Abstract
Adverse drug reaction (ADR) risk-prediction models for use in older adults have been developed, but it is not clear if they are suitable for use in clinical practice. This systematic review aimed to identify and investigate the quality of validated ADR risk-prediction models for use in older adults. Standard computerized databases, the gray literature, bibliographies, and citations were searched (2012) to identify relevant peer-reviewed studies. Studies that developed and validated an ADR prediction model for use in patients over 65 years old, using a multivariable approach in the design and analysis, were included. Data were extracted and their quality assessed by independent reviewers using a standard approach. Of the 13,423 titles identified, only 549 were associated with adverse outcomes of medicines use. Four met the inclusion criteria. All were conducted in inpatient cohorts in Western Europe. None of the models satisfied the four key stages in the creation of a quality risk prediction model; development and validation were completed, but impact and implementation were not assessed. Model performance was modest; area under the receiver operator curve ranged from 0.623 to 0.73. Study quality was difficult to assess due to poor reporting, but inappropriate methods were apparent. Further work needs to be conducted concerning the existing models to enable the development of a robust ADR risk-prediction model that is externally validated, with practical design and good performance. Only then can implementation and impact be assessed with the aim of generating a model of high enough quality to be considered for use in clinical care to prioritize older people at high risk of suffering an ADR.
Keyword: aged, stratified care, prognosis, medication-related harm
Introduction
Adverse drug reactions (ADRs) have long been recognized as a potential outcome of taking medicines, and while the severity of such reactions may vary, a significant proportion of ADRs are responsible for hospital admissions.1 Investigators have strived to identify the key factors that increase a person’s risk of suffering an ADR, especially in older adults, a group nearly seven-times more likely to be hospitalized due to an ADR when compared to younger people.2
We know that the changes in drug pharmacokinetic and pharmacodynamic properties that occur as a result of the aging process often lead to an increased susceptibility to ADRs.3 Polypharmacy, a frequently reported risk factor for ADRs,4 is on the increase as people live longer with multiple chronic conditions, so stratifying an older patient’s risk of suffering an ADR might be attractive.
Risk prediction is a routine component of everyday medicine in both specific areas (for example, approaches used to determine stroke risk in patients with atrial fibrillation)5 as well as more generally, to identify patients at risk of hospital admission.6 ADR risk stratification in older adults could assist in case prioritization, supporting clinicians and patients to make informed decisions about treatments and for the delivery of a more efficient health care service.
Accurate risk prediction models are the result of four key stages: development, validation, impact, and implementation.7 It is recognized that often only the first two stages (ie, development and validation) are completed, the methods and outcomes of which are often poorly reported. 7 Furthermore, to be of practical use, these models should use clearly defined easily obtainable data, have good predictive power, be tested in a large sample representative of the target population, and have high reliability and face validity.7 A recent systematic review emphasized that failure to consider risk prediction in a clinical setting can result in poor care.8 With regard to the prediction of medication risk in older adults, as no systematic review of this area has been undertaken, we aim to identify and assess the quality of validated ADR risk-prediction models for use in adults over 65 years of age in order to determine their potential benefit to clinical practice.
Method
Information sources and search
A systematic search for published material was performed, up to November 30, 2012, using standard databases (Embase, Medline, Cochrane Library, BNI, CINAHL, NeLM, IPA) to identify relevant studies as well as those associated with policy documents and unpublished work (Department of Health, King’s Fund, Worldcat, Open Grey, Google Scholar). For the key studies, the bibliographies and citations were reviewed, and an author search was performed, to identify any additional studies.
Our search strategies for each database included no restrictions and used standard terms based around three key concepts: older people; medication-related problems; and clinical prediction models. The full Embase search strategy is provided in Table S1.
Inclusion criteria and selection
Two researchers (JMS and SDE) independently screened titles, abstracts, and, where necessary, full texts in order to identify studies that potentially satisfied the following inclusion criteria:
Majority of patients ≥65 years old
Included patients who experienced an adverse drug event (ADE) or ADR but excluded prescription errors
A multivariable approach in design and analysis was followed
The model had been validated.
Data extraction
Data were extracted (by JMS) to provide details of the population characteristics, study design, process of model development and validation, and performance of the model, as presented in Tables 1 and 2. This was confirmed by secondary reviewers (SDE and ATP) and, where disagreement occurred, this was resolved through discussion.
Table 1.
Summary table of population characteristics of included studies
| Author | Development
|
Validation
|
|||||
|---|---|---|---|---|---|---|---|
| Population and setting | Number of patients (n) and common comorbidities (%) | Number of drugs | Primary outcome measure and rate | Drugs most frequently associated with primary outcome (%) | Most frequent body systems affected by ADRs (%) | Population and setting | |
| McElnay et al12 | Age: 65–98 years Location: acute hospital (UK) Year: NR Sex: F 49.5% Ethnicity: NR |
n=929 Comorbidities not reported |
Mean: 4.3 (range: 1–15) (SD: NR) |
ADE – ADR and adherence 16% |
Digoxin ACE inhibitors Antidepressants Insulin |
NR | n=204 (number ADRs unknown) Cohort similar to development cohort |
| Tangiisuran13 | Age: 85±7.9 years Location: acute hospital (UK) Year: 2007 and 2008 Sex: F 61% Ethnicity: white |
n=690 HTN (73) Infection (44) Anemia (41) MSK(41) |
Mean: 7 (range: 5–10) (SD: NR) |
ADR 12.5% |
Cardiovascular (34%) Analgesics (16%) Antidiabetics (13%) Antibiotics (13%) |
GI (21.1%) CV (20%) Neuropsychiatric (14.7%) Endocrine (13.7%) Metabolic/renal (11.6%) |
n=483 (56 suffered ADR) Number of drugs 11.0±7.0 Cohort similar – drawn from four European countries (UK, Belgium, Italy, and the Netherlands) |
| Onder et al14 | Age: 78±7.2 years Location: acute and community hospitals (Italy) Year: 1993–1997 Sex: NR Ethnicity: NR |
n=5936 HTN (24)31 CHD (21)31 Diabetes (16)31 COPD (14)31 CVD (13)31 |
Mean: 6.3 (range: NR) (SD: 3.6) |
ADR 6.5% |
Antineoplastics (19.5%)31 NSAIDs (5.2%)31 Antipsychotics (4.4%)31 Antibiotics (3.9%)31 Corticosteroids (3.3%)31 |
GI (18%) CV (25.3%) Neuropsychiatric (17.8%) Dermatologic (11.7%) |
n=483 (56 suffered ADR) Number of drugs 11.0±7.0 Cohort similar – drawn from four European countries (UK, Belgium, Italy, and the Netherlands) |
|
Secondary study21 n=513 (135 ADRs) Number of drugs 7 (IQR 7–10) Cohort similar to development cohort – drawn from Republic of Ireland |
|||||||
| Trivalle et al15 | Age: 83.6±7.9 years Location: 16 rehabilitation hospitals (France) Year: NR Sex: F 72% Ethnicity: NR |
n=576 CV (72) MSK (48) GI (36) GU (29) Neuro (26) |
Mean: 9.4 (range: NR) (SD: 4.24) |
ADE 39% (not all were included in the study) |
Psychotropics (23%) Antihypertensives (17%) Anticoagulants (14%) Analgesics (13%) |
GI (25%) Biological abnormalities (22%) Other (20%) Neuropsychiatrie (12%) |
Bootstrapping n=NR Validation cohort similar to developmental cohort |
Abbreviations: ACE, angiotensin converting enzyme; ADE, adverse drug event; ADR, adverse drug reaction; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; CVD, cerebrovascular disease; F, female; GI, gastrointestinal; GU, genitourinary; HTN, hypertension; IQR, interquartile range; MSK, musculoskeletal; Neuro, neurological comorbidity; NR, not recorded; NSAIDs, non-steroidal anti-inflammatory drug; SD, standard deviation.
