Abstract
The development of dialysis was a dramatic step forward in medicine, allowing people who would soon have died because of lack of kidney function to remain alive for years. We have since found, however, that the “artificial kidney” does not live up fully to its name. Dialysis keeps patients alive but not well. Part of the residual illness that dialysis patients experience is caused by retained waste solutes that dialysis does not remove as well as native kidney function does. New means are available to identify these toxic solutes, about which we currently know remarkably little, and knowledge of these solutes would help us to improve therapy. This review summarizes our current knowledge of toxic solutes and highlights methods being explored to identify additional toxic solutes and to enhance the clearance of these solutes to improve patient outcomes.
Keywords: chronic dialysis, uremia, urea
Uremia today is different from the fatal illness described by Addis1 and Schreiner and Maher.2 Two million people worldwide who would have died a uremic death are today being kept alive by dialysis. But these people suffer a new illness, which Depner3 has aptly named the residual syndrome. This new illness comprises the ill effects of retained organic waste solutes along with the complications of treatment and continued inorganic ion disturbances. In many patients, the residual syndrome is combined with the effects of advancing age and systemic diseases responsible for kidney failure.
We tend now to restrict the label “uremia” to that part of the residual syndrome caused by retained waste solutes. This usage is in keeping with the word's etymology, from the Greek ouron (urine) and haima (blood). But beyond saying that people get sick when things that belong in the urine accumulate in the blood, we know remarkably little. Despite a recent renewal of interest in uremic toxicity, as chronicled by Duranton et al.4 we cannot yet name with confidence any single solute or group of solutes that contribute importantly to uremic illness.
Not knowing which uremic solutes are toxic limits our ability to improve therapy. The contribution of retained solutes to the illness experienced by dialysis patients is difficult to dissect, but we believe it is large. We know that if dialysis is withheld, accumulation of waste solutes will cause confusion, coma, and then death. We believe that this would happen even if the extracellular volume and inorganic ion concentrations were kept normal, if erythropoietin and 1,25-hydroxycholecalciferol were replaced, and if patients were protected from all other insults. We start dialysis when the eGFR has declined to between 5 and 10 ml/min per 1.73 m2 and symptoms have advanced to the point at which treatment is expected to effect some improvement. Patients avoid death by undergoing a burdensome treatment that prolongs their uremic illness—in effect maintaining them in a state of severe renal insufficiency. We could do better if we identified toxic solutes and lowered their levels. The benefit afforded by renal transplantation provides the strongest evidence that reduction in solute levels would benefit patients. Successful transplantation, which can restore solute clearances to more than half normal, improves overall quality of life and enhances specific functions, including sleep, cognition, exercise capacity, and growth in children.5–9
The Multiplicity of Uremic Solutes
The identification of solutes that cause illness has been made difficult by the multiplicity of uremic solutes. The most comprehensive reviews, prepared by the European Uremic Toxin Work Group investigators, include 132 compounds.4,10 Advances in chromatography and mass spectrometry are rapidly increasing this number, and recent papers bring the total to more than 200. High-resolution mass spectrometry has revealed the accumulation of many compounds that do not appear in lists of known metabolites and are characterized only by molecular mass.11–16 With the inclusion of such compounds, the number of reported uremic solutes will soon rise above 500.
The identification of solutes that have ill effects has also been made difficult by the complexity of uremic illness. Some of the most common features of this illness are listed in Table 1. It is important to emphasize that different solutes may have different ill effects. Solutes that contribute to accelerated vascular disease, for instance, may be different from those that impair mental or sexual function. And solutes that did not contribute significantly to the uremic illness when untreated renal failure led rapidly to death may have important ill effects in patients kept alive for years by dialysis.
Table 1.
Common features of uremia
| Neural and Muscular | Endocrine and Metabolic |
|---|---|
| Loss of energy | Amenorrhea and sexual dysfunctiona |
| Decreased mental acuity | Insulin resistancea |
| Anorexia and nausea | Reduced resting energy expenditurea |
| Restless legs | Increased protein/muscle catabolisma |
| Defective taste and smell | Other |
| Peripheral neuropathya | Pruritus |
| Sleep disturbancesa | Decreased red cell survivala |
| Reduced muscle membrane potentiala | Platelet dysfunctiona |
| Oxidant stressa |
The table lists features of illness commonly observed in patients maintained on conventional dialysis which may be caused by the accumulation of uremic solutes but is not a complete list. The list does not vomiting, seizures, coma, serositis, or other features of uremia that have been made much less common by current treatment.
