Abstract
Compound heterozygous and homozygous (comp/hom) mutations in solute carrier family 34, member 3 (SLC34A3), the gene encoding the sodium (Na+)-dependent phosphate cotransporter 2c (NPT2c), cause hereditary hypophosphatemic rickets with hypercalciuria (HHRH), a disorder characterized by renal phosphate wasting resulting in hypophosphatemia, correspondingly elevated 1,25(OH)2 vitamin D levels, hypercalciuria, and rickets/osteomalacia. Similar, albeit less severe, biochemical changes are observed in heterozygous (het) carriers and indistinguishable from those changes encountered in idiopathic hypercalciuria (IH). Here, we report a review of clinical and laboratory records of 133 individuals from 27 kindreds, including 5 previously unreported HHRH kindreds and two cases with IH, in which known and novel SLC34A3 mutations (c.1357delTTC [p.F453del]; c.G1369A [p.G457S]; c.367delC) were identified. Individuals with mutations affecting both SLC34A3 alleles had a significantly increased risk of kidney stone formation or medullary nephrocalcinosis, namely 46% compared with 6% observed in healthy family members carrying only the wild-type SLC34A3 allele (P=0.005) or 5.64% in the general population (P<0.001). Renal calcifications were also more frequent in het carriers (16%; P=0.003 compared with the general population) and were more likely to occur in comp/hom and het individuals with decreased serum phosphate (odds ratio [OR], 0.75, 95% confidence interval [95% CI], 0.59 to 0.96; P=0.02), decreased tubular reabsorption of phosphate (OR, 0.41; 95% CI, 0.23 to 0.72; P=0.002), and increased serum 1,25(OH)2 vitamin D (OR, 1.22; 95% CI, 1.05 to 1.41; P=0.008). Additional studies are needed to determine whether these biochemical parameters are independent of genotype and can guide therapy to prevent nephrocalcinosis, nephrolithiasis, and potentially, CKD.
Inactivating mutations on both parental alleles of the solute carrier family 34, member 3 (SLC34A3), the gene encoding the sodium (Na+)-dependent phosphate cotransporter 2c (NPT2c), are the cause of hereditary hypophosphatemic rickets with hypercalciuria (HHRH; OMIM: 241530)1–3—an autosomal recessive renal phosphate-wasting disorder that was originally described by Tieder et al.4,5 Individuals affected by HHRH who carry compound heterozygous or homozygous (comp/hom) SLC34A3/NPT2c mutations show increased urinary phosphate excretion leading to hypophosphatemic rickets, bowing, and short stature as well as appropriately elevated 1,25(OH)2D levels. Elevated 1,25(OH)2D levels, in turn, result in hypercalciuria because of enhanced intestinal calcium absorption and reduced parathyroid hormone (PTH) -dependent calcium reabsorption in the distal renal tubules. Even heterozygous SLC34A3/NPT2c mutations are frequently associated with hypercalciuria, but none of the carriers of SLC34A3/NPT2c mutations in the originally described HHRH patients were reported to have renal calcifications and kidney stones.4,5 Subsequent investigations, however, revealed that these complications affecting the kidneys were observed in numerous patients with comp/hom SLC34A3/NPT2c mutations.3,6–11 However, the small size of HHRH kindreds and the relatively high prevalence of renal calcifications in the general population (5.64%)12,13 have, thus far, prevented segregation-based statistical approaches to determine whether SLC34A3/NPT2c mutations do increase the risk of developing kidney stones or nephrocalcinosis. Likewise, it is unknown whether loss of NPT2c can lead to additional proximal tubular phenotypes such as Fanconi syndrome, which has been described in two patients with homozygous SLC34A3/NPT2a mutations14 who developed CKD later in life.
The presence of hypercalciuria, kidney stones, and nephrocalcinosis observed in HHRH kindreds is different from the findings in fibroblast growth factor 23 (FGF23)–dependent hypophosphatemic disorders, such as X-linked hypophosphatemia (XLH; mutant PHEX),15 autosomal dominant hypophosphatemic rickets (ADHR; mutant FGF23),16 or autosomal recessive hypophosphatemic rickets (ARHR; mutant DMP1, ENPP1, or FAM20C),17–20 in which affected individuals show, before treatment with oral phosphate and 1,25(OH)2D, inappropriately normal or suppressed 1,25(OH)2D levels despite significant hypophosphatemia and thus, no increase in urinary calcium excretion. Oral phosphate supplements combined with active vitamin D analogs are generally recommended for treatment of FGF23-dependent hypophosphatemic disorders.21 In contrast, HHRH is thought to require phosphate supplements alone,4,5 in part because endogenously elevated 1,25(OH)2D levels are predicted to prevent an increase in PTH secretion triggered by intermittent elevations in serum phosphate. However, long-term studies are lacking that determine whether oral phosphate supplementation alone of HHRH patients is sufficient for prevention of renal calcifications and bone loss. It is likewise unknown how therapy should be monitored, whether secondary hyperparathyroidism can develop as observed in FGF23-dependent hypophosphatemic disorders,15 and whether phosphate requirements decrease with age, which has been reported for ADHR.16
In the current study, we investigated five new HHRH kindreds and two new cases with idiopathic hypercalciuria (IH), in whom we discovered known and novel homozygous or compounded heterozygous SLC34A3/NPT2c mutations. Review of the clinical and laboratory findings along with those findings reported for 22 previously published kindreds suggests that renal calcifications and/or kidney stones may be important, often unrecognized initial findings suggestive of comp/hom SLC34A3/NPT2c mutations. Importantly, heterozygous carriers also show an increased frequency of renal calcifications and biochemical profiles in plasma and urine that are intermediate to those profiles of individuals without SLC34A3/NPT2c mutations and comp/hom changes. Our data suggest that serum phosphate, tubular reabsorption of phosphate (TRP), and serum 1,25(OH)2D levels predict the development of renal calcifications. However, additional studies are needed to determine whether these biochemical parameters are independent of genotype and can guide therapy to prevent renal calcifications and potentially, CKD.