Table 2.
Summary of quality assessment of included studies
| Standard criteria | McElnay et al12 | Tangiisuran13 | Onder et al14 | Trivalle et al15 | |
|---|---|---|---|---|---|
| Study design | Prospective cohort (development and validation) | Prospective cohort (development and validation) | Retrospective cohort (development) Prospective (validation) |
Prospective cohort (development) Retrospective cohort (validation) |
|
| Participant recruitment | Clear inclusion criteria | Yes Development – Nonelective admissions – Medical, surgical, cardiac and geriatric, wards in a single hospital – >65 years old – Taking medicines Validation as above |
Yes Development – Admitted to one of four care of the elderly wards in a teaching hospital – >80 years old Validation – Admitted to one of four European hospitals – ≥65 years old – Taking medicines |
Yes Development – Selected community-and university-based hospital admissions – ≥65 years old – Taking medicines Validation as above except admitted to one of four European hospitals |
Yes Development – Consecutive admissions to 16 geriatric rehabilitation centers Validation as above |
| Evidence that patient selection was not biased | Unsure Data only collected from 50% of development-phase and 42% of validation-phase patients recruited who underwent interview |
Yes All patient exclusions were for appropriately assessed reasons |
Unsure An unknown number of patients were excluded due to incomplete data 61 cancer patients excluded |
Unsure Data from 71 patients were excluded (these patients were either part of an intervention arm or not present for the whole 4 weeks of the study) |
|
| Acceptably low rates of loss to follow-up | Yes Data from all patients who underwent interview were used in development and validation of model |
Yes No patients lost to follow-up |
Yes No patients lost to follow-up |
Yes No patients lost to follow-up |
|
| Candidate predictor variablesa | Clear methods used to measure predictors | Partly 2/7 identified variables were not easily quantifiable (ie, “GI problems” and “patient thinks drugs are responsible for hospital admission”) |
Mostly Data on 17 potential variables assessed Not clear how comorbidity, liver disease, previous history of ADR, or known allergy to medication were defined |
Partly A trained physician completed a questionnaire for each patient, but unclear how key variables (comorbid conditions, liver disease, previous ADR) were defined or consistently applied between assessors |
Partly Where candidate predictors were reported, they could be clearly described Potential candidate predictors that were not included in the model are unknown |
| Blinding to outcome | Yes Data collected prospectively |
Yes Data collected prospectively |
Partly Blinding is not reported for the development phase Physicians collecting data for the validation phase were blinded |
Yes Data collected prospectively |
|
| Conformity with linear gradient | Not reported | Not reported | Not reported | Yes Linearity was checked where possible |
|
| Test for colinearity | Partly Outlined in method but not mentioned in results |
Partly Outlined in method but not mentioned in results |
Not reported | Yes High-correlation risk factors were identified and examined in separate models |
|
| Outcomeb | Appropriate methods used to measure outcomes | Partly Data sourced from patient records and interviews ADE as defined by: – ADR (measured using modified Naranjo scale) – Adherence (self-reported) |
Partly Medical information and health care staff reviewed daily using standardized checklist Suspected ADRs assessed for causality, preventability, and severity using Naranjo algorithm, Hallas criteria, and a confidence in causality Likert scale |
Partly Wards visited daily, and nursing and medical records examined daily Causality was assessed based on Naranjo algorithm |
Partly A combination of approaches used to identify ADEs: a self-generated standardized 32-item checklist was completed by nursing staff. Incident reporting and weekly chart review were also conducted. Four criteria were used to assign likelihood of causality |
| Statistical power | Sufficient events per variable (ie, > 10) | No Unable to determine exact number, but < 10 |
No 86 ADRs/34 candidate predictor variables =2.5 |
Yes Unable to determine exact number but > 10 |
Not possible to determine |
| Selection of predictor variables | Method of selection reported for independent variables | Partly Screened in univariate analysis and entered into model if P<0.25 Applicable to >5% of population |
Yes Screened in univariate analysis and entered into model if P<0.05 Variables identified from other studies entered into model if P<0.25 Applicable to >5% of population |
Yes Screened in univariate analysis and entered into model if P≤0.10 |
Yes Screened in univariate analysis and entered into model if P<0.05 Applicable to >5% of population |
| Fitting procedure reported | Yes Stepwise backward-elimination procedures (using maximum likelihood method) Preliminary removal of variables at P=0.15 then P=0.05 |
Yes Multivariate logistic regression using backward-elimination procedure and forward selection Removal criteria P=0.10 |
Partly Stepwise logistic regression Added and retained variables if P≤0.1 Methods of variable elimination and retention were unclear |
Partly Stepwise logistic regression Retained variables if P<0.05 Methods of variable elimination and retention were unclear |
|
| Model performance | Development phase reported | No | Yes Discrimination as AUROC reported with CI Calibration as Hosmer-Lemeshow and Nagelkerke R2 Sensitivity and specificity reported |
Partly Discrimination as AUROC reported with CI Sensitivity and specificity reported |
No |
| Validation phase reported | Partly Only overall accuracy, sensitivity, and specificity reported |
Yes Discrimination as AUROC reported with CI Sensitivity and specificity reported |
Partly Discrimination as AUROC reported with CI |
Partly Discrimination as AUROC reported with CI |
|
Notes:
Interactions and coding were not dealt with in any of the studies.