Features of illness that can be assessed by test methods independent of volitional patient responses.
Our Reliance on Urea
Faced with the complexity of uremia, we continue to assess the efficacy of treatment by measuring levels of urea. Urea is the solute excreted in largest quantity by the kidneys, and the blood urea level was in use to measure kidney function before dialysis was developed. It was thus natural to prescribe dialysis to maintain the blood urea below a specified level. The National Cooperative Dialysis Study, completed in 1981, was designed to identify the proper level. The initial analysis showed that the patients with time-averaged BUN levels of approximately 50 mg/dl fared better than those with time-averaged BUN levels of approximately 80 mg/dl.17 A retrospective analysis by Gotch and Sargent,18 however, showed that Kt/Vurea predicted outcomes better than did the absolute urea level. Their result taught us that the time-averaged urea clearance should be maintained above a minimum level even when the blood urea level is low.
The development of Kt/Vurea remains the major, evidence-based step forward in prescribing dialysis. In using Kt/Vurea, we acknowledge that urea itself is not very toxic and that toxic solutes continue to be produced when urea production is low. But the development of Kt/Vurea did not, as has sometimes been supposed, actually make it possible to measure the detoxification of patients without identifying the toxins. Use of Kt/Vurea indeed encourages physicians prescribing dialysis to make several assumptions about uremic toxins that have not been verified and seem unlikely to be true. It suggests that important uremic toxins behave like urea, so that they are similarly affected by changes in the dialysis prescription. It further suggests that nonrenal clearance for these toxins is negligible and that they are produced at a stable rate that bears the same proportion to body water volume in all patients.
The Behavior of Solutes Other Than Urea
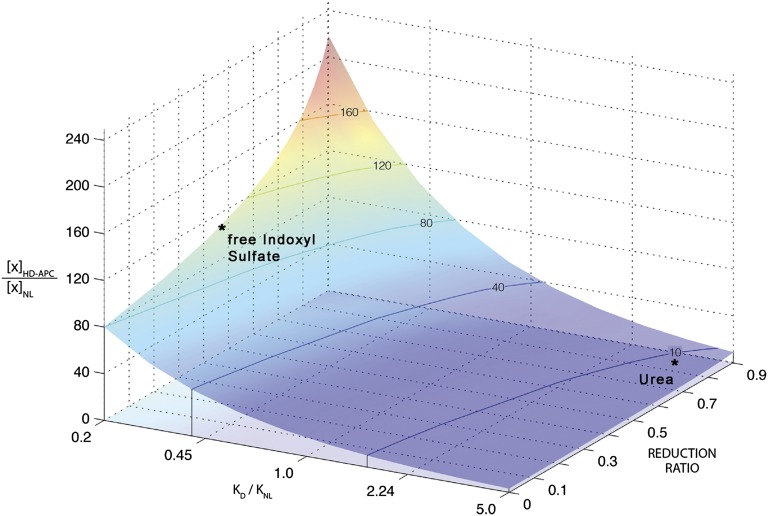
Recent reports have made clear that treatment prescribed to remove urea is much less effective in controlling the levels of other solutes. Indeed, the use of urea as an index solute has had the unintended consequence of maximizing the apparent efficacy of treatment. Urea is cleared at a uniquely high rate by hemodialysis because it diffuses rapidly from both red cells and plasma.19 In the native kidney, by contrast, urea undergoes tubular reabsorption and so is cleared less rapidly than many other solutes. As a result, conventional hemodialysis provides a time-averaged urea clearance that is about one fourth of that provided by the native kidneys. The efficacy of dialysis is reduced by intermittency, but predialysis blood urea values are still maintained within 5–10 times normal. The levels of many other small solutes remain much higher, with pretreatment levels of individual solutes remaining as high as 100 times normal.4,20–22 The elevation of solute levels is determined by the ratio of dialytic clearance to native kidney clearance and by the reduction in solute levels during intermittent treatment, as illustrated in Figure 1.16
Figure 1.
