Abstract
We have previously shown that kava and its flavokavain-free Fraction B completely blocked 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice with a preferential reduction in NNK-induced O 6-methylguanine (O 6-mG). In this study, we first identified natural (+)-dihydromethysticin (DHM) as a lead compound through evaluating the in vivo efficacy of five major compounds in Fraction B on reducing O 6-mG in lung tissues. (+)-DHM demonstrated outstanding chemopreventive activity against NNK-induced lung tumorigenesis in A/J mice with 97% reduction of adenoma multiplicity at a dose of 0.05mg/g of diet (50 ppm). Synthetic (±)-DHM was equally effective as the natural (+)-DHM in these bioassays while a structurally similar analog, (+)-dihydrokavain (DHK), was completely inactive, revealing a sharp in vivo structure–activity relationship. Analyses of an expanded panel of NNK-induced DNA adducts revealed that DHM reduced a subset of DNA adducts in lung tissues derived from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL, the active metabolite of NNK). Preliminary 17-week safety studies of DHM in A/J mice at a dose of 0.5mg/g of diet (at least 10× its minimum effective dose) revealed no adverse effects, suggesting that DHM is likely free of kava’s hepatotoxic risk. These results demonstrate the outstanding efficacy and promising safety margin of DHM in preventing NNK-induced lung tumorigenesis in A/J mice, with a unique mechanism of action and high target specificity.
Introduction
Lung cancer causes ~160 000 deaths in the USA (1,2) and 1.4 million deaths worldwide annually (3). Due to the limited success in treatment (4), prevention is of paramount importance. Tobacco cessation would, without a doubt, be the ideal strategy and should be highly promoted since cigarette smoking causes 90% of lung cancers (5). Quitting, however, is very challenging because of the addictive nature of nicotine in tobacco products; many smokers will not succeed even after multiple attempts and with the best cessation support. These individuals contribute significantly to the estimated 200 000 lung cancer incidence in the USA each year in association with an estimated annual medical cost of $12 billion (1,2,6). Chemopreventive agents hence need to be developed for these high-risk populations, besides further optimizing tobacco cessation methods. During the past few decades, a number of compounds have been identified as potential lung cancer chemopreventive agents and several of them have been evaluated in the clinic, but unfortunately with no success (7). The lack of clinical effectiveness is at least partly due to the moderate in vivo efficacy of some of the leads. Novel entities with superior efficacy and unique mechanisms, therefore, need to be developed.
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a tobacco-specific and highly potent pulmonary carcinogen, selectively inducing lung adenoma and adenocarcinoma in various species (8). Substantial evidence also suggests that NNK in cigarette smoke contributes to pulmonary adenocarcinoma in the United States smokers. The incidence of lung adenocarcinoma has increased in the USA during the past few decades (9–13). Such a change has been observed only among smokers, suggesting its association with cigarette related factors. One study noted an increase in NNK levels in the mainstream smoke of a leading United States non-filter cigarette between 1978 and 1992 while levels of benzo[a]pyrene (BaP), another well-characterized pulmonary carcinogen, decreased in the same cigarette from 1959 to 1992 (13). Further evidence supporting a role for NNK in human lung adenocarcinoma derives from the comparison of NNK content in cigarette smoke and adenocarcinoma rates among the USA, UK, Canada and Australia (10).
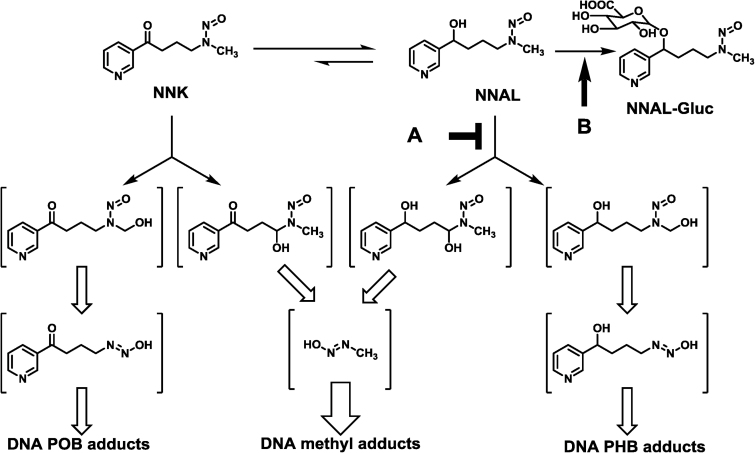
Mechanistically, NNK is metabolically activated via α-hydroxylation to generate two reactive species, leading to two types of DNA modifications—methylation and pyridyloxobutylation (5,14) (Figure 1). The extent of DNA damage can be characterized by quantifying different DNA adducts—O 6-methylguanine (O 6-mG) and 7-methyl guanine (7-mG) for methylation and 7-[4-(3-pyridyl)-4-oxobut-1-yl]guanine (7-pobG), O 6-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxyguanosine (O 6-pobdG) and O 2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine (O 2-pobdT) for pyridyloxobutylation. NNK is also metabolically converted to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) (Figure 1). NNAL can be activated via α-hydroxylation as well to generate two reactive species, resulting in two types of DNA modifications—methylation and pyridylhydroxybutylation. The extent of pyridylhydroxybutylation can be characterized by quantifying 7-[4-(3-pyridyl)-4-hydroxobut-1-yl]guanine (7-phbG), O 6-[4-(3-pyridyl)-4-hydroxobut-1-yl]-2′-deoxyguanosine (O 6-phbdG) and O 2-[4-(3-pyridyl)-4-hydroxobut-1-yl]thymidine (O 2-phbdT). DNA damage by NNK and NNAL has been well established as one major underlying mechanism for NNK-induced lung tumorigenesis (8). Reducing such DNA damage is, therefore, a plausible strategy for chemoprevention of lung cancer.
Fig. 1.
Metabolic pathways of NNK leading to formation of DNA adducts and hypothetic points of action by DHM for its selective reduction in a subset of NNK-induced DNA damage in A/J mouse lung—preferential inhibition of NNAL activation via hydroxylation (A) or enhancing detoxification of NNAL via glucuronidation (B).
