Abstract
Aims
Atrial fibrillation ablation requires peri-procedural oral anticoagulation (OAC) to prevent thromboembolic events. There are several options for OAC. We evaluate peri-procedural AF ablation complications using a variety of peri-procedural OACs.
Methods and results
We examined peri-procedural OAC and groin, bleeding, and thromboembolic complications for 2334 consecutive AF ablations using open irrigated-tip radiofrequency (RF) catheters. Pre-ablation OAC was warfarin in 1113 (47.7%), dabigatran 426 (18.3%), rivaroxaban 187 (8.0%), aspirin 472 (20.2%), and none 136 (5.8%). Oral anticoagulation was always interrupted and intraprocedural anticoagulation was unfractionated heparin (activated clotting time, ACT = 237 ± 26 s). Pre- and post-OAC drugs were the same for 1591 (68.2%) and were different for 743 (31.8%). Following ablation, 693 (29.7%) were treated with dabigatran and 291 (12.5%) were treated with rivaroxaban. There were no problems changing from one OAC pre-ablation to another post-ablation. Complications included 12 (0.51%) pericardial tamponades [no differences for dabigatran (P = 0.457) or rivaroxaban (P = 0.163) compared with warfarin], 12 (0.51%) groin complications [no differences for rivaroxaban (P = 0.709) and fewer for dabigatran (P = 0.041) compared with warfarin]. Only 5 of 2334 (0.21%) required blood transfusions. There were two strokes (0.086%) and no transient ischaemic attacks (TIAs) in the first 48 h post-ablation. Three additional strokes (0.13%), and two TIAs (0.086%) occurred from 48 h to 30 days. Only one stroke had a residual deficit. Compared with warfarin, the neurologic event rate was not different for dabigatran (P = 0.684) or rivaroxaban (P = 0.612).
Conclusion
Using interrupted OAC, low target intraprocedural ACT, and irrigated-tip RF, the rate of peri-procedural groin, haemorrhagic, and thromboembolic complications was extremely low. There were only minimal differences between OACs. Low-risk patients may remain on aspirin/no OAC pre-ablation. There are no problems changing from one OAC pre-ablation to another post-ablation.
Keywords: Atrial fibrillation, Ablation, Dabigatran, Rivaroxaban, Warfarin, Anticoagulation
What's new?
Atrial fibrillation ablation requires peri-procedural oral anticoagulation (OAC) to prevent thromboembolic events.
There are now several options for OAC, both pre-procedure and post-procedure.
We found that low-risk patients did not need to be on any OAC pre-ablation.
There were no problems changing from one OAC pre-ablation to another post-ablation.
The overall incidence of bleeding and thromboembolic events was extremely low and there were minimal differences in bleeding and thromboembolic events when the novel oral anticoagulants (NOACs) were compared with warfarin.
Introduction
Anticoagulation strategy is an important component of atrial fibrillation (AF) ablation procedures.1,2 Pre-ablation anticoagulation strategies vary. Some centres begin warfarin 30 days pre-ablation with enoxaparin bridging in all patients regardless of cerebrovascular accident (CVA) risk.3 Other centres stop oral anticoagullants (OACs) pre-ablation and bridge with enoxaparin only in those patients on warfarin.4 Uninterrupted warfarin, continued at a therapeutic international normalized ratio (INR), has gained popularity recently with some studies suggesting that it may reduce procedural stroke risk.5–8 However, a recent multicentre study found more major complications with uninterrupted warfarin compared with both interrupted warfarin and dabigatran.9 All intraprocedural anticoagulation strategies use systemic unfractionated heparin guided by activated clotting time (ACT) levels most frequently targeted at >300–350 s;10 however, with open irrigated-tip ablation catheters, a target ACT of 225 is safe and reduces bleeding complications without increasing thromboembolic risks.11,12 Most post-ablation strategies use one of the OACs for several months post-procedure, although aspirin alone may be safe in low-risk patients.13
The introduction of the novel oral anticoagulants (NOACs) (dabigatran, rivaroxaban, and apixaban) provides new options for peri-procedural anticoagulation.14 Two meta-analyses15,16 show that warfarin and dabigatran are equivalent in safety and efficacy, while another suggests an increased risk of thromboembolism with dabigatran.17 Considerably less is known about rivaroxaban, although one study suggests that it is comparable with uninterrupted warfarin.18 This study reports our experience with interrupted warfarin, dabigatran, and rivaroxaban prior to and following AF ablation with regard to bleeding/thromboembolic events and changing OAC regimens at the time of ablation.