The predicted pretreatment solute level in patients receiving conventional thrice-weekly hemodialysis relative to the level in healthy persons ([X]HD-APC/[X]NL) is plotted on the vertical axis. The ratio varies widely among solutes depending on the ratio of the dialytic clearance to normal kidney clearance (KD/KNL) and on the concentration reduction ratio during treatment, defined as the pre- minus post-treatment concentration divided by the pretreatment concentration. Because the ratio of dialytic clearance to normal kidney clearance for urea is very high, its average pretreatment level remains <10-fold normal. The ratio of dialytic clearance to normal clearance is lower for other solutes, and their plasma levels therefore rise higher. This is particularly the case for solutes that are secreted by the native kidney, and levels of some of these solutes, including free, unbound indoxyl sulfate, remain >100-fold normal in patients receiving conventional treatment. Their high plasma levels are a predictable consequence of replacing the native kidney's secretory function by a treatment that clears solutes by diffusion. The figure is reproduced from Sirich et al.,16 which further describes the modeling procedure and measured values for solute levels.
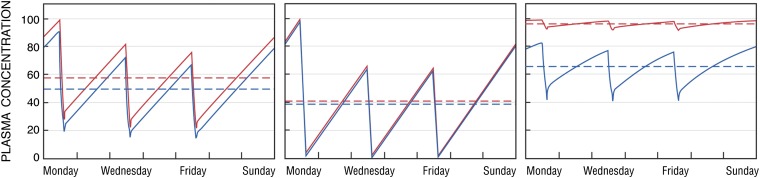
Differences in solute behavior may have contributed to our failure to improve outcomes by increasing Kt/Vurea. After Gotch and Sargent18 showed that better outcomes were associated with a Kt/Vurea≥0.9, hopeful physicians gradually increased their patients' Kt/Vurea values until the Hemodialysis (HEMO) study showed that an increase from 1.3 to 1.7 provided little or no benefit.23 HEMO's increase in Kt/Vurea was achieved largely by increases in blood flow and treatment time. The predicted effect, as confirmed by the study, was to reduce pretreatment urea levels by 15%–20%. But the same increase in Kt/Vurea would be predicted to have different effects on solutes that are differently distributed in the body. In particular, as illustrated in Figure 2, increasing Kt/Vurea would be much less effective in reducing the concentrations of solutes that are efficiently cleared by dialysis but, unlike urea, are confined to the extracellular space.
Figure 2.
The predicted effect of increases in time-averaged clearance on plasma levels of different types of solutes. In each panel, solute levels are normalized and the pretreatment level obtained with the lower time-averaged clearance is assigned a value of 100. The left panel depicts the effect of increasing Kt/Vurea on levels of urea. The red line depicts levels obtained with a conventional dialysis prescription designed to achieve spKt/Vurea of 1.4 during dialysis sessions lasting 3.5 hours. The blue line depicts the predicted effect on urea levels of increasing the urea clearance by 15% and the session length to 4 hours so that spKt/Vurea is increased by close to 30%. This increase is similar in magnitude to that achieved in the “high dose” group of the HEMO study, and its effect is to reduce time-averaged urea levels by 15%–20%. The middle panel depicts the effect of the same two prescriptions on levels of a hypothetical small, unbound solute that is distributed only in the extracellular fluid. Because the clearance is high relative to the volume of distribution, the conventional prescription removes the solute almost completely. The increases in session length and clearance, which together provide a 30% higher “dose” of dialysis as measured by Kt/Vurea, reduce the time-averaged solute concentration by <10% (blue line). The right panel depicts the predicted effect on β2-microglobulin levels of increasing β2-microglobulin's dialytic clearance from 3.4 ml/min to 34 ml/min during dialysis sessions lasting 3.5 hours. This 10-fold increase was the difference between the “high flux” and “low flux” groups of the HEMO Study. The large increase in clearance resulted in a much smaller relative decrease in β2-microglobulin levels, due largely to the presence of nonrenal, nondialytic β2-microglobulin clearance, and average β2-microglobulin levels remained >20-fold normal. Adapted from reference 65 with added calculations based on reference 25; additional discussion of the kinetics of solutes other than urea is provided in references 16 and 65.
The extent to which an increase in time-averaged dialytic clearance lowers solute levels can also be limited by the presence of nonrenal solute clearance. Although urea has been used as a representative small solute, it has long been recognized that large solutes behave differently because they diffuse less readily through dialysis membranes. The 2×2 design of HEMO therefore tested the effect of increasing the β2-microglobulin clearance from an average of 3.4–34 ml/min as well as the effect of increasing Kt/Vurea.23 Because β2-microglobulin has a nonrenal clearance of about 3 ml/min, and because its diffusion from the interstitium to the plasma during treatment is limited, this 10-fold increase in dialytic clearance reduced pretreatment β2-microglobulin levels by only 20%, as further shown in Figure 2.24,25 There is also evidence for nonrenal clearance of other large solutes, and there may be nonrenal clearance of small solutes.
