Abstract
The kidneys contribute to calcium homeostasis by adjusting the reabsorption and excretion of filtered calcium through processes that are regulated by parathyroid hormone (PTH) and 1α,25-dihydroxyvitamin D3 (1α,25[OH]2D3). Most of the filtered calcium is reabsorbed in the proximal tubule, primarily by paracellular mechanisms that are not sensitive to calcium-regulating hormones in physiologically relevant ways. In the distal tubule, however, calcium is reabsorbed by channels and transporters, the activity or expression of which is highly regulated and increased by PTH and 1α,25(OH)2D3. Recent research suggests that other, heretofore unrecognized factors, such as the osteocyte-specific protein sclerostin, also regulate renal calcium excretion. Clues in this regard have come from the study of humans and mice with inactivating mutations of the sclerostin gene that both have increased skeletal density, which would necessitate an increase in intestinal absorption and/or renal reabsorption of calcium. Deletion of the sclerostin gene in mice significantly diminishes urinary calcium excretion and increases fractional renal calcium reabsorption. This is associated with increased circulating 1α,25(OH)2D3 levels, whereas sclerostin directly suppresses 1α-hydroxylase in immortalized proximal tubular cells. Thus, evidence is accumulating that sclerostin directly or indirectly reduces renal calcium reabsorption, suggesting the presence of a novel calcium-excreting bone-kidney axis.
Keywords: calcium, vitamin D, parathyroid hormone
Introduction
In this review we discuss the role of an osteocyte-derived protein, sclerostin, in the regulation of calcium homeostasis and the mechanisms by which it alters renal calcium transport. The impetus for such studies has come from the study of patients with sclerosteosis and its milder variant, van Buchem disease.1–3 Affected individuals have exceptionally dense bones and skeletal overgrowth that often constrict cranial nerve foramina and the foramen magnum, resulting in premature death. Genetic studies have demonstrated that sclerosteosis is due to inactivating mutations of the sclerostin (SOST) gene, which encodes a novel cystine-knot protein that inhibits osteoblast function.4 The milder van Buchem disease is due to a deletion of a downstream enhancer element of the sclerostin gene.5 A key question is how the increased amount of calcium needed for the deposition in the skeleton is accreted. Are increases in renal calcium reabsorption and intestinal calcium transport responsible for the required augmented calcium retention? If so, what are the mechanisms by which this increase occurs? Mouse models of sclerosteosis have increases in skeletal mass similar to those found in patients with the disease.6–9 By using a Sost gene knockout model generated in our laboratory,6 we have demonstrated that sclerostin, either directly or indirectly, through an alteration in the synthesis of 1α,25-dihydroxyvitamin D (1α,25[OH]2D), influences renal calcium reabsorption in the kidney.
Overview of Calcium Homeostasis
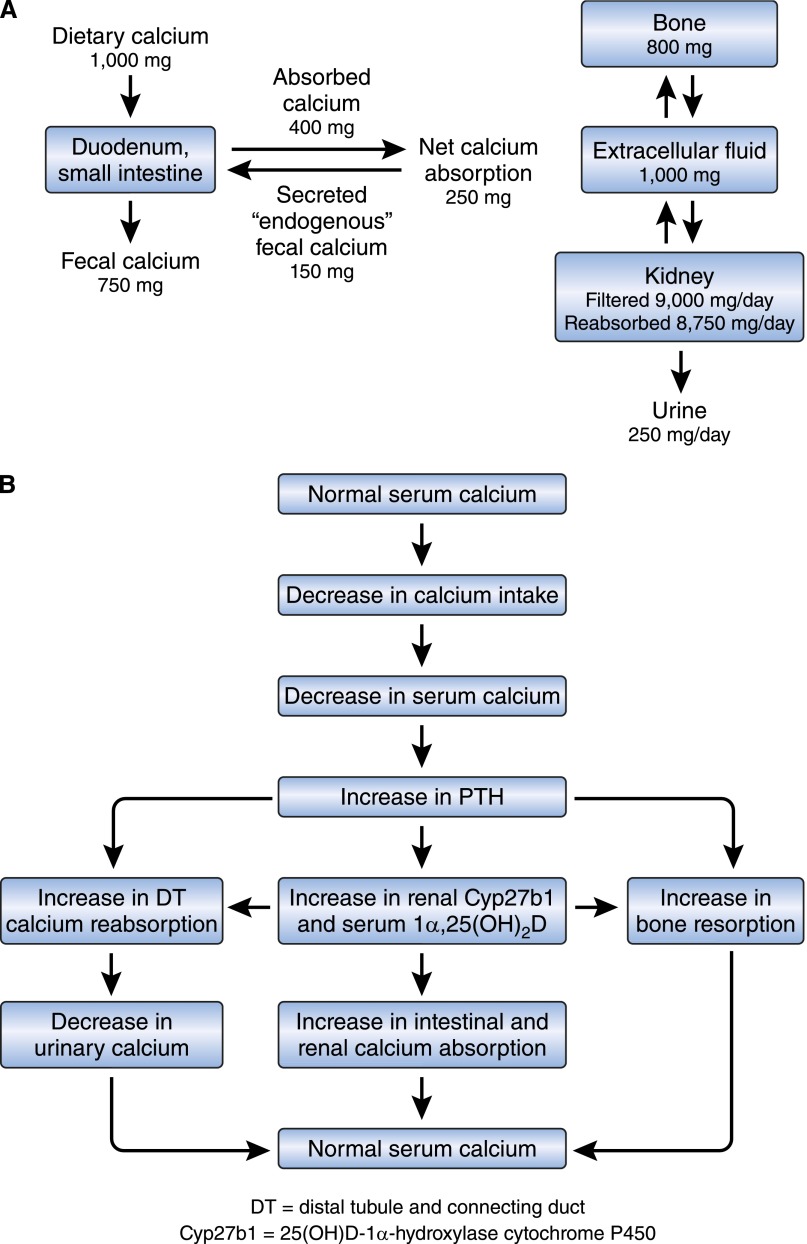
The pivotal role of calcium in biology is well known. Numerous biochemical and physiologic processes, including nerve conduction and function, muscle contraction, blood coagulation, enzyme activity, exocytosis, and bone mineralization, are critically dependent on normal calcium concentrations in intracellular and extracellular fluid.10–12 Not unexpectedly, finely tuned and rapidly responsive mechanisms exist to maintain intracellular and extracellular fluid calcium concentrations within a narrow range.13,14 The intestine and kidney are important in the absorption and excretion of calcium. In states of neutral calcium balance, the amount of calcium excreted by the kidney is equivalent to the net amount of dietary calcium absorbed by the intestine (net calcium absorbed=calcium absorbed–calcium secreted) (Figure 1A).14 The kidney, intestine, and vitamin D–parathyroid hormone (PTH)–endocrine system play important roles in the adaptation to variations in dietary calcium and phosphorus intakes.13,15–19 Figure 1B summarizes current information regarding changes that occur in response to decreasing dietary calcium intake. As shown, the vitamin D-PTH-endocrine system is essential for appropriate adaptations to alterations in dietary calcium intake.13,15,20 In the intestine, increases in circulating and tissue 1α,25(OH)2D enhance active calcium transport processes in response to decreased calcium intake. Calcium reabsorption in the kidney is increased by PTH21–23 and 1α,25(OH)2D3.24–28
Figure 1.