All studies collapsed continuous categorical data into binary outcomes.
Abbreviations: ADE, adverse drug event; ADR, adverse drug reaction; AUROC, area under the receiver operator curve; CI, confidence interval; GI, gastrointestinal
Quality assessment
All papers were initially reviewed (SDE and JMS) using a standard approach for developing and testing clinical prediction models to satisfy a range of criteria representing four stages: development (identification of candidate predictor variables and model design); validation (testing the performance of the model); impact (measurement of usefulness in the clinical setting); and implementation (widespread acceptance and adoption in clinical practice).7
As no standardized quality assessment for risk-prediction models is available, each study was analyzed using criteria derived from the published literature.8–11 Candidate predictor variables were grouped into three categories to allow for comparison between studies: demographic factors; medical factors (eg, comorbidities); and medication factors (eg, class of medicine). Event rate was calculated as percentage ADR/ADE rate where it was not reported by the authors in this form. Quality of design and reporting of the studies was compared based on ability to comply with the standard criteria (Table S2). The overall performance of the models was determined by review of their accuracy, discrimination, and calibration through internal or external validation, as described in detail in Table S2.
Results
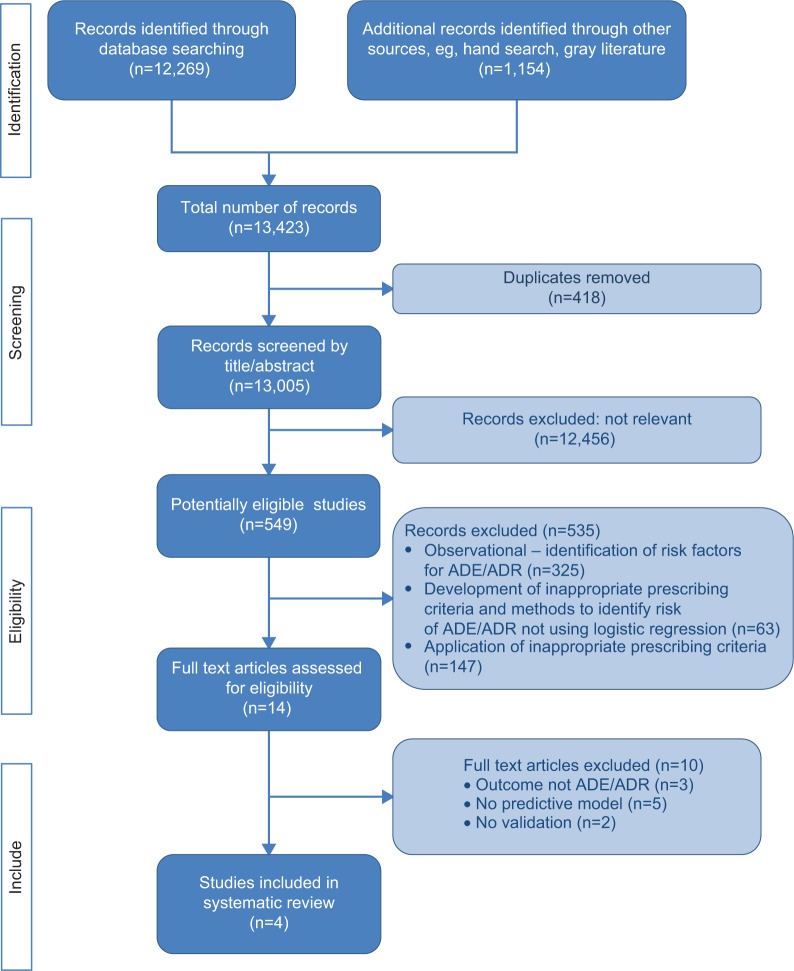
A total of 13,423 potentially relevant titles were identified from the literature, of which only 549 were associated with adverse outcomes of medicines use. The majority of these (535) were excluded on review of their abstract as they were not associated with the design of a risk prediction model; many of these were observational (see Figure 1). Full papers were requested for the remaining 14 articles for further scrutiny, and four met the inclusion criteria and were subjected to a full evaluation.12–15
Figure 1.
PRISMA32 flow diagram.
Abbreviations: ADE, adverse drug event; ADR, adverse drug reaction.
Excluded papers
The 535 articles excluded could be categorized into observational studies (325), those in which indicators to support quality prescribing were developed (63 studies; for example Beers’ criteria16), and those applying the prescribing indicators (147 studies) to determine any association between inappropriate medicines and adverse outcomes.
Included papers
Population characteristics
All included studies were conducted in Western Europe, and only in the hospital setting (acute, community, and rehabilitation hospitals) (Table 1).12–15 Two studies represented the very elderly (aged over 80 years).13,15 Patient functionality was reported by Onder et al14 Tangiisuran,13 and McElnay et al12 and was measured using patient-perceived health status, Katz Index, and Barthel Index.
The primary outcome in all of the studies was ADR,17 with one study using ADE synonymously15 and another12 including ineffective treatment in an extended definition. The proportion of patients who experienced an ADR/ADE ranged from 6.5% to 39%, with gastrointestinal, cardiovascular, and nervous systems being those most frequently affected. Medications most frequently associated with ADRs/ADEs included psychotropics, anticoagulants, and analgesics.
Quality assessment – overview
Whilst all models included the development and validation phases, none addressed the impact and implementation phases.
