Summary
We show herein using both a short-term breast cancer (4T1) and long-term prostate cancer (Transgenic Adenocarcinoma of the Mouse Prostate) mouse model that a combination of a low carbohydrate, high protein diet with celecoxib substantially reduces metastasis.
Abstract
We recently demonstrated that both murine and human carcinomas grow significantly slower in mice on low carbohydrate (CHO), high protein diets than on isocaloric Western diets and that a further reduction in tumor growth rates occur when the low CHO diets are combined with the cyclooxygenase-2 inhibitor, celecoxib. Following upon these studies, we asked herein what effect low CHO, high protein diets, with or without celecoxib, might have on tumor metastasis. In the highly metastatic 4T1 mouse mammary tumor model, a 15% CHO, high protein diet supplemented with celecoxib (1 g/kg chow) markedly reduced lung metastases. Moreover, in longer-term studies using male Transgenic Adenocarcinoma of the Mouse Prostate mice, which are predisposed to metastatic prostate cancer, the 15% CHO diet, with and without celecoxib (0.3 g/kg chow), gave the lowest incidence of metastases, but a more moderate 25% CHO diet containing celecoxib led to the best survival. Metabolic studies with 4T1 tumors suggested that the low CHO, high protein diets may be forcing tumors to become dependent on amino acid catabolism for survival/growth. Taken together, our results suggest that a combination of a low CHO, high protein diet with celecoxib substantially reduces metastasis.
Introduction
Because rapidly growing tumors rely heavily on glycolysis, they may require higher than normal blood glucose (BG) levels to generate sufficient adenosine triphosphate (ATP) and macromolecules for growth and survival. In an attempt to exploit this, we recently designed low carbohydrate (CHO), high protein diets that were isocaloric with Western diets to see whether we could limit BG sufficiently to slow tumor growth. High protein was chosen rather than high fat because of the reported tumor-promoting effects of high fat (1,2) and the reported benefits of amino acid supplementation on immune (3,4) and BG homeostasis (5). We found that subcutaneously injected rapidly growing murine and human carcinomas grew significantly slower in mice on 15% CHO diets than on a Western diet, with little to no effect on mouse weights (6). Moreover, in long-term studies with these diets in mice genetically predisposed to breast cancer, almost half of the mice on a Western diet developed mammary tumors by 1 year of age, whereas none of the mice on the 15% CHO diet, which weighed on average 15% less at this age, developed cancer. Importantly, the low CHO, high protein diets did not induce ketosis, consistent with recent studies showing that ketosis requires high dietary fat (7). Thus, the reduced tumor growth rates and tumor incidence we observed (6) likely reflect reduced insulin and BG levels rather than direct inhibition by ketone bodies (8). Consistent with this, we found that tumor-bearing mice on these low CHO diets exhibited lower BG and lower blood insulin levels, an effect presumably mediated by the higher protein content of the low CHO diet, and also observed by Gunnerud et al in healthy human volunteers consuming high protein diets (5). Taken together, these results suggested that insulin and BG levels could be lowered sufficiently via diet alone (without caloric restriction) to significantly impact both tumor cell growth and tumor incidence.
We also found that these low CHO diets were additive with the cyclooxygenase (COX)-2 inhibitor, celecoxib (brand name, Celebrex) (6). Related to this, it has been shown that COX-2 is overexpressed in many human cancers and that COX-2 inhibitors may be beneficial in preventing/slowing colon, breast (9), and prostate cancers (10,11) by blocking both the metabolism of proinflammatory, omega 6 fatty acids (10) and tumor-induced angiogenesis (12).
These low CHO diets also lowered blood lactate levels, suggesting that these diets, by reducing BG levels, may be causing the primary tumor cells to switch to some extent from glycolysis to oxidative phosphorylation (OXPHOS). This is important because glycolysis leads to the secretion of lactic acid and this can lower the local extracellular pH, from 7.4 to 6.0 within a poorly perfused tumor. This in turn promotes metastasis by inducing normal cell death, angiogenesis, extracellular matrix degradation, and inhibition of tumor antigen-specific immune responses (13).
Because metastasis is the primary cause of cancer-related mortality, we asked herein if low CHO diets, with or without celecoxib, could significantly reduce the metastatic potential of primary tumors. Similar to our previous studies, we wanted to test the effect of low CHO, high protein diets on both injected and spontaneously arising models of cancer. To do so, we used the 4T1 mammary carcinoma and the TRAMP (Transgenic Adenocarcinoma of the Mouse Prostate) mouse as our short- and long-term models, respectively. The 4T1 mammary carcinoma model is a well-established model of metastatic breast cancer that resembles the histopathology and natural history of triple-negative human breast cancer by spontaneously metastasizing to the lung, liver, bone, and brain via the bloodstream (14). The TRAMP model recapitulates the pathological stages of human prostate cancer, at the onset of puberty, through the expression of the SV40 T antigen under the control of an androgen-responsive prostate-specific promoter (14,15) Our results suggest that low CHO, high protein diets coupled with celecoxib indeed markedly lower the levels of metastasis.
Materials and methods
Mice and tumor cell injections
Mice were housed in the Animal Resource Centre of the British Columbia Cancer Research Centre under specific pathogen-free conditions and according to approved and ethical treatment of animal standards of the University of British Columbia. Animals were euthanized by CO2 asphyxiation. Nine- to 10-week-old BALB/c mice, from Simonsen Laboratories (Gilroy, CA), were housed three or four mice/cage in high-top Allentown cages on static racks, with twice/week bedding changes. Unless otherwise stated, 105 4T1 mouse mammary tumor cells (American Type Culture Collection) in 50 μl phosphate-buffered saline were implanted orthotopically into the mammary fat pads of female BALB/c mice. These cells were cultured in vitro in RPMI + 10% fetal calf serum + N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (2.38g/l) + d-glucose (2.5g/l) + 0.05% 1 N HCl + 1 mM sodium pyruvate. The mice were fed 5053 chow (TestDiet, St Louis, MO) from weaning until tumor cell injections. Immediately after 4T1 cell injections, the mice were put on the different diets (typically 6–8 mice/diet). Tumors were measured 2–3 times/week using manual calipers, and their volumes determined using the formula: (Length × Width × Height) × π/6.
