Summary
Here, we show that the ING1b tumor suppressor is SUMOylated on lysine 193 by the PIAS4 E3 SUMO ligase. SUMOylation regulates binding of ING1 to the ISG15 and DGCR8 promoters, implicating SUMOylation of ING1b in transcriptional regulation.
Abstract
The INhibitor of Growth (ING) proteins are encoded as multiple isoforms in five ING genes (ING1 –5) and act as type II tumor suppressors. They are growth inhibitory when overexpressed and are frequently mislocalized or downregulated in several forms of cancer. ING1 and ING2 are stoichiometric members of histone deacetylase complexes, whereas ING3–5 are stoichiometric components of different histone acetyltransferase complexes. The INGs target these complexes to histone marks, thus acting as epigenetic regulators. ING proteins affect angiogenesis, apoptosis, DNA repair, metastasis and senescence, but how the proteins themselves are regulated is not yet clear. Here, we find a small ubiquitin-like modification (SUMOylation) of the ING1b protein and identify lysine 193 (K193) as the preferred ING1b SUMO acceptor site. We also show that PIAS4 is the E3 SUMO ligase responsible for ING1b SUMOylation on K193. Sequence alignment reveals that the SUMO consensus site on ING1b contains a phosphorylation-dependent SUMOylation motif (PDSM) and our data indicate that the SUMOylation on K193 is enhanced by the S199D phosphomimic mutant. Using an ING1b protein mutated at the major SUMOylation site (ING1b E195A), we further demonstrate that ING1b SUMOylation regulates the binding of ING1b to the ISG15 and DGCR8 promoters, consequently regulating ISG15 and DGCR8 transcription. These results suggest a role for ING1b SUMOylation in the regulation of gene transcription.
Introduction
The first member of the INhibitor of Growth (ING) family of epigenetic regulators, ING1b, was isolated using a technique based on subtractive hybridization followed by an in vivo screen for genes with characteristics of tumor suppressors (1). Subsequent analyses revealed loss of ING1b expression in 44% of breast cancer tissues and in 10 of 10 breast cancer cell lines examined, further supporting its role as a tumor suppressor (2). Subsequently, four other members of this family, ING2 –5, were identified by homology search (3–6). Phylogenetic and structural analyses revealed the presence of a highly conserved plant homeodomain (PHD), which binds lysine 4 of histone 3 (H3K4) in a methylation-dependent manner with the highest affinity being for H3K4me3 (7,8). ING1b is a stoichiometric component of Sin3a-histone deacetylase 1/2 (HDAC1/2) complexes (9) and also binds SIRT1 (10). ING1b recruits SIRT1 and this interaction results in the inhibition of Sin3a-HDAC-mediated transcriptional repression (11). ING1b interacts with the Sin3a-HDAC complex through its N-terminus to recruit these complexes onto chromatin to regulate gene transcription. Although ING1b and ING2 function as the targeting modules of the Sin3a-HDAC1/2 complexes, they also play additional roles in the cell through regulating small non-coding RNA expression by regulating RNA processing protein DGCR8 (12) and also interact with the ATP-dependent nucleosome remodeling machinery (9,13).
Small ubiquitin-like modification (SUMO) proteins belong to the ubiquitin-like (Ubl) protein family and is conjugated to target proteins on lysine residues. The SUMO protein family is comprised of SUMO1–4, which have molecular weights of ~12 kDa. SUMO2, 3 and 4 are almost identical, however, SUMO1 shares only ~50% identity with other SUMO family members. SUMO proteins are translated in a precursor form. SUMO-specific proteases cleave the precursor SUMO protein into a mature form with a diglycine motif on its C-terminus, which eventually gets conjugated to the lysine residue of target proteins. Mature SUMO is conjugated to target proteins in three steps: (i) activation, where a thioester bond is formed between SUMO and a cysteine residue of Uba2 by a heterodimer containing E1 activation enzymes Aos1 and Uba2; (ii) conjugation, where Uba2-SUMO transfers SUMO to Ubc9, the only known SUMO E2 conjugation protein. A thioester bond is formed between the C-terminal GG motif of SUMO and cysteine 93 of Ubc9. Ubc9-SUMO then interacts with and transfers SUMO to protein substrates (14). One consensus site for SUMOylation contains a hydrophobic amino acid (ψ), a lysine for SUMO conjugation (K) and an acidic amino acid (E/D) on its first, second or fourth position (ψKXE/D where X is any amino acid) and (iii) ligation, the final step. Transferring SUMO to target proteins is often stabilized or facilitated by another class of proteins, the SUMO E3 ligases. Unlike E1 and E2 enzymes, there are many SUMO E3 ligases. SUMOylation can result in disruption of protein–protein interactions, promote protein–protein interactions or result in structural changes. SUMOylation of transcription factors and chromatin remodeling proteins has often been linked to gene repression (15) and, in a few instances, to gene activation (16) and loss of repression (17).
Although roles for several of the ING proteins have been described in diverse cellular processes (18), few reports exist describing regulation of the INGs by posttranslational modifications (PTMs) (19–23). Exceptions to this are for the role of ING1b phosphorylation at two different serine residues; serine 126, which affects protein stability (23) and serine 199, which affects subcellular localization (24). Also, src-mediated ING1b phosphorylation affects protein stability and ING1b levels (19), ING2 SUMOylation mediates ING2-Sin3a interaction (21) and ING4 citrullination affects ING4-p53 interactions (22). In this study, we find that ING1b is SUMOylated mainly on lysine 193 and that this is catalyzed by the E3 SUMO ligase PIAS4 and E2 SUMO ligase Ubc9. We also find that ING1b SUMOylation regulates the promoter occupancy and expression of the ISG15 and DGCR8 genes.
Materials and methods
Cell culture and transfection
Immortalized human osteosarcoma cells (U2OS) and human embryonic kidney cells (HEK293) were obtained from the American Type Culture Collection (ATCC). U2OS and HEK293 cells were grown in high glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. PEI (Sigma) and Lipofectamine LTX (Invitrogen) reagents were used to transfect plasmids into HEK293 cells and U2OS cells, respectively.
Plasmids
The ING1b mutants, ING1b K193R, ING1b E195A, ING1b S199D, ING1b S199A were generated with a QuickChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) from pcDNA3.1-ING1b. The primers were: 5′-AGCGC TCCAAGGCC AGGGC GGAGC-3′ (sense) and 5′-GCTCCGCCCTGGCCTTGGAGCGCT-3′ (antisense) for ING1b K193R; 5′-GGCCAAGGCGGCGCGAGAGGCGT-3′ (sense) and 5′-ACG CCT CTCG CGCC GCCTTGGCC-3′ (antisense) for ING1b E195A; 5′-GGAGC GAGAGG CGGACC CTGCCGACCTC-3′ (sense), 5′-GAGGTC GGCAGGG TCCGCCTCTCGCTCC-3′ (antisense) for ING1b S199D and 5′-AGCGAGAGG CGGC CCCTGCCGAC-3′ (sense), 5′-GTCGGCAGGGGCCGCCTCTCGCT-3′ (antisense) for ING1b S199A. All mutated ING1b constructs were verified by sequencing. HA/SUMO1, HA/UBC9, HA/UBC9CS, FLAG/PIAS1, 2, 3, 4, FLAG/SUMO1, FLAG/ING1b have been described elsewhere (25).