Methods
Patient population
The subjects were 1745 consecutive patients with symptomatic AF undergoing 2334 radiofrequency (RF) ablations at Sequoia Hospital, Redwood City, CA with initial ablation from April 2006 to May 2013. We also discuss 44 procedures aborted due to aortic perforation, or abnormal transesophageal echocardiogram (TEE). All signed written informed consent. Data collection was prospective and data analysis was retrospective and approved by the Sequoia Hospital Institutional Review Board. The first 123 patients treated with dabigatran are also included in the current study.19 Atrial fibrillation type was categorized as paroxysmal, persistent, and long-standing persistent.1
Ablation protocol
Antiarrhythmics were stopped five half-lives and amiodarone >3 months pre-ablation. General anaesthesia and right groin only venous access were used. A 7F duodecapolar catheter (St. Jude Livewire™) was positioned around the tricuspid annulus with the distal poles in the coronary sinus. A 9F Boston Scientific Ultra Ice™ (Natick, MA) intracardiac ultrasound catheter was used for the transseptal puncture, done using a 71 cm St. Jude BRK™ or Baylis NRG™ needle.20 All patients had a femoral or radial arterial line. The St. Jude NavX™ or Biosense Webster Carto3 mapping system was used with 3.5–4.0 mm open irrigated-tip RF ablation catheters at 50 W and ‘perpetual motion’.21 All patients underwent circumferential pulmonary vein (PV) isolation and a left atrial (LA) roof line ablation. Patients with right or LA isthmus flutter underwent additional linear ablation and some had low posterior LA lines and LA complex fractionated electrograms ablated. Some persistent AF patients underwent ablation of coronary sinus complex fractionated electrograms (at 30–35 W) and superior vena cava isolation. All PVs were mapped to document isolation with a circumferential mapping catheter (7F Webster Lasso™ or St. Jude Reflexion Spiral™), inserted beside the ablation catheter across the single transseptal puncture and sheath. Activation and entrainment mapping were used to ablate concomitant atrial flutters and tachycardias. Isoproterenol was given and non-PV triggers were mapped and ablated.
Anticoagulation
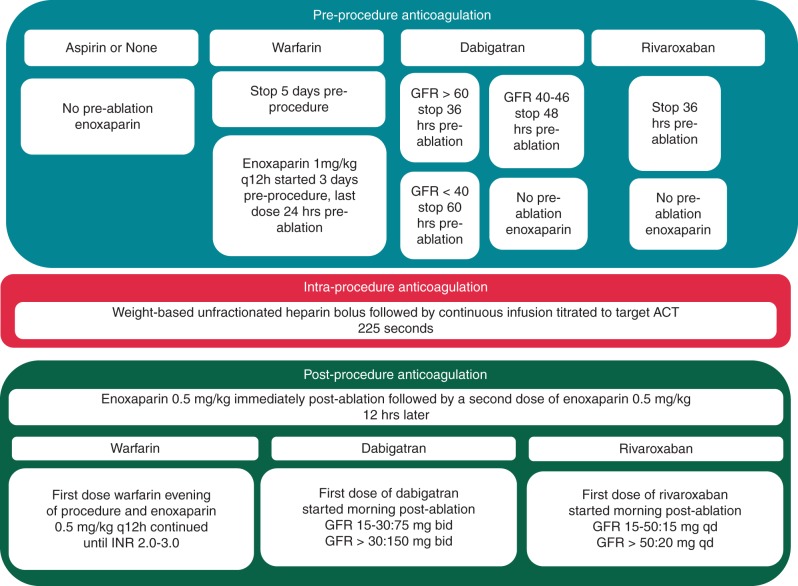
Peri-procedural anticoagulation was based on a predetermined algorithm (Figure 1). Patients receiving warfarin continued it until 5 days pre-procedure. Three days before they began enoxaparin 1 mg/kg every 12 h with the last dose 24 h pre-ablation. Patients receiving dabigatran had the drug stopped pre-procedure based upon their estimated glomerular filtration rate. Patients receiving rivaroxaban had the drug stopped 36 h pre-ablation. Patients receiving dabigatran, rivaroxaban, aspirin, or no OAC pre-ablation were not bridged with enoxaparin. Patients underwent a TEE under general anaesthesia immediately prior to ablation.
Figure 1.
Our algorithm for pre-procedural, intra-procedural, and post-procedural anticoagulation.
Intraprocedural anticoagulation was achieved with a weight-based unfractionated heparin bolus given when the transseptal sheath entered the LA followed by a 1000 unit/h infusion through the transseptal sheath. Additional heparin administration depended upon the ACT, targeted at 225 s.11,12 Heparin was reversed with protamine and the sheaths removed immediately at the end of the procedure. Two doses of enoxaparin 0.5 mg/kg were given, one immediately and one 12 h later. Dabigatran or rivaroxaban was started the morning after the procedure. Patients treated with warfarin post-ablation resumed the drug the evening of the procedure and continued enoxaparin 0.5 mg/kg twice daily until INR ≥ 2.0.
Complications
Major complications were categorized during the first 30 days post-ablation as death, pericardial tamponade, stroke/transient ischaemic attack (TIA) or systemic thromboembolism, atrioesophageal fistula, PV stenosis >50% requiring intervention, groin complications requiring thrombin injections, surgery or transfusion or other bleeding requiring transfusion. CVA/TIAs were subcategorized into those occurring during the ablation or in the first 48 h post-ablation and those occurring from 48 h to 30 days post-ablation.