Finally, changes in solute production may affect the extent to which a change in time-averaged dialytic clearance will alter solute levels. Peritoneal dialysis provides much lower clearances of both large solutes and protein-bound solutes than modern hemodialysis.26,27 Yet levels of the bound solutes indoxyl sulfate and p-cresol sulfate are no higher in patients receiving peritoneal dialysis, suggesting that they are produced at lower rates in these patients.27 We do not know of other changes in solute production in response to treatment. But we should point out that the production rates for uremic solutes have not often been described. In addition, the values we have are based largely on rates of removal in the dialysate and urine without assessment of nonrenal clearance or measurement of solute production by independent means.
More Can Be Known
The multiplicity of uremic solutes and the complexity of their response to treatment make the identification of toxic solutes difficult but not impossible. A logical first step is to compile a more thorough list of uremic solutes, continuing the work of the EUTOX investigators.4,10 Our current list comprises largely compounds that are present in high concentration and could be identified by classic analytic chemistry. Previously unidentified compounds, present in much lower concentration, may be recognized as more toxic. Several approaches could then guide us toward the identification of toxic compounds. One is to seek for solutes associated with poor outcomes. A second is to test whether benefit can be obtained by lowering the levels of particular groups of solutes. A third is further study of native kidney function with particular attention to solutes cleared by secretion. As described below, work is ongoing along all these lines but there is great opportunity for further effort.
Finding Solutes Associated With Outcomes
One means to identify potentially toxic compounds is to identify solutes associated with prominent uremic symptoms or other outcomes. Recent studies using this approach have identified associations between levels of p-cresol sulfate and indoxyl sulfate and cardiovascular disease.28 The accumulated evidence is strongly suggestive but not conclusive. Studies performed to date have examined only one or two candidate solutes because of the limitations of assay methods. Stronger associations with other solutes may therefore have been missed. Developments in mass spectrometry have made it possible to assay numerous solutes simultaneously.11,29 The ability to assay numerous solutes has been accompanied by the development of statistical methods to relate individual solutes to clinical events. Studies combining untargeted mass spectrometry and high dimensional statistical analysis have achieved notable success in other areas of medicine.30,31 These “metabolomic” methods would seem particularly suited to the problem of uremia.
Assessing the Toxicity of Solute Groups
The contribution of various groups of solutes to uremic illness can be assessed by comparing the effects of different treatments. This approach was used by HEMO and by studies testing whether increasing the clearance of large solutes by ultrafiltration affords benefit.23,32–35 A similar approach could be used to assess the toxicity of small solutes that bind to plasma proteins. The clearance of these solutes can be increased selectively by increasing the dialysate flow and dialyzer size.36,37 As is the case with large solutes, more work needs to be done to assess the extent to which increasing the dialytic removal of bound solutes will in fact reduce their plasma levels. Valuable information could be obtained by measuring the levels of representative solutes while prescriptions are changed in patients who embark on dialysis regimens incorporating increases in the frequency and duration of treatment. Valuable information might also be obtained from additional measurements in patients undergoing peritoneal dialysis. According to current guidelines, a much higher time-averaged urea clearance is required for adequacy in hemodialysis than in peritoneal dialysis. Comparison of nonurea solute levels in patients treated with the two modalities provide clues as to which solutes effect clinical well being. The ADEquacy of PD in MEXico (ADEMEX) study in peritoneal dialysis, like the HEMO study, found no benefit in increasing the time-averaged urea clearance.38 Additional measurements could show that as with hemodialysis, increasing time-averaged urea clearance does not effectively reduce the concentrations of all solutes.
Solute levels may be lowered by suppressing solute production as well as by increasing solute removal. A large number of uremic solutes are made by colon microbes. Because these solutes are made in an isolated compartment by processes foreign to mammalian metabolism, their production could prove easier to suppress than that of other solutes.39–41 Pioneering studies have shown that the production of microbially derived solutes in hemodialysis patients can be suppressed by administration of an oral sorbent or by increasing the intake of dietary fiber.42,43 Further studies with such methods could reveal the extent to which microbially-derived solutes contribute to uremic toxicity as a group. New knowledge of the colon microbiome and its metabolism would then potentially allow identification and manipulation of individual compounds.