Western blotting and immunoprecipitation
Cell lysis buffer (20mM Tris–HCl [pH 7.5], 150mM NaCl, 1mM Na2 ethylenediaminetetraacetic acid, 1mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 1% Triton, 2.5mM sodium pyrophosphate, 1mM beta-glycerophosphate, 1mM Na3VO4, 1 µg/ml leupeptin) or radioimmunoprecipitation buffer (20 mM Tris–HCl, pH 7.5, 100 mM NaCl, 5 mM KCl, 1 mM ethylenediaminetetraacetic acid, 0.25% deoxycholate, 0.25% Nonidet P-40, 0.25% Tween-20) containing ethylenediaminetetraacetic acid-free protease tablets (Roche Diagnostics) and 1 mM phenylmethylsulfonyl fluoride was used for protein extraction and immunoprecipitation (IP), respectively. Modified radioimmunoprecipitation buffer containing 0.1% sodium dodecyl sulfate (SDS) and 20 mM N-ethylmaleimide was used for IP of SUMOylated proteins under denaturing conditions. Antibodies were αING1 (26), αHA (Covance), αFLAG (Sigma), αPIAS4, αSIN3a and αACTIN (SCBT). For affinity purification of HA- or FLAG-tagged SUMO-conjugated proteins, αHA affinity matrix (Roche) and anti-FLAG M2 affinity resin (Sigma) were used. For densitometry analysis of western blot bands, Image J (http://imagej.nih.gov/ij/) software was used and graphs were drawn using Graphpad Prism.
Indirect immunofluorescence
Transfection of cells was performed with cells plated on glass coverslips. Twenty-four hours after transfection, immunofluorescence was performed as reported previously. For immunostaining, an undiluted mixture of ING1 monoclonal antibodies (Cabs) (26) was used as primary antibody and images were visualized using a Leica SP8 immunofluorescence microscope.
RNA extraction and real-time PCR analysis
Total RNA from cells was isolated using RNeasy kits (Qiagen), and 1 µg of total RNA was transcribed into cDNA using a First-Strand kit (Applied Biosystems). Real-time PCR was carried out with qPCR MasterMix Plus for SYBR Green (Fermentas) using the company’s standard manual procedure. The primers used for real-time measurement of PCR were as follows: GAPDH, 5′-GTCAGTGGTGGACCTGACCT-3′ and 5′-TGAGCTTGACAAAGTGGTCG-3′; ING1b, 5′-CAACAACGAG AACCGT GAGA-3′ and 5′-GAGACCTGGTTGCACAGACA-3′; ISG15, 5′-AC TCATCTTT GCCAGTACAGGAG-3′ and 5′-CAGCATCTTCACCGTCA GGTC-3′ and DGCR8 are 5′-TGG-AGT-ATG-CAG-TGC-TCG-ATG-3′ and 5′-GGC-TGC-CAA-CAT-ACC-TCG-TA-3′. The expression of each gene was normalized using GAPDH mRNA as an internal control. The relative amounts of each product were calculated using the comparative cycle threshold (2−Δ Δ C t) method described in the ABI 7900HT Fast Real-time PCR System (Applied Biosystems). Results represent differences in ISG15 and DGCR8 relative to ING1b expression.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed using the EpiTect ChIP OneDay Kit (Qiagen, Courtaboeuf, France) following manufacturer’s instructions. Briefly, the cross-linking was performed using 1% formaldehyde solution in phosphate-buffered saline. Before the IP, 1% of each input fraction was saved and used in blots as a positive control. The supernatant was immunoprecipitated with either anti-ING1 or anti-mouse IgG as a negative control at 4°C for 4 h. Then, a mixture of protein A/G agarose beads (Santa Cruz Biotechnology) was added and incubated at 4°C for 1 h. DNA samples were then subjected to quantitative PCR (qPCR), and results were analyzed according to the manufacturer’s instructions. The differential occupancy results were calculated by the normalization of the IP differences (∆∆C t: ∆C t[IP: treated sample] − ∆C t[IP: control sample]). The fold changes in ISG15 or DGCR8 promoter occupancy were calculated following the 2−∆∆Ct method. Primer sequences spanning the upstream region of ISG15 are 5′-AGCATCTCACTGGGGTTTT-3′ and 5′-CTGATGAGGGCATAGCATCC-3′ and DGCR8 are 5′-GACTCTCG TCGCTGTCCG-3′ and 5′-ACACCTTTCCCGCCTGAAG-3′.
Results
ING1b is modified by SUMO1
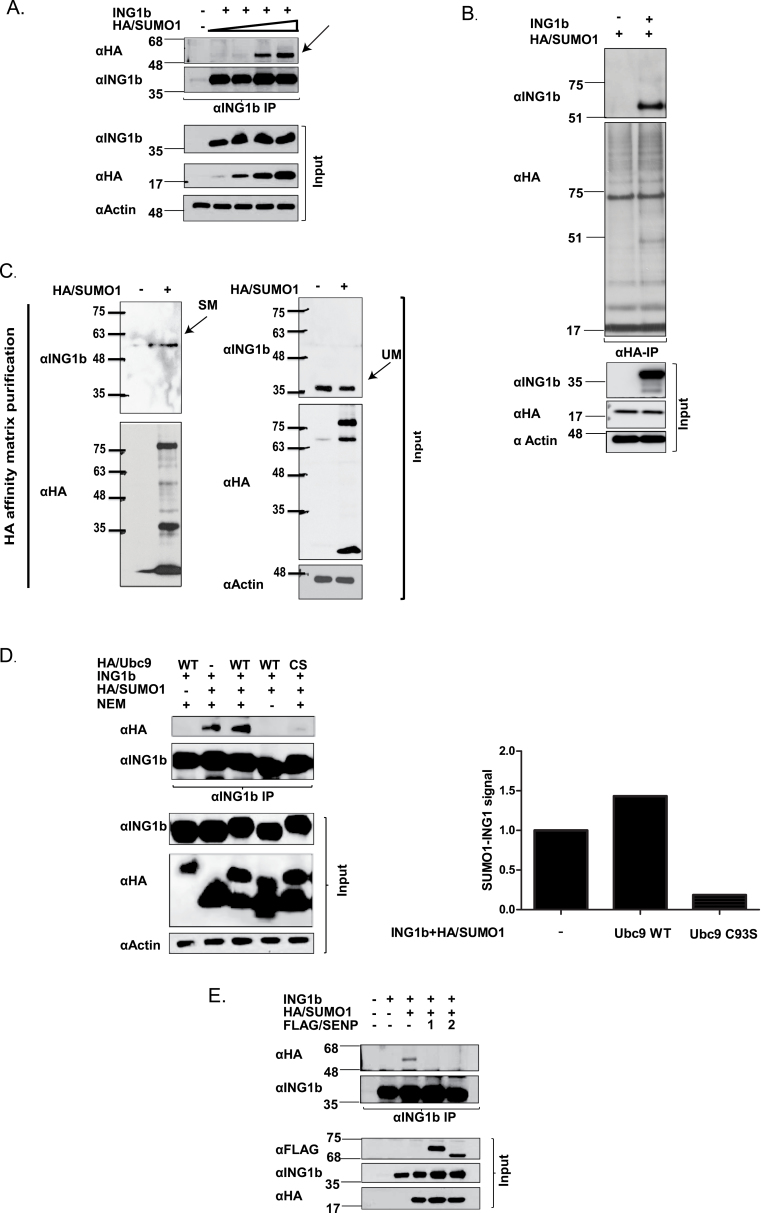
ING1 serves as the targeting component of HDAC complexes and contributes to regulating gene transcription via effects on the histone code. However, the mechanism by which ING1 activation or participation in the complex is regulated is not clear. Regulation of a gene or its product can occur through transcriptional or translational regulation or by PTMs. Indeed, SUMOylation of ING2 was recently reported to increase its occupancy in the Sin3a-HDAC1 complex (21). To test whether ING1b was similarly modified, it was coexpressed with increasing amounts of HA/SUMO1 plasmid in HEK293 cells. As shown in Figure 1A, denaturing IP with αING1 followed by immunoblotting (IB) with αHA revealed an HA-reactive band ~20kDa higher than unmodified ING1b. To determine if ING1b was also SUMOylated in another cell type, HA/SUMO1 was expressed alone or with ING1b expressing plasmid in U2OS cells. As shown in Figure 1B and Supplementary Figure S1A, available at Carcinogenesis Online, SUMOylated ING1b migrated at ~55kDa on 10% SDS–polyacrylamide gel electrophoresis. This is consistent with reports (27) indicating a shift of 15–20kDa with the addition of a single SUMO1 moiety and suggests that ING1b is primarily mono-SUMOylated in different cell types. To further investigate if endogenous ING1b was modified by SUMO1, U2OS cell lysates, with or without the expression of HA/SUMO1, were subjected to IP to detect endogenous sumoylated ING1b. As shown in Figure 1C, modified endogenous ING1b migrated at ~55kDa, consistent with the size of mono-SUMOylated ING1b protein. As shown in Supplementary Figure S1B and C, available at Carcinogenesis Online, the ING1b antibody recognized both the unmodified and modified forms of ING1b with high specificity. To better understand the mechanism by which ING1b was SUMOylated, lysates from cells overexpressing ING1b, HA/SUMO1 and HA/Ubc9 wild-type (HA/Ubc9 WT) were immunoprecipitated with αING1. As shown in Figure 1D and Supplementary Figure S2A, available at Carcinogenesis Online, Ubc9 enhanced ING1b SUMOylation in a dose-dependent manner. The cysteine residue on the 93rd amino acid position of Ubc9 is known to facilitate SUMO conjugation (28), and thus mutation of this cysteine residue to a serine (Ubc9CS) abrogates its conjugation activity. As shown in Figure 1D, expression of HA/Ubc9CS blocked ING1b SUMOylation, suggesting that it can act in a dominant negative fashion. In the absence of N-ethylmaleimide, a SUMO isopeptidase inhibitor, the HA-reactive band was lost, confirming that it is indeed a SUMOylated protein. Furthermore, as shown in Supplementary Figure S2C, available at Carcinogenesis Online, we also observed an interaction between Flag-tagged ING1b and endogenous Ubc9.
