Abstract
Objective
To investigate the diagnostic value of 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in the assessment of myocardial viability in patients with known coronary artery disease (CAD) when compared to 99mTc single photon emission computed tomography (SPECT) and echocardiography, with invasive coronary angiography as the gold standard.
Methods
Thirty patients with diagnosed CAD met the selection criteria, with 10 of them (9 men, mean age 59.5 ± 10.5 years) undergoing all of these imaging procedures consisting of SPECT and PET, echocardiography and invasive angiography. Diagnostic sensitivity of these less invasive modalities for detection of myocardial viability was compared to invasive coronary angiography. Inter- and intra-observer agreement was assessed for diagnostic performance of SPECT and PET.
Results
Of all patients with proven CAD, 50% had triple vessel disease. Diagnostic sensitivity of SPECT, PET and echocardiography was 90%, 100% and 80% at patient-based assessment, respectively. Excellent agreement was achieved between inter-observer and intra-observer agreement of the diagnostic value of SPECT and PET in myocardial viability (k = 0.9).
Conclusion
18F-FDG PET has high diagnostic value in the assessment of myocardial viability in patients with known CAD when compared to SPECT and echocardiography. Further studies based on a large cohort with incorporation of 18F-FDG PET into patient management are warranted.
Keywords: Coronary artery disease, Diagnostic value, Positron emission tomography, Single photon emission computed tomography, Viability
1. Introduction
Cardiac positron emission tomography (PET) utilizing 18F-fluorodeoxyglucose (18F-FDG) is considered the most sensitive modality for detecting hibernating viable myocardium and predicting left ventricular functional recovery post-coronary revascularization.[1]–[4] 18F-FDG PET imaging has been reported to show incremental benefit over 201T1 stress-redistribution/reinjection or 99mTc-sestamibi single photon emission computed tomography (SPECT) in predicting functional recovery in the patients with significantly impaired left ventricular function, while in patients with relatively preserved left ventricular function, the predictive value was similar.[5],[6] Tillisch, et al.[1] in their first pioneering study reported the value of 18F-FDG PET to predict reversibility of cardiac wall motion abnormalities in 17 patients with ischemic cardiomyopathy. The overall sensitivity and specificity of 18F-FDG PET to demonstrate functional recovery after surgery was 95% and 80%, respectively. A meta-analysis of 24 studies has reported a weighted sensitivity and specificity of 92% and 63%, respectively, with a positive and negative predictive value of 74% and 87%, respectively, for the diagnosis of hibernating myocardium and prediction of patient outcomes.[2]
In addition to cardiac PET, there are other diagnostic imaging techniques that are also commonly used to determine myocardial viability such as SPECT, cardiac magnetic resonance imaging (MRI) and echocardiography. The diagnostic accuracy of 18F-FDG PET compares well with other established techniques for viability assessment (SPECT, echocardiography, and cardiac MRI), with sensitivity being superior to other techniques, although cardiac PET is not as widely available as other techniques.[3] A previous health technology assessment identified the pooled estimates of sensitivity and specificity of 18F-FDG PET for predicting wall motion recovery to be 91.5% and 67.8%, respectively.[4]
Despite these promising results, there is still a lack of scientific data with regard to the diagnostic value of cardiac PET in the evaluation of myocardial viability.[7] Thus, the purpose of this study is to investigate the diagnostic accuracy of 18F-FDG PET in the assessment of myocardial viability in patients with known coronary artery disease (CAD) when compared to the routinely performed 99mTc-tetrofosmin SPECT and echocardiography while using invasive coronary angiography as the gold standard. We hypothesized that 18F-FDG PET is superior to SPECT and echocardiography in the diagnostic evaluation of myocardial viability.
2. Methods
2.1. Study population
This was a prospective study that involved consecutive recruitments of patients with previous history of myocardial infarction and left ventricular dysfunction over a period of 6 months at the National Heart Institute, Malaysia and Universiti Kebangsaan Malaysia Medical Centre. All patients were suggested to undergo cardiac SPECT, cardiac PET myocardial viability, echocardiography and invasive coronary angiography examinations with an interval of less than 30 days between the imaging tests. Exclusion criteria included patients with suspected CAD but clinically confirmed to be non-ischemic left ventricular dysfunction, patients refused to participate in the study and patients with pregnancy. Thirty patients met the selection criteria, however, only ten patients (9 men; mean age 59.5 ± 10.5 years) agreed to participate in the study and eventually underwent all of these imaging procedures consisting of invasive coronary angiography, echocardiography, cardiac SPECT and PET. Consent forms were obtained from all patients, and ethical approval was granted from institutional review boards.