The A/J mouse NNK-induced lung tumorigenesis model has been widely used in evaluating lung cancer chemopreventive agents (7) because A/J mice are prone to lung tumorigenesis (15) and the tumors have similar morphological, histological and molecular features as human lung adenocarcinomas (16). With NNK treatment, A/J mice develop lung tumors with a 100% incidence and a high multiplicity (7,16). Among NNK-induced DNA adducts, the quantity of O 6-mG has shown a strong positive correlation with lung tumor multiplicity (17) and A/J mice with an increased DNA repair capacity specific to O 6-mG are less susceptible to NNK-induced lung tumorigenesis (18). In combination with the high miscoding property of O 6-mG (19–22), NNK-induced O 6-mG is believed essential to initiate lung tumorigenesis in A/J mice.
We have recently reported that a commercial kava product efficiently blocks NNK-induced lung tumorigenesis in A/J mice at a dose of 1.25mg/g of diet (23). Fraction B of this product, containing mostly kavalactones (Figure 2A), fully recapitulates its chemopreventive efficacy. Kava and Fraction B reduce NNK-induced DNA damage in the lung with a preference in O 6-mG adduct over the POB adducts while ineffective fractions have no effect on O 6-mG (23). Monitoring the impact of single chemicals in Fraction B on O 6-mG, therefore, would be an economic strategy to identify the active lead(s). It is also mechanistically intriguing regarding the preferential reduction in O 6-mG adduct.
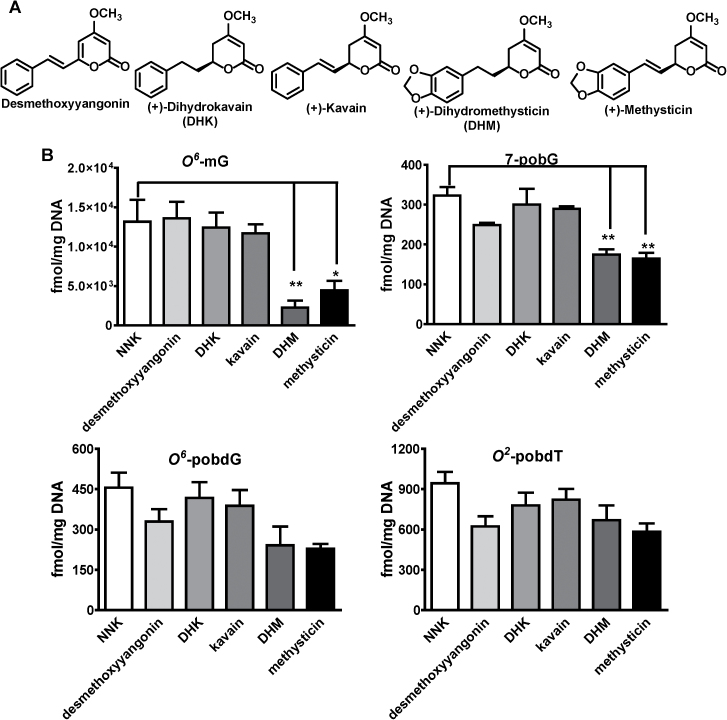
Fig. 2.
Characterization of natural kavalactones on NNK-induced DNA damage in A/J mouse lung tissues. A: Chemical structures of five natural kavalactones isolated from Fraction B. B: Their effects on NNK-induced POB adducts and O 6-mG adduct. Comparison was made with the NNK treatment group by Dunnett’s test when one-way ANOVA was statistically significant. n = 3 each group. *P < 0.05 and **P < 0.01.
This study reports our effort in identifying and confirming dihydromethysticin (DHM) as the active compound. DHM potently and efficiently blocks NNK-induced lung tumorigenesis in A/J mice. We have also investigated the mechanism leading to its differential reduction in NNK-induced DNA damage and provided preliminary long-term safety of DHM in A/J mice upon high-dose exposure.
Materials and methods
Chemicals, reagents and animal diets
NNK was prepared by following a reported procedure (24). [CD3]O 6-mG was purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). [13CD3]7-methylguanine was prepared by following a reported procedure (25). 7-PobG, O 6-pobdG, O 2-pobdT, 7-phbG, O 6-phbdG, O 2-phbdT and the corresponding [pyridine-D4] analogues were synthesized by following reported procedures (26–28). Micrococcal nuclease and phosphodiesterase II were purchased from Worthington Biochemical Corporation (Lakewood, NJ). Alkaline phosphatase was purchased from Roche Molecular Biochemicals (Indianapolis, IN).
Kava was acquired from Gaia Herbs (Brevard, NC) as an ethanol extract of the wild crafted lateral root from Vanuatu. It was standardized to 150mg/ml total kavalactones. The AIN-93 G and M powdered diets were purchased from Harlan Teklad (Madison, WI). For the short-term DNA damage study, the AIN-93 G diet was used during the experimental period. For the long-term lung tumorigenesis study, the AIN-93 G diet started 1 week before the first dose of NNK and ended 1 week after the second dose of NNK. The AIN-93M diet was used during the rest of the experimental period.
Isolation of chemicals in Fraction B
Five kavalactones (desmethoxyyangonin, (+)-dihydrokavain (DHK), (+)-kavain, (+)-DHM and (+)-methysticin, Figure 2A) were isolated from Fraction B by normal phase silica gel chromatography with a gradient mixture of hexane and ethyl acetate as the eluent. Their chemical structures were characterized by 1H-NMR and mass spectrometry. Their purities were estimated >90% by HPLC on a Beckman Coulter System Gold 126 solvent module with a 168 detector. A Clipeus C-18 column (5 μm, 250 mm × 4.6mm) was used for the analyses. The flow rate was 1 ml/min. The mobile phase A was 10mM NH4OAc aqueous solution and B was acetonitrile. The time program used for the analyses was 50% B for 15min.
Synthesis of (±)-DHM
(±)-DHM was synthesized by following a procedure similar as previously reported (29). Its structure and purity were characterized by 1H-NMR, mass spectrometry and HPLC by following the conditions detailed above.
Diet preparation
Kava was reconstituted in absolute ethanol (50 ml) and then mixed with the AIN-93 diet (150g). Similarly, absolute ethanol (50 ml each) reconstituted with kavalactones was mixed with the AIN-93 diet (150g each) for the kavalactone diets. Absolute ethanol (50 ml) was mixed with the AIN-93 diet (150g) for the control diet. The reconstituted diets were dried under vacuum to remove ethanol and then ground into fine powders. All diets were mixed with additional AIN-93 diet to the desired dose.