Results
Novel Mutations in the SLC34A3/NPT2c Gene in Kindreds with HHRH and IH
Five new kindreds were referred to us for genetic evaluation, with the index case presenting with classic HHRH during childhood. Metabolic bone disease was found to be associated with hypophosphatemia, and subsequent laboratory studies were consistent with FGF23-independent renal phosphate wasting. In addition, two unrelated children with IH presented to their pediatrician with renal stones and/or nephrocalcinosis on renal ultrasound and biochemical abnormalities that were consistent with those abnormalities seen in HHRH [i.e., hypercalciuria and elevated 1,25(OH)2D levels]. Apparent bone disease was missing, and mild hypophosphatemia was only noted on more careful evaluation. Nucleotide sequence analysis of SLC34A3/NPT2c revealed known and novel mutations in kindreds A and B and case G (Supplemental Figure 1, A and B, Supplemental Tables 1 and 2). A novel homozygous missense mutation c.1369G>A(p.G457S) was detected in individuals A/IV-1, A/IV-2, A/IV-3, and A/IV-4, whereas both parents were heterozygous for this nucleotide change. The index case B/II-2 inherited the previously described intronic deletion c.560+27_561–38del (g.1440_1469del)22 from his mother (B/I-1) and the novel in-frame deletion c.1357delTTC (p. F453del) from his father (B/I-2); c.1369G>A (p.G457S) and c.1357delTTC (p.F453del) affect highly conserved amino acid residues (Supplemental Table 3). Case G was found to be compound heterozygous for a novel deletion c.367delC, which introduces 14 novel amino acids followed by premature termination of the NPT2c protein after residue p.P48 (WTLPQLKDPWTLPS-Stop) and the known missense mutation c.575C>T(p.S192L).2 All three novel mutations are absent from the 1000 Genome,23 dbSNP,24 and the National Heart, Lung, and Blood Institute Gene Ontology Exome Sequencing Project (http://evs.gs.washington.edu/EVS/) databases, predicted by Polyphen to reduce protein function,25 and therefore, likely disease-causing. Detailed clinical and genotyping information on kindreds A–G is in Supplemental Material, Supplemental Figure 1, A and B, Supplemental Tables 1 and 2.
Meta-Analysis of Clinical Information, Including Previously Reported Kindreds with HHRH
Seven of eleven com/hom carriers of the identified SLC34A3/NPT2c mutations and one of ten heterozygous carriers presented initially with renal calcifications or developed these changes subsequently. To better understand whether this high prevalence of renal calcifications in these not previously reported kindreds can be generalized to all carriers of SLC34A3/NPT2c mutations and to identify possible predictors of renal calcifications, we performed a meta-analysis of the clinical information from 13 reports1–3,6–9,11,22,26–29 and two unpublished families (Simm P, Briody J, Reyes M, Gibbons P, Alexander S, Rauch F, Jüppner H, Bergwitz C, Munns C, manuscript in preparation). Two reports of heterozygous mutations in kindreds with IH and HHRH10,30 were excluded from this analysis, because no mutations on the second allele had been identified; thus, segregation with the renal phenotype in the affected individuals remains uncertain. After including our new cases, this analysis comprised 133 individuals from 27 kindreds (Supplemental Table 2). Genotype information is available for 117 individuals and four obligate heterozygous carriers.
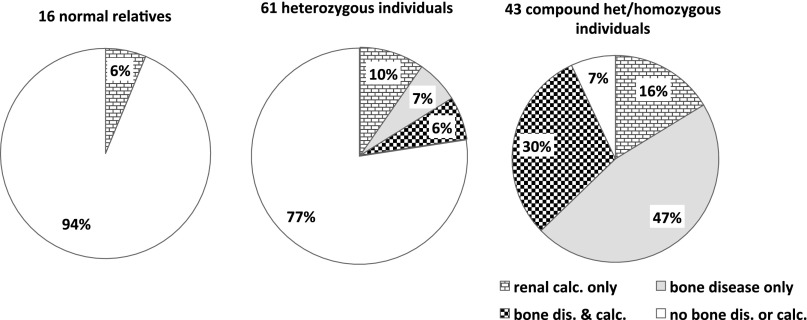
We first evaluated the prevalence of renal calcifications (i.e., nephrolithiasis or nephrocalcinosis) in the comp/hom carriers of SLC34A3/NPT2c mutations. This was significantly increased (20 of 43 [46%] individuals) compared with the available genotyped relatives who carried only the wild-type allele (1 of 16 [6%] individuals; P=0.005) and compared with the heterozygote carriers of SLC34A3/NPT2c mutations (10 of 61 [16%] individuals; P=0.002) (Table 1); 15 of 43 (35%) comp/hom carriers of SLC34A3/NPT2c mutations presented with renal stones (P=0.05), of whom 6 (14%) individuals also had evidence for nephrocalcinosis. Furthermore, renal ultrasound studies showed medullary nephrocalcinosis in 13 of 43 (30%) com/hom individuals (P=0.01) (Table 1). Seven of these individuals (16%) did not have stones. Importantly, 6 of 61 (10%) heterozygous and 7 of 43 (16%) comp/hom carriers of SLC34A3/NPT2c mutations presented with renal calcifications, whereas clinical and laboratory findings suggestive of metabolic bone disease were absent (Figure 1).
Table 1.
Frequency of renal calcifications is increased in carriers of SLC34A3/NPT2c mutations
| Phenotype | Genotype | |||||
|---|---|---|---|---|---|---|
| Normal (n=16) | Heterozygous (n=61) | Comp/Hom (n=43) | ||||
| N | P Value | N | P Value | N | P Value | |
| Renal | ||||||
| Symptomatic | 1 | 1.00 | 10 | 0.44/0.003a | 20 | <0.01/0.002b/<0.001a |
| Nephrolithiasis | 1 | 1.00 | 10 | 0.44/0.003a | 15 | 0.05/0.04b/<0.001a |
| Nephrocalcinosis | 0 | 3 | 1.00 | 13 | 0.01/<0.001b | |
| Asymptomatic | 15 | 51 | 23 | |||
| Bone | ||||||
| Symptomatic | 0 | 8 | 0.19 | 33 | <0.001 | |
| Rickets/osteomalacia | 0 | 8 | 0.19 | 32 | <0.001 | |
| Adult osteopenia/osteoporosis | 0 | 0 | 1 | 1.00 | ||
| Asymptomatic | 16 | 53 | 10 | |||
Number (N) of individuals with renal phenotype who presented with nephrolithiasis or nephrocalcinosis and number of individuals (N) with bone phenotype who presented with rickets/osteomalacia or osteopenia/osteoporosis. P values (Fisher exact t test) are shown compared with normal individuals.
Assuming average global prevalence of stones of 5.64% according to the work by Romero et al.12
P values compared with heterozygous individuals.
Figure 1.