How to Assess Benefit
Recent studies have focused largely on whether improving solute removal can reduce mortality in dialysis patients. This emphasis is natural given the limited life expectancy of patients starting dialysis, particularly in the United States. But there are disadvantages to the use of death and other “hard” endpoints, such as cardiovascular events or hospitalizations. Such events are usually the result of cumulative processes, to which the contribution of elevated solute levels during the study period may be relatively small. In particular, predialysis renal insufficiency and hypertension, comorbid conditions such as diabetes, and the hemodynamic effects of dialysis can all contribute to cardiovascular disease in dialysis patients. Studies designed to test the beneficial effect of reducing solute levels on mortality and hard cardiovascular endpoints must therefore be large and long.
Concentrating on mortality may also cause us to miss opportunities for improving the quality of life. As pointed out by Bargman,44 there is more to living than not dying. And the solutes that impair mental and sexual function may be different from those that contribute to vascular injury. Lowering the levels of these solutes could make dialysis patients feel better even if it does not greatly increase their average life expectancy.
One aspect of the quality of life in dialysis patients that may particularly deserve further study is mental function. Impaired mental function is perhaps the most prominent feature of untreated uremia.2 Early workers hypothesized that uremic solutes acted as false neurotransmitters. It has more recently been appreciated that the kidney and blood-brain barrier function as a two-stage pumping system that keeps the brain exquisitely clean.45–49 Uremic solutes with structural similarities to brain waste compounds could impair the barrier's ability to clean the brain independent of any direct neuronal action.
Given that their unchecked accumulation will cause coma and death, it seems particularly remarkable that we have not identified the uremic solutes responsible for mental dysfunction. One reason, in addition to the multiplicity of uremic solutes, is that the residual defects present in dialysis patients are difficult to measure precisely. Early workers noted that even severely uremic patients could often successfully initiate mental tasks but could not persist in them.2 Modern dialysis patients report lack of “energy,” which is largely restored by successful transplantation. This defect may be missed by standard neuropsychological tests, particularly when tests are kept brief to limit the burden they impose on patients. One potential solution is to use longer tests.50 Another is to use new tests of brain electrical and chemical activity, which may detect defects that are more subtle than those observed by electroencephalography in the early days of dialysis.51
Measurement of the defects in mental function caused by uremia is also made difficult by the confounding effects of age, personal background, and other illnesses.52,53 The problem is analogous to the difficulty we face in distinguishing how much of the cardiovascular disease in dialysis patients is attributable to uremic solutes. In studying mental function, however, we have the advantage that the defects may be reversible, so that patients can be used as their own controls when different treatments are compared. A further approach, as employed by Blake and O'Meara,54 is to study selected high-functioning patients with few comorbidities in whom the effects of uremia may be more easily distinguished. Studies in animals maintained on dialysis should also be considered. Given the difficulty of evaluating different solute removal prescriptions in humans, such studies, while costly, could provide an efficient route to knowledge. Animal studies would also allow procedures that are difficult to perform in patients, such as assay of cerebrospinal fluid and electrical stimulation of the brain. In vitro studies provide a further means to test solute toxicity, but results may be difficult to extrapolate to the human condition.
In studying the effects of uremia on mental function, we must also consider how much function will be improved by the reductions in solute levels we are able to achieve. It is worth considering what would be the effect on mental function of reducing levels of a known neuroactive compound, such as ethanol. Reducing very high ethanol levels by half could restore orientation in a stuporous person, analogous to the effect of initiating dialysis in a severely uremic patient. But the effect of a further 10%–20% reduction in ethanol levels would be harder to detect. This would be particularly the case if we studied patients who had other impairments and reduced the level using a method that imposed a burden. The same problem is encountered in studying other features of uremic illness, and this may have contributed to the failure of HEMO and ADEMEX to identify benefit.23,38 As noted above, we can try to select patients without confounding conditions and use them as their own controls when studying reversible defects. Small studies using experimental methods designed to produce large reductions in solute levels may also yield more information than larger studies using conventional equipment. Demonstration that reducing solute levels was beneficial would spur adoption of an experimental method for widespread use.
Other outcomes to consider are features of uremic illness that can be evaluated by laboratory tests, as listed in Table 1. We presume that these defects, like uremic symptoms, can be rapidly reversed. The appearance of oxidant stress in uremic patients has received particular attention.55 Perhaps the strongest evidence of oxidant activity is an increase in the fraction of albumin, which has undergone oxidation at its free cysteine thiol group. Oxidized albumin is rapidly converted to the reduced form during hemodialysis, suggesting that normal processes of albumin reduction are impaired by the accumulation of uremic solutes.56 Identification of solutes responsible for albumin oxidation or the other defects listed in Table 1 would be a major step forward. It would motivate development of methods to reduce the levels of these solutes and then test whether reducing their levels provides clinical benefit.