Calcium absorption in the intestine and calcium reabsorption in the kidney are influenced by calciotropic hormones. (A) Calcium balance in humans. (B) Physiologic adaptations to a low-calcium diet.
Renal Calcium Excretion Is Regulated by the Calcium-Regulating Hormones, PTH and 1α,25(OH)2D; Calcium-Sensing Receptor; and Klotho in Thick Ascending Limb and Distal Tubule
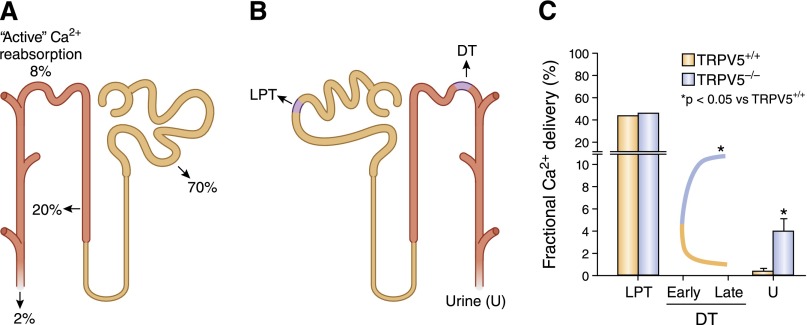
The kidney plays an important role in calcium homeostasis by reabsorbing filtered calcium in amounts that are subject to regulation by calciotropic hormones, PTH and 1α,25(OH)2D.13,16–19,29–31 As a result of reabsorption processes that occur in both the proximal and the distal tubule, only 1%–2% of calcium filtered at the glomerulus appears in the urine18,19,30 (Figure 2A). The amount of calcium reabsorbed in different nephron segments can be determined using micropuncture in which micropipettes are placed in accessible parts of the tubule to sample fluid, and by determining the amount of calcium present in the urine (Figure 2B). About 60%–70% of total plasma calcium is free (not protein bound) and can be filtered at the glomerulus.32,33 A large percentage (approximately 70%) of filtered calcium (Ca2+) is reabsorbed in the proximal tubule mainly by paracellular processes that are linked with sodium (Na+) reabsorption.32,34–37 In this nephron segment, the reabsorption of Na+ and Ca2+ are proportional under a variety of conditions36,38 and are not dissociated following the administration of several factors that are known to alter calcium reabsorption, such as PTH, cyclic AMP, chlorothiazide, furosemide, acetazolamide, or changes in the hydrogen ion content.35,36,39–41 The precise cellular and molecular mechanisms responsible for the movement of Ca2+ from the lumen of the proximal tubule into the interstitial space are not clearly defined. A majority of Ca2+ is believed to move in between cells (paracellular movement) with a smaller, but important, transcellular component. The components of the paracellular pathway might include claudin-2, which has a Ca2+-binding site that competitively binds Na+.42 The Na-K ATPase has been implicated in transcellular Ca2+ transport in the proximal tubule,43 and both the Na+-Ca2+ exchanger44 and isoforms 1 and 4 of the plasma membrane Ca2+ pump45,46 are expressed in the proximal tubule, and could be important in the movement of Ca2+ out of the proximal tubule cell. Undefined Ca2+ channels and Ca2+-binding proteins might also influence the movement of Ca2+ into and across the cell. Suffice it to say that although the proximal tubule reabsorbs large amounts of Ca2+ primarily by paracellular processes, the rate of Ca2+ reabsorption is not influenced by factors or hormones that regulate calcium balance.35,36,39,40 However, in conditions such as volume depletion, where proximal tubule Na+ reabsorption is increased, one also observes enhanced Ca2+ reabsorption that can contribute to the hypercalcemia that is sometimes seen in such situations.
Figure 2.
Regulated calcium reabsorption occurs in the distal convoluted tubule. (A) Segment-specific calcium reabsorption in the kidney. (B) Tubular fluid can be sampled by micropuncture in the late proximal tubule and along the distal tubule, allowing a determination of reabsorption of analytes in the proximal tubule, in the loop of Henle, and along the DT (early to late). The difference between values obtained at late DT and in the ureter (urine) allows estimation of reabsorption in the collecting duct. (C) Deletion of TRPV5 in mice prevents Ca2+ reabsorption along the DT (there is even evidence for Ca2+ leaking back into the lumen, possibly by paracellular routes); TRPV6 may partially compensate in the collecting duct. Data in error bars are presented as mean±SEM. Adapted from reference 76.
The thin descending and thin ascending limbs of the loop of Henle do not transport substantial amounts of Ca2+. 47, 48 Another 20%–25% of filtered Ca2+ is reabsorbed in the thick ascending loop of Henle, primarily by the paracellular route involving claudins 16 and 19.32,47–58 Thick ascending limb cells express the furosemide-sensitive Na-K-Cl cotransporter, NKCC2,59–62 which contributes to the driving force for paracellular Ca2+ transport. Accordingly, mutations of NKCC2 are associated with the common form of Bartter syndrome, which, like the other forms of the syndrome, can be associated with calciuria.63 The calcium-sensing receptor (CaSR) is expressed in the basolateral membrane of the thick ascending limb of the loop of Henle, where its activation inhibits the reabsorption of Ca2+.64,65 In accordance, patients heterozygous (familial benign hypocalciuric hypercalcemia) or homozygous (neonatal severe hyperparathyroidism) for inactivating mutations of the CaSR have hypocalciuria.66 Because these patients have intact parathyroid glands and either detectable (in familial benign hypocalciuric hypercalcemia) or high (in neonatal severe hyperparathyroidism) PTH concentrations, an additional effect of PTH on renal Ca2+ absorption in such patients cannot be ruled out. Mice in which the Casr and Pth genes have been deleted in the germ line, do not dispose of a Ca2+ load as efficiently as mice lacking just the Pth gene demonstrating the importance of the CaSR in facilitating renal calcium excretion.67 Recently, Toka et al. deleted the CaSR just in the kidney and confirmed the expected reduced capacity to excrete Ca2+ in these mice.68 There is considerable species heterogeneity with respect to responses to calcium-regulating hormones by the thick ascending limb; in the mouse, PTH and calcitonin stimulate Ca2+ transport in the cortical thick ascending limb,51,53,69,70 whereas in the rabbit calcitonin stimulates calcium reabsorption in the medullary thick ascending limb but not in the cortical thick ascending limb.56
In the distal convoluted tubule (primarily DCT2) and connecting tubule (together abbreviated as DT), 5%–10% of filtered Ca2+ is reabsorbed71–73 by active transport processes against both an electrical and concentration gradient. Ca2+ reabsorption in this segment of the nephron is regulated by PTH,21–23 calcitonin,69,70 and 1α,25(OH)2D324–28—these hormones increase the efficiency of Ca2+ reabsorption in this nephron segment. Ca2+ reabsorption in the DT occurs via a transcellular pathway. Mediators of Ca2+ transport in the renal DT include apically situated, transient receptor potential cation channels, subfamily V, type 5 and 6 channels (TRPV5, TRPV6), which mediate the increase in Ca2+ uptake from the lumen into the cell19,74–79; micropuncture studies in knockout mice indicated that TRPV5 is the gatekeeper of Ca2+ reabsorption in the accessible DT in mice (Figures 2C and 3, A and B).76 Intracellular Ca2+ binding proteins, such as calbindin D9K and D28K, facilitate the movement of Ca2+ across the cell29,80,81; and the basolateral plasma membrane calcium (PMCA) pump,16,17,29 Na+-Ca2+ exchanger,82–85 and the Na+-Ca2+-K+ exchanger86 increase the rate of extrusion of Ca2+ across the basolateral membrane (Figure 3, A and B). The Na+ gradient for the activity of the Na+-Ca2+ exchanger and the Na+-Ca2+-K+ exchanger is provided by the Na-K ATPase situated at the basolateral cell membrane (not shown).