Fig. 1.
ING1b is SUMOylated. (A) ING1b expression construct was cotransfected with increasing concentrations of HA/SUMO1 expression construct in HEK293 cells. Cells were lysed in the presence of N-ethylmaleimide and lysates were subjected to denaturing αING1 IP and IB with SUMO1 (αHA) and ING1 (Cab1 and Cab5) to detect SUMOylated and unSUMOylated ING1b. (B) U20S cell lysates expressing HA/SUMO1 with or without ING1b were subjected to αHA IP and IB with HA and ING1 antibodies. (C) U2OS cell lysates with or without HA/SUMO1 was subjected to anti-HA purification using HA affinity matrix under denaturing conditions. SDS-Laemmli sample buffer (2×) was used to elute the SUMOylated proteins and eluent was subjected to SDS–polyacrylamide gel electrophoresis electrophoresis. αING1 IBs were performed to detect endogenous SUMOylated ING1b protein followed by αHA to detect purified SUMOylated proteins. αING1 and αHA (SUMO1) and α-actin IB were performed to confirm protein expression and equal loading, respectively. Black arrows depicting SM (sumoylated) and UM (unmodified) denotes SUMO-modified ING1b and unmodified ING1b, respectively. Major SUMOylated endogenous protein species of ~68 and ~85kDa were visualized in the input lysate, whereas a protein of ~85kDa was recovered from the HA affinity matrix. The band at ~20kDa could be free SUMO1. (D) HEK293 cells expressing HA/Ubc9 WT or HA/Ubc9 CS were immunoprecipitated with αING1 followed by IB with αHA and αING1 to detect SUMOylated ING1b, HA/SUMO1, HA/Ubc9 and modified and unmodified ING1, respectively. The associated graph indicates the relative density of SUMOylated ING1b in cells expressing Ubc9 WT or Ubc9 CS mutant. (E) HEK293 cells expressing ING1b and HA/SUMO1 were coexpressed with FLAG-tagged SENP1 or SENP2 and levels of SUMOylated ING1b were assessed by denaturing IP of ING1b and IB against αHA-SUMO. Expression of the different transfected constructs was checked with αFLAG (SENP1 and 2), αHA (SUMO1) and αING1.
SUMOylation is a transient PTM and at steady state, only a very small fraction (1–2%) of proteins are SUMOylated (27). SUMO is cleaved from proteins by SUMO-specific isopeptidases. Of the six described major de-SUMOylation enzymes (SENP1,2,3,5,6,7), SENP1 and SENP2 target both SUMO1 and SUMO2, whereas other SENPs prefer SUMO2 and/or SUMO3. SENP1 and SENP2 localize in nuclear pores and are found in the nucleoplasm as nuclear speckles (29). SENP3 and SENP5 localize in the nucleolus and SENP6 and SENP7 primarily localize in the nucleoplasm (29). Given that ING1b is primarily nuclear and is modified by SUMO1, we examined if SENP1 and 2 regulated ING1b SUMOylation. As shown in Figure 1E, overexpression of Flag-tagged SENP1 or SENP2 efficiently de-SUMOylated ING1b, further suggesting its role in the ING1b SUMOylation pathway. Together, these data suggest that ING1b is SUMOylated by SUMO1 in an Ubc9-dependent manner and is de-SUMOylated by both SENP1 and SENP2 SUMO-specific isopeptidases.
ING1b is SUMOylated by the PIAS4 SUMO E3 ligase
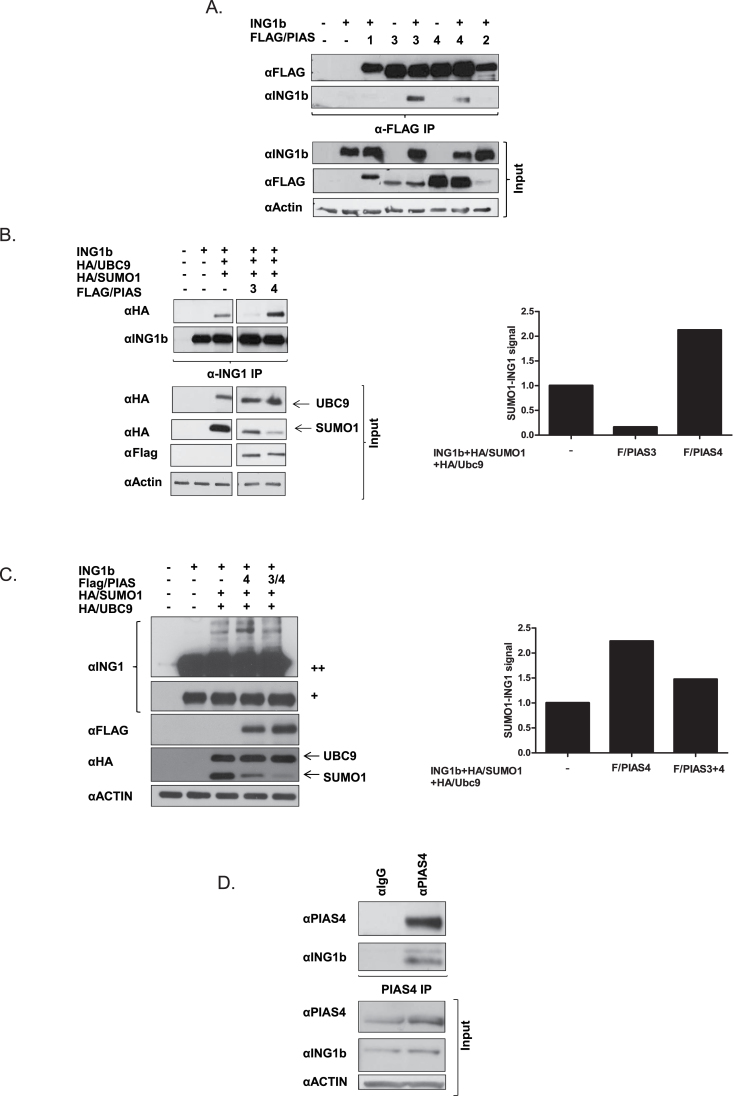
SUMO substrate specificity is regulated by many different SUMO E3 ligases. Although SUMO E3 ligases are dispensable for SUMOylation in vitro where the presence of E1 activation enzyme and E2 conjugation enzyme is sufficient for the transfer of SUMO, SUMO E3 ligases fulfill critical roles in many biological pathways (27). The PIAS (protein inhibitor of activated STAT) protein family is the most widely characterized group of E3 SUMO ligases, facilitating SUMOylation of a variety of chromatin regulators, transcription factors and tumor suppressors. These proteins localize primarily to the nucleus and therefore nuclear proteins are believed to be their major substrates. PIAS1 and PIAS4 have been reported to be involved in SUMOylation of proteins involved in the DNA damage response (30). Given that INGs function in response to one or more types of DNA damage (31,32), and in related stress pathways like senescence and apoptosis where PIAS proteins are known to be involved (30,33), we asked if one or more of the PIAS proteins might act as SUMO E3 ligases for ING1b. ING1b was coexpressed with FLAG-tagged PIAS1, PIAS2α, PIAS3 and PIAS4, and α-FLAG IPs were performed followed by IB with αING1. As shown in Figure 2A, only PIAS3 and PIAS4 immunoprecipitated ING1b. Therefore, we next investigated the involvement of PIAS3 and PIAS4 in