General protocols for animal studies
Female A/J mice (5–6 weeks of age) were purchased from the Jackson Laboratory (Bar Harbor, ME) and handled according to IACUC-approved animal welfare protocols at the University of Minnesota. Upon arrival, the mice were housed in the specific pathogen-free animal facilities of the Research Animal Resources at the University of Minnesota.
General protocols for isolating DNA from lung and liver tissues
DNA was isolated and purified from ~100 mg lung and liver tissues of each mouse, following Genomic-tip 100/G protocol from Qiagen Corp (Valencia, CA).
Assessing the effect of five natural kavalactones in Fraction B on NNK-induced O 6-mG and three POB adducts in A/J mouse lung tissues
After 1-week acclimation, A/J mice were weighed and randomized into seven groups (three mice per group) and switched to the AIN-93 G diet on a date defined as Day 1. Mice in Groups 1 and 2 were given the AIN-93 G diet during Day 1–7. Mice in Groups 3–7 were given the AIN-93 G diet supplemented with desmethoxyyangonin, (+)-DHK, (+)-kavain, (+)-DHM, or (+)-methysticin, respectively, at a dose of 1mg/g of diet during Day 1–7. Mice in Groups 2–7 were given a single dose of NNK at 100mg/kg body weight in saline (0.1ml) via intraperitoneal (i.p.) injection at the beginning of Day 7 while mice in Group 1 were given saline (0.1ml). Mice were euthanized by CO2 overdosing 24h after NNK treatment, and lung tissues were collected and stored at −80°C until DNA isolation. A number of NNK-induced DNA modifications (O 6-mG, 7-pobG, O 6-pobdG, and O 2-pobdT) were quantified by following a reported procedure (23).
Evaluating the dose-response effect of kava, (+)-DHM versus (+)-DHK and synthetic (±)-DHM on NNK-induced DNA adducts in A/J mouse lung and liver tissues
After 1-week acclimation, A/J mice were weighed and randomized into 10 groups (three mice per group except for Group 1 that had one mouse as the negative control) and switched to the AIN-93 G diet on a date defined as Day 1. Mice in Groups 1 and 2 were given the AIN-93 G diet during Day 1–7. Mice in Groups 3–10 were given the AIN-93 G diets supplemented with kava, (+)-DHK, (+)-DHM and synthetic (±)-DHM, respectively, at the specified dose during Day 1–7. Mice in Groups 2–10 were given a single dose of NNK at 100mg/kg body weight in saline (0.1ml) via i.p. injection at the beginning of Day 7 while the mouse in Group 1 was given saline (0.1ml). Mice were euthanized by CO2 overdosing 24h after NNK treatment, and lung and liver tissues were collected and stored at −80°C until DNA isolation. A set of NNK-induced DNA adducts (O 6-mG, 7-mG, 7-pobG, O 6-pobdG, O 2-pobdT, 7-phbG, O 6-phbdG and O 2-phbdT) were quantified by following reported procedures (23,25,28). For 7-mG isolation, DNA (30 µg) was dissolved in 10mM sodium phosphate buffer, pH 7 (500 µl) and spiked with [13C1 2H3]7-mG (10 pmol). Samples were heated to 80°C for 30 min. An aliquot (100 µl) was removed for 7-mG analysis.
Assessing the efficacy of (+)-DHM versus (+)-DHK and synthetic (±)-DHM against NNK-induced lung adenoma formation in A/J mice
After 1-week acclimation, mice were weighed, randomized into eight groups and switched to the AIN-93 G diet on a date defined as Day 1. The number of mice in each group is specified in the Results section (Table I). Mice were fed diets supplemented with different compounds at the dose specified in the Results section during Day 1–14. On Day 7 and 14, mice in the negative control group received 0.1 ml of saline solution while mice in the other groups received NNK (100 and 67mg/kg, respectively, in 0.1 ml of saline solution) via i.p. injection. At the end of Day 21, mice were switched to the AIN-93M diet until the end of the study. The diet was replenished twice weekly. The diet consumption was measured twice weekly and the body weight was monitored weekly. This study was terminated at the end of Day 119. All mice were euthanized with CO2 overdosing. The lungs were collected and tumors on the surface of the lungs were counted under blinded conditions by an A.C.V.P board certified pathologist (M.G. O’S.).
Table I.
Effect of different agents on lung tumor incidence and multiplicity induced by NNK in A/J mice
| Group | No. of mice at termination (initiation) | Body weight at termination (mean ± SD, g/mouse) | Liver weight at termination (mean ± SD, g/mouse) | Lung tumors | P a | ||
|---|---|---|---|---|---|---|---|
| % of mice with tumors | Tumors/mouse (mean ± SD) | Reduction in tumor multiplicity (%) | |||||
| 1 (untreated control) | 5 (5) | 23.8±2.5 | 1.07±0.13 | 20 | 0.2±0.4 | — | — |
| 2 (carcinogen control) | 9 (10) | 22.6±3.1 | 1.07±0.15 | 100 | 13.9±6.9 | — | — |
| 3 (kava, 1.25mg/g) | 5 (5) | 23.7±1.8 | 1.06±0.13 | 80 | 0.8±0.4 | 95.6 | <0.01 |
| 4 ((+)-DHM, 0.5mg/g) | 5 (5) | 23.7±1.5 | 1.08±0.11 | 60 | 0.6±0.5 | 97.1 | <0.01 |
| 5 ((+)-DHM, 0.05mg/g) | 5 (5) | 25.0±0.7 | 1.12±0.05 | 60 | 0.6±0.5 | 97.1 | <0.01 |
| 6 ((+)-DHK, 0.5mg/g) | 5 (5) | 23.2±1.5 | 1.09±0.07 | 100 | 14.8±12.8 | — | >0.05 |
| 7 ((+)-DHK, 0.05mg/g) | 5 (5) | 23.1±0.5 | 1.04±0.04 | 100 | 19.6±5.6 | — | >0.05 |
| 8 ((±)-DHM, 0.5mg/g) | 5 (5) | 23.4±1.5 | 1.07±0.09 | 0 | 0 | 100 | <0.01 |
Female A/J mice in Groups 2–9 were treated with NNK (100 and 67mg/kg body weight on Day 7 and Day 14, respectively) in 0.1ml saline via i.p. injection. Mice were maintained on AIN-93G diet until Day 21 and then shifted to AIN-93M diet for the duration of the experiment. Treatments were between Day 1 and Day 14.
aCompared with Group 2 by Dunnett’s test using one-way ANOVA.