Model development
Study design
During the development phase, all except Onder et al14 used a prospective case-cohort design method, where events accrued over the study period. Onder et al extracted 3 years of data from a historical database, whereas data were extracted over 1–6 months in the other studies. Patient medical notes, in-patient charts, and electronic records were reviewed in the prospective studies.12,13,15 In addition, McElnay12 asked a sample of the patients about aspects of their medicines, while Trivalle et al15 used patient self-reporting as a trigger for further analysis. The validation phase was conducted prospectively for all studies except for that of Trivalle et al where bootstrapping was used.
Participant recruitment
The criteria for inclusion and exclusion as well as any loss to follow-up were clearly described in all studies, although reporting of patient selection was poor (Table 2). An unknown number of patients were excluded by Onder et al due to incomplete data.14
Candidate predictors
The handling of candidate predictor variables was generally poor. In all studies, the description of the variables was inadequate; where Trivalle et al15 did not report the potential candidate variables, McElnay et al12 Tangiisuran13 and Onder et al14 used variables with unclear definitions, eg, “previous ADR”. Despite being labeled as a “bad idea”,18 dichotomization of continuous candidate predictor variables (eg, four or more comorbidities, more than eight medications, previous ADR) was common practice, and may explain the failure to consider conformity to the linear gradient in all12–14 but the Trivalle et al study.15 Interactions were poorly addressed, as was the coding of variables. Insufficient detail in the results made it difficult to establish whether tests that were mentioned in the methods had been implemented; eg, McElnay et al12 reported testing for interactions and colinearity, but this was not followed through to the results. Predictor-variable measurement was blinded for outcome in the development phase in three of the four studies.12,13,15
Outcome
The occurrence of an ADE/ADR was the primary outcome measure for all studies. A validated assessment of causality, in the form of the Naranjo algorithm19 or Hallas criteria,20 was adopted by all but Trivalle et al who used their own checklist.15 The outcome was recorded in the form of continuous categorical data (ie, unlikely, possible, probable, definite) then collapsed to produce a binary outcome. Possible, probable, and definite were combined as a positive outcome. Blinding to the outcome occurred in all four studies during the validation phase.
Statistical power
The poor description of potential candidate predictor variables made it impossible to determine if the studies were adequately powered (Table 2).
Selection of predictor variables
The method of selection of predictor variables for inclusion within the multivariable analysis was described in all of the studies (Table 2). Tangiisuran13 provided the most detailed description, whilst Trivalle et al15 provided the least detailed description. Mixed methods (using the literature, expert opinion, and univariate analysis) were used by Tangiisuran.13 Onder et al appeared to have used univariate analysis alone.14 There was variation in the significance levels used to retain a predictor variable.12–15
Model performance and validation
The area under the receiver operator curve was used to assess discrimination in three of the four studies, and was 0.70–0.74 for the development phase.13–15 Sensitivity and specificity were reported by Tangiisuran,13 Onder et al14 and McElnay et al.12 Calibration was only reported by Tangiisuran,13 for which Hosmer-Lemeshow was satisfactory but Nagelkerke21 was low.
All models underwent the subsequent stage of validation using a second dataset. Internal validation was reported by McElnay et al12 and Trivalle15 in the form of split sample and bootstrapping, retrospectively. External validation was performed by Onder et al14 and Tangiisuran13 in the same European cohort. Another research group (O’Connor et al22) subsequently applied the model developed by Onder et al14 providing additional external validation (Table 1). Area under the receiver operator curve in the validation phase ranged from 0.623 to 0.73 (Table 3). The number of patients involved in the external validation ranged from 204 to 483.12–15,22 Only the study by O’Connor et al22 met the recommended minimum number of events (100 events and nonevents).
Table 3.
Summary of final ADR risk-prediction models
| Author | Significant variables in multivariate analysis | Variable coefficient | OR (CI) | Attributed score | Validation |
|---|---|---|---|---|---|
| McElnay et al12 | Prescribed antidepressants | 1.7569 | 5.7942 (2.12–15.85) | None | Internal (204 patients) |
| Prescribed digoxin | 0.6884 | 1.9905 (1.05–2.33) | Accuracy 63.0% | ||
| Gastrointestinal problems | 0.7704 | 2.1606 (1.13–4.15) | Sensitivity 40.5% | ||
| Abnormal potassium level | 0.9455 | 2.5740 (1.35–4.91) | Specificity 69.0% | ||
| Thinks drugs were responsible | 1.4375 | 4.2103 (2.18–8.14) | |||
| −1.7861 | 0.1676 (0.07–0.42) | ||||
| Experiences angina | 0.8779 | 2.4057 (1.06–5.44) | |||
| Experiences COAD | −1.0997 (constant) | ||||
| Tangiisuran13 | Hyperlipidemia | 1.199 | 3.316 (1.811–6.072) | 1 | External (483 patients) |
| Number of medications ≥8 | 1.194 | 3.300 (1.927–5.651) | 1 | Sensitivity 80.0% | |
| Length of stay ≥12 days | 0.819 | 2.269 (1.345–3.826) | 1 | Specificity 55.0% | |
| Use of hypoglycemic agents | 0.645 | 1.906 (1.040–3.493) | 1 | AUROC 0.73 (95% CI 0.66–0.80) | |
| 0.437 | 1.548 (0.940–2.548) | 1 | |||
| High white blood cell count on admission | −3.628 (constant) | ||||
| Onder et al14 (O’Connor et al)22 | ≥4 comorbidities | Not reported | 1.31 (1.04–1.64) | 1 | External (483 patients) |
| Heart failure | 1.79 (1.39–2.30) | 1 | Sensitivity 68% | ||
| Liver disease | 1.36 (1.06–1.74) | 1 | Specificity 65% | ||
| Number of drugs ≤5 | 1 Reference | – | AUROC 0.70 (95% CI 0.63–0.78) | ||
| Number of drugs 5–7 | 1.9 (1.35–2.68) | 1 | External (513 patients) | ||
| Number of drugs ≥8 | 4.07 (2.93–5.65) | 4 | AUROC 0.623 (95% CI 0.570–0.676) | ||
| Previous ADR | 2.41 (1.79–3.23) | 2 | |||
| Renal failure | 1.21 (0.96–1.51) | 1 | |||
| Trivalle et al15 | Number of medications | Not reported | 1.9 (1.6–2.3) | – | Internal (bootstrap) |
| 0–6 | 2.5 (1.5–4.1) | 0 | AUROC 0.70 (95% CI 0.65–0.74) | ||
| 7–9 | 2.0 (1.1–1.37) | 6 | |||
| 10–12 | 12 | ||||
| ≥13 | 18 | ||||
| Antipsychotic treatment | 9 | ||||
| Recent anticoagulant | 7 | ||||
Abbreviations: ADR, adverse drug reaction; AUROC, area under the receiver operator curve; CI, confidence interval; COAD, chronic obstructive airways disease; OR, odds ratio.