Figure 3.
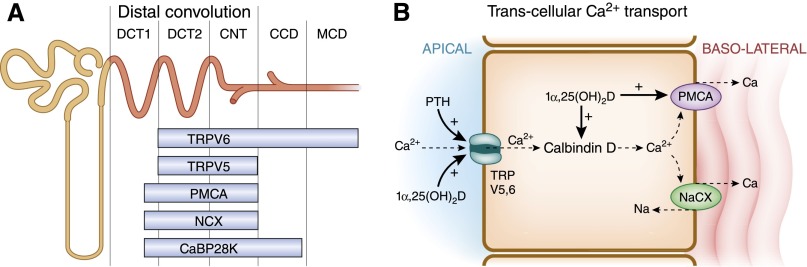
Transcellular calcium transport is mediated by several channels, pumps, and exchangers located at the apical and basolateral portions of the cell. NaCX, Na+-Ca2+. (A) Distribution of 1α,25(OH)2D or PTH-sensitive channels and transporters along the distal convoluted tubule (DCT1 and DCT2), connecting tubule (CNT), and cortical and medullary collecting ducts (CCD and MCD). (B) Calcium transport in the DT occurs by transcellular mechanisms.
The calcium-regulating hormones alter the expression of calcium channels, calcium-binding proteins, calcium pumps, and exchangers by varied mechanisms. PTH increases the activity of TRPV5 channels in the kidney by activating cAMP-protein kinase A signaling, and phosphorylating a threonine residue within the channel, resulting in an increase in the open probability of the channel.86,87 PTH also activates the protein kinase C pathway and increases the numbers of TRPV5 channels on the surface of tubular cells by inhibiting endocytosis of caveolae in which the channels are located.88 1α,25(OH)2D3 enhances the expression of TRPV5 and TRPV6 channels present in the distal and connecting tubule and cortical collecting duct by increasing respective mRNA concentrations through increased binding of the vitamin D receptor to response elements in the gene promoters.78,89 1α,25(OH)2D3 increases the expression of calbindin D9K and D28K and the PMCA pump in the kidney and cultured renal cells.27,46,90–99 The effect of PTH and 1α,25(OH)2D3 is to increase the expression of Ca2+ channels, binding proteins, pumps and exchangers, thereby increasing the retention of calcium by the kidney.
TRPV5-mediated Ca2+ reabsorption is further regulated by Klotho, a protein that exhibits β-glucuronidase activity.74,100–103 A study of the Ca2+-transporting activity of the TRPV5 channel expressed in human embryonic kidney cells found that treatment with Klotho removes carbohydrate moieties from TRPV5, thereby preventing channel internalization. Consistent with a positive influence of Klotho on TRPV5-mediated Ca2+ reabsorption in the kidney, mice lacking the Klotho gene have increased urinary Ca2+ losses and secondary hyperparathyroidism and hypervitaminosis D.100 Other factors, such as kallikrein, alter renal Ca2+ excretion by modulating the amount of TRPV5 channel found on the cell surface.104,105
The Kidney Is the Major Site for Metabolic Transformation of 25(OH)D to 1α,25(OH)2D and 24,25(OH)2D
See references 106–110. The 25(OH)D-1α-hydroxylase111 and the 25(OH)D-24-hydroxylase81 are expressed in the proximal and distal tubule of the kidney and are reciprocally regulated by PTH,112,113 growth factors such as IGF114,115 and fibroblast growth factor-23 (FGF-23),116 1α,25(OH)2D3 itself,117 and perhaps Ca2+ directly.118 Adaptations in hydroxylase activity, which occur with changes in calcium concentrations, are PTH dependent117,118 (see Figure 1B), whereas those that occur following alterations in serum Pi concentrations119 are driven by growth factors such as IGF114,115 and FGF-23.116 1α,25(OH)2D3, synthesized in the kidney by 25(OH)D-1α-hydroxylase, not only increases calcium and phosphate absorption in the intestine106,107,120 and the mobilization of calcium and phosphate from bone,121,122 but also increases the renal reabsorption of Ca2+ in the distal tubule by increasing TRPV5 and TRPV6,78,89 calbindin D,27,94,95,123 and PMCA97,98,124 expression.
Evidence for Regulation of Renal Calcium Excretion by the Bone-Derived Protein Sclerostin
To address how calcium is retained in increased amounts in sclerosteosis, and to determine whether increased renal calcium reabsorption can play a role in this regard, we generated a mouse model of sclerosteosis in which the sclerostin (Sost) gene was deleted (Sost−/− mice).6 As expected, serum sclerostin concentrations were readily detectable in wild-type mice but were not measurable in Sost−/− mice. We found that absolute urinary calcium excretion and renal fractional excretion of calcium were decreased in Sost−/− mice.6 Serum 1α,25(OH)2D concentrations were increased without attendant hypercalcemia; renal 25(OH)D-1α-hydroxylase (Cyp27b1) mRNA and protein expression were likewise increased in Sost−/− mice, strongly suggesting that the increase in serum 1α,25(OH)2D concentrations was due to increased 1α,25(OH)2D synthesis. When recombinant sclerostin was added to cultures of proximal tubular cells, the expression of the messenger RNA for Cyp27b1, the 1α-hydroxylase cytochrome P450, was diminished. Serum 24, 25(OH)2D concentrations were diminished in Sost−/− mice, and PTH concentrations were similar in knockout and wild-type mice. The lack of change in PTH is consistent with previous studies in humans.125 The data suggest that in addition to the hormones traditionally thought to alter calcium reabsorption in the kidney (PTH and 1α,25[OH]2D), sclerostin plays a significant role in altering renal calcium excretion. While PTH and 1α,25(OH)2D decrease fractional excretion of calcium by increasing the efficiency of calcium reabsorption in the DT, sclerostin increases fractional excretion of calcium (the absence of sclerostin expression being associated with a reduced fractional excretion of calcium).6 Thus, the scheme of adaptations to reductions in calcium intake and resultant downstream alterations in hormones (see Figure 1B for current understanding) may need to be amended to include changes in sclerostin expression (Figure 4). In the modified scheme, reduced sclerostin expression, which result from increases in PTH,126–129 would enhance renal Ca2+ reabsorption directly or through changes in 1α,25(OH)2D synthesis (Figure 4). The change in 1α,25(OH)2D synthesis might be direct or mediated through changes in FGF-23 concentrations.
