Abstract
Autosomal dominant polycystic kidney disease (ADPKD), the most common form of polycystic kidney disease (PKD), is a disorder with characteristics of neoplasia. However, it is not known whether renal transplant recipients with PKD have an increased risk of cancer. Data from the Scientific Registry of Transplant Recipients, which contains information on all solid organ transplant recipients in the United States, were linked to 15 population-based cancer registries in the United States. For PKD recipients, we compared overall cancer risk with that in the general population. We also compared cancer incidence in PKD versus non-PKD renal transplant recipients using Poisson regression, and we determined incidence rate ratios (IRRs) adjusted for age, sex, race/ethnicity, dialysis duration, and time since transplantation. The study included 10,166 kidney recipients with PKD and 107,339 without PKD. Cancer incidence in PKD recipients was 1233.6 per 100,000 person-years, 48% higher than expected in the general population (standardized incidence ratio, 1.48; 95% confidence interval [95% CI], 1.37 to 1.60), whereas cancer incidence in non-PKD recipients was 1119.1 per 100,000 person-years. The unadjusted incidence was higher in PKD than in non-PKD recipients (IRR, 1.10; 95% CI, 1.01 to 1.20). However, PKD recipients were older (median age at transplantation, 51 years versus 45 years for non-PKD recipients), and after multivariable adjustment, cancer incidence was lower in PKD recipients than in others (IRR, 0.84; 95% CI, 0.77 to 0.91). The reason for the lower cancer risk in PKD recipients is not known but may relate to biologic characteristics of ADPKD or to cancer risk behaviors associated with ADPKD.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common form of inherited cystic renal disease and the fourth most common cause of ESRD in the United States.1–3 There are currently>16,000 individuals with polycystic kidney disease (PKD, of which ADPKD is by far the most common type) living with a renal transplant in the United States.4
ADPKD is a result of mutations in one of two genes: PKD1 and PKD2.1,5,6 These genes are widely expressed in many tissues, consistent with the multiorgan pathology characterizing ADPKD. A key factor in cyst formation and enlargement in ADPKD is the abnormal proliferation of cyst epithelial cells in a cell-autonomous manner.7,8 This cyst formation is associated with cellular dedifferentiation and is considered a neoplastic process driven by upregulated proto-oncogenes.9–13 While published case reports document the occurrence of renal cell carcinomas (RCCs) in ADPKD-affected kidneys,14,15 these tumors may be partly due to acquired renal cystic disease resulting from long-term dialysis.16 Because there do not appear to be widespread published reports of other cancers in patients with ADPKD, protective mechanisms might exist in ADPKD to prevent malignant transformation. Indeed, many oncogenes that promote cell proliferation also act as potent growth suppressors (e.g., Ras17) or inducers of apoptosis (e.g., Myc18,19). Thus, there is uncertainty whether ADPKD mutations are associated with increased rates of kidney cancer or cancer in general.
We therefore designed a study to compare cancer risk in kidney transplant recipients with PKD versus kidney recipients with other causes of ESRD. Organ transplant recipients are at increased risk of cancer, largely because of immunosuppressive therapy.20 An increased risk of cancer in patients with PKD might be detectable in this high-risk cancer population. Alternatively, if there is no increased risk of cancer in ADPKD, the findings would suggest the need for further study to determine whether protective cellular mechanisms may be at work.
Results
Characteristics of the renal transplant recipients with and without PKD are shown in Table 1. Compared with non-PKD recipients, recipients with PKD were more likely to be female, non-Hispanic white, and older at time of transplant. PKD recipients were slightly more likely to receive a deceased-donor transplant and slightly more likely to receive a first kidney transplant. Furthermore, PKD recipients underwent transplant after a shorter duration on dialysis compared with non-PKD recipients. Initially prescribed maintenance calcineurin inhibitor immunosuppressive therapy did not differ. The median follow-up was 4.12 years after transplant for PKD recipients and 3.64 years for non-PKD recipients.
Table 1.