Data collection and analysis
For each patient we recorded LA size, age, gender, AF duration, AF type, number of antiarrhythmic drugs failed, prior cardioversions, body mass index (BMI), CHADS2 and CHA2DS2-VASC scores, prior CVA/TIA, diabetes, coronary artery disease (CAD), and cardiomyopathy. We documented OAC pre-ablation and OAC prescribed immediately post-ablation. We determined the procedure time (groin access until the sheath removal) and the average ACT during each ablation.
Statistical analysis
Statistical analysis was done using XLSTAT 2012. Continuous data were described as mean ± standard deviation, and categorical data as counts and percentages. Clinical parameters were grouped by pre-ablation OAC. Clinical characteristics and the rates of neurologic events, groin bleeding, pericardial tamponade, and aborted procedures for LA clot for patients whose pre-ablation OAC was not warfarin were compared with patients on warfarin using t-tests or χ2/Fisher's exact tests. All tests were two sided and P < 0.05 was considered statistically significant.
Results
Patient population
Table 1 summarizes baseline demographics of the 1745 patients undergoing 2334 AF ablations. The mean age was 63.6 ± 9.8 years. The CHADS2 score was 1.08 ± 1.06, the CHA2DS2-VASC score was 1.91 ± 1.46 and 7.7% of patients had a prior CVA/TIA. The ablation was an initial ablation in 74.8% of procedures and a redo-ablation in 25.2% with an average of 1.34 ablations per patient. There were no procedure-related deaths, atrioesophageal fistulae, or PV stenoses requiring intervention.
Table 1.
Clinical characteristics of patients
| Number of patients | 1745 |
|---|---|
| LA size (cm) | 4.27 ± 0.68 |
| Age (years) | 63.6 ± 9.8 |
| Gender female | 29.7% |
| Duration of AF (years) | 6.4 ± 7.3 |
| #Drugs failed | 1.26 ± 1.06 |
| CHADS2 score | 1.08 ± 1.06 |
| CHA2DS2-VASC score | 1.91 ± 1.46 |
| Hypertension | 53.28% |
| Diabetes | 11.1% |
| BMI | 29.3 ± 5.5 |
| Paroxysmal AF | 31.4% |
| Persistent AF | 55.6% |
| Long-standing AF | 13.0% |
| Prior cardioversion | 47.3% |
| Coronary artery disease | 14.9% |
| Dilated cardiomyopathy | 9.2% |
| Prior stroke/TIA | 7.7% |
Pre-procedure, intraprocedure, and post-procedure anticoagulation
Table 2 summarizes the pre- and post-ablation OAC regimens. The pre-ablation anticoagulant was warfarin in 1113 (47.7%) ablations, dabigatran in 426 (18.3%), rivaroxaban in 187 (8.0%), and aspirin in 472 (20.2%). There were 136 (5.8%) ablations in patients on no OAC pre-ablation. The mean intraprocedural ACT was 237 ± 26 s with an average procedure time of 119 ± 42 min.
Table 2.
Number of patients on each OAC pre-ablation and post-ablation
| OAC before ablation | Number before ablation | OAC after ablation |
||||
|---|---|---|---|---|---|---|
| Aspirin | Warfarin | None | Dabigatran | Rivaroxaban | ||
| Warfarin | 1113 | 1 | 985 | 0 | 111 | 16 |
| Aspirin | 472 | 3 | 266 | 0 | 128 | 75 |
| None | 136 | 0 | 92 | 0 | 34 | 10 |
| Dabigatran | 426 | 1 | 0 | 0 | 419 | 6 |
| Rivaroxaban | 187 | 1 | 1 | 0 | 1 | 184 |
| Total | 2334 | 6 | 1344 | 0 | 693 | 291 |
OAC, oral anticoagulant.
Following ablation, patients were on a variety of OACs which were not always the same as the pre-ablation OAC (Table 2). Of the 2334 ablations, 1591 (68.2%) were on the same OAC before and after and 743 (31.8%) were on a different OAC post-ablation. Of the 608 patients on aspirin or no pre-ablation OAC, post-ablation, 358 began warfarin, 162 began dabigatran, 85 began rivaroxaban, and 3 remained on aspirin. Of 1726 patients on warfarin, dabigatran, or rivaroxaban pre-ablation, 1588 (92.0%) remained on the same OAC and 138 (8.0%) changed to a different OAC post-ablation. For warfarin, 127 of 1113 (11.4%) changed to dabigatran or rivaroxaban. One patient changed from rivaroxaban to warfarin for cost reasons and seven changed from one NOAC to the other. Post-ablation, 693 (29.7%) were treated with dabigatran and 291 (12.5%) with rivaroxaban. Six patients were discharged only on aspirin: two instances due to warfarin allergy (before NOACs were available) and aspirin was combined with enoxaparin for 1 month, one stroke patient due to concern for haemorrhagic conversion and three low-risk patients were discharged on aspirin alone after experiencing pericardial tamponade, mild gastrointestinal bleeding, and a retroperitoneal bleed.
