence of AER<300 mg/d 10 years after macroalbuminuria diagnosis was 64%, including 17% with AER<30 mg/d (Figure 2A). At this time point, 88%, 85%, and 67% of participants with macroalbuminuria, microalbuminuria, and normoalbuminuria were using RAAS inhibitors, respectively.
Figure 2.
Distributions of prevalent AER and eGFR by time after onset of incident macroalbuminuria among 159 DCCT/EDIC participants. (A) Prevalence of normoalbuminuria, microalbuminuria, macroalbuminuria, and nephrotic syndrome. (B) Prevalence of impaired GFR. RAS, renin-angiotensin system.
GFR Outcomes after Macroalbuminuria Diagnosis
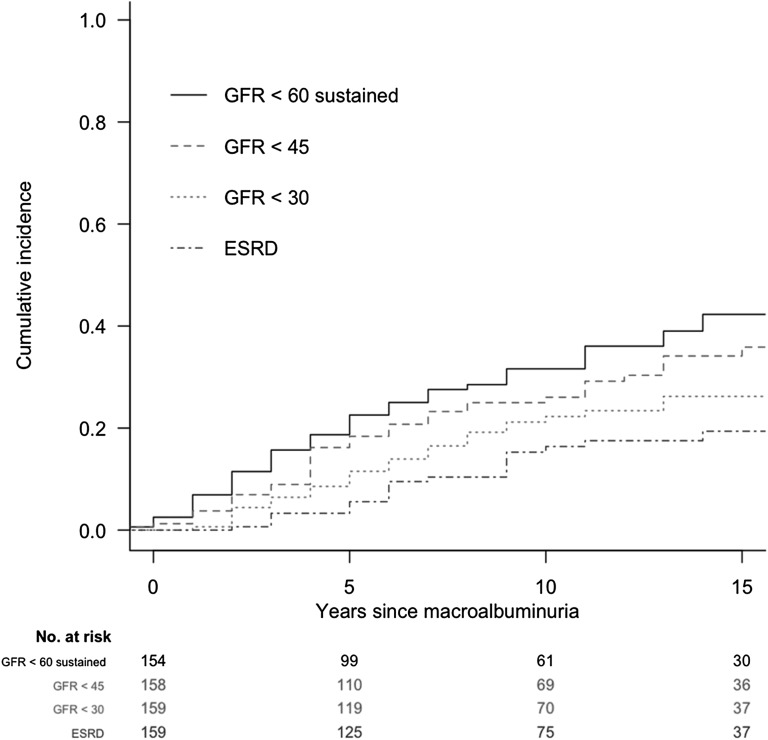
For eGFR, median follow-up after the diagnosis of macroalbuminuria was 9 years, with a maximum follow-up of 25 years (Table 2). Mean (SD) change in eGFR was −5.4 (5.7) ml/min per 1.73 m2 per year. The cumulative incidence of sustained eGFR<60, <45, and <30 ml/min per 1.73 m2 (overlapping categories) 10 years after macroalbuminuria diagnosis was 32%, 26%, and 22%, respectively (Figure 3, Table 2). The cumulative incidence of sustained eGFR<60 ml/min per 1.73 m2 15 years after macroalbuminuria diagnosis was 42%.
Figure 3.
Cumulative incidence of low eGFR among individuals with macroalbuminuria in the DCCT/EDIC study evaluated from the time of macroalbuminuria onset. All four GFR outcomes are depicted simultaneously.
Lower hemoglobin A1c, lower systolic and diastolic BP, and lower AER at the time of macroalbuminuria diagnosis were associated with decreased risk of developing sustained eGFR<60 ml/min per 1.73 m2 after adjustment for age, sex, and eGFR at macroalbuminuria onset (Table 3). Time-updated systolic and diastolic BPs and hemoglobin A1c were also significantly associated with risk of impaired GFR. Participants whose AER returned to <300 mg/d had an 89% lower risk of developing sustained eGFR<60 ml/min per 1.73 m2 (hazard ratio, 0.11; 95% confidence interval [95% CI], 0.04 to 0.31; P<0.001); 22 of 159 participants developed ESRD, with a cumulative incidence of 16% 10 years after macroalbuminuria diagnosis (Table 2).
Table 3.