Figure 4.
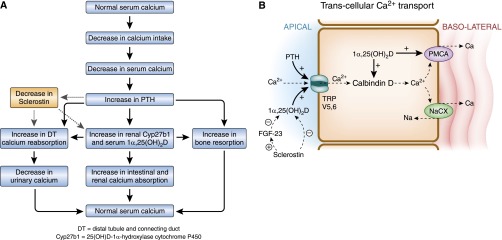
Proposed role of sclerostin in the regulation of calcium reabsorption in the kidney. (A) Proposed physiologic adaptations to a low-calcium diet. The sequence of changes in hormones is similar to that shown in Figure 1B, except that the effects of PTH on sclerostin concentrations, and the effect of sclerostin on tubular transport and 1α,25(OH)2D synthesis, are now superimposed. (B) The mechanism by which sclerostin influences calcium reabsorption in the distal tubule.
Unanswered Questions regarding the Mechanism of Action of Sclerostin in the Kidney
Several unanswered questions remain regarding the manner in which sclerostin affects kidney functions. For example, do patients with inactivating mutations of the SOST gene also have increased serum 1α,25(OH)2D concentrations? In what nephron segment does sclerostin alter Ca2+ transport? What Ca2+ channels and Ca2+ transporters/exchangers mediate the effect of sclerostin on renal Ca2+ reabsorption? Is the inhibition of 1α,25(OH)2D synthesis essential for sclerostin’s action on renal tubular Ca2+ transport? Does FGF-23 mediate the inhibitory effect of sclerostin on 1α,25(OH)2D synthesis? Are other signaling pathways, such as the Wnt/β-catenin pathway (which mediates some of the effects of sclerostin in bone), responsible for the action of sclerostin in the renal tubules? Are serum sclerostin concentrations altered by changes in dietary calcium, and do such changes in serum sclerostin help regulate calcium balance?
It will be important to ascertain where sclerostin functions along the nephron. The effects of sclerostin probably occur in the DT because this is the nephron segment in which hormone-regulated Ca2+ transport occurs. It is plausible, although unproven, that the effects of sclerostin on the tubule are direct and not through any intermediary molecules. Alternatively, or in addition, the effects of sclerostin on renal Ca2+ transport may be indirect and mediated through changes in the synthesis of 1α,25(OH)2D. Because 1α,25(OH)2D increases the reabsorption of Ca2+ in the kidney, it is conceivable that a calciuric effect of sclerostin is mediated through its inhibitory effects on 1α,25(OH)2D synthesis. It is likely that sclerostin-induced changes in renal Ca2+ handling result from changes in the expression of various transporters responsible for the reabsorption of calcium in the DT. Preliminary (unpublished) microarray data suggest that Sost−/− mice have increased renal expression of mRNAs for TrpV5, the 1α,25(OH)2D-regulated gate keeper of DT calcium transport (Figure 2C), and the basolateral Na+-Ca2+-K+ exchanger, member 3 (NCKX3, Slc24a3) (R. Kumar, unpublished data).
How does sclerostin alter 1α,25(OH)2D concentrations? Because the addition of sclerostin to immortalized proximal tubular cells decreases Cyp27b1 expression,6 there is a known direct effect of sclerostin on the expression of renal Cyp27b1, and thus renal 1α,25(OH)2D synthesis. Sclerostin may have an additional indirect inhibitory effect on Cyp27b1 expression since FGF-23 concentrations are diminished in Sost−/− mice along with an increase in serum Pi concentrations.6 The bone-derived FGF-23 is a known suppressor of 25(OH)D-1α-hydroxylase,116 suggesting that the increase in 1α,25(OH)2D concentrations in the absence of sclerostin may have resulted from lower FGF-23 levels. In other words, sclerostin may stimulate FGF-23 release from bone, which suppresses renal 1α,25(OH)2D synthesis. Whether plasma levels of sclerostin are regulated (e.g., stimulated by dietary calcium) and can directly affect and regulate kidney functions, however, remains to be determined. Additional information is needed regarding the regulation of sclerostin in humans by dietary calcium, phosphorus, and vitamin D. As noted earlier, PTH appears to inhibit sclerostin concentrations.126–139 Care, however, needs to be exercised in interpreting sclerostin concentrations in humans because available assays give different values when serum or plasma is used to measure the protein and reproducibility in the lower range of the assay is poor.130
The molecular signaling mechanisms by which sclerostin affects renal tubule function are unknown, but an alteration of Wnt signaling appears attractive. A simplified description of “canonical” Wnt signaling begins with Wnts binding to receptor complexes consisting of Lrp5/6 and Frizzled proteins.131 The canonical pathway involves β-catenin as a key intermediate signaling molecule. Several laboratories have demonstrated that canonical Wnt signaling and β-catenin expression are blocked by sclerostin in bone and osteoblasts through the sequestration of the LRP 5/6 coreceptor by sclerostin.9,132–136 Increased β-catenin in bone of Sost knockout mice is associated with enhanced fracture repair.137 “Noncanonical” Wnt pathways may also play a role in sclerostin signaling in bone. Lrp5/6 also stimulates signaling via Rac1, mTorc2/Akt, and other molecules142 suggesting that sclerostin may function by pathways other than the classic β-catenin pathway. Sclerostin also binds to BMP6, BMP2, noggin, and several other proteins133–135,139–145 in bone, and it is plausible but undetermined that BMP signaling might help regulate sclerostin-mediated changes in renal Ca2+ transport.
The studies outlined introduce sclerostin as part of a novel “bone-kidney” axis that suppresses renal 25(OH)D-1α-hydroxylase and 1α,25(OH)2D3 synthesis and enhances renal calcium excretion. Further studies are required to delineate whether and how sclerostin is regulated in bone and the circulation, how this protein affects the kidney (including the involved molecular mechanisms), and whether sclerostin might be involved in the pathogenesis and/or has therapeutic potential in disorders associated with renal calcium wasting and associated bone loss.146
Disclosures
None.