Comparison of patient baseline characteristics by pre-ablation oral anticoagulation
Table 3 shows the baseline clinical characteristics of the patients grouped by pre-ablation OAC. Compared with the warfarin pre-ablation patients, there were major differences in baseline characteristics for patient's receiving aspirin or no OAC pre-ablation. The aspirin/no OAC patients had smaller LA size, were younger, failed fewer antiarrhythmic drugs, had lower CHADS2 and CHA2DS2-VASC scores, had less hypertension, diabetes, CAD, dilated cardiomyopathies, fewer prior CVA/TIAs, lower BMIs, and more paroxysmal AF. Compared with the warfarin patients, those receiving dabigatran had smaller LA size, higher CHADS2 scores, more diabetes, hypertension and dilated cardiomyopathies and more persistent though less long-standing persistent AF. Compared with warfarin patients, rivaroxaban patients had failed fewer prior drugs, had higher CHADS2 and CHA2DS2-VASC scores and had more hypertension and less long-standing persistent AF.
Table 3.
Baseline patient clinical characteristics by pre-ablation OAC
| OAC before ablation | Warfarin | Aspirin |
None |
Dabigatran |
Rivaroxaban |
||||
|---|---|---|---|---|---|---|---|---|---|
| Number of ablations | 1113 | 472 | 136 | 426 | 187 | ||||
| LA size (cm) | 4.44 ± 0.66 | 4.01 ± 0.62 | <0.0005* | 4.04 ± 0.74 | <0.0005* | 4.36 ± 0.63 | =0.031* | 4.34 ± 0.84 | =0.123 |
| Age (years) | 64.5 ± 9.5 | 60.6 ± 9.6 | <0 .0005* | 58.4 ± 11.8 | <0.0005* | 64.6 ± 8.1 | <0.848 | 65.8 ± 9.6 | =0.084 |
| Gender female | 28.9% | 29.4% | =0.856 | 33.1% | =0.136 | 27.5% | =0.571 | 30.5% | =0.728 |
| Duration of AF (years) | 6.6 ± 7.7 | 6.7 ± 6.9 | =0.808 | 5.8 ± 6.4 | =0.181 | 5.8 ± 7.2 | =0.064 | 6.4 ± 7.0 | =0.739 |
| # Drugs failed | 1.36 ± 1.12 | 1.13 ± 0.98 | <0.0005* | 1.13 ± 0.86 | =0.004* | 1.32 ± 1.09 | =0.528 | 1.18 ± 0.96 | =0.021* |
| CHADS2 score | 1.12 ± 1.05 | 0.61 ± 0.81 | <0.0005* | 0.58 ± 0.84 | <0.0005* | 1.28 ± 1.07 | =0.0008 | 1.55 + 1.04 | <0.0005* |
| CHA2DS2-VASC score | 1.98 ± 1.44 | 1.26 ± 1.18 | <0.0005* | 1.24 ± 1.20 | <0.0005* | 2.13 ± 1.45 | =0.068 | 2.48 + 1.46 | <0.0005* |
| Hypertension | 54.8% | 38.3% | <0.0005* | 34.6% | <0.0005* | 63.3% | =0.003* | 67.4% | =0.001* |
| Diabetes | 11.6% | 4.2% | <0.0005* | 4.4% | =0.012* | 16.0% | =0.026* | 16.0% | =0.09 |
| BMI | 30.1 ± 5.4 | 28.8 ± 4.9 | <0.0005* | 27.9 ± 5.2 | <0.0005* | 29.9 ± 6.1 | =0.531 | 29.3 ± 5.4 | =0.061 |
| Paroxysmal AF | 21.7% | 48.1% | <0.0005* | 48.9% | <0.0005* | 21.7% | =0.944 | 26.5% | =0.189 |
| Persistent AF | 56.8% | 46.5% | <0.0005* | 45.9% | =0.017* | 64.1% | =0.013* | 63.5% | =0.0885 |
| Long-standing AF | 21.5% | 5.1% | <0.0005* | 5.2% | <0.0005* | 14.2% | =0.002* | 9.9% | =0.001* |
| Prior cardioversion | 55.9% | 27.3% | <0.0005* | 30.9% | <0.0005* | 57.0% | =0.688 | 50.8% | =0.204 |
| Coronary artery disease | 16.6% | 11.2% | =0.007* | 8.8% | =0.024* | 16.2% | =0.878 | 15.6% | =0.596 |
| Dilated cardiomyopathy | 10.8% | 4.2% | <0.0005* | 2.9% | =0.003* | 15.0% | =0.023* | 8.0% | =0.30 |
| Prior stroke/TIA | 10.3% | 1.9% | <0.0005* | 2.3% | =0.002* | 9.1% | =0.452 | 6.4% | 0.087 |
AF, atrial fibrillation; TIA, transient ischaemic attack; OAC, oral anticoagulant.
