Abstract
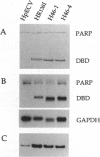
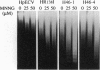
Poly(ADP-ribose) polymerase [PARP; NAD+ ADP-ribosyltransferase; NAD+:poly(adenosine-diphosphate-D-ribosyl)-acceptor ADP-D-ribosyltransferase, EC 2.4.2.30] is a zinc-dependent eukaryotic DNA-binding protein that specifically recognizes DNA strand breaks produced by various genotoxic agents. To study the biological function of this enzyme, we have established stable HeLa cell lines that constitutively produce the 46-kDa DNA-binding domain of human PARP (PARP-DBD), leading to the trans-dominant inhibition of resident PARP activity. As a control, a cell line was constructed, producing a point-mutated version of the DBD, which has no affinity for DNA in vitro. Expression of the PARP-DBD had only a slight effect on undamaged cells but had drastic consequences for cells treated with genotoxic agents. Exposure of cell lines expressing the wild-type (wt) or the mutated PARP-DBD, with low doses of N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) resulted in an increase in their doubling time, a G2 + M accumulation, and a marked reduction in cell survival. However, UVC irradiation had no preferential effect on the cell growth or viability of cell lines expressing the PARP-DBD. These PARP-DBD-expressing cells treated with MNNG presented the characteristic nucleosomal DNA ladder, one of the hallmarks of cell death by apoptosis. Moreover, these cells exhibited chromosomal instability as demonstrated by higher frequencies of both spontaneous and MNNG-induced sister chromatid exchanges. Surprisingly, the line producing the mutated DBD had the same behavior as those producing the wt DBD, indicating that the mechanism of action of the dominant-negative mutant involves more than its DNA-binding function. Altogether, these results strongly suggest that PARP is an element of the G2 checkpoint in mammalian cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Richter C. ADP-ribosylation of proteins. Enzymology and biological significance. Mol Biol Biochem Biophys. 1987;37:1–237. [PubMed] [Google Scholar]
- Belt P. B., Groeneveld H., Teubel W. J., van de Putte P., Backendorf C. Construction and properties of an Epstein-Barr-virus-derived cDNA expression vector for human cells. Gene. 1989 Dec 14;84(2):407–417. doi: 10.1016/0378-1119(89)90515-5. [DOI] [PubMed] [Google Scholar]
- Boorstein R. J., Pardee A. B. Factors modifying 3-aminobenzamide cytotoxicity in normal and repair-deficient human fibroblasts. J Cell Physiol. 1984 Sep;120(3):335–344. doi: 10.1002/jcp.1041200312. [DOI] [PubMed] [Google Scholar]
- Cardenas-Corona M. E., Jacobson E. L., Jacobson M. K. Endogenous polymers of ADP-ribose are associated with the nuclear matrix. J Biol Chem. 1987 Nov 5;262(31):14863–14866. [PubMed] [Google Scholar]
- Chatterjee S., Petzold S. J., Berger S. J., Berger N. A. Strategy for selection of cell variants deficient in poly(ADP-ribose) polymerase. Exp Cell Res. 1987 Oct;172(2):245–257. doi: 10.1016/0014-4827(87)90384-3. [DOI] [PubMed] [Google Scholar]
- Cortés F., Piñero J., Ortiz T. Importance of replication fork progression for the induction of chromosome damage and SCE by inhibitors of DNA topoisomerases. Mutat Res. 1993 Oct;303(2):71–76. doi: 10.1016/0165-7992(93)90097-f. [DOI] [PubMed] [Google Scholar]
- Ding R., Pommier Y., Kang V. H., Smulson M. Depletion of poly(ADP-ribose) polymerase by antisense RNA expression results in a delay in DNA strand break rejoining. J Biol Chem. 1992 Jun 25;267(18):12804–12812. [PubMed] [Google Scholar]
- Eki T., Hurwitz J. Influence of poly(ADP-ribose) polymerase on the enzymatic synthesis of SV40 DNA. J Biol Chem. 1991 Feb 15;266(5):3087–3100. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferro A. M., Higgins N. P., Olivera B. M. Poly(ADP-ribosylation) of a DNA topoisomerase. J Biol Chem. 1983 May 25;258(10):6000–6003. [PubMed] [Google Scholar]
- Goldman N., Brown M., Khoury G. Modification of SV40 T antigen by poly ADP-ribosylation. Cell. 1981 May;24(2):567–572. doi: 10.1016/0092-8674(81)90347-0. [DOI] [PubMed] [Google Scholar]
- Gradwohl G., Ménissier de Murcia J. M., Molinete M., Simonin F., Koken M., Hoeijmakers J. H., de Murcia G. The second zinc-finger domain of poly(ADP-ribose) polymerase determines specificity for single-stranded breaks in DNA. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2990–2994. doi: 10.1073/pnas.87.8.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jacobson E. L., Meadows R., Measel J. Cell cycle perturbations following DNA damage in the presence of ADP-ribosylation inhibitors. Carcinogenesis. 1985 May;6(5):711–714. doi: 10.1093/carcin/6.5.711. [DOI] [PubMed] [Google Scholar]