2.2. Invasive coronary angiography and image assessment
Coronary angiography was carried out using the standard Seldinger's technique on an angiographic machine by femoral approach which was performed by cardiologists at the National Heart Institute, Malaysia. A cardiologist with more than 10 years of experience who had no prior knowledge of cardiac SPECT or PET findings quantitatively analysed the severity of coronary stenosis. The minimal lumen diameter was measured in projections showing the most severe narrowing.
The degree of stenosis was classified into four categories: (1) no stenosis, (2) minimal or mild stenosis (≤ 50%), (3) moderate stenosis (50%–70%), and (4) severe stenosis (> 70%). CAD was defined when lumen diameter reduction was greater than 50% (moderate or severe stenosis).
2.3. Echocardiography and quantitative image assessment
Dobutamine echocardiography was performed by a cardiologist (with more than 10 years of experience in cardiac imaging) using a standard protocol. First, resting echocardiography was achieved with the patient placed in the left lateral leaning position. Echocardiographic imaging was then completed through an intravenous infusion of dobutamine, first at a dose of 5 µg/kg per minute, then it was increased at every 3 min to 7.5, 10, 20, 30, and 40 µg/kg per minute, respectively. Images were acquired in the normal parasternal long-axis and short-axis views, with particular attention paid to determining the regional cardiac function. Through dobutamine infusion, the 12-lead electrocardiograph (ECG) and blood pressure were observed every minute. The cardiologist was blinded to the results of other imaging modalities.
Calculations of the regional wall motion were assumed off line using a 16-segment model according to the American Society of Echocardiography.[7]–[9] These 16 segments were classified as 0 = normal; 1 = hypokinetic; 2 = akinetic; and 3 = dyskinetic. Only segments within the infarction-related coronary segment were analyzed for wall motion. From this territory, the regional wall motion score was calculated.
2.4. Cardiac SPECT and quantitative image assessment
All patients underwent a gated-SPECT 99mTc tetrofosmin (GE Ventri, GE Healthcare, Florida, USA). After a minimum of 4 h of fasting, nitrates were stopped for 12 h prior to the study. Following intravenous cannulation, 450 MBq (12 mCi) of 99mTc tetrofosmin was administered intravenously under resting conditions. About 45–60 min after radiotracer injection, a resting SPECT study was performed under double-headed gamma camera equipped with high-resolution collimators. Data were acquired in 64 × 64 matrix, with 32 projections, and 8 frames per cardiac cycle, and were used in association with a 20% window centred on the 140-keV photon peak.
All data of the 99mTc-tetrofosmin SPECT studies were reoriented into short-axis and horizontal and vertical long-axis sections. Quantitative analysis was performed using a commercially available gated-cardiac software package (4D-MSPECT; University of Michigan Medical Center) for assessing left ventricular regional wall motion.[10] The left ventricular wall motion was visually classified into 4 categories (0 = normal, 1 = mild hypokinetic, 2 = moderate or severe hypokinetic, and 3 = akinetic or dyskinetic) using a 17-segment model.
2.5. Cardiac PET and quantitative image assessment
All of the patients were informed of fasting for at least 6 h before the scan and baseline blood sugar was checked. About 45–60 min after injection of the 50–75 g of glucose loading, blood sugar was checked. If it was < 140 mg/dL, 444 MBq (12 mCi) of 18F-FDG was injected intravenously. If it was > 140 mg/dL, regular insulin was injected intravenously according to blood glucose level (2, 3 and 5 U of regular insulin for 140–160, 160–180, and 180–200 mg/dL of blood glucose, respectively). About 45–60 min after 18F-FDG injection myocardial 18F-FDG PET study was performed in a PET scanner in a 3D mode (Siemens Medical Systems, Erlangen, Germany). PET acquisition parameters were as follows: myocardium was covered in one bed position (5 min per bed position) with ECG gating (8 frames/RR cycle).