The P values compare each clinical characteristic for each non-warfarin OAC with the patients on warfarin.
*Statistically significant compared with the warfarin group.
Haemorrhagic, groin and thromboembolic complications
Table 4 summarizes haemorrhagic, groin and thromboembolic events categorized by pre-ablation OAC. There were 12 of 2334 (0.51%) pericardial tamponades. Eight were treated with emergent pericardiocentesis and two with surgical drainage. Two underwent semi-elective pericardiocentesis post-hospital discharge. When compared with warfarin patients, there were no differences in the rate of tamponade for either dabigatran (P = 0.457) or rivaroxaban (P = 0.163). Groin complications occurred in 12 of 2334 (0.51%) ablations. This included nine groin pseudoaneurysms or AV fistulae, six treated with ultrasound-guided compression or local thrombin injections and three requiring surgical repair. Two groin haematomas and one retroperitoneal bleed required transfusion. Eleven of 12 patients with groin complications were on warfarin pre- and post-ablation. One patient with a retroperitoneal bleed was on rivaroxaban pre-ablation and aspirin post-ablation. Compared with the patients on warfarin, there were no differences in the rate of groin complications for rivaroxaban (P = 0.709) and there were fewer groin complications for dabigatran (P = 0.041).
Table 4.
Major haemorrhagic, groin, and thromboembolic complications by pre-ablation OAC
| OAC before ablation | Number before ablation | Groin, haemorrhagic, and thromboembolic complications |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pericardial tamponade | Groin complications | Strokes <48 h | TIAs <48 h | Strokes (48 h to 30 days) | TIAs (48 h to 30 days) | Other bleeding | DVT/pulmonary embolus | ||
| Warfarin | 1113 | 7 (0.63%) | 11 (0.99%) | 1 (0.090%) | 0 | 3 (0.27%) | 1 | 1 (0.090%) | 0 |
| Aspirin | 472 | 1 (0.21%) | 0 | 0 | 0 | 0 | 1 | 1 (0.21%) | 0 |
| None | 136 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dabigatran | 426 | 1 (0.23%) | 0 | 1 (0.23%) | 0 | 0 | 0 | 0 | 1 (0.23%) |
| Rivaroxaban | 187 | 3 (1.60%) | 1 (0.53%) | 0 | 0 | 0 | 0 | 1 (0.53%) | 0 |
| Total | 2334 | 12 (0.51%) | 12 (0.51%) | 2 (0.086%) | 0 | 3 (0.13%) | 2 (0.086%) | 3 (0.128%) | 1 (0.043%) |
OAC, oral anticoagulant; TIA, transient ischaemic attack; DVT, deep venous thrombosis.
There were three major non-groin bleeding complications: one traumatic urinary catheter insertion requiring blood transfusion in a warfarin patient; one intubation-related tongue haematoma; and one ruptured cerebral aneurysm on Day 7 post-ablation in a rivaroxaban patient who had also been on rivaroxaban pre-ablation. After a failed catheter attempt to seal the aneurysm, the patient underwent surgical treatment and expired on Day 31 post-ablation. One patient on dabigatran had a small pulmonary embolus several weeks post-ablation which resolved without sequelae. Only 5 of 2334 (0.21%) ablations were associated with a complication requiring blood transfusion.
There were two intraprocedural strokes (0.086%) and no TIA's in the first 48 h post-ablation. One stroke resolved over time and the other left a residual deficit. Three additional strokes (0.13%)—all resolved without permanent deficit—and two TIAs (0.086%) occurred from 48 h to 30 days post-ablation. One stroke occurred in a warfarin patient with a mechanical valve several weeks post-ablation, one occurred 4 days post-procedure in a patient with a gastrointestinal bleed requiring OAC to be withheld, and the third stroke occurred 2 weeks post-procedure when OAC was held at another centre in the presence of ongoing AF to perform a pacemaker implantation. The total 30-day stroke rate was 5 of 2334 (0.21%) only one of whom had a significant residual deficit and the total 30-day TIA rate was 2 of 2334 (0.086%). Compared with warfarin, the rate of neurologic events was similar for dabigatran (P = 0.684) and rivaroxaban (P = 0.612).