Characteristics of the study sample
| Characteristic | Kidney Transplant Recipients, n (%) | |
|---|---|---|
| PKD (n=10,166) | No PKD (n=107,339) | |
| Men | 5656 (55.6) | 64,327 (59.9) |
| Age at transplantation | ||
| 0–17 yr | 265 (2.6) | 6621 (6.2) |
| 18−34 yr | 435 (4.3) | 23,758 (22.1) |
| 35−49 yr | 3672 (36.1) | 34,707 (32.3) |
| 50−64 yr | 4896 (48.2) | 32,515 (30.3) |
| ≥ 65 yr | 898 (8.8) | 9738 (9.1) |
| Race/ethnicity | ||
| Non-Hispanic white | 7953 (78.2) | 55,461 (51.7) |
| Non-Hispanic black | 846 (8.3) | 24,965 (23.3) |
| Hispanic | 1049 (10.3) | 19,381 (18.1) |
| Asian/Pacific Islander | 318 (3.1) | 7532 (7.0) |
| Donor type | ||
| Deceased | 6685 (65.8) | 68,743 (64.0) |
| Living | 3481 (34.2) | 38,596 (36.0) |
| Kidney transplant number | ||
| First | 9541 (93.9) | 97,237 (90.6) |
| Second | 588 (5.8) | 9342 (8.7) |
| Third or higher | 37 (0.4) | 760 (0.7) |
| Calendar year of transplant | ||
| 1987–1994 | 1812 (17.8) | 20,541 (19.1) |
| 1995–1999 | 2549 (25.1) | 27,465 (25.6) |
| 2000–2004 | 3341 (32.9) | 34,218 (31.9) |
| 2005–2009 | 2464 (24.2) | 25,115 (23.4) |
| Initial dialysis duration | ||
| 0−5 mo | 3043 (29.9) | 20,113 (18.7) |
| 6−11 mo | 2918 (28.7) | 33,587 (31.3) |
| 12−23 mo | 2047 (20.1) | 24,903 (23.2) |
| ≥24 mo | 1453 (14.3) | 21,863 (20.4) |
| Unknown | 705 (6.9) | 6873 (6.4) |
| Maintenance therapy with CNI | ||
| Cyclosporine | 4643 (45.7) | 49,110 (45.8) |
| Tacrolimus | 4417 (43.5) | 46,178 (43.0) |
| Both | 37 (0.4) | 500 (0.5) |
| Neither | 1069 (10.5) | 11,551 (10.8) |
CNI, calcineurin inhibitor.
Among the 10,166 renal transplant recipients with PKD, there were 622 cancers, for a rate of 1233.6 cancers per 100,000 person-years; among the 107,339 recipients without PKD, there were 5330 cancers, for a rate of 1119.1 per 100,000 person-years. Compared with the general population, overall cancer risk was increased 48% in PKD recipients (standardized incidence ratio, 1.48; 95% confidence interval [95% CI], 1.37 to 1.60; P<0.001), while the overall cancer risk in non-PKD recipients was increased 86% (standardized incidence ratio, 1.86; 95% CI, 1.81 to 1.91; P<0.001).
For individual cancers, unadjusted incidence rates are also shown in Table 2. The incidence of most cancers did not differ significantly between PKD and non-PKD recipients. In these analyses, cancers that occurred significantly more frequently in PKD recipients were soft tissue tumors, including those of the heart (incidence rate ratio [IRR], 2.36; P=0.05), melanoma (IRR, 2.01; P<0.001), and other nonepithelial skin cancers (IRR, 1.77; P=0.03).
Table 2.