ING1b SUMOylation. We coexpressed ING1b, HA/SUMO1 and HA/Ubc9 with or without FLAG-tagged PIAS3 or PIAS4. HEK293 cell lysates were then subjected to ING1b IP. As shown in Figure 2B, PIAS4 enhanced ING1b SUMOylation. In contrast, PIAS3, which also interacted strongly with ING1b, had a negative effect on ING1b SUMOylation. Consequently, we asked whether PIAS3 acted in a dominant negative manner, competing with PIAS4 and blocking its ability to SUMOylate ING1b. We coexpressed ING1b, HA/SUMO1 and HA/Ubc9 with FLAG/PIAS4, with PIAS3 or with both, in both U20S and in HEK293 cells. As shown in Figure 2C and Supplementary Figure S3, available at Carcinogenesis Online, PIAS4 enhanced ING1b SUMOylation. However, coexpression of PIAS3 with PIAS4 inhibited PIAS4-mediated ING1b SUMOylation to levels similar to those seen in the absence of exogenous PIAS4. As shown in Figure 2D, a physical interaction occurs between endogenous ING1b and PIAS4, further supporting the idea that PIAS4 functions as a major SUMO1 E3 ligase for ING1b.
Fig. 2.
PIAS4 promotes ING1b SUMOylation. (A) HEK293 cell lysates coexpressing FLAG/PIAS1, 2, 3 and 4 and ING1b were co-immunoprecipitated with αFLAG and subjected to ING1 IB. Cell lysates were immunoblotted with αING1, αFLAG (PIAS1–4) and α-actin to confirm equal loading. (B) FLAG/PIAS3 and 4, ING1b, HA/Ubc9 and HA/SUMO1 were coexpressed in HEK293 and cell lysates were subjected to denaturing IP with αING1 and immunoblotted with αHA (SUMO1 and Ubc9). The associated graph indicates the relative density of SUMOylated ING1b in cells expressing FLAG/PIAS3 or FLAG/PIAS4. (C) U2OS cell lysates coexpressing ING1b, HA/Ubc9, HA/SUMO1 with PIAS4 and PIAS3 and 4 were directly subjected to SDS–polyacrylamide gel electrophoresis electrophoresis and αING1 to detect modified and unmodified ING1b. SUMO1-modified ING1b is marked by the arrow. (++) represents a higher exposure of the panel marked (+). The associated graph indicates the density of SUMOylated ING1b in cells expression FLAG/PIAS4 alone or PIAS3 and FLAG/PIAS4 together with ING1b, HA/SUMO1 and HA/Ubc9. (D) U2OS cell lysates were subjected to IgG or αPIAS4 IP followed by αING1 IB. Cell lysates were probed for αING1, αPIAS4 and α-actin to confirm protein expression and equal protein loading.
Lysine 193 is the major SUMO1 acceptor on ING1b
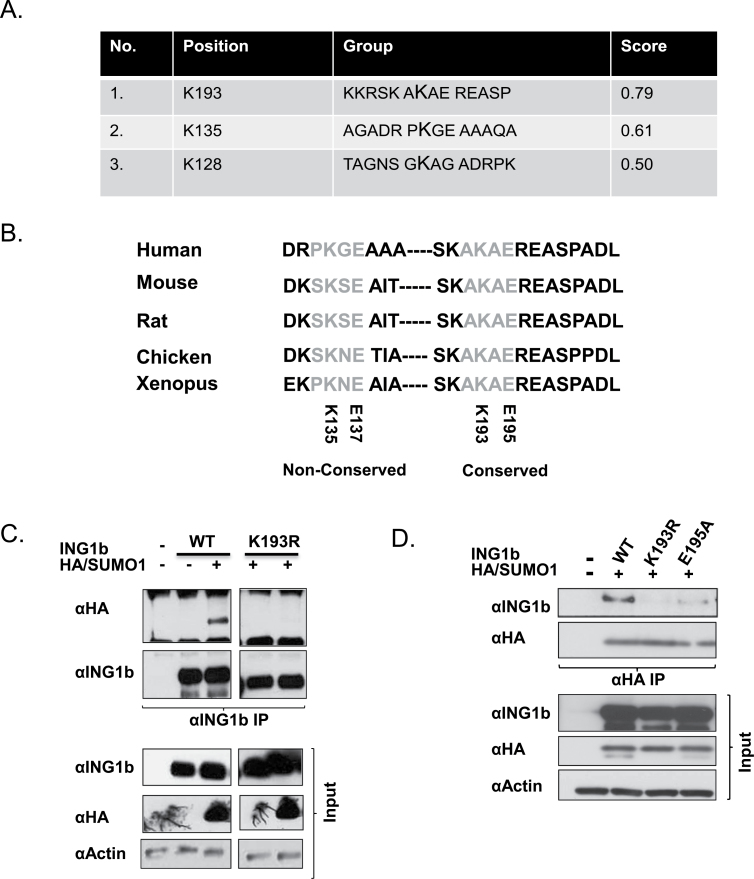
SUMO1 is often conjugated to a short consensus sequence consisting of ΨKXD/E where Ψ is a hydrophobic residue and X is any amino acid. The SUMO consensus motifs (ΨKXD/E) serve as recognition modules enabling Ubc9 to interact with target proteins. Lysine within this module serves as the SUMO acceptor site and the acidic amino acid residue is important for Ubc9 interaction (14). Bioinformatics analysis using the SUMOplot program (http://www.abgent.com/sumoplot) confirmed the presence of three SUMO consensus motifs within ING1b, however, sequence conservation analysis using the ClustalW program (http://www.ebi.ac.uk/Tools/msa/clustalw2/) revealed that the motif containing lysine 193 (Figure 3A) was the highest scoring SUMO consensus motif and was highly conserved between human, mouse, rat and other vertebrates (Figure 3B). To test if lysine 193 was the major SUMO acceptor on ING1b, lysine 193 was mutated to arginine, another basic amino acid but one that could not be SUMOlated (ING1b K193R). ING1b and ING1b K193R were coexpressed with HA/SUMO1 in HEK293 cells and were subjected to denaturing ING1 IP. As observed in Figure 3C, SUMO1 was not conjugated onto the ING1b K193R mutant, suggesting that lysine 193 was the major ING1b SUMO acceptor site, or perhaps the only one. Lysine 193 on ING1b could be the target of a multitude of different lysine-specific PTMs like acetylation, ubiquitination, methylation and other modifications that could interfere with our analysis of SUMOylation. Therefore, we mutated the glutamic acid residue at position 195 to alanine (ING1b E195A), a manipulation that is predicted to only influence SUMOylation (14). This disrupts the sumoylation consensus motif and thus should selectively prevent SUMOylation, but not other modifications on K193. As shown in Figure 3D, consistent with ΨKXD/E being the SUMO consensus site and also being important for Ubc9 and target protein interaction, site-directed mutagenesis of glutamic acid 195 strongly inhibited, but did not totally abrogate SUMOylation.