Frequency of renal calcifications is increased in carriers of SLC34A3/NPT2c mutations. Shown here is the prevalence of renal calcifications (i.e., nephrolithiasis and/or nephrocalcinosis) and bone disease (i.e., rickets/osteomalacia and/or osteopenia/osteoporosis) among individuals with two normal alleles as well as heterozygous and comp/hom carriers of SLC34A3/NPT2c mutations. Calc., calcification; dis., disease.
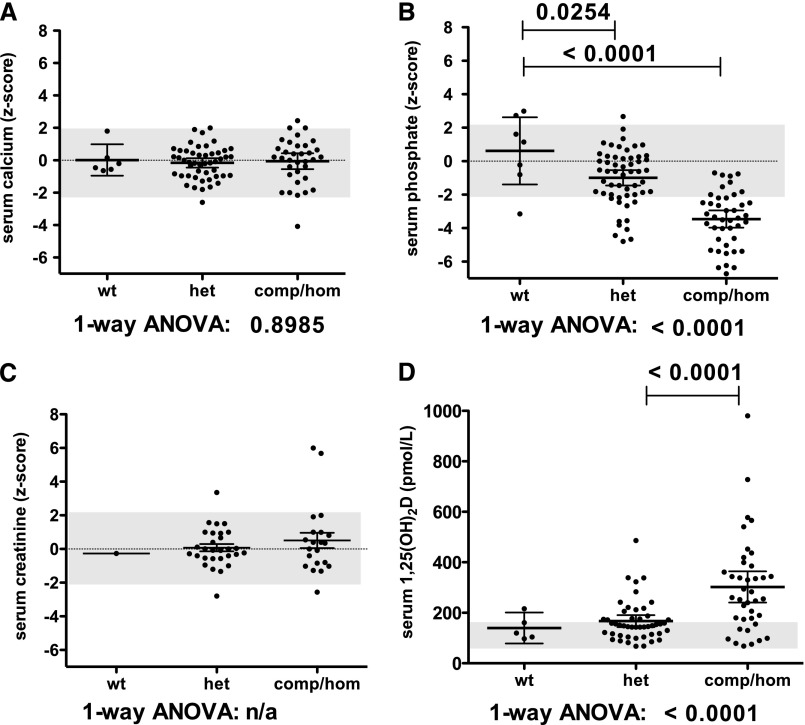
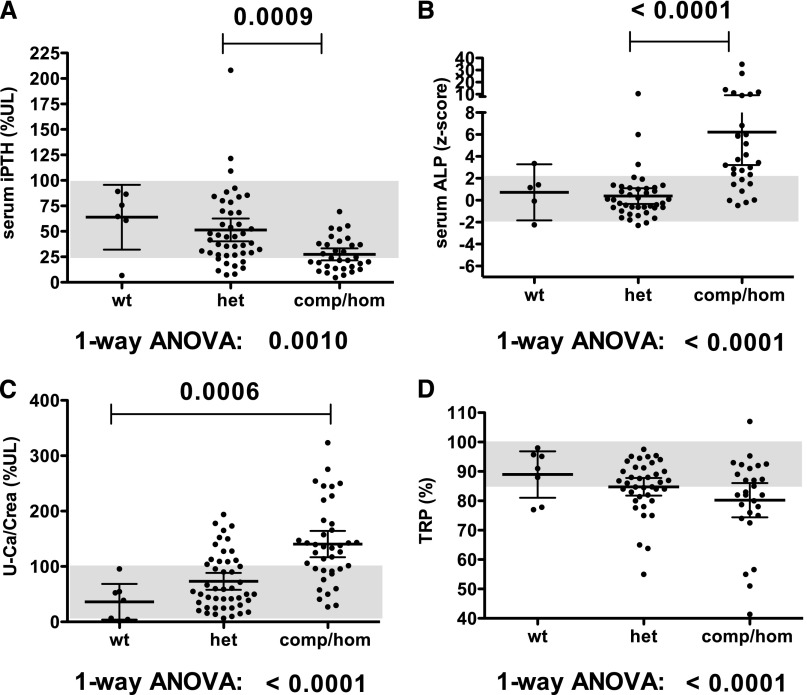
Prevalence of renal calcifications was increased 3-fold in heterozygous carriers of SLC34A3/NPT2c, which is significant when compared with the prevalence of 5.64% reported in large cohorts of healthy controls12,13 (P=0.003) (Table 1). Consistent with an intermediate frequency of renal calcifications, the biochemical findings were, furthermore, intermediate for heterozygous carriers of SLC34A3/NPT2c mutations. A combined analysis of the initial biochemical findings in our new HHRH families along with previously published kindreds (Figures 2 and 3) showed reduced mean serum P, TRP, and intact PTH in carriers of SLC34A3/NPT2c mutations, whereas serum Ca, 1,25(OH)2D, and urinary calcium excretion (uCa/uCrea) ratio were increased. Differences were significant based on one-way ANOVA for these analytes when comparing wild-type, heterozygous, and comp/hom carriers. Despite the small number of healthy siblings, serum P was also significantly reduced in heterozygous carriers compared with healthy siblings (P<0.03), and heterozygous means for 1,25(OH)2D and TRP were above and below the normal range, respectively. No clear difference in severity was observed for individual mutations (Supplemental Figure 2). Renal function was normal in all but one heterozygous and two comp/hom carriers of SLC34A3/NPT2c mutations at initial presentation (Figure 2C).
Figure 2.
Heterozygous carriers of SLC34A3/NPT2c mutations have intermediate biochemical findings. Summary of biochemical values of the presented and published kindreds analyzed with respect to genotype. Individual values with mean±95% confidence interval are shown for (A) normalized serum calcium, (B) normalized serum phosphorus, (C) normalized serum creatinine, and (D) serum 1,25(OH)2 vitamin D. het, heterozygous; n/a, not applicable; wt, wild-type.
Figure 3.
Heterozygous carriers of SLC34A3/NPT2c mutations have intermediate biochemical findings. Summary of biochemical values of presented kindreds and published kindreds analyzed with respect to genotype. Individual values with mean±95% confidence interval are shown for (A) serum PTH levels, (B) normalized ALP, (C) uCa/uCrea, and (D) TRP. %UL, percent upper limit of normal; het, heterozygous; wt, wild-type.
Several of the above biochemical parameters have been implicated as predictors of renal calcifications31 and metabolic bone disease.32 To identify predictors in our HHRH kindreds, we first performed a univariate logistic regression analysis in individuals with all genotypes (n=133) (Table 2). As expected, renal calcifications were more likely to occur in carriers of SLC34A3/NPT2c mutations. Furthermore, individuals with renal calcifications had decreased serum P and TRP but increased serum 1,25(OH)2D. However, only TRP remained significant after adjusting for genotype in a multivariate model, and 43 cases were not sufficient to identify predictors of renal calcifications when limiting the univariate logistic regression analysis to individuals with two mutant SLC34A3/NPT2c alleles.