The American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC) recommend a semi-quantitative analysis of PET studies based on a validated segmental scoring system.[11],[12] A 17-segment model analysis was proposed using a 5-point scale system in direct proportion to the observed count density of the segment: 0 = normal perfusion, 1 = mild defect, 2 = moderate defect, 3 = severe defect and 4 = absent uptake.
2.6. Cardiac SPECT, PET and echocardiography qualitative image assessment
The data were independently analyzed by two radiologists (with more than 10 years of experience in cardiac imaging). Both radiologists used a 17-segment model to analyze myocardial viability as observed on SPECT and PET images and analysis was based on a 5-point scale examining the segment of three main coronary arteries, namely: left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA): 0 = normal perfusion, 1 = mild defect, 2 = moderate defect, 3 = severe defect and 4 = absent uptake. For echocardiography, the assessment used was according to the 16-segment model as recommended by the American Society of Echocardiography.
2.7. Statistical analysis
All data were entered into SPSS V 20.0 for statistical analysis (SPSS, Chicago, ILL). Continuous variables were expressed as mean ± SD, while categorical variables were presented as percentages. P < 0.05 was considered statistically significant difference.
Agreement in qualitative measurements between two radiologists (both intra- and inter-observer variability) and between different methods was compared using kappa statistics (κ) and classified as follows: poor (κ = 0.20); fair (κ = 0.21–0.40); moderate (κ = 0.41–0.60); good (κ = 0.61–0.80) and excellent agreement (κ = 0.81–1.00). Echocardiography, 99mTc tetrofosmin SPECT and 18F-FDG PET were calculated for diagnostic value when compared to invasive coronary angiography which was regarded as the gold standard.
3. Results
A total of 10 patients were included in this study and patient's characteristics are shown in Table 1. All patients had proven CAD but in 50% of the patients the principal diagnosis had triple vessel disease involving LAD, LCX and RCA with ≥ 50% stenosis. A total of 340 segments in 10 patients were analysed for cardiac SPECT and PET imaging examinations, while for echocardiography there were 160 segments that were analyzed in comparison with cardiac SPECT and PET findings.
Table 1. Patient demographics (n = 10).
| Age, yrs | 59.5 ± 10.5 |
| Male/Female | 9/1 |
| Principal diagnosis (%) | |
| Ischaemic heart disease | 10% |
| Ischemic dilated cardiomyopathy | 20% |
| Inferior STEMI thrombolysed | 20% |
| Two vessel disease | 30% |
| Triple vessel disease | 50% |
| Non-ST segment elevation myocardial infarction | 10% |
| Cardiac risk factors (%) | |
| Diabetes | 40% |
| Hypertension | 80% |
| Smoking history | 20% |
| Dyslipidemia | 30% |
| Obesity | 10% |
| Patient management (%) | |
| Control risk factors | 50% |
| Referral for coronary bypass surgery | 50% |
| Percutaneous coronary intervention | 20% |
| Implantable cardioverter defibrillator | 10% |
| Medical therapy | 10% |
| International normalised ratio | 20% |
STEMI: ST segment elevation myocardial infarction.
3.1. Diagnostic accuracy at per-patient assessment
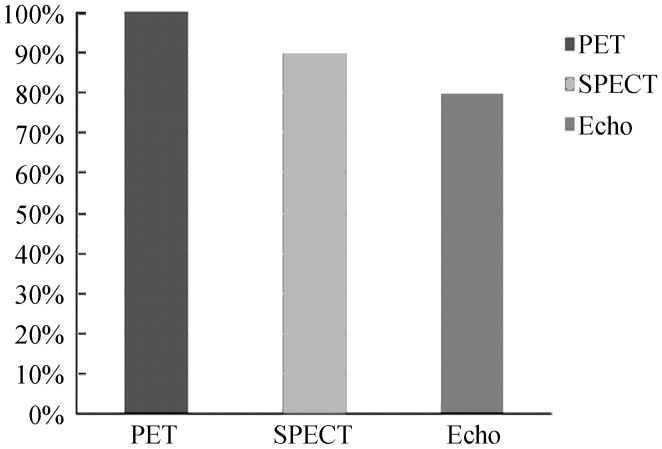
The diagnostic sensitivity of cardiac SPECT, PET and echocardiography at per-patient assessment was 90%, 100% and 80%, respectively, as shown in Figure 1. Since all patients had confirmed CAD, specificity was not analysed (true negative value was zero).