Measurement of 4T1 lung metastases
Lungs from each of the six female BALB/c mice/diet were finely minced with crossed scalpels before agitation for 40min at 37°C with 5 ml of 0.5% trypsin and 0.08% collagenase in phosphate-buffered saline. DNase (0.06%) was then added and the cell suspension gently vortexed and filtered through 40-µm nylon mesh. Cell suspensions were treated with NH4Cl to lyse erythrocytes. For clonogenic assays, cells derived from lung tissue were washed in phosphate-buffered saline, resuspended in RPMI 1640 medium, and aliquots of 3 × 103 to 106 cells plated in triplicate in tissue culture plates in 10 ml RPMI 1640 + 10% fetal calf serum + 60 μM 6-thioguanine (to select for 4T1 cells). After 10 days at 37°C, the medium was removed and the cells stained with malachite green for 10min. The plates were then rinsed 2–3 times with 4°C distilled water and left to dry overnight. Macroscopic green colonies (≥50 cells) were counted.
For histology, lungs from six replicate mice were formalin fixed, paraffin-embedded and then H&E-stained step (100 μm steps) sections were examined for metastatic foci.
TRAMP model
Heterozygous TRAMP male mice from Jackson Laboratories (Bar Harbor, MN) were housed with wild-type C57B/6 mice (8/cage) in a rat cage enriched with an exercise wheel and given water and food ad libitum. Mice were fed 5053 diet before changing to their target diets at 8 weeks of age. Weights were measured weekly, and the development of abdominal distension was monitored daily. TRAMP mice were euthanized when abdominal distension impeded movement and was firm upon palpation (assessed by a person unfamiliar with the different mouse groups, i.e. in a blind fashion). Mortality as a result of abdominal distension was classified as tumor-related mortality. Animals were examined, postmortem, for lung and liver metastases. Visible nodules on either organ were scored as a metastatic event.
Measurement of BG, lactate and insulin
BG was measured via tail vein using a OneTouch Ultraglucose meter and LifeScan test strips. Insulin and lactate levels were determined by enzyme-linked immunosorbent assay (Mercodia, Uppsala, Sweden, #10-1247-01) and Lactate Assay Kits (BioVision, Mountain View, CA, #K607-100), respectively, using plasma from CO2-euthanized mice.
Measurement of primary tumor metabolites
4T1 tumors were excised and flash frozen on dry ice immediately after mice were euthanized. Tumors were kept at -80°C before gas chromatography–mass spectrometry analysis. Equal amounts of tumor tissue were taken for analysis, as per Ref. (16) to obtain tumor metabolite levels.
Measurement of plasma celecoxib levels
Frozen plasma samples were thawed and 10 µl transferred to individual Eppendorf tubes. Internal standard, 4 µl of 0.5 µM enzalutamide was added followed by 4 µl methanol (MeOH) and 22 µl of acetonitrile. Samples were vortexed 5–10 s and centrifuged for 5 min at 20 000g to sediment precipitated protein. The supernatant was transferred to LC vials for analysis. Standards were prepared in a similar fashion using mouse plasma from mice on celecoxib-free chows and also in parallel using 50% methanol to characterize any matrix effects. Optima grade (Fisher) solvents and 18 MΩ water (Millipore) were used for sample preparation and subsequent liquid chromatography–mass spectrometry analysis.
Analysis was performed with an Acquity UPLC coupled to a Quattro Premier (Waters). A 100-mm BEH C18, 1.7 µ column (Waters) was used for separations with a 60–100% acetonitrile gradient from 0.2 to 2 min (0.3 ml/min) followed by a 1-min acetonitrile flush and a 2-min re-equilibration for a 5-min run length (0.1% formic acid present throughout). All mass spectrometry data were collected in ES+ at unit resolution with the following instrument parameters: capillary, 3.5 kV; extractor and RF lens, 5 and 0.1 V; source and desolvation temperatures, 120 and 350ºC; desolvation and cone (N2) flow, 900 and 50 l/h; collision gas (Ar) flow, 0.15 ml/min (7.3 e- 3 m bar). Compounds were detected using multiple reaction monitoring with m/z 382.2 > 362.2 for celecoxib and m/z 465.3 > 209.1 for enzalutamide (40 V/27 V cone/collision volt combinations for both) with 0.1 s dwell each. Retention times for celecoxib and enzalutamide were 1.85 min and 1.5 min, respectively.
Quanlynx (Waters) was used for analysis of data using celecoxib/enzalutamide AUC ratio for quantification. Calibration standards ranged from 0.004 to 8 µM (6 points, serum equivalent level) with R 2 > 0.99 with all % deviation from nominal <15%. Comparisons with spiked plasma using neat standards indicated suppression of about 10% and extraction efficiencies of >85%.
Reagents
All the diets were isocaloric, given ad libitum and prepared by TestDiet (St Louis, MO) (see Supplementary Table 1, available at Carcinogenesis Online, for details). Celecoxib (Pfizer) was formulated into the chows by TestDiet. All other reagents were from Sigma Chemical Co (St Louis, MO), unless otherwise stated.
Statistical analyses
GraphPad Prism (GraphPad Software) was used for statistical analyses. In brief, differences in tumor sizes and metastases were tested for statistical significance using a one-tailed t-test. Log-rank and Fisher’s exact were also used in determining differences in cancer-induced mortality rates and metastatic incidence among diet groups for the TRAMP study.