What Physiology Can Tell Us
The study of native kidney function can provide clues to the identity of toxic solutes. Over the past 70 years, these clues have been largely neglected as physiologists paid more attention to salt and water balance than to waste solute disposal. One potential clue to solute toxicity is tubular secretion. Tubular secretion allows the kidney to clear solutes at rates greatly exceeding the GFR. When combined with plasma protein binding, it allows solute clearances to exceed the renal plasma flow, as first recognized by Marshall57 and recently confirmed.16 Presumably, this process evolved to keep the levels of toxic solutes very low. Yet we cannot now name the secreted toxins. It is sometimes suggested that secretion evolved to eliminate xenobiotics. The similar size and function of the kidneys in herbivores and carnivores, however, argue against the importance of naturally occurring xenobiotics, which are derived largely from plants.58,59 We thus presume that important uremic toxins may be found among the set of compounds secreted by the renal tubules and derived from either mammalian metabolism or the colon microbiome. Molecular mechanisms of tubular secretion have been identified, and manipulation of these mechanisms provides a potential means to identify toxic solutes.60,61 One weakness in the hypothesis that toxic solutes are secreted must be mentioned. In patients receiving conventional hemodialysis, we would expect the plasma levels of such compounds to rise as residual function is lost, and cannot say that a corresponding exacerbation of uremic toxicity has been observed.
Another potential clue to solute toxicity is compensatory renal hypertrophy. When nephron number is reduced, remnant nephrons increase in size.62,63 The remnant kidney grows by about 80% following uninephrectomized mammals, and remnant nephrons can grow to three times their normal size following more severe reductions in nephron number. These increases in size are accompanied by increases in solute clearances.64 It has long been supposed that increases in remnant kidney size and function are triggered by a circulating factor whose levels rise when its clearance is reduced by nephron loss. No such factor, however, has been identified. It is tempting to speculate that the unsolved problems of compensatory renal hypertrophy and uremic toxicity are closely linked, and that the unknown factor triggering kidney growth is a toxic solute normally cleared by the kidney.
Conclusion
We know remarkably little about the solutes that cause illness when the kidneys fail. While new scientific methods have led to rapid progress in other areas of renal medicine, uremic toxicity has received relatively little attention. Looking back, it appears that the success of dialysis in keeping people alive without kidneys may have slowed efforts to identify uremic toxins. But the residual illness we now recognize in dialysis patients should motivate new efforts to identify the compounds that make them sick.
Disclosures
None.
Acknowledgments
Support was provided by the National Institutes of Health (R21-DK84439 and R01-DK80123 to T.W.M. and R01-DK80123 to T.H.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Addis T: Glomerular Nephritis, Diagnosis and Treatment, New York, MacMillan, 1949 [Google Scholar]
- 2.Schreiner G,, Maher J: Biochemistry of uremia. In: Uremia. Springfield, IL, Charles C. Thomas, 1960, pp 55–85 [Google Scholar]
- 3.Depner TA: Uremic toxicity: Urea and beyond. Semin Dial 14: 246–251, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, European Uremic Toxin Work Group : Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimmel PL, Patel SS: Quality of life in patients with chronic kidney disease: Focus on end-stage renal disease treated with hemodialysis. Semin Nephrol 26: 68–79, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Perl J, Unruh ML, Chan CT: Sleep disorders in end-stage renal disease: ‘Markers of inadequate dialysis’? Kidney Int 70: 1687–1693, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Nielens H, Lejeune TM, Lalaoui A, Squifflet JP, Pirson Y, Goffin E: Increase of physical activity level after successful renal transplantation: A 5 year follow-up study. Nephrol Dial Transplant 16: 134–140, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto N, Ichimura S, Hamaoka T, Osada T, Hattori M, Miyakawa S: Impaired muscle oxygen metabolism in uremic children: Improved after renal transplantation. Am J Kidney Dis 48: 473–480, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M, Dębska-Ślizień A, Rutkowski B: Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int 79: 1353–1360, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi K, Itoh Y, Tateoka R, Ezawa A, Murakami K, Niwa T: Metabolomic analysis of uremic toxins by liquid chromatography/electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878: 