Score development
Predictor variables within the final models (Table 3) were attributed a points-based score, which was simplified for practical application.13–15 McElnay et al did not proceed to this stage due to the poor performance of their model.12 The score developed by Onder et al14 was on a points-based system derived from the odds ratio. There was no assessment to determine if any of the predictive ability was lost in this simplification. Tangiisuran13 assigned one point to each predictor variable based on the “variable coefficient being of the same magnitude”. It is unclear how Trivalle et al15 assigned the values to each predictor variable.
Impact and implementation
The impact and implementation of these models have not been published, perhaps reflecting their poor to modest performance. McElnay et al recognized the limitation of their level of performance,12 and both Tangiisuran and Onder et al called for further external validation of their models.13,14 However, Trivalle et al15 concluded that their model could be applied in clinical practice alongside other tools, eg, Mini Mental State Exam. It is also worth considering some of the difficulties highlighted by O’Connor et al22 in the application of Onder et al’s14 model that are due to unclear definition of predictor variables.14,22 Similar challenges are likely to arise when applying results from Tangiisuran, Trivalle et al and McElnay et al given the poorly defined predictor variables.12,13,15 The use of variables such as length of stay would also make prospective risk stratification impossible.
Discussion
Our review suggests that the four models identified, which were designed to predict the risk of older patients suffering an ADR, are not yet suitable for use in clinical practice. While only two (Tangiisuran and Onder et al) were externally validated, their ability to discriminate between those who had experienced an ADR and those who had not was only modest.13,14 This could result in a failure to identify patients at high risk of experiencing an ADR. Furthermore, none were subjected to the investigational rigor required when producing a risk-prediction model; in particular, none reported the findings of impact and implementation stages, thus widening the gap between research potential and clinical application. Pressures within health care systems are driving a need for robust clinical risk-prediction models to inform care provision, but, to be useful, these models must be of high statistical quality and be clinically relevant.
All four studies had limitations commonly reported in the prognostic research literature.7 Three failed to provide sufficient information relating to events-per-variable ratio12,14,15 and one was insufficiently powered (Tangiisuran),13 so the risk of a type II error (false negative finding) was more likely.23 All studies dichotomized their predictor variables (eg, when categorizing the number of medicines) and outcomes (eg, collapsing a continuous ADR causality scale), despite this practice being suboptimal.18,23 The use of unrepresentative samples and the management of missing data were also problematic, regardless of whether a retrospective or prospective design was used. In addition, there was often a lack of reporting of candidate predictor variables, which could hinder replication by others.24
So, if the current risk prediction models have shortcomings, what can we do to limit older adults experiencing ADEs? Although research investigating medication risk in older adults is widespread, the 535 titles identified in our initial search were often associated with other, mainly system-based, approaches to managing risk, and a substantial proportion were observational in nature. This body of evidence documents the complexity of medication risk in older adults and highlights the multidimensional nature of this field, which includes: clinical aspects, such as the changes in drug handling demonstrated in older age; social risk factors, especially during the transfer of care between different settings; and high-risk medicines, where the risk of medicines are considered but not always balanced against the potential benefits. Furthermore, the difficulty in determining whether a patient has experienced an ADR is challenging given the progressive nature of aging, where functional decline and loss of independence are common. Unfortunately, as older adults are often excluded from clinical trials, this limits our understanding of medicine risk in this population, and can result in inappropriate extrapolation of clinical guidelines, often based on research in younger patients.
So, is there a place for risk models in this care setting? A more common strategy is to adopt a systems approach to medicines use where pharmacological appropriateness is monitored, usually by applying a list of prescribing indicators: for example, Beer’s criteria.16 The recognized limitations of such an approach are that it is time-consuming if used in routine care and can be viewed as one-dimensional. This focus on specific medicines often restricts, due to formulary and licensing issues, value in an international context. Perhaps the way forward is a hybrid whereby risk models bring a multidimensional perspective to guide clinical intervention, delivered as part of an integrated system built around the principles of medication safety. If models can map this complex interplay between clinical, social, and medication-related variables to stratify an individual’s risk of a future ADE, they may become a useful decision support tool for clinicians and patients to be used alongside systems-based approaches. This approach could help prioritize interventions for those patients at highest risk. Ultimately, the variables associated with medication risk, eg, polypharmacy and renal impairment, are inherent in clinical decisions and form part of a clinician’s intuitive risk assessment when prescribing medicines. Furthermore, clinicians often modify decisions based on individual variability, whereas a statistical model may not be able to accommodate the clinical nuances and overcome the gerontological phenomenon of age heterogeneity.25 While risk prediction models are not intended to replace clinicians’ decisions, they should not stratify patients less accurately than clinicians. It would be helpful if future work could compare a clinician’s risk stratification against that of an ADR risk-prediction model. This work would help inform the clinical relevance of the model and contribute to the impact and implementation research that is thus far lacking. In the meantime, useful strategies that clinicians may adopt to prevent ADRs occurring are: ensuring that reliable medicines reconciliation is undertaken; avoiding the prescribing cascade (where a drug is prescribed to manage the problem caused by another); and the routine optimization of drug use in line with renal and liver function.