Acknowledgments
The authors were supported by grants provided by the National Institutes of Health (DK56248, HL094728, and P30-DK079337 to V.V., AR060869 and U01-DK066013 to R.K.), the Department of Veterans Affairs (V.V.), and the Ralph & Marion C. Falk Medical Research Trust (R.K.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Beighton P, Barnard A, Hamersma H, van der Wouden A: The syndromic status of sclerosteosis and van Buchem disease. Clin Genet 25: 175–181, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Beighton P, Davidson J, Durr L, Hamersma H: Sclerosteosis - an autosomal recessive disorder. Clin Genet 11: 1–7, 1977 [DOI] [PubMed] [Google Scholar]
- 3.Beighton P, Durr L, Hamersma H: The clinical features of sclerosteosis. A review of the manifestations in twenty-five affected individuals. Ann Intern Med 84: 393–397, 1976 [DOI] [PubMed] [Google Scholar]
- 4.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J: Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68: 577–589, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W: Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39: 91–97, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP, Westendorf JJ, Singh RJ, Kumar R: Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A 110: 6199–6204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C: Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23: 860–869, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, Rubin EM: Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 15: 928–935, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L: Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 24: 1651–1661, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Clapham DE: Calcium signaling. Cell 131: 1047–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Brini M, Carafoli E: Calcium pumps in health and disease. Physiol Rev 89: 1341–1378, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Carafoli E: Intracellular calcium homeostasis. Annu Rev Biochem 56: 395–433, 1987 [DOI] [PubMed] [Google Scholar]
- 13.DeLuca HF, Schnoes HK: Vitamin D: recent advances. Annu Rev Biochem 52: 411–439, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Kumar R: Vitamin D metabolism and mechanisms of calcium transport. J Am Soc Nephrol 1: 30–42, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Wasserman RH, Smith CA, Brindak ME, De Talamoni N, Fullmer CS, Penniston JT, Kumar R: Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology 102: 886–894, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Borke JL, Minami J, Verma A, Penniston JT, Kumar R: Monoclonal antibodies to human erythrocyte membrane Ca++-Mg++ adenosine triphosphatase pump recognize an epitope in the basolateral membrane of human kidney distal tubule cells. J Clin Invest 80: 1225–1231, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar R, Penniston JT, Borke JL: Ca2+-Mg2+-ATPase calcium pumps in the kidney. News Physiol Sci 3: 219–222, 1988 [Google Scholar]
- 18.Dimke H, Hoenderop JG, Bindels RJ: Molecular basis of epithelial Ca2+ and Mg2+ transport: Insights from the TRP channel family. J Physiol 589: 1535–1542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambers TT, Bindels RJ, Hoenderop JG: Coordinated control of renal Ca2+ handling. Kidney Int 69: 650–654, 2006 [DOI] [PubMed] [Google Scholar]
- 20.DeLuca HF, Schnoes HK: Metabolism and mechanism of action of vitamin D. Annu Rev Biochem 45: 631–666, 1976 [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M, Kawanobe Y, Takahashi H, Shimazawa E, Kimura S, Ogata E: Vitamin D deficiency and renal calcium transport in the rat. J Clin Invest 74: 507–513, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hugi K, Bonjour JP, Fleisch H: Renal handling of calcium: Influence of parathyroid hormone and 1,25-dihydroxyvitamin D3. Am J Physiol 236: F349–F356, 1979 [DOI] [PubMed] [Google Scholar]
- 23.Peacock M, Robertson WG, Nordin BE: Relation between serum and urinary calcium with particular reference to parathyroid activity. Lancet 1: 384–386, 1969 [DOI] [PubMed] [Google Scholar]
- 24.Puschett JB, Beck WS, Jr, Jelonek A, Fernandez PC: Study of the renal tubular interactions of thyrocalcitonin, cyclic adenosine 3′,5′-monophosphate, 25-hydroxycholecalciferol, and calcium ion. J Clin Invest 53: 756–767, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puschett JB, Fernandez PC, Boyle IT, Gray RW, Omdahl JL, DeLuca HF: The acute renal tubular effects of 1,25-dihydroxycholecalciferol. Proc Soc Exp Biol Med 141: 379–384, 1972 [DOI] [PubMed] [Google Scholar]
- 26.Bindels RJ, Hartog A, Timmermans J, Van Os CH: Active Ca2+ transport in primary cultures of rabbit kidney CCD: Stimulation by 1,25-dihydroxyvitamin D3 and PTH. Am J Physiol 261: F799–F807, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Hoenderop JG, Dardenne O, Van Abel M, Van Der Kemp AW, Van Os CH, St -Arnaud R, Bindels RJ: Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J 16: 1398–1406, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ: Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 274: 8375–8378, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Borke JL, Minami J, Verma AK, Penniston JT, Kumar R: Co-localization of erythrocyte Ca++-Mg++ ATPase and vitamin D-dependent 28-kDa-calcium binding protein. Kidney Int 34: 262–267, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Borke JL, Penniston JT, Kumar R: Recent advances in calcium transport by the kidney. Semin Nephrol 10: 15–23, 1990 [PubMed] [Google Scholar]
- 31.Kumar R, Tebben PJ, Thompson JR: Vitamin D and the kidney. Arch Biochem Biophys 523: 77–86, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassiter WE, Gottschalk CW, Mylle M: Micropuncture study of renal tubular reabsorption of calcium in normal rodents. Am J Physiol 204: 771–775, 1963 [Google Scholar]
- 33.Harris CA, Baer PG, Chirito E, Dirks JH: Composition of mammalian glomerular filtrate. Am J Physiol 227: 972–976, 1974 [DOI] [PubMed] [Google Scholar]
- 34.Duarte CG, Watson JF: Calcium reabsorption in proximal tubule of the dog nephron. Am J Physiol 212: 1355–1360, 1967 [DOI] [PubMed] [Google Scholar]
- 35.Agus ZS, Gardner LB, Beck LH, Goldberg M: Effects of parathyroid hormone on renal tubular reabsorption of calcium, sodium, and phosphate. Am J Physiol 224: 1143–1148, 1973 [DOI] [PubMed] [Google Scholar]
- 36.Edwards BR, Baer PG, Sutton RA, Dirks JH: Micropuncture study of diuretic effects on sodium and calcium reabsorption in the dog nephron. J Clin Invest 52: 2418–2427, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards BR, Sutton RA, Dirks JH: Effect of calcium infusion on renal tubular reabsorption in the dog. Am J Physiol 227: 13–18, 1974 [DOI] [PubMed] [Google Scholar]
- 38.Agus ZS, Chiu PJ, Goldberg M: Regulation of urinary calcium excretion in the rat. Am J Physiol 232: F545–F549, 1977 [DOI] [PubMed] [Google Scholar]
- 39.Beck LH, Goldberg M: Effects of acetazolamide and parathyroidectomy on renal transport of sodium, calcium, and phosphate. Am J Physiol 224: 1136–1142, 1973 [DOI] [PubMed] [Google Scholar]