1662–1668, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Toyohara T, Akiyama Y, Suzuki T, Takeuchi Y, Mishima E, Tanemoto M, Momose A, Toki N, Sato H, Nakayama M, Hozawa A, Tsuji I, Ito S, Soga T, Abe T: Metabolomic profiling of uremic solutes in CKD patients. Hypertens Res 33: 944–952, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Sato E, Kohno M, Yamamoto M, Fujisawa T, Fujiwara K, Tanaka N: Metabolomic analysis of human plasma from haemodialysis patients. Eur J Clin Invest 41: 241–255, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boelaert J, t'Kindt R, Schepers E, Jorge L, Glorieux G, Neirynck N, Lynen F, Sandra P, Vanholder R, Sandra K: State-of-the-are non-targeted metabolomics in the study of chronic kidney disease. Metabolomics 10: 425–442, 2014 [Google Scholar]
- 16.Sirich TL, Funk BA, Plummer NS, Hostetter TH, Meyer TW: Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol 25: 615–622, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowrie EG, Laird NM, Parker TF, Sargent JA: Effect of the hemodialysis prescription of patient morbidity: Report from the National Cooperative Dialysis Study. N Engl J Med 305: 1176–1181, 1981 [DOI] [PubMed] [Google Scholar]
- 18.Gotch FA, Sargent JA: A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int 28: 526–534, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Schneditz D, Platzer D, Daugirdas JT: A diffusion-adjusted regional blood flow model to predict solute kinetics during haemodialysis. Nephrol Dial Transplant 24: 2218–2224, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Itoh Y, Ezawa A, Kikuchi K, Tsuruta Y, Niwa T: Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal Bioanal Chem 403: 1841–1850, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Rhee EP, Souza A, Farrell L, Pollak MR, Lewis GD, Steele DJ, Thadhani R, Clish CB, Greka A, Gerszten RE: Metabolite profiling identifies markers of uremia. J Am Soc Nephrol 21: 1041–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW: Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84: 585–590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R, Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, Agodoa L, Bailey J, Beck GJ, Clark W, Levey AS, Ornt DB, Schulman G, Schwab S, Teehan B, Eknoyan G: Serum beta-2 microglobulin levels predict mortality in dialysis patients: Results of the HEMO study. J Am Soc Nephrol 17: 546–555, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Ward RA, Greene T, Hartmann B, Samtleben W: Resistance to intercompartmental mass transfer limits beta2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int 69: 1431–1437, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y: Superior dialytic clearance of beta(2)-microglobulin and p-cresol by high-flux hemodialysis as compared to peritoneal dialysis. Kidney Int 70: 794–799, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Pham NM, Recht NS, Hostetter TH, Meyer TW: Removal of the protein-bound solutes indican and p-cresol sulfate by peritoneal dialysis. Clin J Am Soc Nephrol 3: 85–90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirich TL, Meyer TW, Gondouin B, Brunet P, Niwa T: Protein-bound molecules: A large family with a bad character. Semin Nephrol 34: 106–117, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Want EJ, Nordström A, Morita H, Siuzdak G: From exogenous to endogenous: The inevitable imprint of mass spectrometry in metabolomics. J Proteome Res 6: 459–468, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE: Metabolite profiles and the risk of developing diabetes. Nat Med 17: 448–453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grooteman MP, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, den Hoedt CH, van der Tweel I, Lévesque R, Nubé MJ, ter Wee PM, Blankestijn PJ, CONTRAST Investigators : Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol 23: 1087–1096, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maduell F, Moreso F, Pons M, Ramos R, Mora-Macià J, Carreras J, Soler J, Torres F, Campistol JM, Martinez-Castelao A, ESHOL Study Group : High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 24: 487–497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R, Membrane Permeability Outcome (MPO) Study Group : Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 20: 645–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, Hur E, Demirci MS, Demirci C, Duman S, Basci A, Adam SM, Isik IO, Zengin M, Suleymanlar G, Yilmaz ME, Ozkahya M: Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: Results from the Turkish OL-HDF Study. Nephrol Dial Transplant 28: 192–202, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Luo FJ, Patel KP, Marquez IO, Plummer NS, Hostetter TH, Meyer TW: Effect of increasing dialyzer mass transfer area coefficient and dialysate flow on clearance of protein-bound solutes: a pilot crossover trial. Am J Kidney Dis 53: 1042–1049, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sirich