Risk factors for the development of impaired GFR (sustained eGFR<60 ml/min per 1.73m2) among DCCT/EDIC study participants who developed macroalbuminuria
| Variables | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Baseline variables | ||
| Age (yr) | 0.99 (0.95 to 1.02) | 0.48 |
| Women | 1.19 (0.64 to 2.23) | 0.59 |
| Diabetes duration (yr) | 1.02 (0.95 to 1.09) | 0.61 |
| Smoker | 1.11 (0.59 to 2.10) | 0.74 |
| Current use of RAAS blockers | 1.42 (0.68 to 2.95) | 0.35 |
| Use of lipid-lowering agents | 1.41 (0.77 to 2.57) | 0.26 |
| Body mass index (1 kg/m2) | 1.04 (0.97 to 1.12) | 0.30 |
| SBP (per 10 mmHg) | 1.44 (1.20 to 1.74) | <0.001 |
| DBP (per 10 mmHg) | 1.62 (1.19 to 2.22) | 0.002 |
| Hemoglobin A1c (%) | 1.50 (1.20 to 1.88) | <0.001 |
| eGFR (ml/min per 1.73 m2) | 0.95 (0.94 to 0.97) | <0.001 |
| AER≥1000 mg/d | 3.25 (1.69 to 6.25) | <0.001 |
| Time-dependent variables | ||
| SBP (per 10 mmHg) | 1.70 (1.45 to 1.99) | <0.001 |
| DBP (per 10 mmHg) | 2.14 (1.59 to 2.88) | <0.001 |
| Hemoglobin A1c (per %) | 1.22 (1.01 to 1.48) | 0.04 |
| AER<300 versus AER≥300 mg/d | 0.11 (0.04 to 0.31) | <0.001 |
Discrete Cox models for sustained eGFR<60 ml/min per 1.73 m2 adjusted for age, sex, and eGFR at time of incident macroalbuminuria.
The point prevalence of sustained eGFR<60, <45, and <30 ml/min per 1.73 m2 (overlapping categories) 10 years after macroalbuminuria diagnosis was 29%, 20%, and 15%, respectively (Figure 2B).
Nephrotic-Range Albuminuria
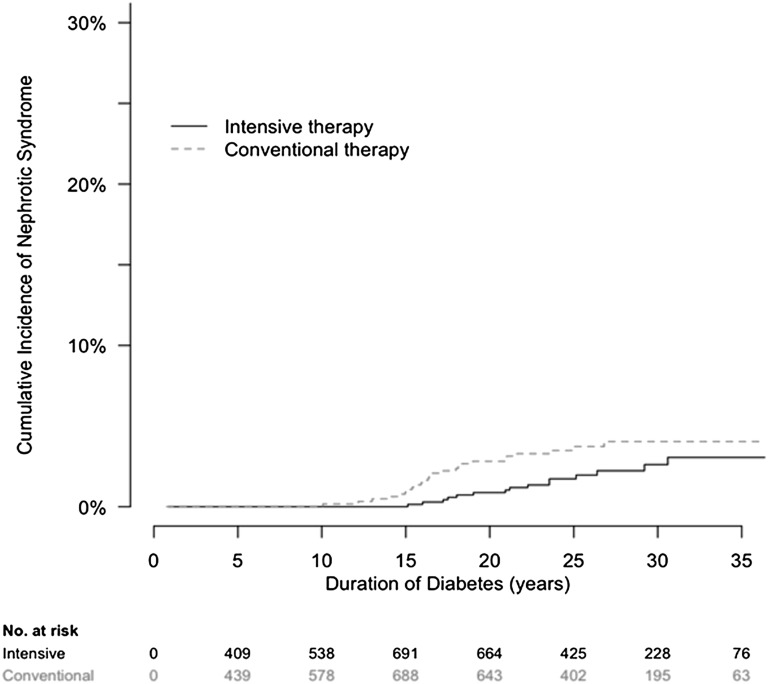
Forty participants developed nephrotic-range albuminuria (AER≥3000 mg/d). For 34 (85%) of 40 participants, this albuminuria occurred more than 15 years after diabetes onset (Figure 4). The cumulative incidence of nephrotic-range albuminuria after 30 years of diabetes was 2.6% and 4.0% among participants assigned to DCCT intensive or conventional diabetes therapy, respectively. Of 40 participants, 25 (63%) were men, and 25 (63%) had been assigned to conventional diabetes therapy. The mean (SD) age was 38.4 (8.2) years, mean (SD) duration of diabetes was 20 (4.9) years, mean time from onset of macroalbuminuria was 3.5 years; median (interquartile range) AER was 4504 (3734–6004) mg/d; mean (SD) eGFR was 76 (31) ml/min per 1.73 m2, and 12 (30%) of 40 participants had eGFR<60ml/min per 1.73 m2 at the time of onset of nephrotic-range albuminuria.