Results
4T1 primary tumors grow slower in mice treated with celecoxib
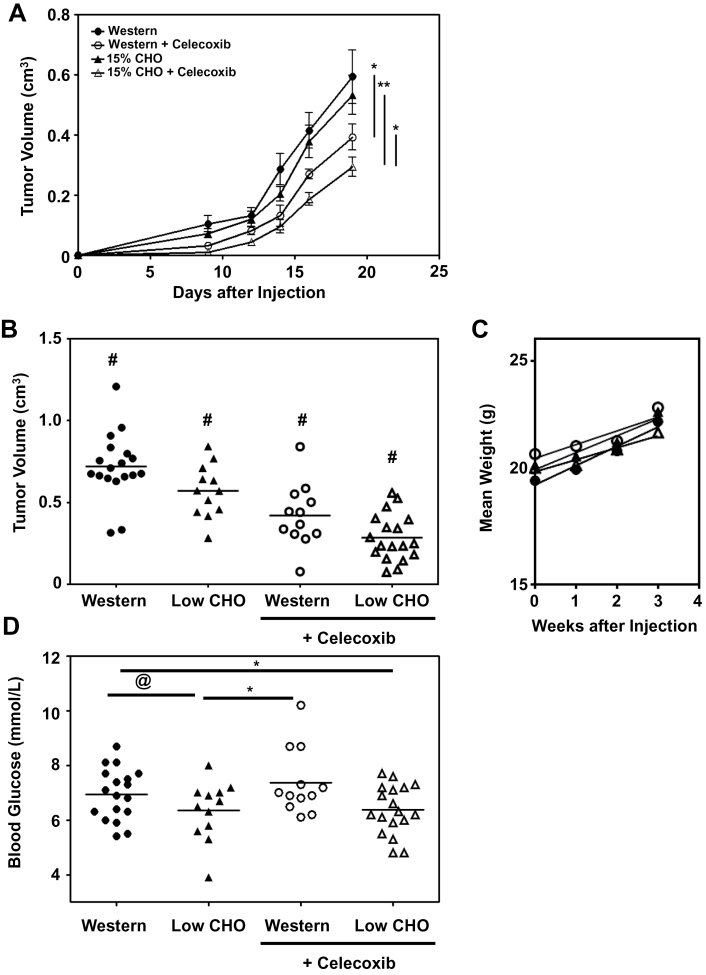
We first compared the primary tumor growth rate of orthotopically injected 4T1 tumor cells into 9- to 10-week-old female BALB/c mice fed a Western versus a 15% CHO, high protein diet (Figure 1A). Although the 15% CHO chow slightly reduced the growth rate of these primary tumors, the difference was not statistically significant and not as pronounced as that obtained in our previous studies with male mice (6). This is consistent with our finding that female mice do not lower their BG as markedly as male mice on low CHO diets (6), and the reported BG buffering effects of estrogen (17). However, the addition of 1 g of celecoxib/kg chow significantly (P < 0.05) reduced the growth rate of 4T1 primary tumors on both diets (Figure 1A), and the combination of celecoxib with the low CHO diet resulted in the lowest primary tumor growth rate, which was significantly (P < 0.01) slower than the Western diet with celecoxib. We next examined pooled data from three independent studies and compared primary tumor sizes of mice on the four different diets after 3 weeks. With an increased sample size, we found that the 4T1 primary tumors grew statistically slower (P < 0.05) in the 15% CHO group than in the Western group and the addition of celecoxib further reduced tumor growth rates (Figure 1B). Of note, there was no significant difference in the weights of these mice over the course of the study (Figure 1C), thus ruling out that differences in tumor growth rates were due to caloric restriction. This is important because caloric restriction, which causes cancer cells to switch, via AMPK activation, to OXPHOS to generate more ATP for survival (18), has been shown to slow tumor growth (19). As reported earlier for male mice, mean BG levels were higher in the Western diet group than the low CHO group (Figure 1D), although, as expected, this was less pronounced than that seen with male mice (6). The addition of celecoxib to the chows had no effect on BG levels (Figure 1D).
Fig. 1.
Low CHO, high protein diets containing celecoxib reduce the growth rate of primary 4T1 Tumors. (A) Growth curve (mean tumor volume ± SEM) of injected 4T1 tumors in BALB/c mice (6 mice/group) on Western or 15% CHO chows (± 0.1% wt/wt celecoxib in the chow). (B) Size of 4T1 tumors 3 weeks postimplantation. Pooled results of three independent studies (mean indicated by line) totaling 18 mice on the Western, 12 on the 15% CHO, 12 on the Western + 0.1% celecoxib and 18 on the 15% CHO + 0.1% celecoxib chow. (C) Mean weights of 4T1 tumor-bearing mice shown in panel (A). (D) Non-fasting BG readings of ad libitum-fed mice. Pooled results from three independent studies. *P ≤ 0.05 in a t-test comparing the two indicated groups; **P ≤ 0.01 in a t-test comparing the two indicated groups; # P ≤ 0.05 in a t-test against any other group; @ P ≤ 0.06 in a t-test comparing the two indicated groups.
4T1 lung metastases are markedly reduced when mice are fed 15% CHO diets containing celecoxib
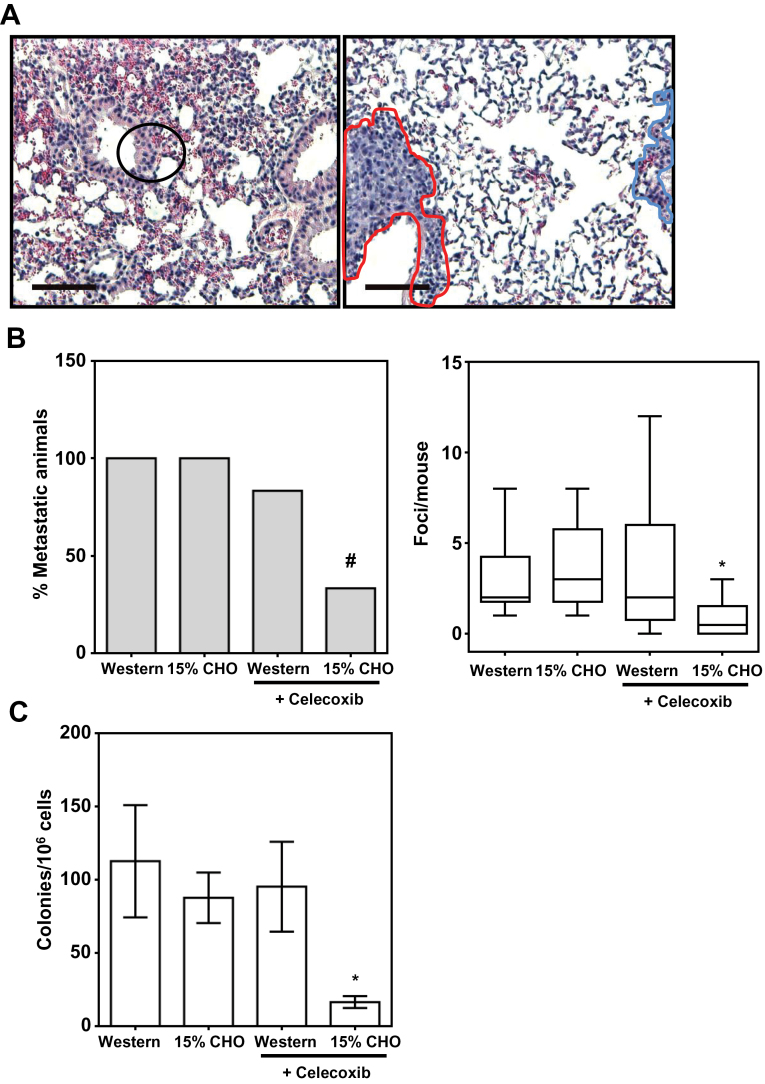
To assess the ability of the 4T1 primary tumors to metastasize, we sectioned formalin-fixed lungs from tumor-bearing mice and quantitated metastatic foci after H&E staining. Normal lung cells were distinguishable from metastatic 4T1 cells by their smaller size, orderly tissue structure, and cytoplasmic eosin staining (Figure 2A, left panel). Metastatic 4T1 tumor cells, on the other hand, exhibited a disordered, hyperplastic tissue morphology and a characteristic hematoxylin staining of both the cytoplasm and nuclei (Figure 2A, right panel).