Figure 1. Diagnostic sensitivity of PET, SPECT and echocardiography in myocardial viability when compared to invasive coronary angiography at per-patient based assessment.
Echo: echocardiography; PET: positron emission tomography; SPECT: single photon emission computed tomography.
3.2. Diagnostic accuracy at per-vessel assessment
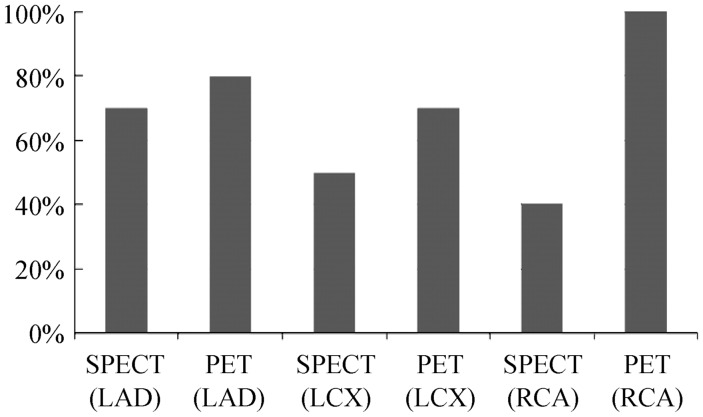
Comparison was also performed between the cardiac PET and SPECT according to vessel-based assessment, as shown in Figure 2. Of the 30 coronary arteries (LAD, LCX, and RCA), 22 were found to have significant coronary stenosis or occlusion (> 70% lumen stenosis), with mild to moderate stenosis observed in six coronary arteries on invasive coronary angiography. Of these diseased coronary arteries, eight were found in the LAD and LCX, and six in the RCA. Interestingly, for two patients documented mild to moderate coronary stenosis by invasive coronary angiography, the three functional modalities, echocardiography, SPECT and PET all showed abnormal changes with PET revealing severely reduced FGD uptake in different regions. Results showed that cardiac PET has the highest diagnostic value in the assessment of all of the three main coronary arteries, while SPECT has moderate diagnostic value in LAD, but low diagnostic performance in the other two coronary arteries, RCA and LCX.
Figure 2. Diagnostic sensitivity of SPECT and PET in myocardial viability at per-vessel based assessment.
As shown in the figure, diagnostic value of PET is superior to that of SPECT in all of the three main coronary arteries. LAD: left anterior descending; LCX: left circumflex; PET: positron emission tomography; RCA: right coronary artery; SPECT: single photon emission computed tomography.
3.3. Qualitative image assessment by echocardiography, SPECT and PET
The mean score for assessment of cardiac function and myocardial viability by echocardiography, SPECT and PET at per-patient based analysis was 2.8 ± 0.42, 3.8 ± 0.63, 4.0, respectively. The ejection fraction of left ventricle measured by echocardiography was less than 40% in all of the patients, while the corresponding SPECT and PET scans showed severely reduced and no uptake of radiopharmaceuticals in more than 80% of the patients.
The mean score for assessment of myocardial viability by SPECT and PET at per-vessel based analysis was 3.4 ± 0.96 and 3.6 ± 0.84, 2.5 ± 1.64 and 3.8 ± 0.42, 2.9 ± 1.28 and 4.0, corresponding to the LAD, LCX and RCA coronary arteries, respectively. The inter-observer assessment, namely SPECT observer 1, SPECT observer 2, PET observer 1 and PET observer 2 were analyzed with regard to the diagnostic evaluation of myocardial viability. Results showed that PET observer1 has high diagnostic value, while the diagnostic performance of PET observer 2 is the same as SPECT observer 1 and SPECT observer 2, although this did not reach significant difference. Excellent agreement was reached on the sensitivity of PET observer 1 and PET observer2, SPECT observer 1 and SPECT observer 2 with kappa value of 0.9.