Figure 4.
Cumulative incidence of nephrotic-range albuminuria by treatment assignment in the DCCT/EDIC study by duration of type 1 diabetes. The cumulative incidence of nephrotic-range albuminuria, defined as AER≥3000 mg/d, is shown according to the group to which participants had been randomly assigned in the DCCT.
Sensitivity Analyses in the Subgroup with Persistent Macroalbuminuria
Of 159 participants with incident macroalbuminuria, 123 participants had persistent macroalbuminuria, defined as AER≥300 mg/d, on two consecutive study visits. Clinical and demographic characteristics in the participants with persistent macroalbuminuria were comparable with those characteristics of the entire incident macroalbuminuria group (Table 1). The cumulative incidences of sustained eGFR<60ml/min per 1.73 m2 and ESRD 10 years after the diagnosis of persistent macroalbuminuria were 34% and 18%, respectively, compared with 32% and 16% in the entire group with incident macroalbuminuria (including participants with AER≥300 mg/d on a single occasion). In the subgroup with persistent macroalbuminuria, risk factors for progression to impaired GFR were similar to those risk factors in the entire group with incident macroalbuminuria. For example, the hazard ratio for progression to impaired GFR for time-updated AER<300 mg/d was 0.09 (95% CI, 0.03 to 0.31; P<0.001) compared with 0.11 (95% CI, 0.04 to 0.31) in the entire incident macroalbuminuria group (n=159). Albuminuria outcomes were also similar in both groups: among 123 participants with persistent macroalbuminuria, the prevalences of AER<300 mg/d and <30 mg/d 10 years after macroalbuminuria diagnosis were 58% and 12%, respectively, compared with 64% and 17% in the entire group of 159 participants with macroalbuminuria.
Discussion
This study describes the incidence of macroalbuminuria as well as its long-term renal outcomes in type 1 diabetes. Macroalbuminuria occurred with a cumulative incidence of 6% and 17% in the intensive and conventional arms of the DCCT/EDIC cohort, respectively, after 25 years of type 1 diabetes. The cumulative incidences of impaired GFR (<60 ml/min per 1.73 m2) and ESRD 10 years after the macroalbuminuria diagnosis were 32% and 16%, respectively. However, the remaining majority maintained eGFR≥60 ml/min per 1.73 m2, and the 10-year cumulative incidence of albuminuria reduction to <300 mg/d was 52%. Lower hemoglobin A1c and BP and reduction of AER to <300 mg/d were associated with decreased risk of developing impaired GFR.
The substantial risk of progression from macroalbuminuria to impaired GFR observed in this study supports the current paradigm that macroalbuminuria is a heightened risk state for progression of kidney disease. The cumulative incidence of progression to eGFR<60 ml/min per 1.73 m2 was somewhat lower than previously reported values for people with type 1 diabetes and macroalbuminuria.6,7 For example, compared with the 42% 15-year cumulative incidence of eGFR<60 ml/min per 1.73 m2 reported here, 49% and 53% of people with type 1 diabetes, macroalbuminuria, and normal eGFR progressed to eGFR<60 ml/min per 1.73 m2 over 15 years of follow-up in the Pittsburgh Epidemiology of Diabetes Complications Study6 and the Joslin proteinuria cohort, respectively.7 Given the relatively frequent monitoring of AER in the DCCT/EDIC study (every 1–2 years), our slightly lower values may reflect earlier diagnosis of macroalbuminuria. The cumulative incidence of ESRD in this study falls between those values observed in prior cohorts. Compared with the 16% 10-year cumulative incidence of ESRD reported here, the 10-year cumulative incidence of ESRD was 3%–14% and 13%–30% among participants with macroalbuminuria and baseline eGFR≥60 ml/min per 1.73 m2 in the FinnDiane and Joslin type 1 diabetes cohorts, respectively.8,9
However, the risk of progression is not absolute: the majority of people with incident macroalbuminuria maintained an eGFR≥60 ml/min per 1.73 m2 through 15 years of follow-up, suggesting that macroalbuminuria does not represent an intractable course to GFR loss. Data from other type 1 diabetes cohorts spanning the last two decades also support stability of GFR in a substantial fraction of people with macroalbuminuria and intact GFR.6,8,9 This finding is in contrast to earlier type 1 diabetes cohorts, which reported up to 75% progression to ESRD over 15 years of follow-up,2,3 highlighting a change in the clinical course of diabetic kidney disease under current standards of care.