Assessing A/J mouse safety upon 17-week continuous exposure of (+)-DHM at a dose of 0.5mg/g of diet
After 1-week acclimation, A/J mice were weighed and randomized into two groups (five mice in the control group and ten mice in (+)-DHM treatment group). Mice in the control group were maintained on the AIN-93 G diet for 3 weeks and then switched to the AIN-93M diet until the end of Week 17. Mice in (+)-DHM treatment group were maintained on the same diet as the control group with supplementation of (+)-DHM at a dose of 0.5mg/g of diet. Food was replenished twice weekly. Food intake was monitored twice a week and mouse body weight was monitored once a week. At the end of Week 8, serum samples from each mouse were prepared from blood collected via a facial vein and analyzed for a panel of clinical chemistry analytes. At the end of Week 17, mice were euthanized by CO2 overdosing. Blood was collected with one portion analyzed for hematology and the other portion processed for serum and analyzed for a full panel of clinical chemistry analytes. Individual animal body weights and major organ weights (lung, liver, heart, spleen and kidney) were recorded. Lung, liver, heart, spleen, kidney and pancreas were fixed in 10% neutral-buffered formalin solution. Appropriately fixed tissues were processed into paraffin blocks using standard histological techniques, and 5 µm sections were cut and stained with hematoxylin and eosin (H&E). Histological slides were examined using light microscopy by an experienced A.C.V.P. board certified pathologist (M.G.O’S.).
Statistical analyses
Data on lung adenoma multiplicity were reported as mean ± SD (n = 5–9). One-way analysis of variance (ANOVA) was used to compare means among the NNK and NNK/treatment groups. Dunnett’s test was used for comparisons of the number of tumors between the NNK control and treatment groups when the one-way ANOVA analysis was statistically significant. P-value ≤ 0.05 was considered statistically significant. For DNA damage studies, one-way ANOVA was used to compare means ± SD (n = 3). Dunnett’s test was used for comparisons between the NNK control and treatment groups when one-way ANOVA analysis was statistically significant. P-value ≤ 0.05 was considered statistically significant. For the safety study, an unpaired Student’s t-test was used for comparison between the control and (+)-DHM treatment groups. A two-tailed P-value ≤ 0.05 was considered statistically significant. All analyses were conducted using GraphPad Prism 4 from GraphPad Software (La Jolla, CA).
Results
The effects of five kavalactones in Fraction B on NNK-induced O 6-mG, 7-pobG, O 6-pobdG and O 2-pobdT in A/J mouse lung tissues
Since our previous work showed that the reduction of O 6-mG in lung tissues by different kava entities correlated positively with their lung tumor prevention efficacy and Fraction B recapitulated kava’s chemopreventive effect on NNK-induced lung tumorigenesis in A/J mice (23), this experiment was designed to identify the key compound(s) in Fraction B that reduced NNK-induced O 6-mG, which may be the active chemopreventive agent.
Five known kavalactones have been isolated from Fraction B as its major components (Figure 2A), accounting for 93% of its mass balance (23). These five compounds were investigated for their effect on NNK-induced DNA damage in lung tissues, including O 6-mG and three POB adducts (7-pobG, O 6-pobdG and O 2-pobdT) (Figure 2B). Two compounds, (+)-DHM and (+)-methysticin, significantly reduced NNK-induced O 6-mG while the other compounds did not, suggesting (+)-DHM and (+)-methysticin as the potential active compounds and the importance of the methylenedioxy functional group. All five compounds had much weaker reduction in the POB adducts (Figure 2B), consistent with that of Fraction B (23). Although not statistically significant, the extent of O 6-mG reduction by (+)-DHM was greater than that by (+)-methysticin. We, therefore, selected (+)-DHM as the lead for further investigation with DHK as a negative control because of its higher structural similarity to (+)-DHM relative to desmethoxyyangonin and (+)-kavain.
The dose-response effect of (+)-DHM on an expanded panel of NNK-induced DNA damage in comparison with kava, (+)-DHK and synthetic (±)-DHM in A/J mouse lung tissues
This study was designed to address three questions. First, what would be the dose of (+)-DHM to induce a similar extent of O 6-mG reduction as kava did at a dose of 1.25mg/g of diet, which effectively blocked NNK-induced lung tumor formation (23)? Kava was evaluated at two dosages—5 and 1.25mg/g of diet, respectively, while (+)-DHM was evaluated at four dosages—1, 0.25, 0.1 and 0.01mg/g of diet with (+)-DHK evaluated at a dose of 1mg/g of diet as a control. Secondly, given that the isolated (+)-DHM contained impurities, how can the impurity be ruled out as the active compound? Synthetic (±)-DHM therefore was prepared and evaluated at a dose of 1mg/g of diet. Lastly, what is the mechanism leading to (+)-DHM’s preferential reduction in O 6-mG over POB adducts in lung tissues? Based on the molecular basis of NNK metabolism and DNA damage (Figure 1) (8), there are four possible mechanisms: (i) preferential inhibition of NNK methylene hydroxylation over its methyl hydroxylation; (ii) an increased O 6-alkylguanine-DNA alkyltransferase (AGT)-mediated O 6-mG repair (30,31); (iii) preferential inhibition of NNAL hydroxylation over NNK hydroxylation; or (iv) increased detoxification of NNAL. These mechanisms could be differentiated by characterizing the effect of DHM on an expanded panel of DNA adducts, including POB DNA adducts (7-pobG, O 6-pobdG and O 2-pobdT), PHB DNA adducts (7-phbG, O 6-phbdG and O 2-phbdT) and methyl DNA adducts (O 6-mG and 7-mG).