Table 2.
Univariate and multivariate regression model identifies association of genotype and biochemical variables with renal calcifications
| Variable | Unit | Renal Calcifications | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted, All | Adjusted, All (for Genotype or Sex/Agea) | Unadjusted, Comp/Hom Only | ||||||||||||||
| N | OR | 95% CI | P Value | N | Adjusted OR | 95% CI | P Value | N | OR | 95% CI | P Value | |||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||||||
| NPT2c mutation | Alleles | 122 | 3.90 | 1.31 | 11.63 | 0.02 | 89 | 12.2a | 2.04 | 72.3 | <0.01 | |||||
| Women | Yes | 133 | 0.83 | 0.41 | 1.69 | 0.61 | 43 | 0.58 | 0.14 | 2.36 | 0.45 | |||||
| Age | Year | 94 | 1.00 | 0.97 | 1.03 | 0.86 | 41 | 1.02 | 0.96 | 1.10 | 0.51 | |||||
| Serum 25(OH)D | 50 nmol/L | 71 | 1.46 | 0.52 | 4.12 | 0.47 | 71 | 2.23 | 0.85 | 5.87 | 0.11 | 33 | 2.72 | 0.70 | 10.59 | 0.15 |
| Serum calcium | 1 SD | 79 | 0.91 | 0.60 | 1.37 | 0.64 | 77 | 0.86 | 0.57 | 1.31 | 0.49 | 33 | 0.95 | 0.62 | 1.46 | 0.82 |
| Serum phosphate | 1 SD | 100 | 0.75 | 0.59 | 0.96 | 0.02 | 98 | 0.98 | 0.71 | 1.37 | 0.92 | 42 | 0.93 | 0.58 | 1.47 | 0.75 |
| Serum intact PTH | 10% UL | 78 | 0.80 | 0.59 | 1.07 | 0.13 | 76 | 0.97 | 0.71 | 1.32 | 0.83 | 32 | 0.92 | 0.61 | 1.40 | 0.71 |
| Serum 1,25(OH)2D | 50 pmol/L | 86 | 1.22 | 1.05 | 1.41 | <0.01 | 85 | 1.09 | 0.96 | 1.25 | 0.19 | 39 | 1.11 | 0.97 | 1.26 | 0.12 |
| uCa/uCrea | 50% UL | 88 | 1.39 | 0.94 | 2.05 | 0.10 | 85 | 0.92 | 0.59 | 1.45 | 0.73 | 38 | 0.87 | 0.51 | 1.45 | 0.58 |
| TRP (%) | 10% | 68 | 0.41 | 0.23 | 0.72 | 0.002 | 66 | 0.54 | 0.31 | 0.92 | 0.02 | 27 | 0.65 | 0.38 | 1.10 | 0.11 |
| Serum ALP | 1 SD | 73 | 1.04 | 0.95 | 1.15 | 0.40 | 72 | 0.97 | 0.90 | 1.04 | 0.37 | 29 | 0.96 | 0.89 | 1.04 | 0.30 |
| Bone disease | Yes | 133 | 2.90 | 1.24 | 6.77 | 0.01 | 122 | 1.42 | 0.39 | 5.22 | 0.60 | 43 | 0.43 | 0.13 | 1.40 | 0.16 |
A univariate logistic regression model (GENMOD; SAS Institute, Inc.) was applied to estimate the unadjusted odds ratios (ORs) and those ORs were further adjusted for genotype (adjusted OR) as well as sex and age using a multivariate logistic regression model. An OR of one indicates that renal or bone involvement is equally likely to occur in individuals with a given variable. An OR greater than one indicates that renal or bone involvement is more likely to occur in individuals with high measurements of a given variable at the indicated units. An OR less than one indicates that renal or bone involvement is less likely to occur with high measurements of a given variable at the indicated units. 95% CI, 95% confidence interval.
Odds ratios were further adjusted for sex and age by using a multivariate logistic regression model.
Genotype was likewise the strongest predictor of metabolic bone disease (Table 3). Furthermore, decreased serum P and TRP and increased serum 1,25(OH)2D and serum alkaline phosphatase (ALP) were significantly associated with bone involvement, even after adjusting for genotype in a multivariate model; 1,25(OH)2D, TRP, and ALP continued to be significant when limiting the univariate logistic regression analysis to individuals with two mutant SLC34A3/NPT2c alleles.
Table 3.
Univariate and multivariate regression model identifies association of genotype and biochemical variables with bone disease
| Variable | Unit | Bone Disease | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted, All (for Genotype or Sex and Agea) | Unadjusted, Comp/Hom Only | ||||||||||||||
| N | OR | 95% CI | P Value | N | Adjusted OR | 95% CI | P Value | N | OR | 95% CI | P Value | |||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||||||
| NPT2c mutation | Alleles | 122 | 20.8 | 7.84 | 55.09 | <0.001 | 89 | 33.9a | 9.52 | 120.9 | <0.001 | |||||
| Women | Yes | 133 | 0.71 | 0.33 | 1.55 | 0.39 | 43 | 0.43 | 0.08 | 2.41 | 0.34 | |||||
| Age | Year | 94 | 0.96 | 0.92 | 0.99 | 0.02 | 41 | 1.02 | 0.97 | 1.08 | 0.45 | |||||
| Serum 25(OH)D | 50 nmol/L | 71 | 0.74 | 0.31 | 1.79 | 0.51 | 71 | 1.18 | 0.40 | 3.49 | 0.77 | 33 | 1.22 | 0.22 | 6.79 | 0.82 |
| Serum calcium | 1 SD | 79 | 1.32 | 0.80 | 2.18 | 0.28 | 77 | 1.41 | 0.87 | 2.28 | 0.16 | 33 | 1.19 | 0.75 | 1.88 | 0.45 |
| Serum phosphate | 1 SD | 100 | 0.39 | 0.24 | 0.63 | <0.001 | 98 | 0.50 | 0.30 | 0.83 | <0.01 | 42 | 0.45 | 0.19 | 1.03 | 0.06 |
| Serum intact PTH | 10% UL | 78 | 0.73 | 0.62 | 0.87 | <0.001 | 76 | 0.94 | 0.78 | 1.14 | 0.51 | 32 | 1.16 | 0.71 | 1.88 | 0.56 |
| Serum 1,25(OH)2D | 50 pmol/L | 86 | 1.62 | 1.20 | 2.20 | 0.002 | 85 | 1.32 | 1.04 | 1.69 | 0.02 | 39 | 1.43 | 1.01 | 2.02 | 0.04 |
| uCa/uCrea | 50% UL | 88 | 2.62 | 1.36 | 5.05 | 0.004 | 85 | 1.45 | 0.66 | 3.21 | 0.35 | 38 | 1.38 | 0.51 | 3.72 | 0.52 |
| TRP(%) | 10% | 68 | 0.23 | 0.13 | 0.40 | <0.001 | 66 | 0.21 | 0.08 | 0.56 | 0.002 | 27 | 0.02 | 0.00 | 0.94 | 0.05 |
| Serum ALP | 1 SD | 73 | 2.46 | 1.73 | 3.50 | <0.001 | 72 | 2.00 | 1.24 | 3.20 | 0.004 | 29 | 1.45 | 1.04 | 2.02 | 0.03 |
| Renal calcifications | Yes | 133 | 2.90 | 1.24 | 6.77 | 0.01 | 122 | 1.39 | 0.36 | 5.33 | 0.63 | 43 | 0.43 | 0.13 | 1.40 | 0.16 |
A univariate logistic regression model (GENMOD; SAS Institute, Inc.) was applied to estimate the unadjusted odds ratios (ORs) and those ORs were further adjusted for genotype (adjusted OR) as well as sex and age using a multivariate logistic regression model. An OR of one indicates that renal or bone involvement is equally likely to occur in individuals with a given variable. An OR greater than one indicates that renal or bone involvement is more likely to occur in individuals with high measurements of a given variable at the indicated units. An OR less than one indicates that renal or bone involvement is less likely to occur with high measurements of a given variable at the indicated units. 95% CI, 95% confidence interval.