In the DCCT/EDIC cohort, reductions in AER were also common, with more than one half of participants who developed macroalbuminuria regressing to persistent AER<300 mg/d 10 years after macroalbuminuria diagnosis. This finding suggests that the change in clinical course includes a frequent reduction and at times, complete regression of albuminuria. Some, but not all, of this reduction occurred during treatment with RAAS inhibitors, which could reduce AER through hemodynamic mechanisms without improving underlying pathology. Taken together, incident macroalbuminuria is a stage of disease associated with not only progression but also, significant potential for stability and even regression, thus presenting an important opportunity for intensive therapeutic intervention.
Among DCCT/EDIC participants with incident macroalbuminuria, lower hemoglobin A1c, systolic and diastolic BPs, and AER were each associated with reduced risk of progression to impaired GFR (<60 ml/min per 1.73 m2). Intensive glycemic control in the DCCT/EDIC cohort has been shown to reduce incidence of microalbuminuria, macroalbuminuria, and impaired GFR,5,10,11 highlighting the potential importance of hyperglycemia as a modifiable risk factor for all major manifestations of kidney disease in type 1 diabetes. The observed association of elevated BPs with progression of kidney disease is well supported by prior work and consistent with clinical trials showing the beneficial effect of BP control on rate of GFR loss in the setting of macroalbuminuria.12–15 The association of higher urine albumin excretion with progression of diabetic kidney disease observed here is also consistent with prior data.16–18
Only 31% of participants with incidence macroalbuminuria were using RAAS inhibitors at the time of macroalbuminuria diagnosis. This result is likely, in part, because some cases of macroalbuminuria occurred during the DCCT, when RAAS inhibitors were proscribed, and some macroalbuminuria cases were previously undiagnosed. RAAS inhibitor use increased substantially after macroalbuminuria diagnosis, consistent with current clinical guidelines.19,20 Of note, RAAS inhibitor use was associated with a nonsignificant trend to increased risk of impaired GFR, which was previously noted in the DCCT/EDIC study.5 This result is likely because of confounding by indication (i.e., participants inherently more likely to lose GFR were selectively treated with RAAS inhibitors) rather than true adverse effects of RAAS inhibitors. In addition, it may also reflect the reversible RAAS inhibitor reduction of GFR by hemodynamic effects.
Historically, clinical trials of novel therapeutic agents for treatment of diabetic nephropathy in types 1 and 2 diabetes have targeted patients with macroalbuminuria.12,21–23 The high rates of impaired GFR and ESRD that we observed in those individuals who developed macroalbuminuria support the focus on this high-risk population. In addition, the striking variability in renal outcomes after macroalbuminuria diagnosis suggests that it is a disease stage particularly appropriate for identification of novel diagnostic and therapeutic tools. Identifying biomarkers that would enable additional risk stratification of this population for propensity to GFR loss and ESRD would help focus more intensive therapeutic interventions on patients at the highest risk of progressive kidney disease. Such biomarkers may also be used to identify new molecular targets and focus clinical trials on participants with the highest risks of progressive disease. Examples of such biomarkers would be components of the pathways already incriminated in the pathophysiology of kidney disease (e.g., RAAS,24 bone morphogenetic protein,25–27 wingless-type mouse mammary tumour virus integration site,28,29 Notch,30 vascular endothelial growth factor, and protein kinase C pathways, to name a few).
Albuminuria exceeding 3 g/d, defined as nephrotic-range albuminuria, has been associated with the most rapid decline in GFR.31–33 Among DCCT/EDIC participants with macroalbuminuria, 40 progressed to nephrotic-range proteinuria after an average of 20 years of diabetes. The cumulative incidence of nephrotic-range proteinuria was lower in participants assigned to intensive diabetes therapy.