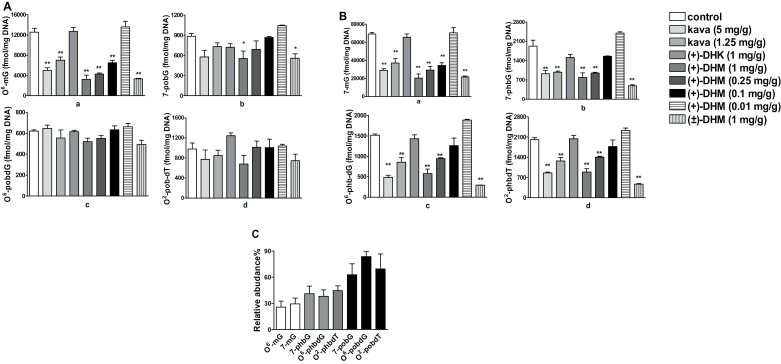
Consistent with our previous results, kava preferentially and dose-dependently reduced O 6-mG in lung tissues (Figure 3A.a) while it had minimal effects on POB adducts (Figure 3A.b–d). (+)-DHM also preferentially and dose-dependently reduced O 6-mG (Figure 3A.a) with weaker effects on POB adducts (Figure 3A.b–d) whereas (+)-DHK did not reduce any of these adducts even at a dose of 1mg/g of diet. The extent of reduction in O 6-mG by (+)-DHM at a dose of 0.1mg/g of diet was comparable with that induced by kava at a dose of 1.25mg/g of diet (Figure 3A.a). Since (+)-DHM at a dose of 0.01mg/g of diet had no effect on O 6-mG, the minimum chemopreventive dose of DHM would be between 0.01 and 0.1mg/g of diet. (+)-DHM also dose-dependently reduced 7-mG (Figure 3B.a) with the extents of reduction similar to those in O 6-mG (Figure 3A.a). These results indicate that AGT-mediated O 6-mG repair is not involved in DHM’s DNA damage reduction. Surprisingly, (+)-DHM dose-dependently reduced PHB adducts (Figure 3B.b–d) in lung tissues as well, again with the extents of reduction similar to those in O 6-mG and 7-mG (Figures 3A.a and 3.a). Since (+)-DHM has minimal effect on POB adducts, these results suggest that its reduction in NNK-induced DNA damage is likely mediated through the NNAL pathway, either by inhibiting its activation or enhancing its detoxification (Figure 1A and B). The synthetic (±)-DHM at 1mg/g of diet had similar effects in reducing DNA damage as the natural (+)-DHM (Figure 3A and B), confirm DHM as the active compound.
Fig. 3.
Characterization of the effect of different agents on NNK-induced DNA damage in A/J mouse lung tissues. A: Dose-response effect of natural (+)-DHM on NNK-induced O 6-mG (a) and three POB adducts (b–d) in comparison with kava, (+)-DHK and synthetic (±)-DHM. B: Dose-response effect of natural (+)-DHM on NNK-induced 7-mG (a) and three PHB adducts (b–d) in comparison to kava, (+)-DHK and synthetic (±)-DHM. C: Percentage of different DNA adducts from mice treated with (+)-DHM (1mg/g of diet) relative to that from NNK-treated control mice. For A and B, comparison was made with NNK treatment group by Dunnett’s test when one-way ANOVA was statistically significant. n = 3 each group. *P < 0.05 and **P < 0.01. For C, one-way ANOVA was not statistically significant.
The lack of effect of kava, (+)-DHM, (+)-DHK and synthetic (±)-DHM on NNK-induced DNA damage in A/J mouse liver tissues
Since liver is the major metabolizing organ of the body, we also characterized the effect of kava, (+)-DHM, (+)-DHK and (±)-DHM on NNK-induced DNA damage in liver tissues. Surprisingly, none of the treatments had significant effects on NNK-induced DNA damage in the liver tissues (data not shown).
The efficacy of kava, (+)-DHM, (+)-DHK and synthetic (±)-DHM on NNK-induced lung adenoma formation in A/J mice
To validate the chemopreventive potential of DHM, we carried out an NNK-induced lung adenoma assay as shown in Table I. A/J mice without NNK treatment had low adenoma incidence (20%) and low adenoma multiplicity (0.2±0.4 lung adenoma/mouse, Group 1) while NNK-treated A/J mice had 100% adenoma incidence and high adenoma multiplicity (13.9±6.9 lung adenoma/mouse, Group 2). Kava at a dose of 1.25mg/g of diet slightly reduced adenoma incidence by 20% but significantly reduced adenoma multiplicity by 95.6% (to 0.8±0.4, Group 3). Based on the estimated minimum effective dose of (+)-DHM between 0.01 and 0.1mg/g of diet, we tested it at 0.5 and 0.05mg/g of diet (Groups 4 and 5). (+)-DHM at both dosages reduced adenoma incidence by 40% and significantly reduced adenoma multiplicity by 97.1% (to 0.6±0.5, Groups 4 and 5), suggesting that its minimum effective dose has not been reached. All mice were adenoma free upon (±)-DHM treatment at a dose of 0.5mg/g of diet (Group 8). (+)-DHK, as a negative control, showed no reduction at all at 0.5 or 0.05mg/g of diet (Groups 6 and 7). Overall, these data established DHM as a lung cancer chemopreventive agent with a minimum effective dose lower than 0.05mg/g (50 ppm).