Unadjusted incidence rates and IRRs for cancer type, by PKD status
| Cancer Type | PKD Recipients | Non-PKD Recipients | IRR (95% CI) | ||
|---|---|---|---|---|---|
| Count (n) | Incidence Ratea | Count (n) | Incidence Ratea | ||
| Lip | 13 | 25.8 | 74 | 15.5 | 1.66 (0.88 to 2.89) |
| Salivary gland | 7 | 13.9 | 30 | 6.3 | 2.20 (0.89 to 4.73) |
| Oropharynx | 7 | 13.9 | 39 | 8.2 | 1.70 (0.69 to 3.56) |
| Other oral cavity and pharynx | 5 | 9.9 | 75 | 15.7 | 0.63 (0.22 to 1.41) |
| Nasopharynxb | 0 | 0 | 7 | 1.5 | 0 |
| Esophagus | 5 | 9.9 | 47 | 9.9 | 1.00 (0.35 to 2.29) |
| Stomach | 8 | 15.9 | 91 | 19.1 | 0.83 (0.37 to 1.60) |
| Small intestine | 2 | 4.0 | 19 | 4.0 | 0.99 (0.16 to 3.42) |
| Colon and rectum | 25 | 49.6 | 328 | 68.9 | 0.72 (0.47 to 1.06) |
| Anus | 3 | 5.9 | 51 | 10.7 | 0.56 (0.14 to 1.51) |
| Liver | 4 | 7.9 | 51 | 10.7 | 0.74 (0.22 to 1.81) |
| Intrahepatic bile duct | 2 | 4.0 | 3 | 0.6 | 6.30 (0.83 to 37.99) |
| Gallbladderb | 0 | 0 | 7 | 1.5 | 0 |
| Other biliary | 4 | 7.9 | 11 | 2.3 | 3.43 (0.95 to 10.04) |
| Pancreas | 14 | 27.8 | 84 | 17.6 | 1.57 (0.86 to 2.68) |
| Larynx | 2 | 4.0 | 45 | 9.4 | 0.42 (0.07 to 1.36) |
| Lung | 69 | 136.8 | 582 | 122.2 | 1.12 (0.86 to 1.43) |
| Bones and jointsb | 0 | 0 | 11 | 2.3 | 0 |
| Soft tissue including heart | 8 | 15.9 | 32 | 6.7 | 2.36 (1.01 to 4.87) |
| Melanoma | 48 | 95.2 | 225 | 47.2 | 2.01 (1.46 to 2.72) |
| Other nonepithelial skin | 20 | 39.7 | 107 | 22.5 | 1.77 (1.06 to 2.78) |
| Breast | 37 | 73.4 | 299 | 62.8 | 1.17 (0.82 to 1.62) |
| Cervixb | 0 | 0 | 27 | 13.9 | 0 |
| Uterus | 12 | 53.2 | 60 | 30.8 | 1.89 (0.97 to 3.38) |
| Ovary | 4 | 17.7 | 26 | 13.3 | 1.45 (0.43 to 3.73) |
| Vaginab | 0 | 0 | 8 | 4.1 | 0 |
| Vulva | 6 | 26.6 | 34 | 17.5 | 1.67 (0.63 to 3.69) |
| Prostate | 74 | 265.6 | 555 | 197.2 | 1.26 (0.98 to 1.59) |
| Testis | 5 | 17.9 | 26 | 9.2 | 1.82 (0.61 to 4.35) |
| Penisb | 0 | 0 | 15 | 5.3 | 0 |
| Bladder | 13 | 25.8 | 142 | 29.8 | 0.86 (0.47 to 1.47) |
| Kidney | 49 | 97.2 | 610 | 128.1 | 0.76 (0.56 to 1.00) |
| RCCc | 45 | 89.2 | 505 | 106.0 | 0.84 (0.61 to 1.13) |
| Renal pelvis | 1 | 2.0 | 15 | 3.1 | 0.63 (0.03 to 3.11) |
| Eye and orbit | 4 | 7.9 | 12 | 2.5 | 3.15 (0.88 to 9.04) |
| Brain | 5 | 9.9 | 29 | 6.1 | 1.63 (0.55 to 3.85) |
| Thyroid | 16 | 31.7 | 168 | 35.3 | 0.90 (0.52 to 1.45) |
| Hodgkin lymphoma | 5 | 9.9 | 49 | 10.3 | 0.96 (0.33 to 2.19) |
| Non-Hodgkin lymphoma | 74 | 146.8 | 672 | 141.1 | 1.04 (0.81 to 1.31) |
| Plasma cell neoplasms | 8 | 15.9 | 73 | 15.3 | 1.03 (0.46 to 2.02) |