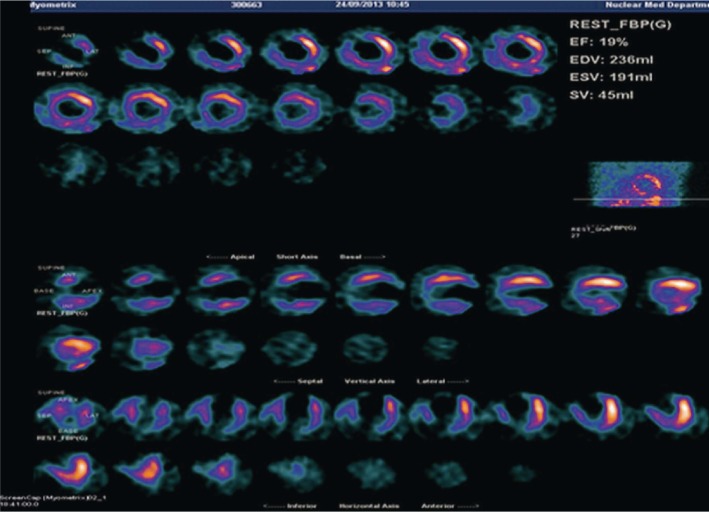
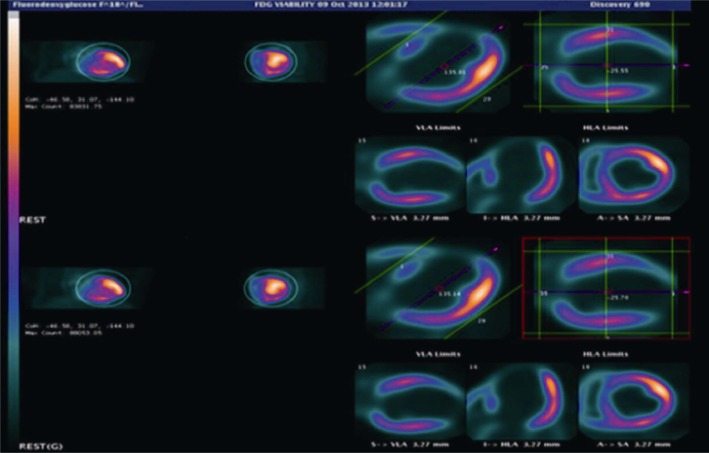
Figure 3 is an example of cardiac SPECT imaging in a patient diagnosed with significant CAD, while Figure 4 is another example of cardiac PET imaging in the same patient with CAD. Both cardiac SPECT and PET detected abnormal myocardial changes, although cardiac PET is superior to cardiac SPECT in terms of image quality with more accurate assessment of all of the segments.
Figure 3. Gated 99mT-tetrofosmin SPECT viability imaging shows marked reduced myocardial thickening and akinetic wall motion at the apex, anterior apical, anteroseptal mid and inferior mid segments.
SPECT: single photon emission computed tomography.
Figure 4. Gated 18F-FDG PET viability imaging shows severely reduced 18FDG uptake in the inferior apical, inferior mid and inferior basal segments.
18F-FDG PET: 18F-fluorodeoxyglucose positron emission tomography.
4. Discussion
This prospective study investigates the diagnostic value of non-invasive cardiac modalities through comparing 18F-FDG PET with 99mTc tetrofosmin SPECT and echocardiography with the aim of determining myocardial viability in patients with known CAD with invasive coronary angiography as the gold standard. Our results showed that cardiac 18F-FDG PET has superior diagnostic value in patients with CAD, when compared to SPECT and echocardiography either at per-patient or per-vessel based analysis. Although based on a small number of patients, results of this study highlight the high diagnostic value of 18F-FDG PET in the detection of myocardial viability.
The assessment of myocardial viability with 18F-FDG PET is based on its ability to distinguish the two main pathogenic mechanisms for chronic myocardial dysfunction in ischemic cardiomyopathy: irreversible loss of myocardium due to prior myocardial infarction (scar), and at least partially reversible loss of contractility owing to chronic or repetitive ischemia (hibernating myocardium).[2] The distinctive feature of these two mechanisms is that revascularization has the potential to restore contractile function of the hibernating myocardium but not scar.[13] This distinction may be of paramount importance in clinical decision-making because of the upfront morbidity and mortality associated with revascularization procedures in patients with severe left ventricular dysfunction.