Fig. 2.
Low CHO, high protein diets containing celecoxib reduce 4T1 breast cancer metastasis. (A) Representative H&E-stained images of normal lung tissue (left panel) and a metastatic focus (right panel) from 4T1 tumor-bearing BALB/c mice at ×100 magnification. A metastatic focus is demarcated with a red outline in contrast to normal tissue on the same slide (blue outline). (B) Pulmonary metastases of 4T1 primary tumors quantified by counting metastatic foci in H&E-stained slides represented in (A) from 1 lung of each of six mice/diet group. (C) Clonogenic assay results showing the number of colony-forming 4T1 tumor cells in the lungs from six replicate mice (mean ± SD).
*P ≤ 0.05 in a t-test against any other group; # P ≤ 0.05 in a Fisher’s exact test compared with the Western group.
We found that although the 15% CHO diet alone did not have a significant impact on metastasis, the addition of celecoxib (1g/kg chow) to the low CHO chow markedly reduced the number of animals that developed metastases from 100 to 33% (P < 0.05 in a Fisher’s exact test) (Figure 2B, left panel), the number of metastatic foci/mouse from 3.00 to 0.83 (Figure 2B, right panel), and the number of total metastatic foci (pooled by treatment group) by 72% (data not shown). In contrast, the addition of celecoxib to the Western diet only caused a modest reduction in the number of animals that developed metastases and had an insignificant effect on the number of metastatic foci/mouse or the total number of metastatic foci (Figure 2B, left and right panels, and data not shown).
Interestingly, the combination of the 15% CHO chow with celecoxib reduced by more than 80% the number of colony-forming pulmonary metastatic clones, in agreement with the H&E staining results (Figure 2C). In contrast, the number of colony-forming metastatic cells was not significantly reduced by adding celecoxib to the Western diet or by feeding the mice the 15% CHO alone. These results suggest that celecoxib in combination with low CHO diets targets metastatic progenitor cells.
Because celecoxib had such a profound effect at reducing metastasis in mice on a 15% CHO but not a Western diet, it was important to determine whether this was simply because the different diets affected the levels of celecoxib in the plasma of these mice. We therefore tested the plasma levels of celecoxib in BalbC mice fed Western + celecoxib (1g/kg) versus 15% CHO + celecoxib (1 g/kg) chow. As shown in Supplementary Figure 1A, available at Carcinogenesis Online, there was no statistically significant difference in their celecoxib levels.
Metastatic incidents and cancer-related mortality in TRAMP mice are markedly delayed with low CHO diets containing celecoxib
Having demonstrated that a low CHO diet containing celecoxib was capable of reducing the metastasis of primary 4T1 mammary tumors in short-term studies, we asked what effects these same diets would have, long term, on the incidence and spread of a spontaneous model of metastatic cancer. For this, we used male TRAMP mice which are genetically predisposed to spontaneously developing metastatic prostate cancer (15). Because male mice exhibit a more marked reduction in BG levels in response to low CHO diets than female mice (6,17), we not only compared the effects of the Western and 15% CHO diets used in our short-term 4T1 cell studies, but added a 25% CHO diet (± celecoxib) (Supplementary Table 1, available at Carcinogenesis Online) to assess the efficacy of this more moderate diet. As well, given that high celecoxib levels can lead to cardiovascular events (20), we used 0.03% rather than 0.1% celecoxib in the chows for these long-term TRAMP studies.
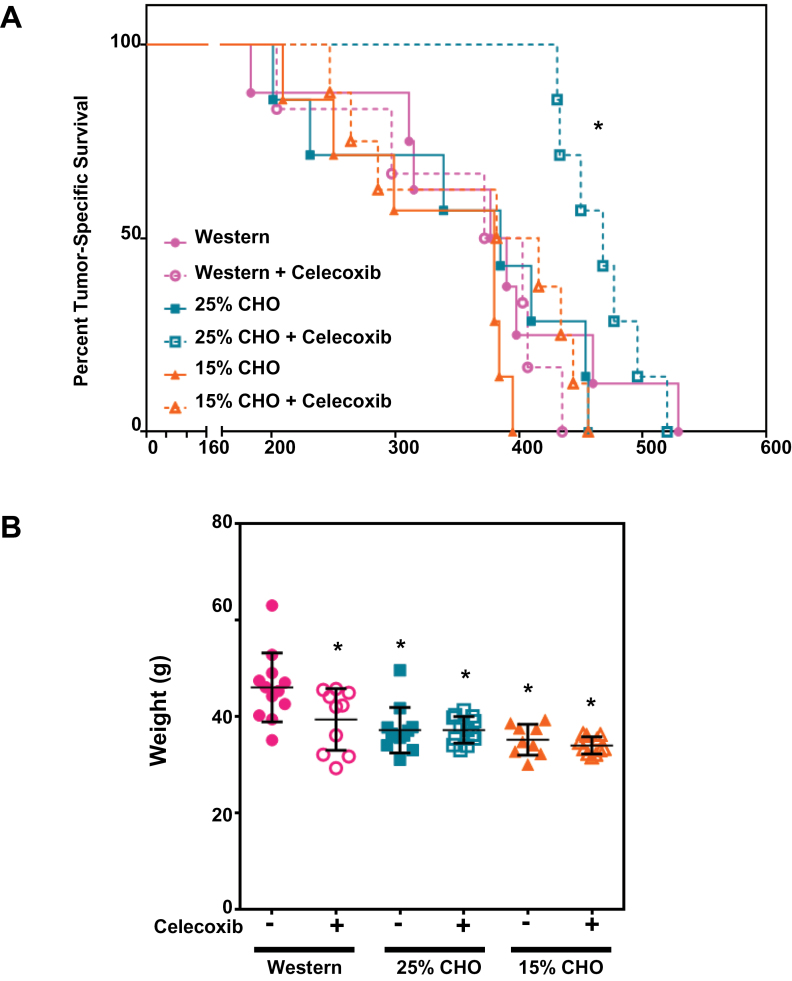
We first examined cancer-related mortality, defined herein as severe abdominal distension requiring euthanasia and deaths associated with metastatic events. We found that the combination of the 25% CHO diet with +0.03% celecoxib markedly reduced cancer-related mortality when measured up to 420 days on the diets (P < 0.05 for both the log-rank and Fisher’s exact tests) from 75% in the Western diet to 0% in the 25% CHO + celecoxib diet (Figure 3A). Nonetheless, the mice on the 25% CHO + celecoxib diet eventually succumbed but there was a significant delay (Gehan Breslow Wilcoxon P < 0.05) in their cancer-related mortality compared with that of the Western diet group. Of interest, all the mice eventually died of cancer-related causes except two of the eight mice in the 15% CHO group, which died without abdominal distension or evidence of metastasis. This may suggest that severe CHO restriction may be deleterious to long-term health.