| Acute lymphocytic leukemia | 1 | 2.0 | 8 | 1.7 | 1.18 (0.06 to 6.44) |
| Chronic lymphocytic leukemia | 1 | 2.0 | 14 | 2.9 | 0.67 (0.04 to 3.36) |
| Acute myeloid leukemia | 7 | 13.9 | 36 | 7.6 | 1.84 (0.75 to 3.88) |
| Chronic myeloid leukemia | 3 | 5.9 | 20 | 4.2 | 1.42 (0.33 to 4.13) |
| Acute monocytic leukemiab | 0 | 0 | 1 | 0.2 | 0 |
| Other acute leukemiab | 0 | 0 | 4 | 0.8 | 0 |
| Mesothelioma | 1 | 2.0 | 12 | 2.5 | 0.79 (0.04 to 3.99) |
| Kaposi sarcoma | 6 | 11.9 | 73 | 15.3 | 0.78 (0.30 to 1.64) |
| Miscellaneous | 37 | 73.4 | 327 | 68.7 | 1.07 (0.75 to 1.48) |
| Tumors of poorly specified morphology | 7 | 13.9 | 96 | 20.2 | 0.69 (0.29 to 1.38) |
| All cancers, combined | 622 | 1233.6 | 5330 | 1119.1 | 1.10 (1.01 to 1.20) |
| All cancers excluding kidney, melanoma, and nonepithelial skin | 505 | 1001.6 | 4388 | 921.3 | 1.09 (0.99 to 1.19) |
Incidence rate is per 100,000 person-years.
Because there were no cases among PKD recipients, the Poisson regression model did not converge.
RCCs are shown separately in the table, but because all were located in the kidney, they do not contribute to the total.
For skin cancers, we assessed associations restricted to non-Hispanic whites in order to remove confounding by race. The unadjusted associations were attenuated in this subgroup and no longer significant (melanoma: IRR, 1.33 [95% CI, 0.95 to 1.81]; other nonepithelial skin cancers: IRR, 1.36 [95% CI, 0.84 to 2.19]). Furthermore, no association was apparent among whites, after adjustment for age, sex, dialysis duration, and time since transplant, for melanoma (IRR, 1.03 [95% CI, 0.73 to 1.41]) or other nonepithelial skin cancers (IRR, 1.02 [95% CI, 0.63 to 1.67]).
As shown in Table 2, cancers of the kidney tended to occur less often in PKD recipients (IRR, 0.76; P=0.053), although the difference was less apparent for RCC specifically (IRR, 0.84; P=0.26). In regression models adjusted for age, sex, race/ethnicity, dialysis duration, and time since transplant, risk of kidney cancer in PKD recipients was significantly decreased (adjusted IRR, 0.70; 95% CI, 0.51 to 0.93; P=0.013), but the association remained nonsignificant for RCC (IRR, 0.78; 95% CI, 0.56 to 1.05; P=0.11).
In unadjusted analyses, PKD was associated with an increased risk of cancer overall (IRR, 1.10; 95% CI, 1.01 to 1.20; P=0.024) (Table 2). After exclusion of kidney cancers (and therefore RCCs, since all RCCs were in the kidney), melanomas, and nonepithelial skin cell cancers, PKD recipients still tended toward having an increased cancer risk (IRR, 1.09; 95% CI, 0.99 to 1.19; P=0.08).