Oral anticoagulation changes during blanking period
The initial post-ablation OAC was changed to another OAC during the 3-month post-ablation blanking period in 43 of 2334 (1.84%) instances. The reasons for these changes were gastrointestinal side effects in 14, minor bleeding in 7, physician/patient preference in 7, other minor side effects in 5, rash in 2, unstable INR in 2, and unknown in 4. The only common themes to these OAC changes were that 12 of 693 (1.73%) on dabigatran changed OACs for gastrointestinal side effects, 7 of 1344 (0.52%) warfarin patients changed to newer OACs because of patient/physician preference, and 6 of 291 (2.1%) rivaroxaban patients changed OACs for minor bleeding problems. Stopping dabigatran for gastrointestinal side effects was more frequent than for either warfarin (P < 0.0005) or rivaroxaban (P = 0.01). The number of rivaroxaban patients changing OACs because of minor bleeding was greater than with either warfarin (P < 0.0005) or dabigatran (P = 0.01).
Aborted ablations including those with left atrial thrombus
Ablations were aborted in 44 instances in addition to the 2334 ablations. Two patients had inadvertent aortic puncture with the transseptal sheath entering the aortic root. Both patients were transported to the operating room with the sheaths in the aorta. With cardiovascular surgical standby, the sheaths were pulled under TEE monitoring. Both puncture wounds clotted and sealed immediately without sequelae. Three patients had procedures cancelled because of TEE anatomical findings (1 LA myxoma, 1 cor-triatriatum, and 1 mitral regurgitation referred for mitral valve repair and LA Maze). There were 39 of 2373 (1.64%) ablations aborted because of LA appendage thrombus visualized on TEE. The rates of procedures aborted for LA/LAA thrombus on TEE were: warfarin 16 of 1129 (1.42%), aspirin 3 of 457 (0.63%), no OAC 0 of 136 (0.0%), dabigatran 11 of 437 (2.52%), and rivaroxaban 9 of 196 (4.6%). The rate of aborted procedures was not different from warfarin for aspirin (P = 0.216), no OAC (P = 0.244), or dabigatran (P = 0.192) but was greater for rivaroxaban (P = 0.007). Despite our intentions for the patients on rivaroxaban to be off anticoagulation for only 36 h pre-ablation, of the nine patients with clot noted on TEE, four had been off rivaroxaban for 60 h, four had been off for 48 h, and only one had been off for the planned 36 h.
Discussion
In this study, we report our experience with 2334 ablations in 1745 patients with symptomatic AF. We evaluated all ablations after April 2006, when we switched to using open irrigated-tip RF catheters at a power setting of 50 W20 and lowered our target intraprocedural ACT to 225 s.11,12 The findings of this study are (i) using the open irrigated-tip RF catheter, interrupted OAC can be associated with a very low incidence of bleeding/thromboembolic events; (ii) interrupted OAC permits safe transitioning of patients from one OAC pre-ablation to another post-ablation; (iii) low-risk patients on aspirin or no OAC pre-ablation do not need to begin OAC prior to AF ablation; and (iv) there were minimal differences between the use of warfarin, dabigatran, or rivaroxaban.
Despite a recent trend towards uninterrupted OAC for AF ablation,5–8 interrupted OAC provides several advantages. Many low-risk patients are on aspirin or no OAC pre-ablation and do not require either OAC or the risks of finding a therapeutic warfarin dose pre-procedure. Some patients on uninterrupted warfarin will not have a stable INR pre-ablation and may arrive with a subtherapeutic or supratherapeutic INR. With the NOACs, there is no test to verify patient compliance pre-ablation and the lack of proven reversal agents may add risk if they are not interrupted. Bleeding complications are easier to handle with interrupted OAC, illustrated by our two patients with aortic perforation. They were unanticoagulated at the time of perforation when the sheath was withdrawn without sequela. Finally, the majority of our current patients are warfarin naïve and already on a NOAC pre-ablation and are uninterested in taking warfarin to facilitate uninterrupted anticoagulation.
Uninterrupted warfarin does not completely eliminate stroke risk or peri-procedural anticoagulation issues. Hussein, et al.5 reported 3052 patients receiving uninterrupted warfarin with three intraprocedural ischaemic strokes (0.098%), virtually identical to our rate of 0.086%. Di Biase et al.22 reported no intraprocedural strokes in 2618 patients undergoing uninterrupted warfarin using open irrigated-tip RF; however, they excluded patients with subtherapeutic or supratherapeutic INRs on the day of the ablation so the ‘intention to treat’ outcome of uninterrupted warfarin is unknown. Patients with tamponade required more fresh frozen plasma and transfusions and had more pericardial blood aspirated. Another study examining uninterrupted warfarin7 had 2 of 87 (2.3%) cases cancelled because of supratherapeutic INRs and reported a procedure-related stroke in 1.15%. Another study8 suggesting that TEEs were unnecessary with uninterrupted warfarin, had 5 of 89 (5.6%) with a supratherapeutic INR on the day of the procedure and 6 of 89 (6.7%) with a subtherapeutic INR. A stroke occurred in 1.12% despite uninterrupted warfarin.