While conducting this systematic review, we could not assess for publication bias using conventional methods such as funnel plots due to the small number of studies available.26 Publication bias in favor of positive results has been raised as a significant problem in the area of cancer risk-prediction research, and it is likely to be present in this area in which negative results remain unpublished.27 The proposal to develop reporting guidelines that stipulate registration of all risk-prediction research should go some way in reducing future reporting bias.28 These guidelines could also be applied to protocols and manuscripts when designing or publishing risk-prediction research, and may be a more suitable tool for quality assessment in the future.29 In the absence of a consensus guideline, we used an amalgamation of standards for reporting risk-prediction research to carry out this review. This approach should reduce the likelihood of any important quality measures being excluded. In the future, recommendations developed by the Cochrane Prognosis Methods Group and the Prognosis Research Strategy Partnership should assist investigators in combating the challenges present when conducting risk-prediction research.23,28,29
Conclusion
Risk stratification is attractive, especially in older patients where the population is growing and placing an increased demand on the health care service, a service that is woefully underprepared for the projected global growth to over 2 billion people over the age of 60 years by 2050.30 We identified four ADR risk-prediction models with poor to modest performance and raised questions about their overall quality, a finding not uncommon in the area of risk-prediction research. If these models are to be embraced as part of routine clinical care, further work needs to be conducted so that external validity can be assured and a practical approach upheld. Only then can implementation and impact be assessed with the view to adoption as part of a systems approach within routine clinical care.
Supplementary materials
Table S1.
Embase search strategy indicating the order in which the terms were entered and how they were combined
| Risk tool |
| 1. risk assessment.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 2. exp prediction/ |
| 3. exp scoring system/ |
| 4. exp clinical assessment tool/ |
| 5. exp risk factor/ |
| 6. exp risk management/ |
| 7. exp decision support system/ |
| 8. risk stratification.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8a |
| Medication related problem |
| 10. exp adverse drug reaction/ |
| 11. adverse drug event*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 12. adverse drug reaction*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 13. medication related problem*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 14. drug related problem*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 15. exp medication therapy management/ |
| 16. drug/ae [Adverse Drug Reaction] |
| 17. exp polypharmacy/ |
| 18. exp medication error/ae, pc [Adverse Drug Reaction, Prevention] |
| 19. inappropriate prescri*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 20. (readmission and drugs).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 21. patient compliance.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 22. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21a |
| Elderly |
| 23. aged/ |
| 24. exp aging/ |
| 25. exp elderly care/ |
| 26. older people.mp. |
| 27. older person.mp. |
| 28. aged over 80.mp. |
| 29. 23 or 24 or 25 or 26 or 27 or 28 or 29a |
| Combined terms |
| 30. 9 and 22 and 29a |
Notes:
The numbers demonstrate how search terms have been combined ie, all of the terms for the risk tool were combined in Step 9 of the search. Then these combined terms were combined with those from all those relating to medication related problems ie, Step 22 and with terms relating to elderly ie, Step 29. This resulted in a combined search of the terms listed in Steps 9 and 22 and 29.
Abbreviations: exp, explode all trees; mp, multiple posting.
Table S2.
Criteria to consider when evaluating the quality of risk prediction models
| Standard criteriaa | Explanation | Example |
|---|---|---|
| Study design | Prospective: allows optimal collection of potential candidate variables; smaller dataset often generated. Retrospective: enables use of large previously collected datasets; quality of candidate variable data may be compromised due to missing data, which rarely occurs at random. |
Prospective study design, n=690, all exclusions were for appropriate reasons.1 Retrospective study design, n=5,936, unknown number of exclusions due to missing data.2 |
| Participant recruitment | Inclusion and exclusion criteria should be clearly described to allow full assessment of patient population studied. Any systematic variation in recruitment of patients should be viewed with caution due to risk of sampling bias. There is no predetermined satisfactory number for loss to follow-up; however, it should be considered that missing data impacts on the statistical power of the study. |
Interview data was only collected for half of the patients during the development phase. Patients not wishing to participate in the interview may systematically differ.3 |
| Candidate predictor variables | Variables and their measurement should be clearly defined to allow for replication. Investigators should be blind to outcome to reduce risk of bias. Continuous variables should be assessed for conformity to linear gradient. Not necessary for dichotomous variables; however, dichotomization of continuous variables not recommended as it impacts on the statistical power of the study. Correlation (test for colinearity) between risk variables should be examined and reported. |
Unclear how key variables, eg, liver disease, were defined. To replicate, study investigators would be required to apply their own definition, which may have an impact on reproducibility.2 |
| Outcome | Method of measuring outcome: must be reproducible and, where assessment scales are applied, these should be validated to increase accuracy and reproducibility of the measurement. Dichotomization of continuous outcomes is not recommended as it can affect statistical power. | Investigators generated own causality assessment of unknown validity.4 Applied widely-used validated causality assessment (Naranjo algorithm).2 |
| Statistical power | Sample size is calculated based on number of outcome events per variable, where ten events per variable is often recommended. A high number of variables and a rare outcome can result in over-fitting of the model, causing poor generalizability. | Reported 86 ADRs in a sample of 690 patients and assessed 34 candidate predictor variables, resulting in only 2.5 events per variable.1 |
| Selection of variables | Independent variable selection should be described clearly, and can be based on the literature and/or statistical association as determined by univariate analysis with outcome variable. Selection based upon univariate analysis alone increases likelihood of developing an over-fitted model. Inclusion of variables applicable to over 5% of population may help exclude artifact variables. Fitting procedure (entering of variables into model) should be explicitly stated, including removal criteria. |
Variables were entered into multivariate analysis if P<0.05 after univariate analysis, or if P<0.25 for variables identified from other studies. Liver disease was removed as it applied to <5% of population. Backward elimination and forward selection were used with a removal criteria of P=0.10.1 |