Fig. 3.
Identification of ING1b SUMO acceptor sites. (A) SUMOplot software (Abgent) predicts the presence of three consensuses SUMO sites from the primary protein sequence of ING1b and a score is assigned based on the hydrophobicity of the first residue and the presence of an acidic amino acid on the fourth position of the consensus motif. (B) Sequence analysis revealed that the site with the highest probability containing lysine 193 is completely conserved in other vertebrates. (C) αING1b denaturing IP was performed with HEK293 cell lysates expressing ING1b wild-type (WT) and the ING1 K193R (K193R) point mutant with and without HA/SUMO1. Expression of the transfected constructs and equal loading were confirmed using αING1 and αHA (SUMO1) and α-actin, respectively. (D) A reciprocal αHA IP was performed with U2OS cell lysates expressing ING1b WT, K193R and E195A (SUMOylation specific point mutant) and HA/SUMO1 to confirm the presence of a functional SUMO consensus site (AKAE) containing lysine 193 and glutamic acid 195.
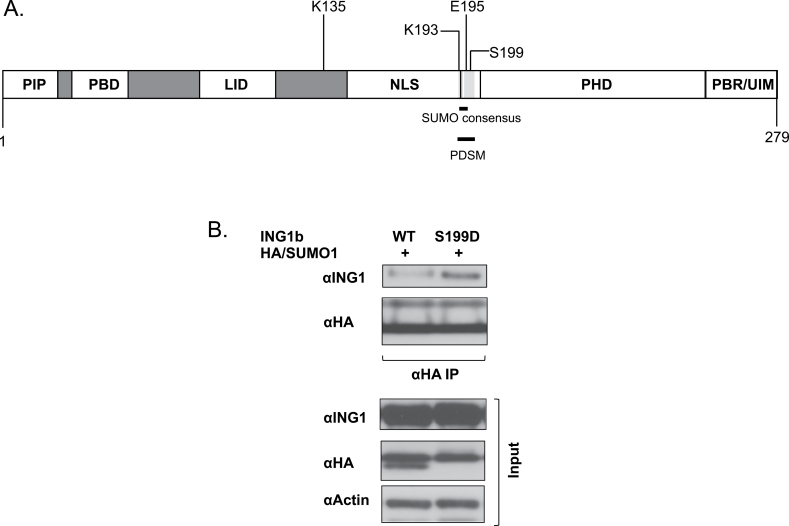
ING1b K193 is also a PDSM
The phosphorylation-dependent SUMOylation motif (PDSM) contains a SUMOylation target residue governed by the phosphorylation of a serine residue downstream. The consensus PDSM is of the sequence ΨKXEXXSP where ΨKXD/E is the SUMO consensus motif and the downstream serine is the phospho-acceptor residue (34). ING1b possesses the sequence AK(193)XE(195)REAS(199)P that is very similar to a PDSM and contains the target K193 residue. The ING1b PDSM is well conserved within vertebrates and lies between the nuclear localization signal (NLS) and PHD of ING1b as indicated in Figure 4A. The PDSM in ING1b overlaps with its 14-3-3 binding motif and 14-3-3 binding to ING1b is dependent on the phosphorylation status of serine 199 (24). To test whether phosphorylation of ING1b on this site affected its sumoylation, mutagenesis was performed to mutate serine 199 to glutamic acid (ING1 S199D) which should serve as a phosphomimic. As presented in Figure 4B, denaturing αHA IPs of cell lysates expressing ING1b WT or ING1b S199D and HA/SUMO1, showed that the S199D phosphomimic mutant showed a significant increase in ING1b SUMOylation, suggesting that phosphorylation of S199 promotes SUMOylation of K193. However, we saw no difference in ING1b SUMOylation when the serine was mutated to alanine as shown in Supplementary Figure S4A, available at Carcinogenesis Online.
Fig. 4.
Identification of a novel PDSM in ING1b. (A) Lysine 193 of the SUMO consensus is the second last amino acid within the NLS, however, the rest of the SUMO consensus and PDSM lies between the NLS and PHD domains of ING1b. (B) U2OS cell lysates expressing ING1b WT or ING1b S199D mutant and HA/SUMO1 were subjected to αHA IP followed by IB with αING1, αHA (SUMO1) and α-actin for detecting SUMO-modified ING1, protein expression and protein loading controls, respectively.
ING1 SUMOylation does not alter its subcellular localization
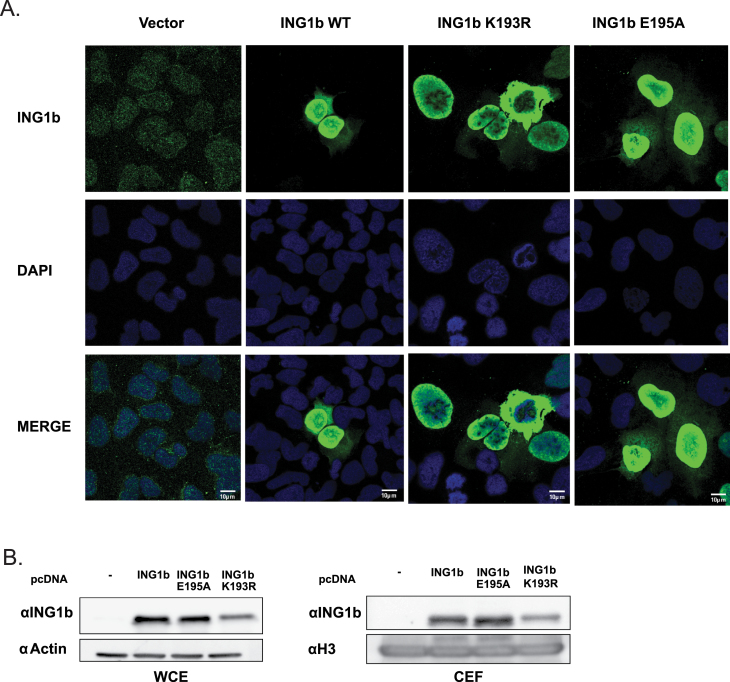
SUMOylation has been widely linked to protein relocalization. Attachment of a SUMO moiety can influence translocation or recruitment of proteins to different subcellular compartments or to macromolecular protein complexes. Consequently, sumoylation could alter the interaction of ING1b with transport machinery proteins such as 14-3-3η (24). To test whether ING1b SUMOylation affected its localization, we transfected cells with ING1b, ING1b K193R or ING1b E195A and performed indirect immunofluorescence. As shown in Figure 5A, ING1b WT and SUMOylation-deficient mutants ING1b K193R and ING1b E195A localized similarly in the nucleus, suggesting that there was no significant role for SUMOylation in subcellular relocalization under unstressed conditions. However, ING1b binds to chromatin and plays a role in apoptosis in response to exogenous stress (7,35,36). To ask if SUMOylation of ING1b affected chromatin binding, we performed experiments with ING1b WT, ING1b K193R and ING1b E195A. We purified a chromatin-enriched fraction from cells transfected with ING1b WT or the mutants and probed for chromatin bound ING1b. The E195A or K193R mutants did not appear to bind chromatin markedly differentially compared with ING1b WT as shown in Figure 5B.
Fig. 5.