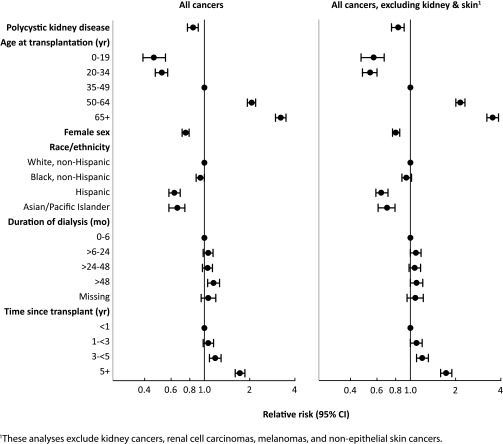
However, after adjustment for potential confounders, the direction of the association was reversed: the adjusted IRR was 0.84 (95% CI, 0.77 to 0.91; P<0.001) for all cancers combined and 0.83 (95% CI, 0.75 to 0.91; P<0.001) for all cancers excluding kidney cancers, melanomas, and nonepithelial skin cancers (Table 3). The point estimates of these IRRs and the associated 95% CIs are shown graphically in Figure 1. Adjustment for age alone accounted for most of the effect of adjustment for the combined cancer outcomes (age-adjusted IRR, 0.88 [95% CI, 0.81 to 0.96; P=0.003] for all cancers; IRR, 0.87 [95% CI, 0.80 to 0.96; P=0.004] for all cancers excluding kidney cancers, melanomas, and nonepithelial skin cancers).
Table 3.
Adjusted IRRs for combined cancer outcomes in PKD versus non-PKD kidney transplant recipients
| Characteristic | All Cancers | All Cancers, Excluding Kidney and Skina | ||
|---|---|---|---|---|
| Multivariate IRR (95% CI)b | P Value | Multivariate IRR (95% CI)b | P Value | |
| PKD | 0.84 (0.77 to 0.91) | <0.001 | 0.83 (0.75 to 0.91) | <0.001 |
| Age at transplantation | <0.001 | <0.001 | ||
| 0–19 yr | 0.46 (0.39 to 0.55) | 0.57 (0.47 to 0.67) | ||
| 20–34 yr | 0.52 (0.47 to 0.57) | 0.54 (0.48 to 0.60) | ||
| 35–49 yr | Reference | Reference | ||
| 50–64 yr | 2.06 (1.93 to 2.19) | 2.15 (2.01 to 2.31) | ||
| ≥65 yr | 3.22 (2.97 to 3.49) | 3.53 (3.23 to 3.86) | ||
| Female sex | 0.75 (0.71 to 0.79) | <0.001 | 0.80 (0.76 to 0.85) | <0.001 |
| Race/ethnicity | <0.001 | <0.001 | ||
| White, non-Hispanic | Reference | Reference | ||
| Black, non-Hispanic | 0.94 (0.88 to 1.00) | 0.94 (0.88 to 1.02) | ||
| Hispanic | 0.63 (0.58 to 0.69) | 0.64 (0.59 to 0.71) | ||
| Asian/Pacific Islander | 0.66 (0.58 to 0.74) | 0.70 (0.61 to 0.79) | ||
| Duration of dialysis | 0.037 | 0.27 | ||
| 0–6 mo | Reference | Reference | ||
| >6–24 mo | 1.06 (0.98 to 1.14) | 1.09 (1.00 to 1.18) | ||
| >24–48 mo | 1.05 (0.97 to 1.13) | 1.07 (0.98 to 1.17) | ||
| >48 mo | 1.15 (1.05 to 1.26) | 1.10 (1.00 to 1.21) | ||
| Missing mo | 1.06 (0.95 to 1.19) | 1.08 (0.95 to 1.22) | ||
| Time since transplant | <0.001 | <0.001 | ||
| <1 yr | Reference | Reference | ||
| 1 to <3 yr | 1.06 (0.98 to 1.15) | 1.10 (1.00 to 1.20) | ||
| 3 to <5 yr | 1.18 (1.08 to 1.29) | 1.20 (1.10 to 1.32) | ||
| >5 yr | 1.72 (1.60 to 1.86) | 1.73 (1.59 to 1.89) | ||
These analyses exclude kidney cancers, RCCs, melanomas, and nonepithelial skin cancers.
Adjusted for factors in this table.
Figure 1.
The incidence of cancer overall, and of all cancers excluding kidney and skin, is lower in PKD renal transplant recipients compared with non-PKD recipients after statistical adjustment.