Odds ratios were further adjusted for sex and age by using a multivariate logistic regression model.
Discussion
Prevalence of Renal Calcifications in Carriers of SLC34A3/NPT2c Mutations Is Increased
Renal calcifications were not previously thought to be part of the clinical presentation of HHRH.4,5 More recently, however, an increased frequency of medullary nephrocalcinosis and nephrolithiasis has been recognized3,6,7,9,11 in comp/hom carriers. These observations are further substantiated in the current report, because the index cases in three of five new kindreds showed renal calcifications on initial presentation. Furthermore, evaluation of 27 available kindreds indicates that approximately one half of all comp/hom carriers of SLC34A3/NPT2c mutations present with renal calcifications. Renal calcifications, furthermore, are the only presenting sign in 16%, whereas bone disease is (apparently) absent. Even heterozygous carriers of SLC34A3/NPT2c mutations show an approximately 3-fold higher incidence of renal calcifications, which may be related to their intermediate biochemical profile (Figures 2 and 3). Therefore, all affected individuals and their first-degree relatives should be examined for renal calcifications. The biochemical profile may help identify, in addition to genetic analysis of SLC34A3/NPT2c, those first-degree members in HHRH families who are at risk for developing renal complications as further discussed below.
Although prevalence of renal calcifications in heterozygous carriers in this initial survey is only significant compared with the prevalence reported in large cohorts of healthy controls,12,13 it may be underestimated, because most asymptomatic individuals have not had imaging studies. A particularly important strength of our study is the analysis of heterozygous carriers of only those mutations that are clearly disease-causing when combined with another mutation on the second allele or homozygously present. Systematic evaluation of these heterozygous individuals will be an important aspect of future investigations.
Several genes that cause rare monogenic disorders have been associated with hypercalciuric nephrolithiasis (i.e., CLCN5, CASR, CLDN16, CLDN19, ADCY10, SLC34A1, SLC9A3R1, GLUT2, HSPG2, and FN1),31,33 whereas variants of uromodulin and fetuin seem to be protective.34 Some of these genes affect tubular handling of calcium and phosphate in a way that is similar to what is observed in our patients with SLC34A3/NPT2c mutations. More recently, FAM20A mutations in enamel renal syndrome were found to be associated with renal calcifications, but these individuals do not seem to fit the biochemical profile of IH20; thus, it is more likely that loss of function of the extracellular kinase encoded by FAM20A affects local tissue factors that contribute to the development of renal calcifications. Furthermore, mutations in CYP24A1, the gene encoding the 24-hydroxylase, which is the key enzyme leading to inactivation of 1,25(OH)2D, have been reported as a cause of hypercalciuric nephrolithiasis.35 It should be noted that, thus far, genome-wide association studies for uric acid nephrolithiasis,36 serum phosphate levels,37 and CKD34 have not supported an association of hypercalciuric stone disease with the SLC34A3/NPT2c locus. However, our meta-analysis clearly suggests that SLC34A3/NPT2c should be added to the above list of hypercalciuric stone disease genes. Our finding may have important implications for the general population, because heterozygous nucleotide changes that alter the amino acid sequence or introduce deletions/insertions are relatively frequent in NPT2c, but it is currently unknown whether they are of biologic significance. Improved clinical characterization may provide a rationale for genetic analysis of SLC34A3/NPT2c in a subpopulation of hypercalciuric stone patients with clinical and biochemical profiles that resemble the profiles of HHRH.
To increase sample size, we decided to combine nephrocalcinosis and nephrolithiasis for statistical analysis, because these clinical findings are both associated with the increased urinary calcium excretion observed in HHRH. However, it should be noted that 13 of 43 (30%) comp/hom individuals showed nephrocalcinosis on ultrasound, which reaches statistical significance, compared with normal and heterozygous family members (Table 1), and 7 of these individuals did not have evidence for renal stones. Future studies may be able to evaluate the possibility that nephrocalcinosis is a unique feature of the hypercalciuria and hyperphosphaturia caused by loss of NPT2c in the proximal tubule, which raises interesting pathophysiological questions.