Fig. 3.
Low CHO, high protein diets containing celecoxib reduce prostate cancer progression in the TRAMP mouse model. (A) Cancer-related mortality rates of TRAMP mice on Western, 25 and 15% CHO diets (±0.03% wt/wt celecoxib) with time on the various diets (in days). (B) Weights of mice after 47 weeks on the various diets. *P ≤ 0.05 in a Fisher’s Exact or log rank tests against the Western diet group for (A) and t-tests against the Western diet for (B).
Similar to the 4T1 study, we compared the plasma levels of celecoxib in the TRAMP mice on the three different celecoxib-containing chows and found no statistically significant difference in their levels (Supplementary Figure 1B, available at Carcinogenesis Online).
We also examined the long-term impact of these diets on mouse weights and found that Western diet–fed mice were significantly (P < 0.05) heavier than mice in any other diet group (Figure 3B).
To rule out that the 25% CHO + celecoxib diet was reducing tumor-related deaths by simply improving the overall health of these mice, we put wild-type C57B/6 male littermates on a Western versus 25% CHO + celecoxib diet. As shown in Supplementary Figure 2, available at Carcinogenesis Online, there was no discernible difference in the overall health or mortality of these mice for up to 2 years of age, at which time they were euthanized.
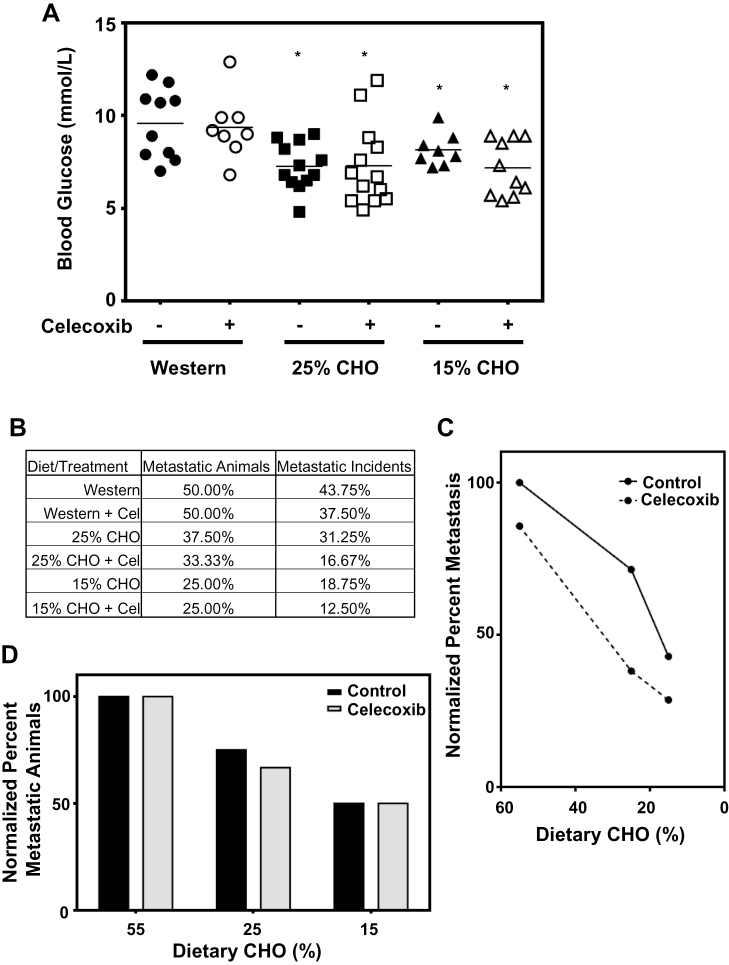
As with the female 4T1 mouse model, the male TRAMP mice had lower BG levels on the 15% CHO diet than on the Western diet, and the addition of celecoxib had no significant effect on BG levels (Figure 4A). However, the drop in BG levels in these male mice was far more pronounced, with a mean average reduction of 2.5mM, compared with 0.6mM in the female 4T1 mice. Interestingly, mice on the 25% CHO diets, with or without celecoxib, had BG levels similar to those on the 15% CHO diet, suggesting this more moderate diet may be as effective at lowering BG levels, at least in these mice. Importantly, although it was difficult to assess primary tumor sizes with this TRAMP model, the low CHO diets significantly reduced the number of metastatic animals (i.e. animals that bore either lung or liver tumor nodules), with the 15% diet being most effective. Interestingly, although the number of absolute metastatic incidents (i.e. the presence (+ or -) of tumor nodules in either the lungs or the liver) was also reduced with the low CHO diets, this reduction was far greater with celecoxib (assessed using a Fisher’s exact test of pooled data) (Figure 4B). Specifically, although the addition of celecoxib to any of the diets did not reduce the number of metastatic animals, it had an additive effect with the low CHO diets in reducing metastatic incidents (Figure 4B). Also of note is a proportional relationship between dietary CHO levels and metastatic incidents, with the 25% CHO diet and the 15% CHO diet reducing metastatic incidents by more than 30 and 50%, respectively, compared to mice fed a Western diet (Figure 4C). The 25% CHO diet with celecoxib and the 15% CHO diet with celecoxib reduced metastatic incidents by 60 and 70%, respectively, compared with the Western-fed mice (Figure 4C). A similar dose–dependent reduction in the number of metastasis-bearing mice was observed with the low CHO diets alone, but, unlike our results with metastatic incidents, celecoxib did not further reduce the number of mice that developed metastases (Figure 4D).
Fig. 4.