TL, Luo FJ, Plummer NS, Hostetter TH, Meyer TW: Selectively increasing the clearance of protein-bound uremic solutes. Nephrol Dial Transplant 27: 1574–1579, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S, Mexican Nephrology Collaborative Study Group : Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13: 1307–1320, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Evenepoel P, Meijers BK, Bammens BR, Verbeke K: Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl S12–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Schepers E, Glorieux G, Vanholder R: The gut: The forgotten organ in uremia? Blood Purif 29: 130–136, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Meyer TW, Hostetter TH: Uremic solutes from colon microbes. Kidney Int 81: 949–954, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Niwa T, Emoto Y, Maeda K, Uehara Y, Yamada N, Shibata M: Oral sorbent suppresses accumulation of albumin-bound indoxyl sulphate in serum of haemodialysis patients. Nephrol Dial Transplant 6: 105–109, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P: p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 25: 219–224, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Bargman JM: Is there more to living than not dying? A reflection on survival studies in dialysis. Semin Dial 20: 50–52, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Müting D: Studies on the pathogenesis of uremia. Comparative determinations of glucuronic acid, indican, free and bound phenols in the serum, cerebrospinal fluid, and urine of renal diseases with and without uremia. Clin Chim Acta 12: 551–554, 1965 [DOI] [PubMed] [Google Scholar]
- 46.Porter RD, Cathcart-Rake WF, Wan SH, Whittier FC, Grantham JJ: Secretory activity and aryl acid content of serum, urine, and cerebrospinal fluid in normal and uremic man. J Lab Clin Med 85: 723–731, 1975 [PubMed] [Google Scholar]
- 47.Spector R, Snodgrass SR: The effect of uremia on penicillin flux between blood and cerebrospinal fluid. J Lab Clin Med 87: 749–759, 1976 [PubMed] [Google Scholar]
- 48.Deguchi T, Isozaki K, Yousuke K, Terasaki T, Otagiri M: Involvement of organic anion transporters in the efflux of uremic toxins across the blood-brain barrier. J Neurochem 96: 1051–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Hosoya KI, Tachikawa M: Roles of organic anion/cation transporters at the blood-brain and blood-cerebrospinal fluid barriers involving uremic toxins. Clin Exp Nephrol 15: 478–485, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Lieberman HR: Cognitive methods for assessing mental energy. Nutr Neurosci 10: 229–242, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Teschan PE: Electroencephalographic and other neurophysiological abnormalities in uremia. Kidney Int Suppl 210–216, 1975 [PubMed] [Google Scholar]
- 52.Kurella Tamura M, Yaffe K: Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int 79: 14–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madero M, Gul A, Sarnak MJ: Cognitive function in chronic kidney disease. Semin Dial 21: 29–37, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Blake C, O’Meara YM: Subjective and objective physical limitations in high-functioning renal dialysis patients. Nephrol Dial Transplant 19: 3124–3129, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Himmelfarb J: Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial 22: 636–643, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Himmelfarb J, McMonagle E, McMenamin E: Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int 58: 2571–2578, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Marshall EK, Jr: Two lectures on renal physiology. Physiologist 9: 367–384, 1966 [PubMed] [Google Scholar]
- 58.Singer MA, Morton AR: Mouse to elephant: Biological scaling and Kt/V. Am J Kidney Dis 35: 306–309, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Beuchat CA: Structure and concentrating ability of the mammalian kidney: Correlations with habitat. Am J Physiol 271: R157–R179, 1996 [DOI] [PubMed] [Google Scholar]
- 60.Anzai N, Kanai Y, Endou H: Organic anion transporter family: Current knowledge. J Pharmacol Sci 100: 411–426, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Wikoff WR, Nagle MA, Kouznetsova VL, Tsigelny IF, Nigam SK: Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1). J Proteome Res 10: 2842–2851, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fine L: The biology of renal hypertrophy. Kidney Int 29: 619–634, 1986 [DOI] [PubMed] [Google Scholar]
- 63.Wesson LG: Compensatory growth and other growth responses of the kidney. Nephron 51: 149–184, 1989 [DOI] [PubMed] [Google Scholar]
- 64.Hayslett JP: Functional adaptation to reduction in renal mass. Physiol Rev 59: 137–164, 1979 [DOI] [PubMed] [Google Scholar]
- 65.Meyer TW, Sirich TL, Hostetter TH: Dialysis cannot be dosed. Semin Dial 24: 471–479, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]