Serum 1,25(OH)2D, Phosphate, and TRP May Be Predictors of Renal Calcifications in Carriers of SLC34A3/NPT2c Mutations
It is unclear at the moment which genetic and biochemical criteria best predict the risk for renal calcifications in our HHRH kindreds. Based on the current understanding of the pathophysiology of HHRH, the defective sodium–phosphate cotransporter causes renal phosphate wasting, which triggers renal 1α-hydroxylase (CYP27B1) enzyme activity; this activity causes an increase in circulating 1,25(OH)2D levels, which in turn, increases intestinal Ca absorption, leading to an increased renal tubular Ca load and hypercalciuria. Thus, hypercalciuria, hyperphosphaturia, and possibly, tissue-specific effects of 1,25(OH)2D levels may lead to renal calcifications and stones. Hypercalciuria is the most common risk factor of kidney stones, which is present in 40%–50% of adults with recurrent calcium stones and 75%–80% of children with kidney stones. This disease is often referred to as IH because of increased intestinal absorption of calcium, and it can be associated with a mild renal phosphate leak, despite the lack of parathyroid hyperactivity.38 Association of increased 1,25(OH)2D levels with renal calcifications was observed in mouse models that lack Fgf2339 or Klotho40 or individuals with CYP24A1 mutations,35 even in the setting of low or normal urinary phosphate excretion. Finally, excessive phosphaturia, even with normal or low urinary calcium, can cause nephrocalcinosis in humans as is seen in XLH41 or in phosphate enema–induced nephrocalcinosis.42 Consistent with the pathophysiology of HHRH, we found that increased serum 1,25(OH)2D, low serum P, and decreased TRP may be positive predictors of renal calcifications. Only TRP remained associated with renal calcifications after controlling for genotype; however, serum P, 1,25(OH)2D, TRP, and ALP continued to be significant predictors of bone involvement. This initial evaluation suggests that decreased serum P levels and increased excretion of phosphate and serum 1,25(OH)2D merit additional evaluation as possible nongenetic predictors of renal calcifications.
We wondered whether specific SLC34A3/NPT2c mutations are associated with an increased serum 1,25(OH)2D, low serum P, decreased TRP, and renal calcifications (Supplemental Figure 2). However, not all individuals with a specific mutation have developed stones, and the numbers are small. It is, therefore, not possible to be certain about a genotype–phenotype effect at the present time.
Our study is limited by its relatively small sample size, because HHRH is a rare condition affecting, thus far, less than 50 individuals worldwide. Furthermore, clinical and biochemical data are often only available for the initial presentation, and family members who are heterozygous for a SLC34A3/NPT2c mutation or carry no mutation have been studied less well. In addition to a systematic evaluation of these individuals, long-term studies are required that determine whether oral phosphate supplementation alone of HHRH patients is sufficient for the prevention of renal calcifications and bone loss. It is likewise unknown how therapy should be monitored, whether secondary hyperparathyroidism can develop as observed in FGF23-dependent hypophosphatemic disorders,15 and whether phosphate requirements decrease with age, which has been reported for ADHR.16 It will also be important to determine whether lack of the NPT2c transporter leads to additional symptoms, such as Fanconi syndrome or CKD, which have been shown for individuals with SLC34A1/NPT2a mutations,14 using either animal models of HHRH or large HHRH kindreds, such as those kindreds described by Tieder et al.4,5
In summary, we here present five previously unreported HHRH kindreds and two individuals with IH in whom SLC34A3/NPT2c nucleotide sequence analysis identified known or novel mutations. Review of the clinical presentation of these kindreds and previously published HHRH kindreds suggests that renal calcifications and/or renal stones may be an important, often unrecognized initial symptom in carriers of comp/hom SLC34A3/NPT2c mutations. Even heterozygous carriers can be affected by nephrocalcinosis and nephrolithiasis, which is consistent with their intermediate biochemical profile. Serum 1,25(OH)2D, phosphate, and TRP may be predictors of renal calcifications, and future studies will help determine whether these biochemical parameters are independent of genotype and can guide therapy to prevent nephrocalcinosis, nephrolithiasis, and potentially CKD.
Concise Methods
Laboratory Assays
With the exception of genetic analyses, all laboratory studies were performed at laboratories used by the different investigators (normal ranges are provided in parentheses after each value). 25-Hydroxy vitamin D levels were measured by liquid chromatography with tandem mass spectroscopy or chemiluminescence immunoassay, and 1,25(OH)2D levels were measured by radioimmunoassay or ELISA. Serum intact PTH levels were determined by electrochemiluminescence immunoassay, and FGF23 levels were measured by an ELISA that detects the intact hormone and C-terminal fragments thereof (Immutopics) as well as intact FGF23 (Kainos). The renal TRP (in percentage) was calculated using the following equation: 100×(1−(urine phosphorus×serum creatinine)/(serum phosphorus×urine creatinine); when serum phosphorus is below the reference range for age, TRP should be above 90. Tubular maximum phosphate reabsorption per GFR (TmP/GFR) was estimated using the Walton and Bijvoet nomogram.43
SLC34A3 Genetic Analyses
Mutational and haplotype analysis of SLC34A3 was performed after informed written consent was obtained using forms approved by the Institutional Review Board of the Massachusetts General Hospital (MGH). The entire SLC34A3 gene, including approximately 800 bp 5′ of the transcriptional start site, all intervening sequences, and approximately 200 bp of the 3′-untranslated region, was amplified by PCR from genomic DNA of the index cases followed by nucleotide sequence analysis at the MGH DNA Sequencing Core Facility or Genewiz, Inc. as described.1 PCR assays to confirm the findings in index cases and analyze family members and controls were performed as described1 using Qiagen reagents or the Expand Long Template PCR System (Roche) at standard PCR cycling conditions followed by restriction enzymatic digest or nucleotide sequence analysis. Primer sequences are listed in Supplemental Table 4, and conditions for amplification and detection of the mutations are given in Supplemental Table 1. Searches of the National Center for Biotechnology Information–dbSNP24 and the 1000 Genomes Project23 were negative for the identified novel mutations, with the exception of Het.c.413C>T(p.S138F) (HGMD: CM060480), which was identified as a rare single nucleotide polymorphism rs141734934, Het: 0.001 based on nucleotide sequence analysis of 4352 chromosomes (1000 Genomes). GenBank accession numbers for SLC34A3 are as follows: genomic contig, NT024000.15; cDNA, NM080877.1; protein, NP543153.1.