A/J mouse safety assessment upon 17-week continuous exposure of (+)-DHM at a dose of 0.5mg/g of diet
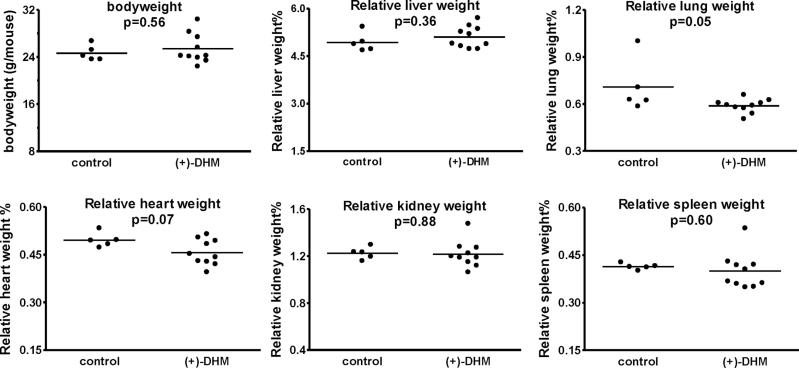
Cognizant of the hepatotoxic concerns associated with kava (32,33), we characterized a number of parameters to assess the safety of (+)-DHM. Mice with (+)-DHM feeding started with a slightly higher average body weight and remained heavier than the control mice through the study, but none of the differences were statistically significant (Supplementary Figure S1, available at Carcinogenesis Online). The food intake was similar as well (Supplementary Figure S1, available at Carcinogenesis Online). The clinical chemistry results for the serum samples collected at the end of Week 8 are summarized in Supplementary Figure S2A, available at Carcinogenesis Online, and they included alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), albumin, total bilirubin, creatine kinase, glucose, cholesterol and amylase. All of these parameters reflect liver functions, except for amylase (a marker for pancreas). No significant differences were detected in any of these parameters between the control group and the (+)-DHM group. The clinical chemistry results of the serum samples collected at the end of Week 17 are summarized in Supplementary Figure S2B, available at Carcinogenesis Online. Same as the 8-week outcome, there were no significant differences for any of these parameters between the control group and the (+)-DHM group. The time-course changes of these parameters for each mouse also revealed no obvious difference between the control and (+)-DHM groups (data not shown). On Week 17, the concentration of various salts in the serum was quantified as well; these data were used to calculate serum osmolality and anion gap, which revealed no differences between the control and (+)-DHM groups either (data not shown). The blood samples on Week 17 were also analyzed for full-panel hematology (one blood sample from a control mouse clotted and could not be analyzed, which resulted in four data points for the control group, Supplementary Figure S3, available at Carcinogenesis Online). None of these parameters were significantly different between the control and (+)-DHM groups. Lastly, the final body weight and the relative weights of five organs (Figure 4) were not significantly different between the control and (+)-DHM groups except for the lung tissues. The average relative lung weight for the control group was significantly higher than that for the (+)-DHM group (P = 0.05). An inspection of the data from each mouse revealed that one mouse in the control group had an increased lung weight that was attributed to multifocal hemorrhage within the lung at the time of euthanasia. Excluding this animal, there were no significant differences between the control and (+)-DHM groups (P = 0.10). Histopathological examination of liver, lung, heart, kidney, spleen and pancreas tissues revealed no significant differences between the control and (+)-DHM groups (data not shown).
Fig. 4.
Body weight and major organs from the control mice and mice with (+)-DHM exposure at a dose of 0.5mg/g of diet at Week 17. P-values were given with comparison between the control group (n = 5) and (+)-DHM treatment group (n = 10) using a two-tailed Student’s t-test.
Discussion and conclusion
Our results have unambiguously identified DHM as a potent chemopreventive agent that safely and completely blocks NNK-induced lung tumorigenesis in A/J mice at a dietary intake dose of 0.05mg/g (50 ppm). We first identified (+)-DHM as the lead among five structurally similar compounds based on their efficacy to reduce O 6-mG in lung tissues in a short-term assay (Figure 2B). In a follow-up dose-response experiment using DNA adduct reduction as the readouts, we estimated its minimum effective dose between 0.01 and 0.1mg/g of diet (Figure 3). We therefore tested (+)-DHM at doses of 0.5mg/g of diet (500 ppm) and 0.05mg/g (50 ppm) with a 2-week exposure window during the NNK initiation period only (Table I, Groups 4 and 5). Since (+)-DHM at 0.05mg/g reduced NNK-induced lung adenoma multiplicity by 97% and was as effective as (+)-DHM at 0.5mg/g, its minimum effective dose would likely be lower than 0.05mg/g. An extrapolation of an effective dose of 0.05mg/g of diet for a mouse of 20g (daily food intake 3g) would be equivalent to a human dose of 47mg/day (based on 75kg human body weight) according to the body surface area normalization method (34). Thus, from the dose aspect, (+)-DHM is highly feasible for human usage. The results of the synthetic (±)-DHM are also informative. First, they confirmed DHM as the active chemopreventive agent. Second, it appeared that the synthetic racemic forms outperformed the natural (+)-DHM in blocking NNK-induced DNA adducts and lung adenoma formation at the tested dose of 1 and 0.5mg/g, respectively, although the differences were not statistically significant. It remains to be determined whether the synthetic (−)-DHM may be more efficacious than the natural (+)-DHM in a dose range close to its minimum effective dose.
It is interesting to note that (+)-DHK, a structural analog of (+)-DHM (Figure 2A), was completely inactive in blocking DNA damage (Figures 2 and 3) or lung adenoma formation (Table I). Such a sharp in vivo structure–activity relationship (SAR) between (+)-DHM and (+)-DHK suggests a high target specificity of (+)-DHM. It also highlights the importance of the methylenedioxy functional group in (+)-DHM for its efficacy. Such a five-member ring may be critical for (+)-DHM to specifically interact with its molecular target, which remains to be identified, leading to its outstanding chemopreventive efficacy. Alternatively (+)-DHM may be metabolized via the methylenedioxy functional group to generate the in vivo active form. Further investigation is needed to differentiate these possibilities.
Mechanistically (+)-DHM dose-dependently reduced O 6-mG, 7-mG, 7-phbG, O 6-phbdG and O 2-phbdT in the lung tissues with similar extents of reduction (Figure 3A.a and B.a–d) while it had much weaker effect on 7-pobG, O 6-pobdG and O 2-pobdT (Figure 3A.b–d). These data suggest that DHM may preferentially block NNAL-mediated DNA damage, possibly via inhibiting NNAL activation (Figure 1A) or increasing NNAL detoxification (Figure 1B). A closer inspection of the extent of reduction in methyl and PHB adducts reveals a slightly higher reduction in methyl adducts, although the difference was not statistically significant (Figure 3C showed a representative set of data with (+)-DHM treatment at a dose of 1mg/g of diet). Since the extent of reduction in POB and PHB adducts are all less than those of the methyl adducts, DHM may preferentially inhibit methylene hydroxylation over methyl hydroxylation of NNK and NNAL as well. The lack of effect of DHM on NNK-induced DNA damage in the liver is intriguing, which may be mediated via a potential DHM abundance difference in lung and liver tissues. Alternatively, lung tissues may contain DHM-interacting biomolecules involved in NNAL activation/detoxification that are absent in liver tissues.