Metastasis of prostate cancer in the TRAMP mouse model is reduced with low CHO, high protein diets containing celecoxib. (A) Non-fasting BG readings of ad libitum-fed mice. (B) Percent of mice in each diet group that develop metastases, and the percent of potential metastatic sites that develop metastasis (2 incidents/mouse, i.e.—in the liver and in the lungs) in each group. (C) Metastatic incidence, normalized to that of the Western diet group as a function of dietary CHO content (± 0.03% wt/wt celecoxib). (D) Number of mice on the low CHO diets that develop metastases, normalized to the Western diet group (± 0.03% wt/wt celecoxib).
Low CHO diets and celecoxib alter 4T1 primary tumor metabolism
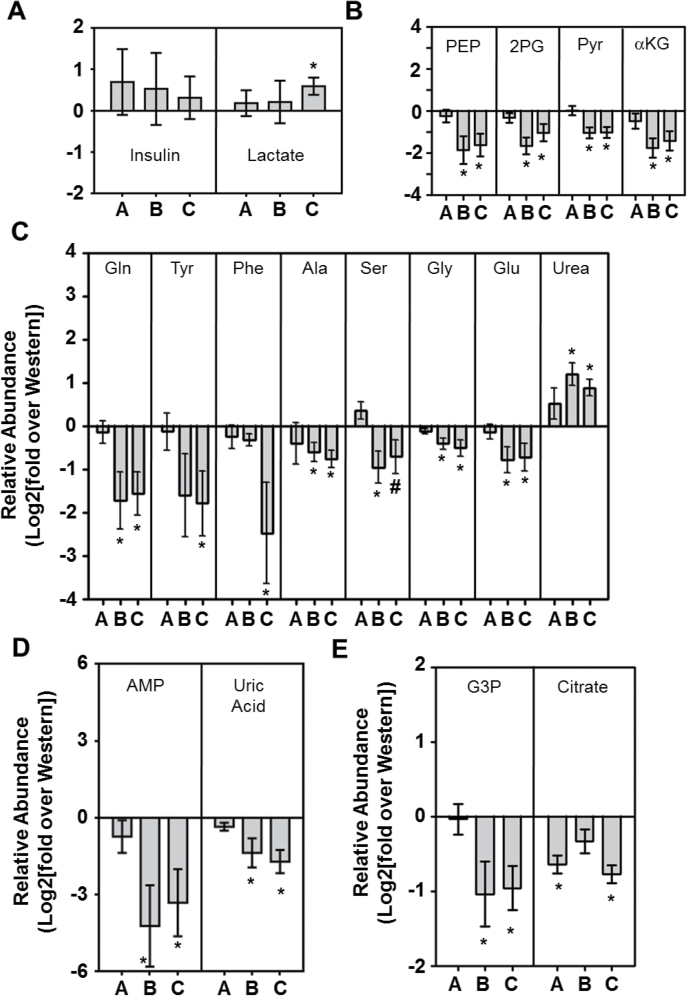
To gain some insight into the mechanisms responsible for the lower levels of metastases in mice on low CHO diets, with and without celecoxib, we monitored insulin and various metabolite levels in our 4T1 tumor-bearing BALB/c mice (Figure 5). All the values shown are expressed relative to those obtained with mice on a Western diet, which have been given a value of zero in these log plots. Interestingly, mice on the 15% CHO diet, particularly with celecoxib, had slightly increased, albeit not statistically significant, insulin levels (Figure 5A, left panel). We also assayed blood lactate levels to gain some insight into whether the 15% CHO diet, with or without celecoxib, was skewing glucose metabolism away from glycolysis toward OXPHOS. However, unlike in previous studies with squamous cell carcinoma VII cells injected into male C3H mice, we did not see a drop in blood lactate and, in fact, low CHO + celecoxib may have caused a slight increase (Figure 5A, right panel).
Fig. 5.
Primary 4T1 tumor metabolite analysis. (A) Relative levels of insulin and lactate in the plasma of 4T1-tumor bearing BALB/c mice fed a Western diet + 0.1% (wt/wt) celecoxib (A), a 15% CHO diet (B) or a 15% CHO + 0.1% (wt/wt) celecoxib diet (C), expressed as a ratio over levels detected in the plasma of Western diet-fed 4T1 tumor-bearing mice [Log 2 of (mean of experimental group/mean of Western diet group) ± SD of 6 individual mice/group]. (B) Relative abundance of glycolysis and TCA cycle intermediates within the tumors. (C) Relative abundance of amino acids within the tumors. (D) Nucleic acid synthesis intermediates within the tumors and (E) lipid synthesis intermediates within the tumors, as assessed by GC-MS. Levels are expressed as a ratio over levels detected in tumors in Western diet-fed mice. All GC-MS data are expressed as Log 2 of (mean of experimental group/mean of Western diet group) ± SEM of triplicate determinations from six independent mice.*P ≤ 0.05 in a t-test against the Western diet group; # P ≤ 0.06 in a t-test against the Western diet group. GC-MS, gas chromatography–mass spectrometry.
A more thorough examination of glycolysis, using gas chromatography and mass spectroscopy, revealed that at 3 weeks postimplantation, the glycolytic intermediates phosphoenol pyruvate, 2-phosphoglycerate, and pyruvate were all lowered in the tumors of low CHO-fed mice (Figure 5B). Downstream of glycolysis, we found that levels of α-ketoglutarate were similarly lowered, whereas those of other TCA cycle intermediates examined (i.e. succinate, fumarate, malate) did not change with the different diet groups (Figure 5B and data not shown).
Next, we analyzed protein, nucleic acid and lipid building blocks, because of their requirement for rapid cell division, and because they would likely be affected by diet changes (21–23). As shown in Figure 5C, a number of amino acids were lower in tumors from mice fed a 15% CHO diet. Specifically, glutamine, tyrosine, alanine, serine, glycine and glutamic acid were significantly lower in tumors within mice fed the 15% CHO diet, with or without celecoxib, whereas, in contrast, urea levels were increased (Figure 5C). Celecoxib treatment, on the other hand, did not alter the levels of the above-mentioned amino acids or urea but did profoundly reduce phenylalanine levels (Figure 5C). In terms of nucleic acid metabolism, we found that adenosine monophosphate was significantly decreased with low CHO feeding, as was uric acid, a byproduct and indicator of nucleotide turnover (Figure 5D).