The primary strengths of this study derive from the meticulous longitudinal characterization of participant outcomes over 25 years of follow-up in the DCCT/EDIC study. This longitudinal follow-up, combined with the fact that all participants were free of macroalbuminuria at study entry, enabled detection of incident macroalbuminuria within 1–2 years of its onset during the DCCT/EDIC study as well as accurate measurement and ascertainment of subsequent outcomes. An additional strength of the DCCT/EDIC cohort, particularly during the EDIC study phase, is that participants are treated under current standards of care in terms of glycemic control and RAAS inhibitor use, which makes observations made in these cohorts relevant to current clinical practice. This study also has limitations. We evaluated macroalbuminuria primarily in a binary classification. Associations of hemoglobin A1c, BP, and AER with impaired GFR are subject to confounding. Kidney biopsies were not performed as part of the study, and it is possible that cases of nephropathy secondary to causes other than diabetes have been included in our cohort. Finally, we did not assess mortality outcomes because of the low rate of death in the DCCT/EDIC study.
In conclusion, we confirm that macroalbuminuria in type 1 diabetes marks a disease state with high risk of progression to impaired GFR and ESRD. However, stabilization and even regression of disease occur even more frequently. As such, macroalbuminuria presents an important disease stage for intensive delivery of treatments targeted at improving renal outcomes in type 1 diabetes.
Concise Methods
The DCCT/EDIC Study
The DCCT was a multicenter randomized clinical trial in type 1 diabetes mellitus comparing the effects of intensive diabetes therapy aimed at achieving glycemic control as close to the nondiabetic range as safely possible with conventional therapy.10,11 The trial included two cohorts: a primary prevention cohort (1–5 years duration of diabetes, AER<40 mg/24 h, no retinopathy by fundus photography) and a secondary intervention cohort (1–15 years duration, AER≤200 mg/24 h, and at least one microaneurysm in either eye but no more than moderate nonproliferative retinopathy). From 1983 to 1989, 1441 participants between the ages of 13 and 39 years were enrolled and randomly assigned to intensive or conventional diabetes therapy. Intensive therapy included three or more insulin injections daily or use of an insulin pump with the aim of achieving hemoglobin A1c levels<6.05%. The goal of conventional therapy was prevention of symptoms of hyperglycemia and hypoglycemia using one or two daily injections of insulin. Subjects were followed for a mean of 6.5 years until DCCT closeout in 1993.
At the end of the DCCT, all former conventional treatment participants were offered instruction in intensive therapy, and all participants returned to their own health care providers for ongoing diabetes care. All DCCT participants were invited to join the EDIC study, an observational extension of the DCCT, and 1375 (96% of the surviving cohort) agreed to participate. During the EDIC study, mean hemoglobin A1c levels, which had been separated by approximately 2% between conventional and intensive therapy groups during the DCCT, converged from the former treatment groups.10 The DCCT/EDIC study procedures were approved by the institutional review boards of participating centers, and all participants provided written informed consent. The study described herein includes data from the DCCT baseline through EDIC study year 16 (2008–2010).
Macroalbuminuria Cohort
This study focuses on 159 DCCT/EDIC participants who developed macroalbuminuria during the course of DCCT/EDIC study observation. Macroalbuminuria was defined as AER≥300 mg/d. In addition, sensitivity analyses were performed in the subset of 123 participants who developed persistent macroalbuminuria, defined as AER≥300 mg/d, on two consecutive visits. Because few published studies describe the incidence of nephrotic-range albuminuria in type 1 or 2 diabetes, we also described a separate subset of 40 DCCT/EDIC participants who developed AER≥3000 mg/d. AER was measured yearly during the DCCT and every 2 years during the EDIC study. Urine was collected for 4 hours during water diuresis; albumin was measured by fluoroimmunoassay (coefficient of variation of 9.4%).11 Urine creatinine was measured by a variation of the Jaffe method on the Beckman CX3 (coefficient of variation of 2.8%).
Definitions of Long-Term Renal Outcomes
Among the macroalbuminuria cohort, we assessed the following outcomes: sustained microalbuminuria, sustained normoalbuminuria, change in eGFR (slope), development of impaired GFR, and development of ESRD. Sustained microalbuminuria was defined as urine AER<300 mg/d on two successive occasions; sustained normoalbuminuria was defined as urine AER<30 mg/d on two successive occasions. Serum creatinine was measured yearly during the DCCT and the EDIC study using methods traceable to isotope dilution mass spectrometry, with overall interassay coefficients of variation<3%.5 GFR was estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration formula.34 Impaired GFR was defined as eGFR<60 ml/min per 1.73 m2 on two consecutive study visits.5 Incidence rates of lower levels of impaired GFR were also ascertained (eGFR<45 and <30 ml/min per 1.73 m2, each on a single study visit). ESRD was defined as the initiation of maintenance dialysis (n=14) or kidney transplantation (n=8) assessed yearly by questionnaire and adjudicated centrally. We described the distribution of AER over time after macroalbuminuria diagnosis. Renal outcomes were not mutually exclusive.