There have been limited reports characterizing multiple DNA adducts upon NNK treatment in A/J mice in both lung and liver tissues. An early study by Morse et al. showed that phenethyl isothiocyanate (PETIC) reduced O 6-mG in lung tissues, but other DNA adducts and liver tissues were not analyzed (35). A later study by Prokopczyk et al. (36) showed that synthetic 1,4-phenylenebis(methylene)selenocyanate, an analog of PEITC, preferentially reduced O 6-mG in comparison with 7-mG. Crampsie et al. (37) recently reported that phenylbutyl isoselenocyanate (ISC-4), again a synthetic selenium mimic of PEITC, reduced both methyl and POB DNA damage in A/J mouse lung and liver tissues. Another promising lung cancer chemopreventive agent, indole-3-carbinol (I3C) was found to reduce O 6-mG in lung tissues but increased O 6-mG in liver tissues, potentially via increasing liver NNK metabolism (38). Our study herein is the first that characterized all three types of DNA damage in A/J mice and DHM appears to have a different mechanism relative to PEITC- and I3C-based lung cancer chemopreventive agents.
More characterizations have been performed on NNK-induced DNA damage in F344 rat lung and liver tissues and several studies have analyzed the impact of the chirality of NNAL as well (26,28,39,40). Upadhyaya et al. (41) demonstrated that (R)-NNAL preferentially generated PHB adducts, while (S)-NNAL and NNK produced mainly POB adducts. Although there has been no characterization of (R)- and (S)-NNAL-induced DNA damage in A/J mice, these two isomers appeared to have different metabolism and tumorigenicity. It remains to be determined whether the PHB DNA damage in A/J mouse lung tissues mainly derives from (R)-NNAL and whether DHM selectively inhibits the activation of (R)-NNAL, leading to the preferential reduction in PHB DNA adducts in lung tissues. Alternatively, DHM may selectively enhance the detoxification of (R)-NNAL.
Because of the purported hepatotoxic risk of kava, the safety of (+)-DHM is of great importance. A/J mice continuously exposed to (+)-DHM at a dose of 0.5mg/g of diet (at least 10 times its minimum effective dose) for 17 weeks did not present with any adverse effects in the following parameters: average weekly body weight increase, average weekly food intake, clinical chemistry analyses of serum samples collected at Week 8 and 17, hematology analysis at Week 17, final body weight, relative weight of heart, lung, liver, kidney and spleen at Week 17, and the pathology of heart, lung, liver, kidney, spleen and pancreas at Week 17. Since the 0.5mg/g dose appeared to be still below the maximum tolerated dose (MTD, which remains to be determined), (+)-DHM is expected to have a very wide safety margin as a lung cancer chemopreventive agent. We have also identified one non-kavalactone compound in kava that recapitulates its hepatotoxic risk while (+)-DHM under the same conditions revealed no sign of hepatotoxic risk, suggesting that (+)-DHM is likely free of the hepatotoxic concern associated with kava (a separate manuscript is in preparation to disclose these results).
In summary, our data support DHM as a potent and efficacious chemopreventive agent against NNK-induced lung tumorigenesis in A/J mice with a unique mechanism of action relative to several well-known lung cancer chemopreventive agents. Its sharp in vivo SAR suggests high target specificity, which is highly desirable to minimize adverse effects as reflected by its impressive safety profile. DHM is therefore a promising lung cancer chemopreventive agent that warrants further investigation for future translation into humans. Future studies are also needed to evaluate its chemopreventive potential when administered during the post-initiation stage.
Supplementary material
Supplementary Figures 1–3 can be found at http://carcin.oxfordjournals.org/
Funding
R01 CA142649, R01 CA81301, P01 CA138338 by the National Cancer Institute ; R01 AT007395 by National Center for Complementary and Alternative Medicine, National Institutes of Health.
Supplementary Material
Acknowledgements
Margaret Martyr has provided editorial assistance to the manuscript preparation and is acknowledged.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- DHM
dihydromethysticin
- DHK
dihydrokavain
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- BaP
benzo[a]pyrene
- O6-mG
O 6-methylguanine
- 7-mG
7-methylguanine
- 7-pobG
7-[4-(3-pyridyl)-4-oxobut-1-yl]guanine
- O6-pobdG
O 6-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxyguanosine
- 7-phbG
7-[4-(3-pyridyl)-4-hydroxobut-1-yl]guanine
- O6-phbdG
O 6-[4-(3-pyridyl)-4-hydroxobut-1-yl]-2′-deoxyguanosine; SAR, structure–activity relationship
- AGT
O 6-alkylguanine-DNA alkyltransferase
- ANOVA
analysis of variance
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- H&E
hematoxylin and eosin
- MTD
maximum tolerated dose
- PEITC
phenethyl isothiocyanate
- I3C
indole-3-carbinol.