| Model performance | In both development and validation phases, assessment of discrimination and calibration should be reported to determine how well the model distinguishes those who have an ADR from those who have not, as well as how close the prediction is to the observed outcome for that risk group. AUROC >0.7 is often deemed acceptable, but this alone is not sufficient to determine the clinical usefulness of the model.6 Assessment of the generalizability of the model is important to determine the accuracy of predictions in another population and is recommended prior to routine clinical application. Internal validation, by methods such as bootstrapping (data resampling) or split-sample, assesses how well predictors correspond to the outcome, but leads to optimistic estimates of model performance. External validation is more rigorous and enables assessment of accuracy when the model is applied by investigators not involved in the development of the model. |
Discrimination (AUROC) and calibration (Hosmer-Lemeshow) reported in the development and validation phases.1 Trivalle applied bootstrapping.5 Onder applied external validation whereby the model was applied by investigators not involved in the development of the model and in a different geographical location.7 |
References
- 1.Siontis GC, Tzoulaki I, Siontis KC, Ioannidis JP. Comparisons of established risk prediction models for cardiovascular disease: systematic review. BMJ. 2012;344:e3318. doi: 10.1136/bmj.e3318. [DOI] [PubMed] [Google Scholar]
- 2.Nelson EA, Dannefer D. Age heterogeneity: fact or fiction? The fate of diversity in gerontological research. Gerontologist. 1992;32:17–23. doi: 10.1093/geront/32.1.17. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani AD, Windt DA, Riley RD, et al. Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ. 2013;346:e5793. doi: 10.1136/bmj.e5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20:641–654. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 5.Trivalle C, Burlaud A, Ducimetière P, The IMEPAG Group 1 Risk factors for adverse drug events in hospitalised elderly patients: a geriatric score. Eur Geriatr Med. 2011;2:284–289. [Google Scholar]
- 6.Field A. Discovering Statistics Using IBM SPSS Statistics. 4th edition. London: Sage; 2013. [Google Scholar]
- 7.Onder G, Petrovic M, Tangiisuran B, et al. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: The GerontoNet ADR Risk Score. Arch Intern Med. 2010;170:1142–1148. doi: 10.1001/archinternmed.2010.153. [DOI] [PubMed] [Google Scholar]
- 8.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indicies for older adults. A systematic review. JAMA. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54:979–985. doi: 10.1016/s0895-4356(01)00372-9. [DOI] [PubMed] [Google Scholar]
- 10.Critical Appraisal Skills Programme for Clinical Prediction Rules [homepage on the Internet] [Accessed November 9, 2011]. Available from: http://www.casp-uk.net.
- 11.Steyerberg EW. Clinical Prediction Models: a practical approach to development, validation and updating. New York: Springer; 2010. [Google Scholar]
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pirmohammed M, James S, Meakin S, et al. Adverse drug reactions as a cause of admission to hospital: prospective analysis of 18,820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budnitz DS, Pollock DA, Weidenbach KN, et al. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296:1858–1866. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 3.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient episodes. PLoS One. 2009;4:e4439. doi: 10.1371/journal.pone.0004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 6.The John Hopkins ACG® System: International Experience [webpage on the Internet] Baltimore: Johns Hopkins University; 2013. [Accessed April 15, 2013]. Available from: http://acg.jhsph.org/index.php?option=com_content&view=article&id=73&Itemid=138. [Google Scholar]
- 7.Toll DB, Janssen KJM, Vergouwe Y, Moons KG. Validation, updating and impact of clinical prediction rules: a review. J Clin Epidemiol. 2008;61:1085–1094. doi: 10.1016/j.jclinepi.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indicies for older adults. A systematic review. JAMA. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54:979–985. doi: 10.1016/s0895-4356(01)00372-9. [DOI] [PubMed] [Google Scholar]
- 10.Critical Appraisal Skills Programme for Clinical Prediction Rules [homepage on the Internet] [Accessed November 9, 2011]. Available from: http://www.casp-uk.net.
- 11.Steyerberg EW. Clinical Prediction Models: a practical approach to development, validation and updating. New York: Springer; 2010. [Google Scholar]
- 12.McElnay JC, McCallion CR, Al-Deagi F, Scott MG. Development of a risk model for adverse drug events in the elderly. Clin Drug Invest. 1997;13:47–55. [Google Scholar]
- 13.Tangiisuran B. Predicting adverse drug reactions in the hospitalised elderly [PhD Thesis] Brighton: University of Sussex; 2009. [Google Scholar]
- 14.Onder G, Petrovic M, Tangiisuran B, et al. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: The GerontoNet ADR Risk Score. Arch Intern Med. 2010;170:1142–1148. doi: 10.1001/archinternmed.2010.153. [DOI] [PubMed] [Google Scholar]
- 15.Trivalle C, Burlaud A, Ducimetière P, The IMEPAG Group 1 Risk factors for adverse drug events in hospitalised elderly patients: a geriatric score. Eur Geriatr Med. 2011;2:284–289. [Google Scholar]
- 16.American Geriatrics Society 2012 Beers Criteria Update Expert Panel American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . International Drug Monitoring: The Role of National Centres (World Health Organization Technical Report Series No. 498) Geneva: World Health Organization; 1972. [PubMed] [Google Scholar]
- 18.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 19.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 20.Hallas J, Harvald B, Gram LF, et al. Drug related hospital admissions: the role of definitions and intensity of data collection and the possibility of prevention. J Intern Med. 1990;228:83–90. doi: 10.1111/j.1365-2796.1990.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 21.Field A. Discovering Statistics Using IBM SPSS Statistics. 4th edition. London: Sage; 2013. [Google Scholar]
- 22.O’Connor MN, Gallgher P, Byrne S, O’Mahony D. Adverse drug reactions in older patients during hospitalisation: are they predictable? Age Ageing. 2012;41:771–776. doi: 10.1093/ageing/afs046. [DOI] [PubMed] [Google Scholar]
- 23.Hingorani AD, Windt DA, Riley RD, et al. Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ. 2013;346:e5793. doi: 10.1136/bmj.e5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siontis GC, Tzoulaki I, Siontis KC, Ioannidis JP. Comparisons of established risk prediction models for cardiovascular disease: systematic review. BMJ. 2012;344:e3318. doi: 10.1136/bmj.e3318. [DOI] [PubMed] [Google Scholar]
- 25.Nelson EA, Dannefer D. Age heterogeneity: fact or fiction? The fate of diversity in gerontological research. Gerontologist. 1992;32:17–23. doi: 10.1093/geront/32.1.17. [DOI] [PubMed] [Google Scholar]
- 26.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20:641–654. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 27.Hemingway H, Riley RD, Altman DG. Ten steps towards improving prognosis research. BMJ. 2009;339:b4184. doi: 10.1136/bmj.b4184. [DOI] [PubMed] [Google Scholar]