SUMOylation does not alter ING1b localization. (A) U2OS cells transfected with control vector or either ING1b- or SUMO-deficient mutants (K193R or E195A) were stained with αING1 or DAPI to stain ING1b protein and DNA, respectively. Cells expressing ING1b, ING1b K193R and ING1b E195A were imaged with the same exposure time and for cells expressing control vector, exposure time was increased in order to detect endogenous ING1b. (B) The chromatin-enriched fractions (CEFs) or whole cell extracts (WCE) from U2OS cells transfected with empty vector, ING1b WT or SUMO-specific mutant (ING1b E195A) were subjected to IB using αING1 to detect chromatin bound ING1b and αH3 for establishing equal loading of CEFs. WCE was probed for αING1 and with actin to confirm equal protein expression and loading, respectively.
SUMOylation of ING1b regulates its recruitment to the ISG15 and DGCR8 promoters
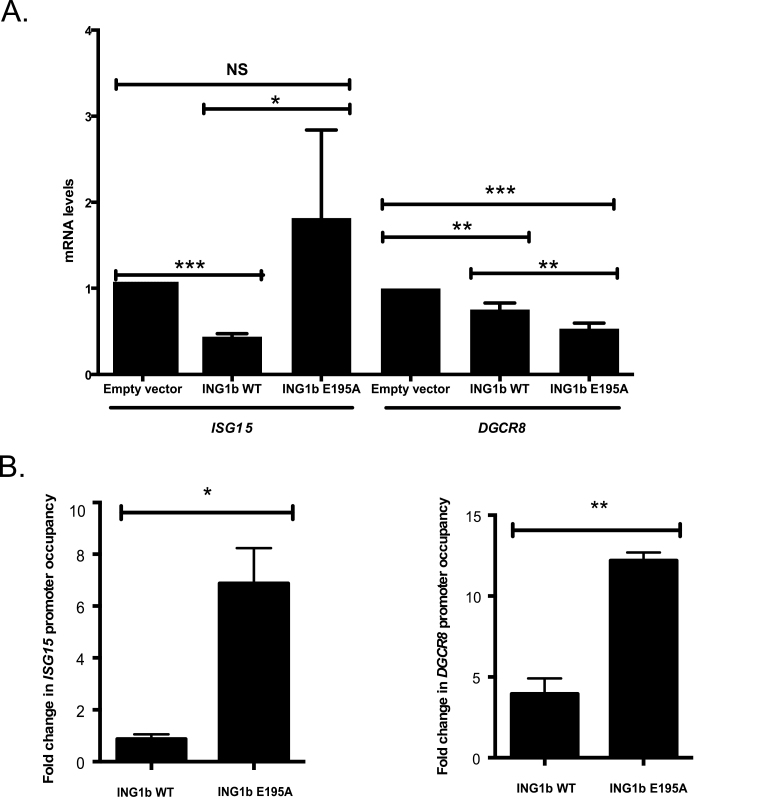
ING1 affects gene expression by regulating the acetylation status of core histones on the promoters of genes such as DGCR8, ITSN1 and mi204a among others (12,38,53). To test the role of ING1b SUMOylation on its ability to regulate transcription, we performed qPCR on 12 different ING1b target genes recently identified in a microarray screen (Chen,J., Tran,U. and Riabowol,K., unpublished data in preparation) and DGCR8, a previously identified ING1b target (12). Among the 13 genes examined (12 genes from microarray screen and DGCR8), we found that ING1b SUMOylation reproducibly affected the expression of only two genes, ISG15, which codes for the ubiquitin-like protein ISG15 and DGCR8, which codes for a protein involved in microRNA processing (39). As shown in Figure 6A, overexpression of wild-type ING1b repressed the expression of ISG15 and DGCR8. Interestingly, SUMOylation-resistant mutant ING1b E195A regulated their transcription differently. ING1b E195A did not repress ISG15 while it was more effective in DGCR8 repression. To further understand how ING1b SUMOylation could regulate ISG15 and DGCR8 transcription, we further analyzed ING1b binding on their promoters by ChIPs. Lysates from cells transfected with either an empty vector, ING1b WT or ING1b E195A were subjected to ChIP. As illustrated in Supplementary Figure S7, available at Carcinogenesis Online, IgG was used as a negative control. Our data showed that the SUMOylation-defective mutant, ING1b E195A, bound more avidly compared with the ING1b WT for both of these genes. However, ING1b E195A failed to repress ISG15 expression, whereas ING1b E195A repressed DGCR8 more efficiently. Given that ING1b is a stoichiometric member of Sin3a/HDAC complexes, we tested if SUMOylation influenced its interaction with this complex. As shown in Supplementary Figure S5, available at Carcinogenesis Online, unlike ING2, ING1b SUMOylation did not appear to affect its interaction with Sin3a. Collectively, our results indicate that SUMOylation of ING1b can have different roles based on which promoter it binds to. Our data are consistent with several reports, suggesting a role for SUMOylation in regulating gene expression (15,25,37,40).
Fig. 6.
ING1b SUMOylation affects ING1b binding to and modulates transcription of ISG15 and DGCR8. (A) ISG15 and DGCR8 mRNA expression was quantified by reverse transcription–qPCR in cells either expressing empty vector, ING1b WT or ING1b E195A. All the gene expression levels were normalized to GAPDH. Results represent differences in ISG15 and DGCR8 relative to ING1b expression ± SD. (*P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant). (B) Binding of ING1b on ISG15 and DGCR8 promoters was analyzed by ChIP. Mouse IgG was used as a negative control. Bar graphs represent fold changes in ING1b on ISG15 and DGCR8 promoter occupancy of cells transfected as in Figure 1A ± SD. (*P < 0.05; **P < 0.01; NS, not significant).
Discussion
The roles of the ING protein family in multiple cellular processes are being widely investigated by many groups. However, how ING proteins are themselves regulated has received very little attention. INGs have been classified as type II tumor suppressors because they are often downregulated or mislocalized, but not frequently mutated in cancers (41). High-throughput proteomic analyses have identified several different types of posttranslational modifications on all of the INGs (20) and understanding the significance of those modifications could substantially add to our understanding of how members of the ING family affect several biological processes. In this study, we report that SUMOylation of ING1b occurs at K193 and is mediated by the SUMO E2 conjugation enzyme Ubc9 and SUMO E3 ligase PIAS4. Our data also indicate that ING1b is modified by SUMO1. ING1b modified by SUMO1 migrates at 55 kDa, which is consistent with other studies reporting an electrophoretic shift of ~20 kDa (27) upon conjugation of one SUMO moiety.
ING1b contains at least six recognizable domains (8) and although the ING1b SUMO consensus lysine is the penultimate amino acid within the NLS, its SUMOylation did not significantly affect ING1b subcellular localization. This does not exclude the possibility of SUMO-mediated ING1b relocalization under stress conditions. We also identified a novel PDSM within ING1b. The PDSM was first identified as ΨKxExxSP in HSF1, HSF4b, GATA1 and MEF2A and was predicted to occur in many other proteins (34). In this study, we show that the sequence AK(193)AE(195)REAS(199)P in ING1b acts as a PDSM. We previously reported that S199 is phosphorylated and that this affects binding to 14-3-3η, regulating ING1b shuttling from the nucleus to the cytoplasm (24). However, under unstressed conditions, ING1b SUMOylation did not modify its subcellular localization. ING1b sumoylation might thus have little effect on its binding to 14-3-3η and consequently no effect on its localization. Interestingly, a PDSM is also conserved in ING2. Serine 201 within the ING2 PDSM is likely to be involved in crosstalk with ING2 SUMOylation at the K197 residue (21) and preliminary experiments suggests that this is also regulated in a similar manner to that of ING1b (data not shown). This may be relevant to the biological functions of ING1b and ING2 since they are both stoichiometric members of HDAC complexes (9).