Discussion
In this study, we sought to determine whether cancer risk following renal transplantation differed between individuals with ADPKD and those without. Although unadjusted analyses suggested a small excess risk of cancer overall, PKD recipients were older than other kidney recipients, and when we adjusted for age and other factors, PKD recipients were actually 16% less likely to develop cancer. This decrement remained apparent after we excluded renal and skin cancers.
Although kidney transplant recipients have an elevated risk of cancer relative to the general population, cancer risk is lower for patients with PKD than for other kidney recipients. This finding was unexpected given that ADPKD is a multiorgan disease process with characteristics of neoplasia.9,21,22 Nonetheless, no published studies that we are aware of indicate that ADPKD is associated with increased rates of cancer. Although PKD mutations clearly cause cyst formation, it is also possible that these mutations somehow trigger protective cellular mechanisms that prevent cells from undergoing malignant transformation. Potential support for this comes from a recently reported population study by Ward et al.23 in which germline mutations in PKHD1 (the gene responsible for autosomal recessive PKD) were protective against colorectal cancer. This group speculated that a reduction of fibrocystin activity might increase mitotic instability, “paradoxically” inhibiting carcinogenesis.
Alternatively, another possibility for our observation of an inverse association between PKD and cancer invokes patterns of healthy behaviors or medical care that may distinguish patients with ADPKD from others with CKD. Those with ADPKD frequently know at a relatively early age that they have a progressive genetic condition. This might prompt them to adopt healthy behaviors, encouraged by providers who see them on a regular long-term basis.
Notably, we found a significantly lower risk of kidney cancer in PKD recipients. We believe this association arises because ADPKD recipients are more likely to have nephrectomies than their non-PKD counterparts. The Scientific Registry of Transplant Recipients (SRTR) does not include data on nephrectomies, so we could not directly examine this possibility. Small case series suggest that the lifetime nephrectomy rate (of at least one kidney) is approximately 20%–30% for patients with ADPKD.24,25 We therefore reviewed nephrectomy data from the University of Kansas Medical Center. Of 90 ADPKD recipients who underwent transplantation there during 2004–2012, 21 (23%) had a nephrectomy before or at the time of kidney transplantation (presumably, this total would be increased slightly in the post-transplant environment). In contrast, almost no pretransplant nephrectomies occurred in non-ADPKD recipients. Nephrectomy rates of 20%–30% could lead to a deficit of RCCs among ADPKD recipients. Although RCCs have been found at higher-than-expected rates in two recent large case series of patients with PKD,14,15 our data do not support a greatly increased risk of RCC associated with PKD following kidney transplantation.
We found a roughly 2-fold increase in melanomas and other nonepithelial skin cancers among ADPKD recipients, but this excess was the result of confounding by race because ADPKD recipients were more likely to be white. Restricting analysis to non-Hispanic whites demonstrated this to be the case, even before subsequent statistical adjustments. These findings are concordant with far higher rates of skin cancers in whites. We cannot draw conclusions about the association of ADPKD with the most common types of skin cancers that arise after transplantation, namely squamous and basal cell carcinomas, because cancer registries do not collect data on these cancers.
Several important study limitations must be considered. First, we only studied individuals following renal transplantation. As such, our analysis is unable to answer the question of whether the natural history for cancer varies between patients with and without PKD who have not received a transplant. Second, many low-frequency cancers occurred infrequently in PKD recipients, rendering some estimates unstable. Third, we made many statistical comparisons, which could have led to some chance associations. Indeed, we believe that the borderline-significant association between PKD and soft tissue tumors was a chance finding.
In conclusion, this was the first large-scale study of cancer in kidney recipients with PKD patients. We found a significantly lower rate compared with non-PKD recipients after adjustment for important potential confounders. The reason for this decreased risk is uncertain, and further study is required to determine how ADPKD mutations might influence development of cancer.
Concise Methods
The Transplant Cancer Match Study has been described elsewhere in detail (http://www.transplantmatch.cancer.gov).26 Briefly, we used data from the SRTR, which contains information on all solid organ transplant recipients in the United States since 1987. This includes information on demographic characteristics, reason for transplantation, and characteristics of transplanted organs.