Our results are consistent with previous findings which reported the high diagnostic accuracy of 18F-FDG PET in viability assessment, and 18F-FDG PET compares well with other established techniques such as SPECT and echocardiography.[14]–[17] Studies using 18F-FDG PET suggested that the presence of viable myocardium by itself carries an increased risk for adverse events compared to patients without viable myocardium.[18]–[20] In a recent meta-analysis, the optimal threshold for the presence of viability required to improve survival with revascularization was estimated to be 25.8% by 18F FDG-PET perfusion mismatch for the assessment of viability, which is much lower than the cut-off values used by stress echocardiography and SPECT.[21] When PET was integrated into clinical patient management, a significant reduction in cardiac events was observed in patients with 18F-FDG PET-assisted management, according to randomized controlled trials.[22],[23] This preliminary study did not address the impact of 18F-FDG PET on patient management, although all of these patients were diagnosed with significant coronary artery disease. Further research of incorporating 18F-FDG PET into patient management deserves to be investigated.
Although 18F-FDG PET is regarded as the gold standard for the diagnosis of myocardial viability,[16] cardiac PET is not widely available as opposed to SPECT. Furthermore, the high cost of PET camera and cyclotron technology limit the extensive use of this technique in many clinical centers. This is the main reason in this study that we compared 18F-FDG PET with SPECT and echocardiography to further clarify its clinical value, despite limited applications. In comparison with PET, SPECT still shows high diagnostic value at per-patient analysis with sensitivity of 90%. This highlights the fact that SPECT can be used as a reliable technique for assessment of myocardial viability due to its wide availability and low cost when 18F-FDG PET is not available.
Invasive coronary angiography is the gold standard for the identification of obstructive coronary lesions, however, it lacks the sensitivity and specificity for the early detection of coronary artery disease, quantitative analysis of plaque composition and cardiac function assessment.[24],[25] The most recently published PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) trial highlights the value of non- or less-invasive imaging modalities to evaluate high-risk plaque for more accurate risk stratification of patients with coronary artery disease, as invasive angiography was found to have poor predictive accuracy in identifying patients with high risk of developing acute cardiac events.[26] This has been confirmed by our study as well. Cardiac SPECT and PET showed more accurate assessment of myocardial viability in some patients with significant coronary stenosis which was only demonstrated as moderate coronary stenosis by invasive coronary angiography. The reason we used invasive coronary angiography as the gold standard in this study is because all of the patients recruited were clinically diagnosed with significant coronary artery disease, thus, they were referred for invasive coronary angiography for the purpose of diagnosis and revascularization. Furthermore, we would like to determine the diagnostic value of invasive coronary angiography in risk stratification, in particular, the accuracy in predicting patients of developing cardiac events. In routine clinical practice, cardiac SPECT or PET is commonly used for the assessment of myocardial metabolism and viability, while invasive coronary angiography is reserved for confirming disease extent and performing revascularization. This study further emphasises the clinical value of SPECT and PET for assessment of myocardial function while highlighting the limitations of lumen assessment by invasive coronary angiography.
There are some limitations in this study that should be acknowledged. Firstly, the sample size is small. This is either due to the number of patients who refused to participate in the study or due to the disease severity of CAD or unstable condition, therefore, most of the patients decided to undergo revascularization treatment instead of further imaging assessment. Secondly, only patients with known or proven CAD were included in this study, thus, our results should be interpreted with caution, in particular the very high diagnostic value of cardiac PET and SPECT in the assessment of myocardial viability. Lastly, although advances in nuclear cardiology imaging procedures have improved the ability to diagnose and treat cardiovascular conditions, there is a lack of patient-level data on radiation exposure over time from these procedures despite a rapid increase in their clinical use. The dosimetry of different radiopharmaceuticals in nuclear cardiology may be quite different; therefore, it is necessary to be aware of the radiation dose associated with cardiac SPECT and PET procedures.[7] The effective dose of PET and SPECT ranges from 2 mSv to 30 mSv, depending on the type and activity of radiopharmaceuticals administered.[10],[27] Furthermore, CT-based attenuation correction is commonly performed during SPECT and PET examinations, thus, radiation dose associated with CT (although low-dose CT protocol is routinely used) should be considered as it contributes to the total radiation exposure to patients. All of the patients in this study are quite old (average age 60 years), however, attention should be paid to younger patients who undergo cardiac PET and SPECT examinations, with dose-reduction strategies being implemented whenever possible to minimize radiation dose. Another aspect that needs to be considered is the cost associated with cardiac SPECT and PET scans when compared to echocardiography. Recently, echocardiography has been increasingly used in the diagnostic evaluation of cardiac structure and function due to dramatic improvements in technology, such as contrast echocardiography, 3D echocardiography and speckle tracking echocardiography.[28] Therefore, echocardiography still continues to play an important role in the current clinical practice in the diagnosis of patients with coronary artery disease.