The use of uninterrupted warfarin followed an era of interrupted warfarin. Thus, published retrospective comparisons were done in two different timeframes of AF ablation. Some of the apparent advantages of uninterrupted warfarin may instead be due to improvements in ablation safety over time. The single meta-analysis comparing uninterrupted vs. interrupted warfarin23 included 11 093 patients with uninterrupted warfarin. However, 9861 (89%) of those patients came from several publications of the co-authors. It is unclear how many unique patients existed and how many contributed data more than once to the meta-analysis. Arshad et al.9 recently compared concurrent peri-ablation anticoagulation strategies at four experienced AF ablation centres. They found that major complications (stroke/TIA, pericardial tamponade, transfusion or major bleeding, and surgical intervention) occurred more frequently in the uninterrupted warfarin group (4.3%) vs. both the dabigatran group (0.8%) and the bridged warfarin group (2.6%) (P < 0.01). Given the extraordinarily low rate of intraprocedural CVA/TIAs in our series, it would be impossible for us to do a randomized trial to prove superiority of either interrupted or uninterrupted OAC and interrupted OAC seems at least as safe as uninterrupted OAC.
We observed relatively few differences in complication rates for patients on warfarin compared with those receiving NOACs. The lower rate of groin complications with dabigatran may be attributable to more frequent use of ultrasound to guide instrumentation later in our series when more dabigatran was used. The high rate of LA appendage thrombus found in rivaroxaban patients may be due to their higher CHADS2 and CHA2DS2-VASc scores, and that they were inadvertently off of rivaroxaban longer than intended. The high rate of LA clot noted with rivaroxaban emphasizes the importance of performing a TEE prior to instrumentation in all AF ablations, especially those at higher risk of thromboembolic events and those on NOAC's where patient adherence cannot be verified.
There are many reasons why patients might want to switch from one OAC to another in the peri-ablation period. Warfarin patients may desire to avoid frequent blood testing. Some on dabigatran experience gastrointestinal symptoms and desire to switch to a different NOAC. Insurance coverage may make it financially advantageous to change OACs. It is convenient and probably safer to leave low-risk patients on aspirin/no OAC pre-ablation. Gomes et al.24 showed the first 30 days after starting warfarin was the most risky with major bleeding of 11.8%/patient year compared with an overall rate of 3.8%/patient year. If low-risk patients remain on aspirin/no OAC pre-ablation, they can be started on a NOAC post-ablation and avoid the high-risk period of warfarin initiation. It is a testimony as to how well patients tolerate NOACs that 603 of 613 (98.4%) patients on dabigatran/rivaroxaban desired to stay on the same drug post-ablation.
Limitations
This was a retrospective single centre study. Although our ablation technique was nearly constant throughout the period of this study, we used groin ultrasound more frequently later in this series and we also introduced the use of a RF needle for transseptal puncture, which reduced the rate of pericardial tamponade.20 All of these patients were enrolled at a single, high-volume centre with expertise in performing AF ablation. We did not include minor bleeding complications as they are difficult to quantify and may be under-reported by patients.
Conclusions
Using interrupted OAC, low target intraprocedural ACTs, and irrigated-tip RF at 50 W, the rates of peri-procedural groin, haemorrhagic, and thromboembolic complications were extremely low. Low-risk patients may remain on aspirin or no OAC pre-ablation and do not need to change to warfarin or one of the NOACs pre-ablation. Patients may safely change from one OAC pre-ablation to another OAC post-ablation and there do not seem to be major differences between warfarin and dabigatran/rivaroxaban with regard to safety and efficacy.
Funding
Funding to pay the Open Access publication charges for this article was provided by Silicon Valley Cardiology.
Acknowledgements
Patricia Barberini, R.N., Cynthia Lebsack, Pharm. D, William Fleming, B.S., and Glenda Rhodes assisted with data and manuscript management.
Conflict of interest: none declared.