The SRTR has been linked to 15 population-based United States cancer registries, covering California, Colorado, Connecticut, Florida, Georgia, Hawaii, Illinois, Iowa, Michigan, New Jersey, New York, North Carolina, Texas, Utah, and the Seattle–Puget Sound area of Washington. Database linkages between the SRTR and these cancer registries used a computer-matching algorithm incorporating name, sex, date of birth, and Social Security number. This was followed by manual review of potential matches in cases of ambiguity. The linkage was approved by the human subjects committees of the National Cancer Institute and, as required, participating cancer registries.
Our study population consisted of renal transplant recipients who resided in the geographic areas covered by the 15 cancer registries (1987–2009); we excluded multiorgan recipients. Transplant recipients were considered at risk for cancer beginning with transplantation or start of cancer registry coverage, whichever came last. Follow-up ended at death, failure of the transplanted kidney, subsequent transplant, loss to follow-up, or last date of cancer registry coverage (whichever came first). We restricted analysis to non-Hispanic whites, non-Hispanic blacks, Hispanics, and Asian/Pacific Islanders.
Invasive cancers were identified using the linked cancer registry data and classified using the Surveillance, Epidemiology, and End Results program “site recode with Kaposi sarcoma and mesothelioma” with minor modifications.26 Cancer registries do not capture basal cell and squamous cell skin cancers, but they record the occurrence of other rare nonmelanoma skin cancers (i.e., nonepithelial skin cancers). Additionally, RCCs were defined by International Classification of Diseases for Oncology (version 3) morphology codes 8260 or 8310 so long as the site was in the kidney (topography code C64.9) or morphology codes 8312, 8316, 8317, or 8318 occurring at any site (although we observed all of these to arise in the kidney). Individuals could develop multiple cancers (i.e., they were not censored at first cancer).
The SRTR records the presence of PKD (most cases of which are ADPKD) as an indication for transplantation. We divided renal transplant recipients into those with PKD and those without. We first compared overall cancer rates in recipients with PKD to those in the general population, using expected rates stratified by sex, age, race/ethnicity, calendar year, and registry, to generate standardized incidence ratios. We then compared the incidence of each cancer type in PKD versus non-PKD renal transplant recipients using Poisson regression. We report unadjusted IRRs. For cancer outcomes where risk differed significantly, we adjusted the IRRs for age, sex, race/ethnicity, dialysis duration, and time since transplantation. For melanoma and the included nonmelanoma skin cancers, we also evaluated Poisson models restricted to non-Hispanic whites.
All analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Disclosures
None.
Acknowledgments
The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California (Tina Clarke), Colorado (Jack Finch), Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa, Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors. This research was supported in part by the Intramural Research Program of the National Cancer Institute.
During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, Michigan (contract HHSH234200537009C). Beginning in September 2010, the SRTR was managed by Minneapolis Medical Research Foundation in Minneapolis, Minnesota (HHSH250201000018C). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5658DP000805-04), Michigan (5U58DP000812-03), New Jersey (1US58/DP0039311-01), New York (U58DP003879), North Carolina (U58DP000832), and Texas (5U58DP000824-04). The following cancer registries were supported by the Surveillance, Epidemiology, and End Result Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201000027C, N01-PC-2010-0027), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN261201000026C). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, New Jersey, New York (Cancer Surveillance Improvement Initiative 14-2491), Texas, and Washington, as well as the Fred Hutchinson Cancer Research Center in Seattle, Washington.