Statistical and Data Analyses
For our meta-analysis, we used clinical and laboratory findings (LFs) obtained at presentation before therapy was initiated. For statistical evaluation, we determined the number of individuals affected by (1) nephrolithiasis who had history of renal colic or based on imaging or stone analysis, (2) nephrocalcinosis, in whom renal mineral deposits were found on imaging studies (i.e., ultrasound or computed tomography), (3) rickets/osteomalacia based on history of bowing, corrective surgery, stress fractures, bone pain, or imaging studies (i.e., skeletal radiographic survey and 99mTc methylene diphosphonate bone scan), and (4) osteopenia/osteoporosis based on World Health Organization bone densitometry criteria. Asymptomatic individuals were negative for the above renal and bone phenotypes either by imaging or based on absence of history of symptoms suggesting the presence of renal calcifications or bone disease. Age-related reference values (RVs; i.e., 95% confidence intervals) were obtained from http://www.mayomedicallaboratories.com/, http://cclnprod.cc.nih.gov/dlm/testguide.nsf/Index/1D336E0232533D3285256B9C0059ECEA, and respective clinical laboratories; age-related RVs for TmP/GFR are in refs. 44–46, and age-related RVs for uCa/uCrea are from ref. 47. Assuming normal distribution, LFs can be transformed into z scores using means of age-appropriate RVs (RVmean) and SDs (where SD=RV/4) according to the formula: z score=(LF−RVmean)/SD.48 z Scores were used for all statistical analyses with the following exceptions: absolute values are presented for 25-hydroxy vitamin D, 1,25(OH)2D, TRP, and TmP/GFR measurements. The data for PTH and age-specific uCa/uCrea are presented as percentage of the upper limit of normal RV. These exceptions are important, because for these assays, upper and lower RVs have not been established in sufficiently large number of normal age-specific controls.49 We used Prism for OSX, version 5.0d (GraphPad Software Inc.), to perform t tests for unpaired comparisons, one-way ANOVAs for multiple comparisons, and linear regression analyses. For univariate and multivariate logistic regression models, we used SAS (GENMOD; SAS Institute, Inc.) to estimate the odds ratios. To increase sample size, we treated all renal phenotypes (nephrolithiasis and nephrocalcinosis) or bone phenotypes (rickets, osteomalacia, and osteopenia/osteoporosis) collectively. Data are expressed as means±95% confidence intervals.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01-DK46718-20 and P01-DK11794, project IV (to H.J.), National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant 5K08-DK078361 (to C.B.), Young Investigator Awards by the National Kidney Foundation, and the American Association for Clinical Investigation (C.B.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101085/-/DCSupplemental.
References
- 1.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Jüppner H: SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78: 179–192, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM: Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78: 193–201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichikawa S, Sorenson AH, Imel EA, Friedman NE, Gertner JM, Econs MJ: Intronic deletions in the SLC34A3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J Clin Endocrinol Metab 91: 4022–4027, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Tieder M, Modai D, Samuel R, Arie R, Halabe A, Bab I, Gabizon D, Liberman UA: Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med 312: 611–617, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Tieder M, Modai D, Shaked U, Samuel R, Arie R, Halabe A, Maor J, Weissgarten J, Averbukh Z, Cohen N, Edelstein S, Liberman UA: “Idiopathic” hypercalciuria and hereditary hypophosphatemic rickets. Two phenotypical expressions of a common genetic defect. N Engl J Med 316: 125–129, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Page K, Bergwitz C, Jaureguiberry G, Harinarayan CV, Insogna K: A patient with hypophosphatemia, a femoral fracture, and recurrent kidney stones: Report of a novel mutation in SLC34A3. Endocr Pract 14: 869–874, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kremke B, Bergwitz C, Ahrens W, Schütt S, Schumacher M, Wagner V, Holterhus PM, Jüppner H, Hiort O: Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/NaPi-IIc can be masked by vitamin D deficiency and can be associated with renal calcifications. Exp Clin Endocrinol Diabetes 117: 49–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phulwani P, Bergwitz C, Jaureguiberry G, Rasoulpour M, Estrada E: Hereditary hypophosphatemic rickets with hypercalciuria and nephrolithiasis-identification of a novel SLC34A3/NaPi-IIc mutation. Am J Med Genet A 155A: 626–633, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaureguiberry G, Carpenter TO, Forman S, Jüppner H, Bergwitz C: A novel missense mutation in SLC34A3 that causes hereditary hypophosphatemic rickets with hypercalciuria in humans identifies threonine 137 as an important determinant of sodium-phosphate cotransport in NaPi-IIc. Am J Physiol Renal Physiol 295: F371–F379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mejia-Gaviria N, Gil-Peña H, Coto E, Pérez-Menéndez TM, Santos F: Genetic and clinical peculiarities in a new family with hereditary hypophosphatemic rickets with hypercalciuria: A case report. Orphanet J Rare Dis 5: 1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tencza AL, Ichikawa S, Dang A, Kenagy D, McCarthy E, Econs MJ, Levine MA: Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/type IIc sodium-phosphate cotransporter: Presentation as hypercalciuria and nephrolithiasis. J Clin Endocrinol Metab 94: 4433–4438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero V, Akpinar H, Assimos DG: Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev Urol 12: e86–e96, 2010 [PMC free article] [PubMed] [Google Scholar]
- 13.Schissel BL, Johnson BK: Renal stones: Evolving epidemiology and management. Pediatr Emerg Care 27: 676–681, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Magen D, Berger L, Coady MJ, Ilivitzki A, Militianu D, Tieder M, Selig S, Lapointe JY, Zelikovic I, Skorecki K: A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med 362: 1102–1109, 2010 [DOI] [PubMed] [Google Scholar]
- 15.The HYP Consortium : A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 11: 130–136, 1995 [DOI] [PubMed] [Google Scholar]