References
- 1. Siegel R., et al. (2012). Cancer statistics, 2012. CA Cancer J. Clin., 62, 10–29 [DOI] [PubMed] [Google Scholar]
- 2. Siegel R., et al. (2013). Cancer statistics, 2013. CA Cancer J. Clin., 63, 11–30 [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J., et al. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer, 127, 2893–2917 [DOI] [PubMed] [Google Scholar]
- 4. Cohen V., et al. (2004). Chemoprevention of lung cancer. Curr. Opin. Pulm. Med., 10, 279–283 [DOI] [PubMed] [Google Scholar]
- 5. Hecht S.S. (2012). Lung carcinogenesis by tobacco smoke. Int. J. Cancer, 131, 2724–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mariotto A.B., et al. (2011). Projections of the cost of cancer care in the United States: 2010–2020. J. Natl. Cancer Inst., 103, 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hecht S.S., et al. (2009). Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat. Rev. Cancer, 9, 476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hecht S.S. (1998). Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol., 11, 559–603 [DOI] [PubMed] [Google Scholar]
- 9. Devesa S.S., et al. (1991). Changing patterns of lung cancer incidence by histological type. Cancer Epidemiol. Biomarkers Prev., 1, 29–34 [PubMed] [Google Scholar]
- 10. Burns D.M., et al. (2011). Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control, 22, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann D., et al. (1996). The biological significance of tobacco-specific N-nitrosamines: smoking and adenocarcinoma of the lung. Crit. Rev. Toxicol., 26, 199–211 [DOI] [PubMed] [Google Scholar]
- 12. Thun M.J., et al. (2013). Smoking-related mortality in the United States. N. Engl. J. Med., 368, 1753. [DOI] [PubMed] [Google Scholar]
- 13. Hoffmann D., et al. (1993). Cigarette smoking and adenocarcinoma of the lung: the relevance of nicotine-derived N-nitrosamines. J. Smok.Relat. Disord., 4, 165–189 [Google Scholar]
- 14. Hecht S.S. (2008). Progress and challenges in selected areas of tobacco carcinogenesis. Chem. Res. Toxicol., 21, 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Donnell E.P., et al. (2006). Quantitative analysis of early chemically-induced pulmonary lesions in mice of varying susceptibilities to lung tumorigenesis. Cancer Lett., 241, 197–202 [DOI] [PubMed] [Google Scholar]
- 16. Malkinson A.M. (2001). Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer, 32, 265–279 [DOI] [PubMed] [Google Scholar]
- 17. Peterson L.A., et al. (1991). O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res., 51, 5557–5564 [PubMed] [Google Scholar]
- 18. Liu L., et al. (1999). Reduced lung tumorigenesis in human methylguanine DNA–methyltransferase transgenic mice achieved by expression of transgene within the target cell. Carcinogenesis, 20, 279–284 [DOI] [PubMed] [Google Scholar]
- 19. Loechler E.L., et al. (1984). In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc. Natl. Acad. Sci. USA, 81, 6271–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Essigmann J.M., et al. (1986). Mutagenesis and repair of O6-substituted guanines. IARC Sci. Publ. 393–399 [PubMed] [Google Scholar]
- 21. Ronai Z.A., et al. (1993). G to A transitions and G to T transversions in codon 12 of the Ki-ras oncogene isolated from mouse lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and related DNA methylating and pyridyloxobutylating agents. Carcinogenesis, 14, 2419–2422 [DOI] [PubMed] [Google Scholar]
- 22. Malkinson AM. (1992). Primary lung tumors in mice: an experimentally manipulable model of human adenocarcinoma. Cancer Res., 52, 2670s–2676s [PubMed] [Google Scholar]
- 23. Leitzman P., et al. (2014). Kava blocks 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in association with reducing O6-methylguanine DNA adduct in A/J mice. Cancer Prev. Res. (Phila)., 7, 86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hecht S.S., et al. (1983). Effects of alpha-deuterium substitution on the mutagenicity of 4-(methyl-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Carcinogenesis, 4, 305–310 [DOI] [PubMed] [Google Scholar]
- 25. Peterson L.A., et al. (2013). Role of aldehydes in the toxic and mutagenic effects of nitrosamines. Chem. Res. Toxicol., 26, 1464–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lao Y., et al. (2006). Quantitation of pyridyloxobutyl DNA adducts of tobacco-specific nitrosamines in rat tissue DNA by high-performance liquid chromatography–electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol., 19, 674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sturla S.J., et al. (2005). Mass spectrometric analysis of relative levels of pyridyloxobutylation adducts formed in the reaction of DNA with a chemically activated form of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Chem. Res. Toxicol., 18, 1048–1055 [DOI] [PubMed] [Google Scholar]
- 28. Upadhyaya P., et al. (2008). Quantitation of pyridylhydroxybutyl-DNA adducts in liver and lung of F-344 rats treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol., 21, 1468–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaik A.A., et al. (2012). Economically viable efficient synthesis of (±)-Methysticin—a component in kava potential responsible for its cancer chemopreventive activity. ARKIVOC, viii, 137–145 [Google Scholar]
- 30. Belinsky S.A., et al. (1988). Cell specific differences in O6-methylguanine-DNA methyltransferase activity and removal of O6-methylguanine in rat pulmonary cells. Carcinogenesis, 9, 2053–2058 [DOI] [PubMed] [Google Scholar]
- 31. Peterson L.A., et al. (1993). Pyridyloxobutyl DNA adducts inhibit the repair of O6-methylguanine. Cancer Res., 53, 2780–2785 [PubMed] [Google Scholar]
- 32. Rowe A., et al. (2012). Are mould hepatotoxins responsible for kava hepatotoxicity? Phytother. Res., 26, 1768–1770 [DOI] [PubMed] [Google Scholar]
- 33. Teschke R., et al. (2013). Contaminant hepatotoxins as culprits for kava hepatotoxicity—fact or fiction? Phytother. Res., 27, 472–474 [DOI] [PubMed] [Google Scholar]
- 34. Reagan-Shaw S., et al. (2008). Dose translation from animal to human studies revisited. FASEB J., 22, 659–661 [DOI] [PubMed] [Google Scholar]
- 35. Morse M.A., et al. (1989). Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Res., 49, 2894–2897 [PubMed] [Google Scholar]
- 36. Prokopczyk B., et al. (1996). Effects of dietary 1,4-phenylenebis(methylene)selenocyanate on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation in lung and liver of A/J mice and F344 rats. Carcinogenesis, 17, 749–753 [DOI] [PubMed] [Google Scholar]
- 37. Crampsie M.A., et al. (2011). Phenylbutyl isoselenocyanate modulates phase I and II enzymes and inhibits 4-(methylnitrosamino)-1-(3-pyridyl)- 1-butanone-induced DNA adducts in mice. Cancer Prev. Res. (Phila)., 4, 1884–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morse M.A., et al. (1990). Effects of indole-3-carbinol on lung tumorigenesis and DNA methylation induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and on the metabolism and disposition of NNK in A/J mice. Cancer Res., 50, 2613–2617 [PubMed] [Google Scholar]
- 39. Lao Y., et al. (2007). Formation and accumulation of pyridyloxobutyl DNA adducts in F344 rats chronically treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol., 20, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Upadhyaya P., et al. (2009). Comparative levels of O6-methylguanine, pyridyloxobutyl-, and pyridylhydroxybutyl-DNA adducts in lung and liver of rats treated chronically with the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Drug Metab. Dispos., 37, 1147–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Upadhyaya P., et al. (1999). Tumorigenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol enantiomers and metabolites in the A/J mouse. Carcinogenesis, 20, 1577–1582 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.