In conclusion, 18F-FDG cardiac PET has high diagnostic value in the assessment of myocardial viability in patients with known coronary artery disease when compared to cardiac SPECT and echocardiography. Further studies based on a large cohort are warranted to verify our preliminary results, and in particular, to determine the clinical impact of cardiac PET in patient management.
Acknowledgments
Authors would like to thank Dr. Ahmad Khairuddin Md Yusof from National Heart Institute, Malaysia, and Dr. Aini AbAziz from Universiti Kebangsaan Malaysia Medical Center for their assistance in this project. The funding support from Saudi Cultural Mission, Australia is greatly appreciated. We declare that there is no conflict of interest in this study.
References
- 1.Tillisch J, Brunken R, Marshall, et al. Reversibility of cardiac wall motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884–888. doi: 10.1056/NEJM198604033141405. [DOI] [PubMed] [Google Scholar]
- 2.Schinkel AF, Bax JJ, Poldermans D, et al. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32:375–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Gaemperli O, Kaufmann PA. PET and PET/CT in cardiovascular disease. Ann N Y Acad Sci. 2011;1228:109–136. doi: 10.1111/j.1749-6632.2011.06030.x. [DOI] [PubMed] [Google Scholar]
- 4.Siebelin HM, Blanksma PK, Crijns HJ, et al. No difference in cardiac event-free survival between positron emission tomography-guided and single-photon emission computed tomography-guided patient management a prospective, randomized comparison of patients with suspicion of jeopardized myocardium. J Am Coll Cardiol. 2001;37:81–88. doi: 10.1016/s0735-1097(00)01087-1. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan G, Kitsiou AN, Bacharach SL, et al. F-18 fluorodeoxyglucose single photon enission computed tomography: Can it replace PET and thallium SPECT for the assessment of myocardial viability? Circulation. 1998;97:843–850. doi: 10.1161/01.cir.97.9.843. [DOI] [PubMed] [Google Scholar]
- 6.Arrhighi JA, CK NG, Dey HM, et al. Effect of left ventricular function on the assessment of myocardial viability by technetium-99m sestamibi and correlation with positron tomography in patients with healed myocardial infarcts of stable angina pectoris, or both. Am J Cardiol. 1997;80:1007–1013. doi: 10.1016/s0002-9149(97)00594-8. [DOI] [PubMed] [Google Scholar]
- 7.Cerqueria MD, Weissman NJ, Dilsizian V, et al. Standardization myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Nuclear Cardiology Imaging guidelines for nuclear cardiology procedures, Part 2. J Nucl Cardiol. 1999;6:G47–G84. [PubMed] [Google Scholar]
- 9.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Aziz A, Md Yusof AK. Cardiac nuclear imaging: current status and future directions. Curr Med Imaging Rev. 2013;9:170–183. [Google Scholar]
- 11.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC Guidelines for the clinical use of cardiac radionuclide imaging-executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to revise the 1995 Guidelines for the clinical use of cardiac radionuclide imaging) Circulation. 2003;108:1404–1418. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 12.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC Guidelines for the clinical use of cardiac radionuclide imaging-executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to revise the 1995 Guidelines for the clinical use of cardiac radionuclide imaging) J Am Coll Cardiol. 2003;42:1318–1333. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh N, Rimoldi OE, Beanlands RS, et al. Assessment of myocardial ischaemia and viability: role of positron emission tomography. Eur Heart J. 2010;31:2984–2995. doi: 10.1093/eurheartj/ehq361. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Wei HX, Yang MF, et al. Phase analysis by gated F-18 FDG PET/CT for left ventricular dyssynchrony assessment: a comparison with gated Tc-99m sestamibi SPECT. Ann Nucl Med. 2013;4:1–10. doi: 10.1007/s12149-013-0691-y. [DOI] [PubMed] [Google Scholar]