- 40.Sutton RAL, Wong NLM, Dirks JH: The hypercalciuria of metabolic acidosis - a specific impairment of distal calcium reabsorption. Clin Res 23: 434A, 1975 [Google Scholar]
- 41.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ: Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115: 1651–1658, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu AS, Cheng MH, Coalson RD: Calcium inhibits paracellular sodium conductance through claudin-2 by competitive binding. J Biol Chem 285: 37060–37069, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ullrich KJ, Rumrich G, Klöss S: Active Ca2+ reabsorption in the proximal tubule of the rat kidney. Dependence on sodium and buffer transport. Pflugers Arch 364: 223–228, 1976 [DOI] [PubMed] [Google Scholar]
- 44.Dominguez JH, Juhaszova M, Kleiboeker SB, Hale CC, Feister HA: Na(+)-Ca2+ exchanger of rat proximal tubule: gene expression and subcellular localization. Am J Physiol 263: F945–F950, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Freeman TC, Howard A, Bentsen BS, Legon S, Walters JR: Cellular and regional expression of transcripts of the plasma membrane calcium pump PMCA1 in rabbit intestine. Am J Physiol 269: G126–G131, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Magosci M, Yamaki M, Penniston JT, Dousa TP: Localization of mRNAs coding for isozymes of plasma membrane Ca(2+)-ATPase pump in rat kidney. Am J Physiol 263: F7–F14, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Rocha AS, Magaldi JB, Kokko JP: Calcium and phosphate transport in isolated segments of rabbit Henle’s loop. J Clin Invest 59: 975–983, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jamison RL, Frey NR, Lacy FB: Calcium reabsorption in the thin loop of Henle. Am J Physiol 227: 745–751, 1974 [DOI] [PubMed] [Google Scholar]
- 49.de Rouffignac C, Morel F, Moss N, Roinel N: Micropuncture study of water and electrolyte movements along the loop of Henle in psammomys with special reference to magnesium, calcium and phosphorus. Pflugers Arch 344: 309–326, 1973 [DOI] [PubMed] [Google Scholar]
- 50.Bourdeau JE, Burg MB: Voltage dependence of calcium transport in the thick ascending limb of Henle’s loop. Am J Physiol 236: F357–F364, 1979 [DOI] [PubMed] [Google Scholar]
- 51.Bourdeau JE, Burg MB: Effect of PTH on calcium transport across the cortical thick ascending limb of Henle’s loop. Am J Physiol 239: F121–F126, 1980 [DOI] [PubMed] [Google Scholar]
- 52.Imai M: Calcium transport across the rabbit thick ascending limb of Henle’s loop perfused in vitro. Pflugers Arch 374: 255–263, 1978 [DOI] [PubMed] [Google Scholar]
- 53.Imai M: Effects of parathyroid hormone and N6,O2′-dibutyryl cyclic AMP on Ca2+ transport across the rabbit distal nephron segments perfused in vitro. Pflugers Arch 390: 145–151, 1981 [DOI] [PubMed] [Google Scholar]
- 54.Shareghi GR, Stoner LC: Calcium transport across segments of the rabbit distal nephron in vitro. Am J Physiol 235: F367–F375, 1978 [DOI] [PubMed] [Google Scholar]
- 55.Suki WN: Calcium transport in the pars recta and the loop of Henle. Adv Exp Med Biol 128: 37–40, 1980 [DOI] [PubMed] [Google Scholar]
- 56.Suki WN, Rouse D: Hormonal regulation of calcium transport in thick ascending limb renal tubules. Am J Physiol 241: F171–F174, 1981 [DOI] [PubMed] [Google Scholar]
- 57.Suki WN, Rouse D, Ng RC, Kokko JP: Calcium transport in the thick ascending limb of Henle. Heterogeneity of function in the medullary and cortical segments. J Clin Invest 66: 1004–1009, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Ananthapanyasut W, Yu AS: Claudins in renal physiology and disease. Pediatr Nephrol 26: 2133–2142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC: Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994 [PubMed] [Google Scholar]
- 60.Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E: Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium, and the glomerular afferent arteriole. J Clin Invest 98: 723–730, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaplan MR, Plotkin MD, Lee WS, Xu ZC, Lytton J, Hebert SC: Apical localization of the Na-K-Cl cotransporter, rBSC1, on rat thick ascending limbs. Kidney Int 49: 40–47, 1996 [DOI] [PubMed] [Google Scholar]
- 62.Plata C, Mount DB, Rubio V, Hebert SC, Gamba G: Isoforms of the Na-K-2Cl cotransporter in murine TAL II. Functional characterization and activation by cAMP. Am J Physiol 276: F359–F366, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Hebert SC: Bartter syndrome. Curr Opin Nephrol Hypertens 12: 527–532, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Aida K, Koishi S, Tawata M, Onaya T: Molecular cloning of a putative Ca(2+)-sensing receptor cDNA from human kidney. Biochem Biophys Res Commun 214: 524–529, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Caride AJ, Chini EN, Homma S, Dousa TP, Penniston JT: mRNAs coding for the calcium-sensing receptor along the rat nephron: Effect of a low-phosphate diet. Kidney Blood Press Res 21: 305–309, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Pearce SH, Trump D, Wooding C, Besser GM, Chew SL, Grant DB, Heath DA, Hughes IA, Paterson CR, Whyte MP, Thakker RV: Calcium-sensing receptor mutations in familial benign hypercalcemia and neonatal hyperparathyroidism. J Clin Invest 96: 2683–2692, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kantham L, Quinn SJ, Egbuna OI, Baxi K, Butters R, Pang JL, Pollak MR, Goltzman D, Brown EM: The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab 297: E915–E923, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toka HR, Al-Romaih K, Koshy JM, DiBartolo S, 3rd, Kos CH, Quinn SJ, Curhan GC, Mount DB, Brown EM, Pollak MR: Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J Am Soc Nephrol 23: 1879–1890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Rouffignac C, Di Stefano A, Wittner M, Roinel N, Elalouf JM: Consequences of differential effects of ADH and other peptide hormones on thick ascending limb of mammalian kidney. Am J Physiol 260: R1023–R1035, 1991 [DOI] [PubMed] [Google Scholar]
- 70.Di Stefano A, Wittner M, Nitschke R, Braitsch R, Greger R, Bailly C, Amiel C, Roinel N, de Rouffignac C: Effects of parathyroid hormone and calcitonin on Na+, Cl-, K+, Mg2+ and Ca2+ transport in cortical and medullary thick ascending limbs of mouse kidney. Pflugers Arch 417: 161–167, 1990 [DOI] [PubMed] [Google Scholar]
- 71.Costanzo LS, Windhager EE: Calcium and sodium transport by the distal convoluted tubule of the rat. Am J Physiol 235: F492–F506, 1978 [DOI] [PubMed] [Google Scholar]
- 72.Costanzo LS, Windhager EE, Ellison DH: Calcium and sodium transport by the distal convoluted tubule of the rat. 1978. J Am Soc Nephrol 11: 1562–1580, 2000 [PubMed] [Google Scholar]
- 73.Dimke H, Hoenderop JG, Bindels RJ: Hereditary tubular transport disorders: Implications for renal handling of Ca2+ and Mg2+. Clin Sci (Lond) 118: 1–18, 2010 [DOI] [PubMed] [Google Scholar]