- Kartasova T., Cornelissen B. J., Belt P., van de Putte P. Effects of UV, 4-NQO and TPA on gene expression in cultured human epidermal keratinocytes. Nucleic Acids Res. 1987 Aug 11;15(15):5945–5962. doi: 10.1093/nar/15.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Khochbin S., Chabanas A., Albert P., Albert J., Lawrence J. J. Application of bromodeoxyuridine incorporation measurements to the determination of cell distribution within the S phase of the cell cycle. Cytometry. 1988 Sep;9(5):499–503. doi: 10.1002/cyto.990090516. [DOI] [PubMed] [Google Scholar]
- Küpper J. H., de Murcia G., Bürkle A. Inhibition of poly(ADP-ribosyl)ation by overexpressing the poly(ADP-ribose) polymerase DNA-binding domain in mammalian cells. J Biol Chem. 1990 Nov 5;265(31):18721–18724. [PubMed] [Google Scholar]
- Le Cam E., Fack F., Ménissier-de Murcia J., Cognet J. A., Barbin A., Sarantoglou V., Révet B., Delain E., de Murcia G. Conformational analysis of a 139 base-pair DNA fragment containing a single-stranded break and its interaction with human poly(ADP-ribose) polymerase. J Mol Biol. 1994 Jan 21;235(3):1062–1071. doi: 10.1006/jmbi.1994.1057. [DOI] [PubMed] [Google Scholar]
- MacLaren R. A., Witmer M. V., Richardson E., Stamato T. D. Isolation of Chinese hamster ovary cells with reduced poly(ADP-ribose) polymerase activity. Mutat Res. 1990 Aug;231(2):265–274. doi: 10.1016/0027-5107(90)90032-y. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam K. M., Cleaver J. E. Inhibitors of poly(adenosine diphosphate-ribose) synthesis: effect on other metabolic processes. Science. 1984 Feb 10;223(4636):589–591. doi: 10.1126/science.6420886. [DOI] [PubMed] [Google Scholar]
- Molinete M., Vermeulen W., Bürkle A., Ménissier-de Murcia J., Küpper J. H., Hoeijmakers J. H., de Murcia G. Overproduction of the poly(ADP-ribose) polymerase DNA-binding domain blocks alkylation-induced DNA repair synthesis in mammalian cells. EMBO J. 1993 May;12(5):2109–2117. doi: 10.1002/j.1460-2075.1993.tb05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A. T., Csukás I., van Zeeland A. A. Contribution of incorporated 5-bromodeoxyuridine in DNA to the frequencies of sister-chromatid exchanges induced by inhibitors of poly-(ADP-ribose)-polymerase. Mutat Res. 1981 Nov;84(1):125–132. doi: 10.1016/0027-5107(81)90056-7. [DOI] [PubMed] [Google Scholar]
- Negri C., Bernardi R., Braghetti A., Ricotti G. C., Scovassi A. I. The effect of the chemotherapeutic drug VP-16 on poly(ADP-ribosylation) in apoptotic HeLa cells. Carcinogenesis. 1993 Dec;14(12):2559–2564. doi: 10.1093/carcin/14.12.2559. [DOI] [PubMed] [Google Scholar]
- Oikawa A., Tohda H., Kanai M., Miwa M., Sugimura T. Inhibitors of poly(adenosine diphosphate ribose) polymerase induce sister chromatid exchanges. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1311–1316. doi: 10.1016/s0006-291x(80)80009-x. [DOI] [PubMed] [Google Scholar]
- Satoh M. S., Poirier G. G., Lindahl T. NAD(+)-dependent repair of damaged DNA by human cell extracts. J Biol Chem. 1993 Mar 15;268(8):5480–5487. [PubMed] [Google Scholar]
- Schreiber V., Molinete M., Boeuf H., de Murcia G., Ménissier-de Murcia J. The human poly(ADP-ribose) polymerase nuclear localization signal is a bipartite element functionally separate from DNA binding and catalytic activity. EMBO J. 1992 Sep;11(9):3263–3269. doi: 10.1002/j.1460-2075.1992.tb05404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbulan C. M., Suzuki M., Izuta S., Sakurai T., Savoysky E., Kojima K., Miyahara K., Shizuta Y., Yoshida S. Poly(ADP-ribose) polymerase stimulates DNA polymerase alpha by physical association. J Biol Chem. 1993 Jan 5;268(1):93–99. [PubMed] [Google Scholar]
- Simonin F., Briand J. P., Muller S., de Murcia G. Detection of poly(ADP ribose) polymerase in crude extracts by activity-blot. Anal Biochem. 1991 Jun;195(2):226–231. doi: 10.1016/0003-2697(91)90321-j. [DOI] [PubMed] [Google Scholar]
- Simonin F., Poch O., Delarue M., de Murcia G. Identification of potential active-site residues in the human poly(ADP-ribose) polymerase. J Biol Chem. 1993 Apr 25;268(12):8529–8535. [PubMed] [Google Scholar]
- Slichenmyer W. J., Nelson W. G., Slebos R. J., Kastan M. B. Loss of a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 1993 Sep 15;53(18):4164–4168. [PubMed] [Google Scholar]
- Yoshihara K., Itaya A., Hironaka T., Sakuramoto S., Tanaka Y., Tsuyuki M., Inada Y., Kamiya T., Ohnishi K., Honma M. Poly(ADP ribose) polymerase-defective mutant cell clone of mouse L1210 cells. Exp Cell Res. 1992 May;200(1):126–134. doi: 10.1016/s0014-4827(05)80080-1. [DOI] [PubMed] [Google Scholar]
- de Murcia G., Jongstra-Bilen J., Ittel M. E., Mandel P., Delain E. Poly(ADP-ribose) polymerase auto-modification and interaction with DNA: electron microscopic visualization. EMBO J. 1983;2(4):543–548. doi: 10.1002/j.1460-2075.1983.tb01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Murcia G., Ménissier de Murcia J. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci. 1994 Apr;19(4):172–176. doi: 10.1016/0968-0004(94)90280-1. [DOI] [PubMed] [Google Scholar]