- 16.ADHR Consortium : Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE: Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38: 1310–1315, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Jüppner H, Strom TM: DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38: 1248–1250, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM: Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet 86: 267–272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaureguiberry G, De la Dure-Molla M, Parry D, Quentric M, Himmerkus N, Koike T, Poulter J, Klootwijk E, Robinette SL, Howie AJ, Patel V, Figueres ML, Stanescu HC, Issler N, Nicholson JK, Bockenhauer D, Laing C, Walsh SB, McCredie DA, Povey S, Asselin A, Picard A, Coulomb A, Medlar AJ, Bailleul-Forestier I, Verloes A, Le Caignec C, Roussey G, Guiol J, Isidor B, Logan C, Shore R, Johnson C, Inglehearn C, Al-Bahlani S, Schmittbuhl M, Clauss F, Huckert M, Laugel V, Ginglinger E, Pajarola S, Spartà G, Bartholdi D, Rauch A, Addor MC, Yamaguti PM, Safatle HP, Acevedo AC, Martelli-Júnior H, dos Santos Netos PE, Coletta RD, Gruessel S, Sandmann C, Ruehmann D, Langman CB, Scheinman SJ, Ozdemir-Ozenen D, Hart TC, Hart PS, Neugebauer U, Schlatter E, Houillier P, Gahl WA, Vikkula M, Bloch-Zupan A, Bleich M, Kitagawa H, Unwin RJ, Mighell A, Berdal A, Kleta R: Nephrocalcinosis (enamel renal syndrome) caused by autosomal recessive FAM20A mutations. Nephron, Physiol 122: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White AJ, Northcutt MJ, Rohrback SE, Carpenter RO, Niehaus-Sauter MM, Gao Y, Wheatly MG, Gillen CM: Characterization of sarcoplasmic calcium binding protein (SCP) variants from freshwater crayfish Procambarus clarkii. Comp Biochem Physiol B Biochem Mol Biol 160: 8–14, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, Sanderson SR, Reyes M, Sharma A, Dunbar N, Srivastava T, Jüppner H, Bergwitz C: Novel NaPi-IIc mutations causing HHRH and idiopathic hypercalciuria in several unrelated families: Long-term follow-up in one kindred. Bone 50: 1100–1106, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA, 1000 Genomes Project Consortium : A map of human genome variation from population-scale sequencing. Nature 467: 1061–1073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Edgar R, Federhen S, Geer LY, Helmberg W, Kapustin Y, Kenton DL, Khovayko O, Lipman DJ, Madden TL, Maglott DR, Ostell J, Pruitt KD, Schuler GD, Schriml LM, Sequeira E, Sherry ST, Sirotkin K, Souvorov A, Starchenko G, Suzek TO, Tatusov R, Tatusova TA, Wagner L, Yaschenko E: Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 34[Database issue]: D173–D180, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR: A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braithwaite V, Pettifor JM, Prentice A: Novel SLC34A3 mutation causing hereditary hypophosphataemic rickets with hypercalciuria in a Gambian family. Bone 53: 216–220, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Areses-Trapote R, López-García JA, Ubetagoyena-Arrieta M, Eizaguirre A, Sáez-Villaverde R: Hereditary hypophosphatemic rickets with hypercalciuria: Case report. Nefrologia 32: 529–534, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Hasani-Ranjbar S, Amoli MM, Ebrahim-Habibi A, Dehghan E, Soltani A, Amiri P, Larijani B: SLC34A3 intronic deletion in a new kindred with hereditary hypophosphatemic rickets with hypercalciuria. J Clin Res Pediatr Endocrinol 4: 89–93, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichikawa S, Tuchman S, Padgett LR, Gray AK, Baluarte HJ, Econs MJ: Intronic deletions in the SLC34A3 gene: A cautionary tale for mutation analysis of hereditary hypophosphatemic rickets with hypercalciuria. Bone 59: 53–56, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto T, Michigami T, Aranami F, Segawa H, Yoh K, Nakajima S, Miyamoto K, Ozono K: Hereditary hypophosphatemic rickets with hypercalciuria: A study for the phosphate transporter gene type IIc and osteoblastic function. J Bone Miner Metab 25: 407–413, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Stechman MJ, Loh NY, Thakker RV: Genetic causes of hypercalciuric nephrolithiasis. Pediatr Nephrol 24: 2321–2332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL: A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res 26: 1381–1388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langman CB: The molecular basis of kidney stones. Curr Opin Pediatr 16: 188–193, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Gudbjartsson DF, Holm H, Indridason OS, Thorleifsson G, Edvardsson V, Sulem P, de Vegt F, d’Ancona FC, den Heijer M, Wetzels JF, Franzson L, Rafnar T, Kristjansson K, Bjornsdottir US, Eyjolfsson GI, Kiemeney LA, Kong A, Palsson R, Thorsteinsdottir U, Stefansson K: Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet 6: e1001039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, Fehrenbach H, Wingen AM, Güran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M: Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365: 410–421, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Tore S, Casula S, Casu G, Concas MP, Pistidda P, Persico I, Sassu A, Maestrale GB, Mele C, Caruso MR, Bonerba B, Usai P, Deiana I, Thornton T, Pirastu M, Forabosco P: Application of a new method for GWAS in a related case/control sample with known pedigree structure: Identification of new loci for nephrolithiasis. PLoS Genet 7: e1001281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kestenbaum B, Glazer NL, Köttgen A, Felix JF, Hwang SJ, Liu Y, Lohman K, Kritchevsky SB, Hausman DB, Petersen AK, Gieger C, Ried JS, Meitinger T, Strom TM, Wichmann HE, Campbell H, Hayward C, Rudan I, de Boer IH, Psaty BM, Rice KM, Chen YD, Li M, Arking DE, Boerwinkle E, Coresh J, Yang Q, Levy D, van Rooij FJ, Dehghan A, Rivadeneira F, Uitterlinden AG, Hofman A, van Duijn CM, Shlipak MG, Kao WH, Witteman JC, Siscovick DS, Fox CS: Common genetic variants associate with serum phosphorus concentration. J Am Soc Nephrol 21: 1223–1232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prié D, Ravery V, Boccon-Gibod L, Friedlander G: Frequency of renal phosphate leak among patients with calcium nephrolithiasis. Kidney Int 60: 272–276, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Hesse M, Fröhlich LF, Zeitz U, Lanske B, Erben RG: Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol 26: 75–84, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL: A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res 26: 1381–1388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonlusen G, Akgun H, Ertan A, Olivero J, Truong LD: Renal failure and nephrocalcinosis associated with oral sodium phosphate bowel cleansing: clinical patterns and renal biopsy findings. Arch Pathol Lab Med 130: 101–106, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Walton RJ, Bijvoet OL: A simple slide-rule method for the assessment of renal tubular reaborption of phosphate in man. Clin Chim Acta 81: 273–276, 1977 [DOI] [PubMed] [Google Scholar]
- 44.Alon U, Hellerstein S: Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol 8: 250–251, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Brodehl J, Gellissen K, Weber HP: Postnatal development of tubular phosphate reabsorption. Clin Nephrol 17: 163–171, 1982 [PubMed] [Google Scholar]
- 46.Kruse K, Kracht U, Göpfert G: Renal threshold phosphate concentration (TmPO4/GFR). Arch Dis Child 57: 217–223, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell DM, Jüppner H: Regulation of calcium homeostasis and bone metabolism in the fetus and neonate. Curr Opin Endocrinol Diabetes Obes 17: 25–30, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Boyd JC, Lacher DA: A multi-stage Gaussian transformation algorithm for clinical laboratory data. Clin Chem 28: 1735–1741, 1982 [PubMed] [Google Scholar]
- 49.Lee WT, Jiang J: The resurgence of the importance of vitamin D in bone health. Asia Pac J Clin Nutr 17[Suppl 1]: 138–142, 2008 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.