We also examined the lipid synthesis precursors glycerol-3-phosphate and citrate and found that glycerol-3-phosphate was lower on a 15% CHO diet, with or without celecoxib, than on a Western diet (with or without celecoxib) (Figure 5E). In contrast, intratumoral citrate levels in mice fed a 15% CHO diet alone were not significantly lower than in mice fed a Western diet but celecoxib treatment alone (i.e. added to the Western diet) or added to the 15% CHO chow significantly decreased it (Figure 5E). Thus, of the metabolites examined, citrate was the only one affected by celecoxib alone. Taken together, our data suggest that mice given 15% CHO diets significantly affect metabolic intermediates in 4T1 primary tumors.
Discussion
Over 80 years ago, Otto Warburg found that rapidly dividing cancer cells, unlike normal cells, rely more heavily on glycolysis than OXPHOS, even under normoxic conditions, to meet their metabolic needs (24). This is likely because glycolysis does not catabolize glucose completely to CO2 for ATP but instead uses the carbon skeletons as building blocks for nucleic acid (i.e. ribose) and protein (i.e. alanine, etc.) synthesis, which are essential for cell proliferation (25). However, because glycolysis is far less efficient at generating ATP, most cancer cells require higher levels of glucose than normal cells to proliferate and survive. Therefore, as long as cancer cells can obtain high levels of glucose, a high glycolytic rate provides both sufficient ATP and glucose-derived carbons, even under hypoxic conditions, for tumor cell survival and proliferation (26). In earlier studies, we demonstrated that we could lower BG, insulin and lactate levels with isocaloric low CHO, high protein diets and that these diets both slowed tumor growth and reduced cancer incidence and this effect was additive with the COX-2 inhibitor, celecoxib (6).
A major novel finding in the current work is that the combination of these low CHO diets with celecoxib markedly reduces metastatic progression in both injected and spontaneous cancer models. Specifically, low CHO diets containing celecoxib reduce both pulmonary metastatic foci and, significantly, the number of colony-forming 4T1 cells in the lungs. The latter result suggests that low CHO diets may act synergistically with celecoxib to restrain the generation of colony-forming metastatic cells. Moreover, the TRAMP mouse results suggest that low CHO diets containing celecoxib are also beneficial in models that recapitulate human cancer progression. Because these are two independent models of cancer with different etiologies, these results suggest that the antimetastasis effect of low CHO + celecoxib may be broadly applicable.
The effectiveness of the moderate (25%) CHO diet also suggests that a less extreme level of CHO restriction can achieve beneficial results on both BG and metastasis. Thus, extremely low CHO interventions (i.e. ketogenic or 10–15% CHO diets) may not be required to achieve an antitumor effect, making this approach a more realistic option for clinical application. The data also suggest that moderate, low CHO diets may be superior to lower CHO diets in terms of overall health, at least in the TRAMP model. In fact, the unexpected deterioration of the TRAMP mice on the 15% diet cautions that long-term, severe CHO restriction may be deleterious, despite its efficacy in limiting metastasis.
Celecoxib is a COX-2 inhibitor that inhibits the prostaglandin synthesis pathway and slows cancer progression, at least in part by reducing the immunosuppressive and angiogenic properties of PGE2 (27–29). In addition to these properties, PGE2 can directly stimulate cancer cell survival/metastasis pathways (i.e. PI3K, Ras, Wnt/β-catenin) by acting directly on cancer cells, or indirectly via aromatase in breast cancer (30,31). Our findings suggest that celecoxib may also have an impact on tumor metabolism, and may be limiting tumor growth, in part, by reducing intracellular citrate. Although low CHO and celecoxib additively slowed primary 4T1 tumor growth, neither alone was sufficient to limit metastasis. Similarly, in the TRAMP model, both low CHO and celecoxib lowered metastatic incidents, but only the combination of the two markedly slowed the onset of cancer-induced mortality.
CHO reduction alters primary tumor metabolism
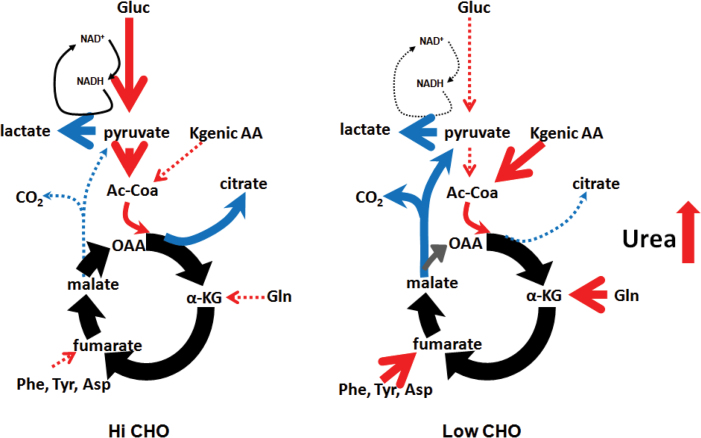
We demonstrate herein that low CHO diets decrease intratumoral amino acid and increase urea levels, indicating an increase in amino acid catabolism. This suggests that tumors may be switching from glucose to amino acids as a carbon source (i.e. for biosynthesis and ATP generation; Figure 6). Furthermore, intratumoral adenosine monophosphate and uric acid levels—substrate and byproduct, respectively, of nucleotide metabolism—are also lower in low CHO-fed mice, suggesting that amino acid carbon skeleton catabolism depletes multiple substrates needed for tumor nucleotide synthesis.
Fig. 6.
Simplified model of tumor metabolism in mice fed high versus low CHO diets. Low CHO diets promote amino acid catabolism and reduced citrate efflux from the Krebs cycle for fatty acid synthesis. Under low CHO conditions, the malic enzyme shunt maintains lactate generation.
Thus, the metabolic data presented herein suggest that low CHO feeding causes a metabolic shift from glucose catabolism (reducing biosynthesis of lipids, proteins and nucleic acids) to utilization of amino acid carbon skeletons and an increase in byproducts of this catabolism (e.g. urea; see Figure 6). We also demonstrate that celecoxib alone can reduce intratumoral citrate levels—a required intermediate for the de novo synthesis of fatty acids—suggesting that the observed synergy between low CHO and celecoxib in reducing metastatic progression may be due to compounding effects on lipid metabolism. Together, the above results support recent findings implicating fatty acid synthesis in the progression of a variety of cancers, including prostate, breast and bone cancer (32–35).
In conclusion, the demonstration herein that low CHO diets containing celecoxib markedly reduce metastasis is a significant step forward in metabolism-based cancer therapeutics and suggests that the sensitization of tumors to therapy through diet manipulation may be a viable therapeutic approach. Given that the anticancer benefits of high-dose celecoxib are offset by its cardiovascular side effects (20), combining it with low CHO diets might allow for a lower, safer dose of celecoxib to be used as an anticancer agent in the clinic.