- 15.Pazhenkottil AP, Buechel RR, Nkoulou R, et al. Left ventricular dyssynchrony assessment by phase analysis from gated PET-FDG scans. J Nucl Cardiol. 2011;18:920–925. doi: 10.1007/s12350-011-9411-y. [DOI] [PubMed] [Google Scholar]
- 16.Raja S, Singh B, Rohit MK, et al. Comparison of nitrate augmented Tc-99m tetrofosmin gated SPECT imaging with FDG PET imaging for the assessment of myocardial viability in patients with severe left ventricular dysfunction. J Nucl Cardiol. 2012;19:1176–1181. doi: 10.1007/s12350-012-9607-9. [DOI] [PubMed] [Google Scholar]
- 17.Almoudi M, Sun Z. A head-head comparison of the coronary calcium score by computed tomography with myocardial perfusion imaging in predicting coronary artery disease. J Geriatr Cardiol. 2012;9:349–354. doi: 10.3724/SP.J.1263.2012.06291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marwick TH, Nemec JJ, Lafont A, et al. Prediction by postexercise fluoro-18 deoxyglucose positron emission tomography of improvement in exercise capacity after revascularization. Am J Cardiol. 1992;69:854–859. doi: 10.1016/0002-9149(92)90782-t. [DOI] [PubMed] [Google Scholar]
- 19.Marwick TH, Zuchowski C, Lauer MS, et al. Functional status and quality of life in patients with heart failure undergoing coronary bypass surgery after assessment of myocardial viability. J Am Coll Cardiol. 1999;33:750–758. doi: 10.1016/s0735-1097(98)00642-1. [DOI] [PubMed] [Google Scholar]
- 20.Di Carli MF, Asgarzadie F, Schelbert HR, et al. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation. 1995;92:3436–3444. doi: 10.1161/01.cir.92.12.3436. [DOI] [PubMed] [Google Scholar]
- 21.Inaba Y, Chen J, Bergmann SR. Quantity of viable myocardium required to improve survival with revascularization in patients with ischemic cardiomyopathy: a meta-analysis. J Nucl Cardiol. 2010;17:646–654. doi: 10.1007/s12350-010-9226-2. [DOI] [PubMed] [Google Scholar]
- 22.Beanlands RS, Nichol G, Huszti E, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized controlled trial (PARR-2) J Am Coll Cardiol. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Abraham A, Nichol G, Williams KA, et al. 18F-FDG PET imaging of myocardial viability in an experienced center with access to 18F-FDG and integration with clinical management teams: the Ottawa-FIVE substudy of the PARR 2 trial. J Nucl Med. 2010;51:567–574. doi: 10.2967/jnumed.109.065938. [DOI] [PubMed] [Google Scholar]
- 24.Chan KH, Ng MKC. Is there a role for coronary angiography in the early detection of the vulnerable plaque? Int J Cardiol. 2013;164:262–266. doi: 10.1016/j.ijcard.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Xu L. Coronary CT angiography in the quantitative assessment of coronary plaques. Biomed Res Int. 2014;2014:346380. doi: 10.1155/2014/346380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourantas CV, Garcia-Garcia HM, Farooq V, et al. Clinical and angiographic characteristics of patients likely to have vulnerable plaques: Analysis from the PROSPECT study. JACC Cardiovasc Imaging. 2013;6:1263–1272. doi: 10.1016/j.jcmg.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Montes C, Tamayo P, Hernandez J, et al. Estimation of the total effective dose from low-dose CT scans and radiopharmaceutical administrations delivered to patients undergoing SPECT/CT explorations. Ann Nucl Med. 2013;27:610–617. doi: 10.1007/s12149-013-0724-6. [DOI] [PubMed] [Google Scholar]
- 28.Shah BN. Echocardiography in the era of multimodality cardiovascular imaging. Biomed Res Int. 2013;2013:310483. doi: 10.1155/2013/310483. [DOI] [PMC free article] [PubMed] [Google Scholar]