- 74.de Groot T, Bindels RJ, Hoenderop JG: TRPV5: An ingeniously controlled calcium channel. Kidney Int 74: 1241–1246, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Hoenderop JG, Nilius B, Bindels RJ: Calcium absorption across epithelia. Physiol Rev 85: 373–422, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Mérillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ: Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112: 1906–1914, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsu YJ, Hoenderop JG, Bindels RJ: TRP channels in kidney disease. Biochim Biophys Acta 1772: 928–936, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ: Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol 14: 2731–2740, 2003 [DOI] [PubMed] [Google Scholar]
- 79.van de Graaf SF, Hoenderop JG, Bindels RJ: Regulation of TRPV5 and TRPV6 by associated proteins. Am J Physiol Renal Physiol 290: F1295–F1302, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Johnson JA, Kumar R: Vitamin D and renal calcium transport. Curr Opin Nephrol Hypertens 3: 424–429, 1994 [DOI] [PubMed] [Google Scholar]
- 81.Kumar R, Schaefer J, Grande JP, Roche PC: Immunolocalization of calcitriol receptor, 24-hydroxylase cytochrome P-450, and calbindin D28k in human kidney. Am J Physiol 266: F477–F485, 1994 [DOI] [PubMed] [Google Scholar]
- 82.Magyar CE, White KE, Rojas R, Apodaca G, Friedman PA: Plasma membrane Ca2+-ATPase and NCX1 Na+/Ca2+ exchanger expression in distal convoluted tubule cells. Am J Physiol Renal Physiol 283: F29–F40, 2002 [DOI] [PubMed] [Google Scholar]
- 83.White KE, Gesek FA, Friedman PA: Structural and functional analysis of Na+/Ca2+ exchange in distal convoluted tubule cells. Am J Physiol 271: F560–F570, 1996 [DOI] [PubMed] [Google Scholar]
- 84.White KE, Gesek FA, Reilly RF, Friedman PA: NCX1 Na/Ca exchanger inhibition by antisense oligonucleotides in mouse distal convoluted tubule cells. Kidney Int 54: 897–906, 1998 [DOI] [PubMed] [Google Scholar]
- 85.Yu AS, Hebert SC, Lee SL, Brenner BM, Lytton J: Identification and localization of renal Na(+)-Ca2+ exchanger by polymerase chain reaction. Am J Physiol 263: F680–F685, 1992 [DOI] [PubMed] [Google Scholar]
- 86.de Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJ, Hoenderop JG: Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol 20: 1693–1704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Abel M, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ: Coordinated control of renal Ca(2+) transport proteins by parathyroid hormone. Kidney Int 68: 1708–1721, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Cha SK, Wu T, Huang CL: Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am J Physiol Renal Physiol 294: F1212–F1221, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Hoenderop JG, Dardenne O, Van Abel M, Van Der Kemp AW, Van Os CH, St Arnaud R, Bindels RJ: Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J 16: 1398–1406, 2002 [DOI] [PubMed] [Google Scholar]
- 90.Christakos S, Brunette MG, Norman AW: Localization of immunoreactive vitamin D-dependent calcium binding protein in chick nephron. Endocrinology 109: 322–324, 1981 [DOI] [PubMed] [Google Scholar]
- 91.Johnson JA, Kumar R: Renal and intestinal calcium transport: Roles of vitamin D and vitamin D-dependent calcium binding proteins. Semin Nephrol 14: 119–128, 1994 [PubMed] [Google Scholar]
- 92.Roth J, Thorens B, Hunziker W, Norman AW, Orci L: Vitamin D-dependent calcium binding protein: Immunocytochemical localization in chick kidney. Science 214: 197–200, 1981 [DOI] [PubMed] [Google Scholar]
- 93.Schreiner DS, Jande SS, Parkes CO, Lawson DE, Thomasset M: Immunocytochemical demonstration of two vitamin D-dependent calcium-binding proteins in mammalian kidney. Acta Anat (Basel) 117: 1–14, 1983 [DOI] [PubMed] [Google Scholar]
- 94.Thomasset M, Desplan C, Warembourg M, Perret C: Vitamin-D dependent 9 kDa calcium-binding protein gene: cDNA cloning, mRNA distribution and regulation. Biochimie 68: 935–940, 1986 [DOI] [PubMed] [Google Scholar]
- 95.Thomasset M, Parkes CO, Cuisinier-Gleizes P: Rat calcium-binding proteins: Distribution, development, and vitamin D dependence. Am J Physiol 243: E483–E488, 1982 [DOI] [PubMed] [Google Scholar]
- 96.Caride AJ, Chini EN, Yamaki M, Dousa TP, Penniston JT: Unique localization of mRNA encoding plasma membrane Ca2+ pump isoform 3 in rat thin descending loop of Henle. Am J Physiol 269: F681–F685, 1995 [DOI] [PubMed] [Google Scholar]
- 97.Glendenning P, Ratajczak T, Dick IM, Prince RL: Calcitriol upregulates expression and activity of the 1b isoform of the plasma membrane calcium pump in immortalized distal kidney tubular cells. Arch Biochem Biophys 380: 126–132, 2000 [DOI] [PubMed] [Google Scholar]
- 98.Kip SN, Strehler EE: Vitamin D3 upregulates plasma membrane Ca2+-ATPase expression and potentiates apico-basal Ca2+ flux in MDCK cells. Am J Physiol Renal Physiol 286: F363–F369, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Stauffer TP, Guerini D, Carafoli E: Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. A study using specific antibodies. J Biol Chem 270: 12184–12190, 1995 [DOI] [PubMed] [Google Scholar]
- 100.Alexander RT, Woudenberg-Vrenken TE, Buurman J, Dijkman H, van der Eerden BC, van Leeuwen JP, Bindels RJ, Hoenderop JG: Klotho prevents renal calcium loss. J Am Soc Nephrol 20: 2371–2379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG: The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310: 490–493, 2005 [DOI] [PubMed] [Google Scholar]
- 102.Mensenkamp AR, Hoenderop JG, Bindels RJ: Recent advances in renal tubular calcium reabsorption. Curr Opin Nephrol Hypertens 15: 524–529, 2006 [DOI] [PubMed] [Google Scholar]
- 103.Topala CN, Bindels RJ, Hoenderop JG: Regulation of the epithelial calcium channel TRPV5 by extracellular factors. Curr Opin Nephrol Hypertens 16: 319–324, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Gkika D, Topala CN, Chang Q, Picard N, Thébault S, Houillier P, Hoenderop JG, Bindels RJ: Tissue kallikrein stimulates Ca(2+) reabsorption via PKC-dependent plasma membrane accumulation of TRPV5. EMBO J 25: 4707–4716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Picard N, Van Abel M, Campone C, Seiler M, Bloch-Faure M, Hoenderop JG, Loffing J, Meneton P, Bindels RJ, Paillard M, Alhenc-Gelas F, Houillier P: Tissue kallikrein-deficient mice display a defect in renal tubular calcium absorption. J Am Soc Nephrol 16: 3602–3610, 2005 [DOI] [PubMed] [Google Scholar]
- 106.Holick MF, Schnoes HK, DeLuca HF, Suda T, Cousins RJ: Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry 10: 2799–2804, 1971 [DOI] [PubMed] [Google Scholar]
- 107.Tanaka Y, DeLuca HF, Omdahl J, Holick MF: Mechanism of action of 1,25-dihydroxycholecalciferol on intestinal calcium transport. Proc Natl Acad Sci U S A 68: 1286–1288, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fraser DR, Kodicek E: Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature 228: 764–766, 1970 [DOI] [PubMed] [Google Scholar]
- 109.Lawson DE, Fraser DR, Kodicek E, Morris HR, Williams DH: Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature 230: 228–230, 1971 [DOI] [PubMed] [Google Scholar]