References
- 1.Calkins H, Brugada J, Packer DL, Capppato R, Chen S, Crijns H, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Europace. 2007;9:335–79. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 2.Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes M, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2011;8:157–76. doi: 10.1016/j.hrthm.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill MD, Wright M, Knecht S, Jais P, Hocini M, Takahashi Y, et al. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur Heart J. 2009;30:1105–12. doi: 10.1093/eurheartj/ehp063. [DOI] [PubMed] [Google Scholar]
- 4.Winkle RA, Mead RH, Engel G, Patrawala RA. Long term results of atrial fibrillation ablation: the importance of all initial ablation failures undergoing a repeat ablation. Am Heart J. 2011;162:193–200. doi: 10.1016/j.ahj.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Hussein AA, Martin DO, Saliba W, Patel D, Karim S, Batal O, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm. 2009;6:1425–9. doi: 10.1016/j.hrthm.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Wazni OM, Heheiry S, Fahmy T, Barrett C, Hoa S, Patel D, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratio. Circulation. 2007;116:2531–4. doi: 10.1161/CIRCULATIONAHA.107.727784. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt M, Segerson NM, Marschang H, Akoum N, Rittger H, Clifford SM, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratios. Pacing Clin Elecrophysiol. 2009;32:995–9. doi: 10.1111/j.1540-8159.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 8.Page SP, Siddiqui MS, Finlay M, Hunter RJ, Abrams DJ, Dhinoja M, et al. Catheter ablation for atrial fibrillation on uninterrupted warfarin: can it be done without echo guidance? J Cardiovasc Electrophysiol. 2011;22:265–70. doi: 10.1111/j.1540-8167.2010.01910.x. [DOI] [PubMed] [Google Scholar]
- 9.Arshad A, Johnson CK, Mittal S, Buch E, Hamam I, Tran T, et al. Comparative safety of peri-ablation anticoagulation strategies for atrial fibrillation: data from a large multicenter study. Pacing Clin Electrophysiol. 2014;37:665–73. doi: 10.1111/pace.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raviele A, Natale A, Calkins H, Camm JA, Cappato R, Chen SA, et al. Venice chart international consensus document on atrial fibrillation ablation: 2011 update. J Cardiovasc Electrophysiol. 2012;23:890–923. doi: 10.1111/j.1540-8167.2012.02381.x. [DOI] [PubMed] [Google Scholar]
- 11.Winkle RA, Mead RH, Engel G, Patrawala RA. Safety of lower activated clotting times during atrial fibrillation ablation using open irrigated tip catheters and a single transseptal puncture. Am J Cardiol. 2011;107:704–8. doi: 10.1016/j.amjcard.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 12.Winkle RA, Mead RH, Engel G, Patrawala RA. Atrial fibrillation ablation using open irrigated-tip RF: experience with intraprocedural ACT <210 seconds. Heart Rhythm. 2014;11:963–8. doi: 10.1016/j.hrthm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, et al. Warfarin is not needed in low-risk patients following atrial fibrillation ablation procedures. J Cardiovasc Electrophysiol. 2009;20:988–93. doi: 10.1111/j.1540-8167.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 14.Winkle RA, Kong MH. Peri-ablation warfarin vs. dabigatran: different but the same. Europace. 2013;15:1385–6. doi: 10.1093/europace/eut295. [DOI] [PubMed] [Google Scholar]
- 15.Hohnloser SH, Camm AJ. Safety and efficacy of dabigatran etexilate during catheter ablation of atrial fibrillation: a meta-analysis of the literature. Europace. 2013;15:1407–11. doi: 10.1093/europace/eut241. [DOI] [PubMed] [Google Scholar]
- 16.Abdulhak AAB, Khan AR, Tleyjeh IM, Spertus JA, Sanders SU, Steigerwarlt KE, et al. Safety and efficacy of interrupted dabigatran for peri-procedural anticoagulation in catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace. 2013;15:1412–20. doi: 10.1093/europace/eut239. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg BA, Hasselblad V, Atwate BD, Bahnson TD, Washam JB, Alexander JH, et al. Dabigatran for periprocedural anticoagulation following radiofrequency ablation for atrial fibrillation: a meta-analysis of observational studies. J Interv Card Electrophysiol. 2013;37:213–21. doi: 10.1007/s10840-013-9813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakkireddy D, Reddy YM, Di Biase L, Vallakati A, Mansour MC, Santangeli P, et al. Feasibility & safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2013;63:982–8. doi: 10.1016/j.jacc.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. The use of dabigatran immediately after atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2011;24:264–8. doi: 10.1111/j.1540-8167.2011.02175.x. [DOI] [PubMed] [Google Scholar]
- 20.Winkle RA, Mead RH, Engel G, Patrawala RA. The use of a radiofrequency needle improves the safety and efficacy of transseptal puncture for atrial fibrillation ablation. Heart Rhythm. 2011;8:1411–5. doi: 10.1016/j.hrthm.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Winkle RA, Mead RH, Engel G, Patrawala RA. Atrial fibrillation ablation: perpetual motion of open irrigated tip catheters at 50 Watts is safe and improves outcomes. Pacing Clin Elecrophysiol. 2011;34:531–9. doi: 10.1111/j.1540-8159.2010.02990.x. [DOI] [PubMed] [Google Scholar]
- 22.Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Horton R, Gallinghouse GJ, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation. Ciculation. 2010;121:2550–6. doi: 10.1161/CIRCULATIONAHA.109.921320. [DOI] [PubMed] [Google Scholar]
- 23.Santangeli P, Di Biase L, Horton R, Burkhardt JD, Sanchez J, Al-Ahmad A, et al. Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications: evidence from a meta-analysis. Circ Arrhythm Electrophysiol. 2012;5:302–11. doi: 10.1161/CIRCEP.111.964916. [DOI] [PubMed] [Google Scholar]
- 24.Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Hellings C, Juurlink DN. Rates of hemorrhage during warfarin therapy for atrial fibrillation. Can Med Assoc J. 2013;185:E121–7. doi: 10.1503/cmaj.121218. [DOI] [PMC free article] [PubMed] [Google Scholar]