- 28.Steyerberg EW, Moons KG, van der Windt DA, et al. Prognosis research strategy (PROGRESS) 3: prognostic model research. PLoS Med. 2013;10:e1001381. doi: 10.1371/journal.pmed.1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geersing G-J, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG. Search filters for finding prognostic and diagnostic prediction studies in medline to enhance systematic reviews. PLoS One. 2012;7:e32844. doi: 10.1371/journal.pone.0032844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization . World Health Day 2012 Toolkit for Organizers (webpage on the Internet) Geneva: World Health Organization; 2012. [Accessed August 30, 2012]. Available from: http://www.who.int/world-health-day/2012/toolkit/background/en/index.html. [Google Scholar]
- 31.Onder G, Pedone C, Landi F, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA) J Am Geriatr Soc. 2002;50(12):1962–1968. doi: 10.1046/j.1532-5415.2002.50607.x. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Embase search strategy indicating the order in which the terms were entered and how they were combined
| Risk tool |
| 1. risk assessment.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 2. exp prediction/ |
| 3. exp scoring system/ |
| 4. exp clinical assessment tool/ |
| 5. exp risk factor/ |
| 6. exp risk management/ |
| 7. exp decision support system/ |
| 8. risk stratification.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8a |
| Medication related problem |
| 10. exp adverse drug reaction/ |
| 11. adverse drug event*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 12. adverse drug reaction*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 13. medication related problem*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 14. drug related problem*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 15. exp medication therapy management/ |
| 16. drug/ae [Adverse Drug Reaction] |
| 17. exp polypharmacy/ |
| 18. exp medication error/ae, pc [Adverse Drug Reaction, Prevention] |
| 19. inappropriate prescri*.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 20. (readmission and drugs).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 21. patient compliance.mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 22. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21a |
| Elderly |
| 23. aged/ |
| 24. exp aging/ |
| 25. exp elderly care/ |
| 26. older people.mp. |
| 27. older person.mp. |
| 28. aged over 80.mp. |
| 29. 23 or 24 or 25 or 26 or 27 or 28 or 29a |
| Combined terms |
| 30. 9 and 22 and 29a |
Notes:
The numbers demonstrate how search terms have been combined ie, all of the terms for the risk tool were combined in Step 9 of the search. Then these combined terms were combined with those from all those relating to medication related problems ie, Step 22 and with terms relating to elderly ie, Step 29. This resulted in a combined search of the terms listed in Steps 9 and 22 and 29.
Abbreviations: exp, explode all trees; mp, multiple posting.
Table S2.
Criteria to consider when evaluating the quality of risk prediction models
| Standard criteriaa | Explanation | Example |
|---|---|---|
| Study design | Prospective: allows optimal collection of potential candidate variables; smaller dataset often generated. Retrospective: enables use of large previously collected datasets; quality of candidate variable data may be compromised due to missing data, which rarely occurs at random. |
Prospective study design, n=690, all exclusions were for appropriate reasons.1 Retrospective study design, n=5,936, unknown number of exclusions due to missing data.2 |
| Participant recruitment | Inclusion and exclusion criteria should be clearly described to allow full assessment of patient population studied. Any systematic variation in recruitment of patients should be viewed with caution due to risk of sampling bias. There is no predetermined satisfactory number for loss to follow-up; however, it should be considered that missing data impacts on the statistical power of the study. |
Interview data was only collected for half of the patients during the development phase. Patients not wishing to participate in the interview may systematically differ.3 |
| Candidate predictor variables | Variables and their measurement should be clearly defined to allow for replication. Investigators should be blind to outcome to reduce risk of bias. Continuous variables should be assessed for conformity to linear gradient. Not necessary for dichotomous variables; however, dichotomization of continuous variables not recommended as it impacts on the statistical power of the study. Correlation (test for colinearity) between risk variables should be examined and reported. |
Unclear how key variables, eg, liver disease, were defined. To replicate, study investigators would be required to apply their own definition, which may have an impact on reproducibility.2 |
| Outcome | Method of measuring outcome: must be reproducible and, where assessment scales are applied, these should be validated to increase accuracy and reproducibility of the measurement. Dichotomization of continuous outcomes is not recommended as it can affect statistical power. | Investigators generated own causality assessment of unknown validity.4 Applied widely-used validated causality assessment (Naranjo algorithm).2 |
| Statistical power | Sample size is calculated based on number of outcome events per variable, where ten events per variable is often recommended. A high number of variables and a rare outcome can result in over-fitting of the model, causing poor generalizability. | Reported 86 ADRs in a sample of 690 patients and assessed 34 candidate predictor variables, resulting in only 2.5 events per variable.1 |
| Selection of variables | Independent variable selection should be described clearly, and can be based on the literature and/or statistical association as determined by univariate analysis with outcome variable. Selection based upon univariate analysis alone increases likelihood of developing an over-fitted model. Inclusion of variables applicable to over 5% of population may help exclude artifact variables. Fitting procedure (entering of variables into model) should be explicitly stated, including removal criteria. |
Variables were entered into multivariate analysis if P<0.05 after univariate analysis, or if P<0.25 for variables identified from other studies. Liver disease was removed as it applied to <5% of population. Backward elimination and forward selection were used with a removal criteria of P=0.10.1 |
| Model performance | In both development and validation phases, assessment of discrimination and calibration should be reported to determine how well the model distinguishes those who have an ADR from those who have not, as well as how close the prediction is to the observed outcome for that risk group. AUROC >0.7 is often deemed acceptable, but this alone is not sufficient to determine the clinical usefulness of the model.6 Assessment of the generalizability of the model is important to determine the accuracy of predictions in another population and is recommended prior to routine clinical application. Internal validation, by methods such as bootstrapping (data resampling) or split-sample, assesses how well predictors correspond to the outcome, but leads to optimistic estimates of model performance. External validation is more rigorous and enables assessment of accuracy when the model is applied by investigators not involved in the development of the model. |
Discrimination (AUROC) and calibration (Hosmer-Lemeshow) reported in the development and validation phases.1 Trivalle applied bootstrapping.5 Onder applied external validation whereby the model was applied by investigators not involved in the development of the model and in a different geographical location.7 |