Covariates
Demographic data and smoking were assessed by self-report. Body mass index was calculated as weight (kilograms) divided by height (meters) squared. BP was measured using the standardized procedure by trained personnel.35 All laboratory measurements were completed at the DCCT/EDIC study Central Biochemistry Laboratory at the University of Minnesota. Hemoglobin A1c was measured using high-performance ion-exchange liquid chromatography (coefficient of variation<4%).36 Plasma lipids were measured using conventional enzymatic methods, with LDL cholesterol calculated using the Friedewald formula.
Medication use was assessed yearly by self-report only during the EDIC study. Use of angiotensin-converting enzyme (ACE) inhibitors was discouraged during the DCCT, and only 6% of participants reported ACE inhibitor use on their first EDIC study visit. Angiotensin receptor blockers were available for clinical use only during the EDIC study. RAAS inhibitors (ACE inhibitors and angiotensin receptor blockers) were combined and analyzed as a class. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors became available during the end of the DCCT (beginning in 1989), and use of a lipid-lowering agent was reported by only 2% of participants at their first EDIC study visit.
Statistical Analyses
To describe the development of macroalbuminuria over the clinical course of type 1 diabetes in our population, we first graphed the cumulative incidence of macroalbuminuria by duration of diabetes in the full DCCT/EDIC cohort using Kaplan–Meier methods.
To examine development of impaired GFR, time at risk began at macroalbuminuria diagnosis and extended to the diagnosis of impaired GFR or the penultimate serum creatinine measurement for censored subjects. For ESRD, time at risk extended to the diagnosis of ESRD or the last study visit for censored subjects. Incidence rates were expressed in person-years, and Kaplan–Meier methods were used to plot cumulative incidence. Discrete Cox proportional hazards models were used to test associations of clinical characteristics with the development of impaired GFR adjusting for age, sex, and eGFR at macroalbuminuria diagnosis. Cox models assessed as exposures both characteristics at the time of macroalbuminuria diagnosis and characteristics assessed at the time of follow-up examination (time-updated variables).
Change in eGFR was estimated using linear mixed models. For participants who developed ESRD, eGFR was defined as 10 ml/min per 1.73 m2 at the time of ESRD and missing thereafter. Random effects were assigned to participants and a fixed effect to time (years).
Among 159 participants who developed incident macroalbuminuria, subsequent distributions of AER and eGFR were examined in 2-year time intervals. Mean values were used if two measurements were available within a 2-year period.
Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Disclosures
None.
Acknowledgments
The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study has been supported by U01 Cooperative Agreement Grants (1982–1993 and 2011–2016) and Contracts (1982–2011) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the Genetic Clinical Research Centers Program (1993–2007), and the Clinical Translational Science Center Program (2006 to present). Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care, Animas, Bayer Diabetes Care, Becton Dickinson, CanAm, Eli Lilly, Lifescan, Medtronic Diabetes, Omron, OmniPod Insulin Management System, Roche Diabetes Care, and Sanofi-Aventis. I.H.d.B. was supported by NIDDK Grants R01-DK087726 and R01-DK088762. M.A. was supported by NIDDK Grant K23-DK089017 and a Norman S. Coplon Extramural Grant from Satellite Healthcare.
A complete list of participants in the DCCT/EDIC Research Group was previously published (DCCT/EDIC Research Group et al., New Engl J Med 365: 2366–2376, 2011).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Are Post-Trial Observational Studies Useful?,” on pages 2148–2150.