ING1b interacts with SUMO E2 conjugation enzyme Ubc9 and SUMO E3 ligase PIAS3 and PIAS4. Ubc9 affected ING1b SUMOylation in a dose-dependent manner however, among the two interacting PIAS proteins, only PIAS4 mediated ING1b sumoylation. This is of particular interest since a role for PIAS4 was found in the DNA damage response (30). PIAS4 mediates SUMO1 conjugation to a variety of different DNA damage repair proteins unlike PIAS1, which mediates SUMO2/3 conjugation. These observations suggest that PIAS4 may have a selective function during the DNA damage response or other forms of genomic instabilities such as UV-mediated stress response or replication stress, in which ING1b has been implicated by numerous studies. For example, ING1b association with the proliferating cell nuclear antigen of DNA repair complexes increases by >10-fold in response to UV-induced DNA damage, and binding occurs through a proliferating cell nuclear antigen-interacting protein (PIP) motif found in ING1b (35). An ability to affect DNA repair in immortalized cells was also seen in an independent study (42). ING1b was also recently reported to affect genomic stability during replication (43) and thus PIAS4-mediated ING1b SUMOylation could be a mechanism for directing its function in response to DNA damage, particularly since proliferating cell nuclear antigen is also known to be SUMOylated at stalled replication forks in response to DNA damage (44).
In this study, we also found that PIAS3 and PIAS4 interact with ING1b. However, PIAS3 overexpression, in contrast to PIAS4 overexpression, decreased ING1b SUMOylation. When PIAS3 and 4 were coexpressed, PIAS3 inhibited ING1b SUMOylation mediated by PIAS4, suggesting that PIAS3 might act as a dominant negative, perhaps through blocking PIAS4 access to ING1b K193. The PIAS family of SUMO E3 ligases contains a SP-RING finger domain, a zinc finger motif that is closely related to the RING finger of ubiquitin E3 ligases. PHD finger motifs very closely resemble RING finger motifs and are also characterized by Cys3HisCys4 which coordinate Zn2+ and that are required for function (45). KAP1, a well-characterized transcription corepressor, was reported to act as a SUMO E3 ligase for its own SUMOylation, and this was mediated through its PHD finger (46), suggesting that this could also occur with the ING1b protein. However, experiments with a PHD 7CA mutant where seven conserved cysteines were mutated to alanine did not have any effect on SUMOylation (data not shown), supporting the idea that ING1b does not auto-SUMOylate and at least one SUMO E3 ligase is required to SUMOylate ING1b. Finally, although the PIAS E3 SUMO ligase family is arguably the best understood, there are many other E3 SUMO ligases known, leaving open the possibility that ING1b is also a substrate of other E3 SUMO ligases.
We also found that ING1b SUMOylation at K193 affects the regulation of ISG15 and DGCR8 transcription. First, we found that ING1b WT repressed ISG15. In addition, consistent with a previous study (12), we saw that ING1b WT overexpression mediated DGCR8 repression. Although ING1b E195A SUMOylation-resistant mutant repressed ISG15 twice as efficiently as WT, ING1b E195A acted differently on DGCR8 and repressed it more than ING1b WT. Although we see a strong enrichment of ING1b E195A on promoters of both these genes, we do not have a clear indication of how ING1b SUMOylation might affect promoters differently. Although we do not see a difference in their binding to Sin3a/HDAC complexes, we cannot rule out a promoter-specific or context-dependent interaction of SUMO-modified ING1b with mSin3a or other HDAC components such as in case of genomic instability, or UV-mediated stress response, or programmed cell death where ING1b has been implicated. Our data are consistent with ING1b repressing the expression of significantly more genes that it induces (47) and suggests that SUMOylation may define a subset of genes that are differentially sensitive to regulation by ING1b. Along with the role of SUMOylated ING1b in DGCR8 repression, we now uncover a new ING1b target gene, ISG15. Given that DGCR8 plays a vital role in microRNA biogenesis, ING1b SUMOylation may play an important role in the processing of microRNA and thereby regulation of multiple processes. The second gene that was significantly repressed by ING1b SUMOylation was ISG15. Interestingly, it is an ubiquitin-like modifier protein (48) induced by type 1 interferons (49). ISG15 is upregulated in a variety of different breast cancer cell lines and primary breast cancer tissues (50). This is particularly interesting because ING1b has been reported to be downregulated in 44% of primary breast cancers and in all the widely used breast cancer cell lines (2). This may underline the role of ING1b in repressing ISG15 expression and preventing tumorigenesis. It was also reported that ISG15 has antiviral activity towards different types of viruses (51,52). A role for ING1b has not been reported in these cellular processes but it opens the interesting possibility of the ING epigenetic regulators being involved in remodeling DNA in response to viral infection and the subsequent transient and longer term immune responses that are elicited.
Supplementary material
Supplementary Material and methods and Figures S1–S7 can be found at http://carcin.oxfordjournals.org/
Funding
Mobilité des doctorants étrangers à Rennes to S.S.; Doctoral fellowship from the French Ministry of Education and Research and from the FRM (Fondation pour la Recherche Médicale, FDT20130928015) to C.G.; Alberta Cancer Foundation graduate studentship to S.T.; Alberta Cancer Foundation (#22183) & the Canadian Institute of Health Research (#192320) to the laboratory of S.B.; Alberta Cancer Foundation, the Canadian Institutes of Health Research, Alberta Innovates Health Solutions and the Canadian Breast Cancer Foundation to the laboratory of K.R.; INSERM, La Ligue Contre le Cancer (Grand Ouest), Association pour la Recherchesur le Cancer (ARC), Rennes Métropole (AIS), ANR program (SAFE 2012, ANR-11-RPIB-0012) and Leucémie Espoir to the laboratory of R.P.
Supplementary Material
Acknowledgements
We thank Donna Boland and the Southern Alberta Antibody Facility for ING1 antibodies, K Shuai for PIAS1-4 plasmids, and Céline Monvoisin and Hélène Guyodo for technical support. We also thank the photonic facility of the Microscopy Rennes Imaging Center (MRic-Photonics) of SFR BIOSIT, Université de Rennes 1.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ChIP
chromatin immunoprecipitation
- HDAC
histone deacetylase
- IB
immunoblotting
- ING
INhibitor of Growth
- IP
immunoprecipitation
- NLS, nuclear localization signal; PHD
plant homeodomain
- PIAS
protein inhibitor of activated STAT
- PTM
posttranslational modification
- qPCR
quantitative PCR
- SDS
sodium dodecyl sulfate
- SUMO
small ubiquitin-like modification.