The authors also thank Delaney Berrini, Ed Constantini, and Susan Everson of the Chronic Disease Research Group for assistance with the graphics.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477–1485, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76: 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman TI: Polycystic kidney disease: A 2011 update. Curr Opin Nephrol Hypertens 21: 189–194, 2012 [DOI] [PubMed] [Google Scholar]
- 4.United States Renal Data System : USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 5.Harris PC, Rossetti S: Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 197–206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pei Y: Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract 118: c19–c30, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Chang MY, Ong AC: Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: Recent progress and future prospects. Nephron Clin Pract 120: c25–c34; discussion c35, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Chang MY, Ong AC: New treatments for autosomal dominant polycystic kidney disease. Br J Clin Pharmacol 76: 524–535, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley BD, Jr, Chadwick LJ, Grantham JJ, Calvet JP: Elevated proto-oncogene expression in polycystic kidneys of the C57BL/6J (cpk) mouse. J Am Soc Nephrol 1: 1048–1053, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Cowley BD, Jr, Smardo FL, Jr, Grantham JJ, Calvet JP: Elevated c-myc protooncogene expression in autosomal recessive polycystic kidney disease. Proc Natl Acad Sci U S A 84: 8394–8398, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding Y, Chen L, Deng FM, Melamed J, Fan R, Bonsib S, Zhou M: Localized cystic disease of the kidney: Distinction from cystic neoplasms and hereditary polycystic diseases. Am J Surg Pathol 37: 506–513, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Witzgall R, Obermüller N, Bölitz U, Calvet JP, Cowley BD, Jr, Walker C, Kriz W, Gretz N, Bonventre JV: Kid-1 expression is high in differentiated renal proximal tubule cells and suppressed in cyst epithelia. Am J Physiol 275: F928–F937, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Calvet JP, Grantham JJ: The genetics and physiology of polycystic kidney disease. Semin Nephrol 21: 107–123, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hajj P, Ferlicot S, Massoud W, Awad A, Hammoudi Y, Charpentier B, Durrbach A, Droupy S, Benoît G: Prevalence of renal cell carcinoma in patients with autosomal dominant polycystic kidney disease and chronic renal failure. Urology 74: 631–634, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Jilg CA, Drendel V, Bacher J, Pisarski P, Neeff H, Drognitz O, Schwardt M, Gläsker S, Malinoc A, Erlic Z, Nunez M, Weber A, Azurmendi P, Schultze-Seemann W, Werner M, Neumann HP: Autosomal dominant polycystic kidney disease: Prevalence of renal neoplasias in surgical kidney specimens. Nephron Clin Pract 123: 13–21, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa I, Hayama S, Morita K, Nakazawa T, Yokoyama H, Honda R, Satoh K, Kakuma T: Long-term natural history of acquired cystic disease of the kidney. Ther Apheresis Dialysis 14: 409-416, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW: Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593–602, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC: Induction of apoptosis in fibroblasts by c-myc protein. Cell 69: 119–128, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Glynn JM, Guilbert LJ, Cotter TG, Bissonnette RP, Green DR: Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science 257: 212–214, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE: Cancer incidence before and after kidney transplantation. JAMA 296: 2823–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A: Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19: 488–493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grantham JJ: Polycystic kidney disease: Neoplasia in disguise. Am J Kidney Dis 15: 110–116, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Ward CJ, Wu Y, Johnson RA, Woollard JR, Bergstralh EJ, Cicek MS, Bakeberg J, Rossetti S, Heyer CM, Petersen GM, Lindor NM, Thibodeau SN, Harris PC, Torres VE, Hogan MC, Boardman LA: Germline PKHD1 mutations are protective against colorectal cancer. Hum Genet 129: 345–349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sulikowski T, Tejchman K, Zietek Z, Rózański J, Domański L, Kamiński M, Sieńko J, Romanowski M, Nowacki M, Pabisiak K, Kaczmarczyk M, Ciechanowski K, Ciechanowicz A, Ostrowski M: Experience with autosomal dominant polycystic kidney disease in patients before and after renal transplantation: A 7-year observation. Transplant Proc 41: 177–180, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Patel P, Horsfield C, Compton F, Taylor J, Koffman G, Olsburgh J: Native nephrectomy in transplant patients with autosomal dominant polycystic kidney disease. Ann R Coll Surg Engl 93: 391–395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M: Spectrum of cancer risk among US solid organ transplant recipients. JAMA 306: 1891–1901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]