- 110.Holick MF, Schnoes HK, DeLuca HF, Gray RW, Boyle IT, Suda T: Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry 11: 4251–4255, 1972 [DOI] [PubMed] [Google Scholar]
- 111.Zehnder D, Bland R, Walker EA, Bradwell AR, Howie AJ, Hewison M, Stewart PM: Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in the human kidney. J Am Soc Nephrol 10: 2465–2473, 1999 [DOI] [PubMed] [Google Scholar]
- 112.Boyle IT, Gray RW, DeLuca HF: Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A 68: 2131–2134, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garabedian M, Holick MF, Deluca HF, Boyle IT: Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A 69: 1673–1676, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Condamine L, Menaa C, Vrtovsnik F, Friedlander G, Garabédian M, Garabedian M: Local action of phosphate depletion and insulin-like growth factor 1 on in vitro production of 1,25-dihydroxyvitamin D by cultured mammalian kidney cells. J Clin Invest 94: 1673–1679, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Menaa C, Vrtovsnik F, Friedlander G, Corvol M, Garabédian M: Insulin-like growth factor I, a unique calcium-dependent stimulator of 1,25-dihydroxyvitamin D3 production. Studies in cultured mouse kidney cells. J Biol Chem 270: 25461–25467, 1995 [DOI] [PubMed] [Google Scholar]
- 116.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T: Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tanaka Y, DeLuca HF: Stimulation of 24,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3. Science 183: 1198–1200, 1974 [DOI] [PubMed] [Google Scholar]
- 118.Kumar R: Metabolism of 1,25-dihydroxyvitamin D3. Physiol Rev 64: 478–504, 1984 [DOI] [PubMed] [Google Scholar]
- 119.Baxter LA, DeLuca HF: Stimulation of 25-hydroxyvitamin D3-1alpha-hydroxylase by phosphate depletion. J Biol Chem 251: 3158–3161, 1976 [PubMed] [Google Scholar]
- 120.Omdahl J, Holick M, Suda T, Tanaka Y, DeLuca HF: Biological activity of 1,25-dihydroxycholecalciferol. Biochemistry 10: 2935–2940, 1971 [DOI] [PubMed] [Google Scholar]
- 121.Tanaka Y, Deluca HF: Bone mineral mobilization activity of 1,25-dihydroxycholecalciferol, a metabolite of vitamin D. Arch Biochem Biophys 146: 574–578, 1971 [DOI] [PubMed] [Google Scholar]
- 122.Raisz LG, Trummel CL, Holick MF, DeLuca HF: 1,25-dihydroxycholecalciferol: A potent stimulator of bone resorption in tissue culture. Science 175: 768–769, 1972 [DOI] [PubMed] [Google Scholar]
- 123.Thomasset M, Desplan C, Parkes O: Rat vitamin-D-dependent calcium-binding proteins. Specificity of mRNAs coding for the 7500-Mr protein from duodenum and the 28000-Mr protein from kidney and cerebellum. Eur J Biochem 129: 519–524, 1983 [PubMed] [Google Scholar]
- 124.Kip SN, Strehler EE: Characterization of PMCA isoforms and their contribution to transcellular Ca2+ flux in MDCK cells. Am J Physiol Renal Physiol 284: F122–F132, 2003 [DOI] [PubMed] [Google Scholar]
- 125.Epstein S, Hamersma H, Beighton P: Endocrine function in sclerosteosis. S Afr Med J 55: 1105–1110, 1979 [PubMed] [Google Scholar]
- 126.Bellido T: Downregulation of SOST/sclerostin by PTH: A novel mechanism of hormonal control of bone formation mediated by osteocytes. J Musculoskelet Neuronal Interact 6: 358–359, 2006 [PubMed] [Google Scholar]
- 127.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL: Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: A novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146: 4577–4583, 2005 [DOI] [PubMed] [Google Scholar]
- 128.Keller H, Kneissel M: SOST is a target gene for PTH in bone. Bone 37: 148–158, 2005 [DOI] [PubMed] [Google Scholar]
- 129.Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H: Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res 22: 1957–1967, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McNulty M, Singh RJ, Li X, Bergstralh EJ, Kumar R: Determination of serum and plasma sclerostin concentrations by enzyme-linked immunoassays. J Clin Endocrinol Metab 96: E1159–E1162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Westendorf JJ, Kahler RA, Schroeder TM: Wnt signaling in osteoblasts and bone diseases. Gene 341: 19–39, 2004 [DOI] [PubMed] [Google Scholar]
- 132.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D: Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280: 19883–19887, 2005 [DOI] [PubMed] [Google Scholar]
- 133.Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R: Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 21: 1738–1749, 2006 [DOI] [PubMed] [Google Scholar]
- 134.Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y: BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development 135: 3801–3811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Löwik CW, van Bezooijen RL: Wnt signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Musculoskelet Neuronal Interact 6: 357, 2006 [PubMed] [Google Scholar]
- 136.Collette NM, Genetos DC, Murugesh D, Harland RM, Loots GG: Genetic evidence that SOST inhibits WNT signaling in the limb. Dev Biol 342: 169–179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McGee-Lawrence ME, Ryan ZC, Carpio LR, Kakar S, Westendorf JJ, Kumar R: Sclerostin deficient mice rapidly heal bone defects by activating β-catenin and increasing intramembranous ossification. Biochem Biophys Res Commun 441: 886–890, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F: WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab 17: 745–755, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A, Thesleff I, Itoh N: Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem 278: 24113–24117, 2003 [DOI] [PubMed] [Google Scholar]
- 140.ten Dijke P, Krause C, de Gorter DJ, Löwik CW, van Bezooijen RL: Osteocyte-derived sclerostin inhibits bone formation: Its role in bone morphogenetic protein and Wnt signaling. J Bone Joint Surg Am 90[Suppl 1]: 31–35, 2008 [DOI] [PubMed] [Google Scholar]
- 141.van Bezooijen RL, Papapoulos SE, Löwik CW: Bone morphogenetic proteins and their antagonists: the sclerostin paradigm. J Endocrinol Invest 28[Suppl]: 15–17, 2005 [PubMed] [Google Scholar]
- 142.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA: Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22: 6267–6276, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Winkler DG, Yu C, Geoghegan JC, Ojala EW, Skonier JE, Shpektor D, Sutherland MK, Latham JA: Noggin and sclerostin bone morphogenetic protein antagonists form a mutually inhibitory complex. J Biol Chem 279: 36293–36298, 2004 [DOI] [PubMed] [Google Scholar]
- 144.Craig TA, Kumar R: Sclerostin-erbB-3 interactions: Modulation of erbB-3 activity by sclerostin. Biochem Biophys Res Commun 402: 421–424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Devarajan-Ketha H, Craig TA, Madden BJ, Robert Bergen H, 3rd, Kumar R: The sclerostin-bone protein interactome. Biochem Biophys Res Commun 417: 830–835, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW: Nephrolithiasis-associated bone disease: Pathogenesis and treatment options. Kidney Int 79: 393–403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]