References
- 1. Garkavtsev I., et al. (1996). Suppression of the novel growth inhibitor p33ING1 promotes neoplastic transformation. Nat. Genet., 14, 415–420 [DOI] [PubMed] [Google Scholar]
- 2. Toyama T., et al. (1999). Suppression of ING1 expression in sporadic breast cancer. Oncogene, 18, 5187–5193 [DOI] [PubMed] [Google Scholar]
- 3. Nagashima M., et al. (2001). DNA damage-inducible gene p33ING2 negatively regulates cell proliferation through acetylation of p53. Proc. Natl Acad. Sci. USA, 98, 9671–9676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagashima M., et al. (2003). A novel PHD-finger motif protein, p47ING3, modulates p53-mediated transcription, cell cycle control, and apoptosis. Oncogene, 22, 343–350 [DOI] [PubMed] [Google Scholar]
- 5. Shiseki M., et al. (2003). p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity. Cancer Res., 63, 2373–2378 [PubMed] [Google Scholar]
- 6. Feng X., et al. (2002). Different HATS of the ING1 gene family. Trends Cell Biol., 12, 532–538 [DOI] [PubMed] [Google Scholar]
- 7. Peña P.V., et al. (2008). Histone H3K4me3 binding is required for the DNA repair and apoptotic activities of ING1 tumor suppressor. J. Mol. Biol., 380, 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He G.H., et al. (2005). Phylogenetic analysis of the ING family of PHD finger proteins. Mol. Biol. Evol., 22, 104–116 [DOI] [PubMed] [Google Scholar]
- 9. Doyon Y., et al. (2006). ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol. Cell, 21, 51–64 [DOI] [PubMed] [Google Scholar]
- 10. Kataoka H., et al. (2003). ING1 represses transcription by direct DNA binding and through effects on p53. Cancer Res., 63, 5785–5792 [PubMed] [Google Scholar]
- 11. Binda O., et al. (2008). SIRT1 negatively regulates HDAC1-dependent transcriptional repression by the RBP1 family of proteins. Oncogene, 27, 3384–3392 [DOI] [PubMed] [Google Scholar]
- 12. Gómez-Cabello D., et al. (2010). Regulation of the microRNA processor DGCR8 by the tumor suppressor ING1. Cancer Res., 70, 1866–1874 [DOI] [PubMed] [Google Scholar]
- 13. Han X., et al. (2008). Tethering by lamin A stabilizes and targets the ING1 tumour suppressor. Nat. Cell Biol., 10, 1333–1340 [DOI] [PubMed] [Google Scholar]
- 14. Bernier-Villamor V., et al. (2002). Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell, 108, 345–356 [DOI] [PubMed] [Google Scholar]
- 15. Yang S.H., et al. (2004). SUMO promotes HDAC-mediated transcriptional repression. Mol. Cell, 13, 611–617 [DOI] [PubMed] [Google Scholar]
- 16. Liu H.W., et al. (2012). Chromatin modification by SUMO-1 stimulates the promoters of translation machinery genes. Nucleic Acids Res., 40, 10172–10186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gómez-del Arco P., et al. (2005). Ikaros SUMOylation: switching out of repression. Mol. Cell. Biol., 25, 2688–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guérillon C., et al. (2013). ING1 and ING2: multifaceted tumor suppressor genes. Cell. Mol. Life Sci., 70, 3753–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu L., et al. (2013). Src regulates the activity of the ING1 tumor suppressor. PLoS ONE, 8, e60943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satpathy S., et al. (2013). RegulatING chromatin regulators: post-translational modification of the ING family of epigenetic regulators. Biochem. J., 450, 433–442 [DOI] [PubMed] [Google Scholar]
- 21. Ythier D., et al. (2010). Sumoylation of ING2 regulates the transcription mediated by Sin3A. Oncogene, 29, 5946–5956 [DOI] [PubMed] [Google Scholar]
- 22. Guo Q., et al. (2011). Citrullination of inhibitor of growth 4 (ING4) by peptidylarginine deminase 4 (PAD4) disrupts the interaction between ING4 and p53. J. Biol. Chem., 286, 17069–17078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garate M., et al. (2007). Phosphorylation of the tumor suppressor p33(ING1b) at Ser-126 influences its protein stability and proliferation of melanoma cells. FASEB J., 21, 3705–3716 [DOI] [PubMed] [Google Scholar]
- 24. Gong W., et al. (2006). Subcellular targeting of p33ING1b by phosphorylation-dependent 14-3-3 binding regulates p21WAF1 expression. Mol. Cell. Biol., 26, 2947–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsu Y.H., et al. (2006). Sumoylated SnoN represses transcription in a promoter-specific manner. J. Biol. Chem., 281, 33008–33018 [DOI] [PubMed] [Google Scholar]
- 26. Suzuki K., et al. (2011). Domain recognition of the ING1 tumor suppressor by a panel of monoclonal antibodies. Hybridoma (Larchmt)., 30, 239–245 [DOI] [PubMed] [Google Scholar]
- 27. Johnson E.S. (2004). Protein modification by SUMO. Annu. Rev. Biochem., 73, 355–382 [DOI] [PubMed] [Google Scholar]
- 28. Schwarz S.E., et al. (1998). The ubiquitin-like proteins SMT3 and SUMO-1 are conjugated by the UBC9 E2 enzyme. Proc. Natl Acad. Sci. USA, 95, 560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hickey C.M., et al. (2012). Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol., 13, 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galanty Y., et al. (2009). Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature, 462, 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo J., et al. (2009). The Caenorhabditis elegans ing-3 gene regulates ionizing radiation-induced germ-cell apoptosis in a p53-associated pathway. Genetics, 181, 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larrieu D., et al. (2009). ING2 controls the progression of DNA replication forks to maintain genome stability. EMBO Rep., 10, 1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bischof O., et al. (2006). The E3 SUMO ligase PIASy is a regulator of cellular senescence and apoptosis. Mol. Cell, 22, 783–794 [DOI] [PubMed] [Google Scholar]
- 34. Hietakangas V., et al. (2006). PDSM, a motif for phosphorylation-dependent SUMO modification. Proc. Natl Acad. Sci. USA, 103, 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scott M., et al. (2001). UV-induced binding of ING1 to PCNA regulates the induction of apoptosis. J. Cell. Sci., 114(Pt 19), 3455–3462 [DOI] [PubMed] [Google Scholar]
- 36. Vieyra D., et al. (2002). ING1 isoforms differentially affect apoptosis in a cell age-dependent manner. Cancer Res., 62, 4445–4452 [PubMed] [Google Scholar]
- 37. Rohira A.D., et al. (2013). Covalent small ubiquitin-like modifier (SUMO) modification of Maf1 protein controls RNA polymerase III-dependent transcription repression. J. Biol. Chem., 288, 19288–19295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rajarajacholan U.K., et al. (2013). The ING1a tumor suppressor regulates endocytosis to induce cellular senescence via the Rb-E2F pathway. PLoS Biol., 11, e1001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han J., et al. (2004). The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev., 18, 3016–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neyret-Kahn H., et al. (2013). Sumoylation at chromatin governs coordinated repression of a transcriptional program essential for cell growth and proliferation. Genome Res., 23, 1563–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ythier D., et al. (2008). The new tumor suppressor genes ING: genomic structure and status in cancer. Int. J. Cancer, 123, 1483–1490 [DOI] [PubMed] [Google Scholar]
- 42. Cheung K.J., Jr, et al. (2001). The tumor suppressor candidate p33(ING1) mediates repair of UV-damaged DNA. Cancer Res., 61, 4974–4977 [PubMed] [Google Scholar]
- 43. Wong R.P., et al. (2011). Tumour suppressor ING1b maintains genomic stability upon replication stress. Nucleic Acids Res., 39, 3632–3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gali H., et al. (2012). Role of SUMO modification of human PCNA at stalled replication fork. Nucleic Acids Res., 40, 6049–6059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bienz M. (2006). The PHD finger, a nuclear protein-interaction domain. Trends Biochem. Sci., 31, 35–40 [DOI] [PubMed] [Google Scholar]
- 46. Ivanov A.V., et al. (2007). PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol. Cell, 28, 823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feng X., et al. (2006). HSP70 induction by ING proteins sensitizes cells to tumor necrosis factor alpha receptor-mediated apoptosis. Mol. Cell. Biol., 26, 9244–9255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loeb K.R., et al. (1992). The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J. Biol. Chem., 267, 7806–7813 [PubMed] [Google Scholar]
- 49. Haas A.L., et al. (1987). Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J. Biol. Chem., 262, 11315–11323 [PubMed] [Google Scholar]
- 50. Bektas N., et al. (2008). The ubiquitin-like molecule interferon-stimulated gene 15 (ISG15) is a potential prognostic marker in human breast cancer. Breast Cancer Res., 10, R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Okumura A., et al. (2006). Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl Acad. Sci. USA, 103, 1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lenschow D.J., et al. (2005). Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo . J. Virol., 79, 13974–13983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen J., et al. (2013) ING1b-inducible microRNA203 inhibits cell proliferation. Br. J. Cancer, 108, 1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.