References
- 1.Mogensen CE, Christensen CK, Vittinghus E: The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 32[Suppl 2]: 64–78, 1983 [DOI] [PubMed] [Google Scholar]
- 2.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR: The changing natural history of nephropathy in type I diabetes. Am J Med 78: 785–794, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T: Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: An epidemiological study. Diabetologia 25: 496–501, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Molitch ME, Steffes M, Sun W, Rutledge B, Cleary P, de Boer IH, Zinman B, Lachin J, Epidemiology of Diabetes Interventions and Complications Study Group : Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 33: 1536–1543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B, DCCT/EDIC Research Group : Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365: 2366–2376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costacou T, Ellis D, Fried L, Orchard TJ: Sequence of progression of albuminuria and decreased GFR in persons with type 1 diabetes: A cohort study. Am J Kidney Dis 50: 721–732, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Skupien J, Warram JH, Smiles AM, Niewczas MA, Gohda T, Pezzolesi MG, Cantarovich D, Stanton R, Krolewski AS: The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int 82: 589–597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsblom C, Harjutsalo V, Thorn LM, Wadén J, Tolonen N, Saraheimo M, Gordin D, Moran JL, Thomas MC, Groop PH, FinnDiane Study Group : Competing-risk analysis of ESRD and death among patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 22: 537–544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS: Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 22: 545–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group : Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: The Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290: 2159–2167, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Diabetes Control and Complications (DCCT) Research Group : Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int 47: 1703–1720, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ, Collaborative Study Group : Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Am J Kidney Dis 34: 809–817, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH: Remission and regression in the nephropathy of type 1 diabetes when blood pressure is controlled aggressively. Kidney Int 60: 277–283, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Mogensen CE: Progression of nephropathy in long-term diabetics with proteinuria and effect of initial anti-hypertensive treatment. Scand J Clin Lab Invest 36: 383–388, 1976 [DOI] [PubMed] [Google Scholar]
- 15.Parving HH, Andersen AR, Smidt UM, Svendsen PA: Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet 1: 1175–1179, 1983 [DOI] [PubMed] [Google Scholar]
- 16.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH: Progression of diabetic nephropathy. Kidney Int 59: 702–709, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Mulec H, Blohmé G, Grände B, Björck S: The effect of metabolic control on rate of decline in renal function in insulin-dependent diabetes mellitus with overt diabetic nephropathy. Nephrol Dial Transplant 13: 651–655, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Breyer JA, Bain RP, Evans JK, Nahman NS, Jr., Lewis EJ, Cooper M, McGill J, Berl T, The Collaborative Study Group : Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. Kidney Int 50: 1651–1658, 1996 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association : Standards of medical care in diabetes—2014. Diabetes Care 37[Suppl 1]: S14–S80, 2014 [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation : KODQI clinical practive guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 60: 850–886, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH: Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int 57: 601–606, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A: Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558–1565, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Dolan V, Hensey C, Brady HR: Diabetic nephropathy: Renal development gone awry? Pediatr Nephrol 18: 75–84, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Zhang Q: Bone morphogenetic protein-7 and Gremlin: New emerging therapeutic targets for diabetic nephropathy. Biochem Biophys Res Commun 383: 1–3, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Wong MG, Perkovic V, Woodward M, Chalmers J, Li Q, Hillis GS, Yaghobian Azari D, Jun M, Poulter N, Hamet P, Williams B, Neal B, Mancia G, Cooper M, Pollock CA: Circulating bone morphogenetic protein-7 and transforming growth factor-β1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int 83: 278–284, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y: New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawakami T, Ren S, Duffield JS: Wnt signalling in kidney diseases: Dual roles in renal injury and repair. J Pathol 229: 221–231, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Bonegio R, Susztak K: Notch signaling in diabetic nephropathy. Exp Cell Res 318: 986–992, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin SM, Lieberman JS, Newton LD, Mejia M, Peters WA, Myers BD: Slope of serial glomerular filtration rate and the progression of diabetic glomerular disease. J Am Soc Nephrol 3: 1358–1370, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Kussman MJ, Goldstein H, Gleason RE: The clinical course of diabetic nephropathy. JAMA 236: 1861–1863, 1976 [PubMed] [Google Scholar]
- 33.Watkins PJ, Blainey JD, Brewer DB, Fitzgerald MG, Malins JM, O’Sullivan DJ, Pinto JA: The natural history of diabetic renal disease. A follow-up study of a series of renal biopsies. Q J Med 41: 437–456, 1972 [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, Lachin JM, Weiss NS, Brunzell JD, Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group : Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med 168: 1867–1873, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steffes M, Cleary P, Goldstein D, Little R, Wiedmeyer HM, Rohlfing C, England J, Bucksa J, Nowicki M: Hemoglobin A1c measurements over nearly two decades: Sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem 51: 753–758, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]