Supplementary material
Supplementary Table 1 and Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
Canadian Breast Cancer Foundation Post-Doctoral Fellowship (M.J.H.). K.L.B. is a Michael Smith Foundation for Health Research Biomedical Research scholar. Lotte & John Hecht Memorial Foundation with core support from the BC Cancer Foundation and the BC Cancer Agency; Terry Fox Foundation (020395); Canadian Institutes of Health Research Institute of Cancer Research (CoP-120229).
Supplementary Material
Acknowledgements
The authors thank Dr Judit Banáth and Nancy LePard for expert technical assistance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ATP
adenosine triphosphate
- BG
blood glucose
- CHO
carbohydrate
- COX-2
cyclooxygenase-2
- OXPHOS
oxidative phosphorylation
- TRAMP
Transgenic Adenocarcinoma of the Mouse Prostate.
References
- 1. Khalid S., et al. (2010). Evidence for a tumor promoting effect of high-fat diet independent of insulin resistance in HER2/Neu mammary carcinogenesis. Breast Cancer Res. Treat., 122, 647–659 [DOI] [PubMed] [Google Scholar]
- 2. VanSaun M.N., et al. (2009). High fat diet induced hepatic steatosis establishes a permissive microenvironment for colorectal metastases and promotes primary dysplasia in a murine model. Am. J. Pathol., 175, 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faber J., et al. (2008). Beneficial immune modulatory effects of a specific nutritional combination in a murine model for cancer cachexia. Br. J. Cancer, 99, 2029–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Srivastava M.K., et al. (2010). Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res., 70, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunnerud U.J., et al. (2012). Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS One, 7, e44731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ho V.W., et al. (2011). A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res., 71, 4484–4493 [DOI] [PubMed] [Google Scholar]
- 7. Bielohuby M., et al. (2011). Induction of ketosis in rats fed low-carbohydrate, high-fat diets depends on the relative abundance of dietary fat and protein. Am. J. Physiol. Endocrinol. Metab., 300, E65–E76 [DOI] [PubMed] [Google Scholar]
- 8. Magee B.A., et al. (1979). The inhibition of malignant cell growth by ketone bodies. Aust. J. Exp. Biol. Med. Sci., 57, 529–539 [DOI] [PubMed] [Google Scholar]
- 9. Basu G.D., et al. (2006). Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J. Immunol., 177, 2391–2402 [DOI] [PubMed] [Google Scholar]
- 10. Brown M.D., et al. (2006). Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. Br. J. Cancer, 94, 842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DuBois R.N. (2006). Cyclooxygenase-2 selective inhibitors and prostate cancer: what is the clinical benefit? J. Clin. Oncol., 24, 2691–2693 [DOI] [PubMed] [Google Scholar]
- 12. Wang D., et al. (2004). Cyclooxygenase 2-derived prostaglandin E2 regulates the angiogenic switch. Proc. Natl. Acad. Sci. USA, 101, 415–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gatenby R.A., et al. (2006). Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res., 66, 5216–5223 [DOI] [PubMed] [Google Scholar]
- 14. Pulaski B.A., et al. (2001). Mouse 4T1 breast tumor model. Curr. Protoc. Immunol., 20, 20.2. [DOI] [PubMed] [Google Scholar]
- 15. Gingrich J.R., et al. (1996). Metastatic prostate cancer in a transgenic mouse. Cancer Res., 56, 4096–4102 [PubMed] [Google Scholar]
- 16. Farshidfar F., et al. (2012). Serum metabolomic profile as a means to distinguish stage of colorectal cancer. Genome Med., 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsuda M., et al. (1996). Effect of estrogen on hyperprolactinemia-induced glucose intolerance in SHN mice. Proc. Soc. Exp. Biol. Med., 212, 243–247 [DOI] [PubMed] [Google Scholar]
- 18. Guarente L. (2008). Mitochondria—a nexus for aging, calorie restriction, and sirtuins? Cell, 132, 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hursting S.D., et al. (2010). Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis, 31, 83–89 [DOI] [PubMed] [Google Scholar]
- 20. Menter D.G., et al. (2010). Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clin. Cancer Res., 16, 1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gameiro P.A., et al. (2013). In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab., 17, 372–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Locasale J.W., et al. (2011). Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet., 43, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santos C.R., et al. (2012). Lipid metabolism in cancer. FEBS J., 279, 2610––2623 [DOI] [PubMed] [Google Scholar]
- 24. Warburg O, et al. (1927). The metabolism of tumors in the body. J. Gen. Physiol., 8, 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kroemer G., et al. (2008). Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell, 13, 472–482 [DOI] [PubMed] [Google Scholar]
- 26. Thomlinson R.H, et al. (1955). The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer, 9, 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W., et al. (2013). Effects of combining Taxol and cyclooxygenase inhibitors on the angiogenesis and apoptosis in human ovarian cancer xenografts. Oncol. Lett., 5, 923–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perroud H.A., et al. (2013). Safety and therapeutic effect of metronomic chemotherapy with cyclophosphamide and celecoxib in advanced breast cancer patients. Future Oncol., 9, 451–462 [DOI] [PubMed] [Google Scholar]
- 29. Zhang B., et al. (2013). Celecoxib enhances the efficacy of 15-hydroxyprostaglandin dehydrogenase gene therapy in treating murine breast cancer. J. Cancer Res. Clin. Oncol., 139, 797–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang D., et al. (2010). Eicosanoids and cancer. Nat. Rev. Cancer, 10, 181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou J., et al. (2005). Interactions between prostaglandin E(2), liver receptor homologue-1, and aromatase in breast cancer. Cancer Res., 65, 657–663 [PubMed] [Google Scholar]
- 32. Long X.H., et al. (2013). Tumor suppressive microRNA-424 inhibits osteosarcoma cell migration and invasion via targeting fatty acid synthase. Exp. Ther. Med., 5, 1048–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Migita T., et al. (2009). Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J. Natl. Cancer Inst., 101, 519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porta R., et al. (2014). Fatty acid synthase expression is strongly related to menopause in early-stage breast cancer patients. Menopause, 21, 188–191 [DOI] [PubMed] [Google Scholar]
- 35. Puig T., et al. (2009). Novel inhibitors of fatty acid synthase with anticancer activity. Clin. Cancer Res., 15, 7608–7615 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.