Abstract
Coronary artery disease remains a major cause of mortality. Presence of atherosclerotic plaques in the coronary artery is responsible for lumen stenosis which is often used as an indicator for determining the severity of coronary artery disease. However, the degree of coronary lumen stenosis is not often related to compromising myocardial blood flow, as most of the cardiac events that are caused by atherosclerotic plaques are the result of vulnerable plaques which are prone to rupture. Thus, identification of vulnerable plaques in coronary arteries has become increasingly important to assist identify patients with high cardiovascular risks. Molecular imaging with use of positron emission tomography (PET) and single photon emission computed tomography (SPECT) has fulfilled this goal by providing functional information about plaque activity which enables accurate assessment of plaque stability. This review article provides an overview of diagnostic applications of molecular imaging techniques in the detection of plaques in coronary arteries with PET and SPECT. New radiopharmaceuticals used in the molecular imaging of coronary plaques and diagnostic applications of integrated PET/CT and PET/MRI in coronary plaques are also discussed.
Keywords: Atherosclerotic plaque, Coronary artery disease, Single photon emission computed tomography, Positron emission tomography, Vulnerability
1. Introduction
It has become well established that plaque composition is a key determinant of plaque stability. High-risk plaques tend to be large, demonstrate positive remodelling and have a large lipid core that occupies 40% or more of the plaque volume. These kinds of plaques are prone to rupture and develop intraplaque hemorrhage in comparison to stable plaques. Thus, the ability to noninvasively detect and analyse these plaques at early stages, especially in asymptomatic and low-risk patients, would improve risk stratification without the need for more invasive procedures.[1] The development of fast CT scanners allows non-invasive coronary artery visualization with high diagnostic accuracy, reliable detection of lumen stenosis, characterization and quantification of atherosclerotic coronary plaque, and even coronary risk stratification.[2],[3] Coronary CT angiography not only allows accurate detection of coronary atherosclerotic plaques (Figure 1), but also enables quantitative analysis of plaque vessel area, lumen area, and plaque burden.[4]–[17] However, these coronary CT angiography-derived indexes of plaque vulnerability demonstrated a high negative predictive value for major adverse cardiac events, but a low positive predictive value, thus making their clinical application rather limited.[18],[19]
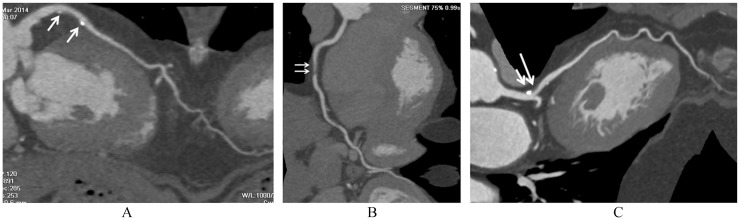
Figure 1. Coronary CT angiography characterization of plaque composition.
(A): calcified plaques at the left anterior descending coronary artery (arrows); (B): non-calcified plaque at the right coronary artery (arrows); (C): mixed plaque at the left anterior descending coronary artery (short arrow refers to calcification, while long arrow points to non-calcified component).
Invasive coronary angiography is still the gold standard for the investigation of coronary lumen changes, but it is unable to detect atherosclerotic plaques that do not protrude into the lumen and does not provide information about plaque composition (Figure 2).[20] Intravascular ultrasound (IVUS) allows direct imaging of plaque and can provide a cross-sectional image of the entire atheroma and vessel wall, with important information on the composition of individual plaques. Advances in IVUS technology, such as spectral analysis of the IVUS backscatter radiofrequency signal have further improved the tissue characterization of plaques, and this technique is referred to as virtual histology intravascular ultrasound (VH-IVUS).[21] VH-IVUS spectral analysis has been reported to correlate well with histopathology with predictive accuracy of 87.1%, 87.1%, 88.3% and 96.5% for fibrous, fibro-fatty, necrotic core, and dense calcium, respectively.[22],[23] Furthermore, VH-IVUS can identify thin cap fibroatheroma and other plaque subtypes, and follow plaque composition after treatment.[24],[25] However, IVUS is an invasive, time-consuming and expensive technique which demands considerable expertise, thus its use is limited to only a few clinical centers. Magnetic resonance imaging (MRI) is capable of detecting plaque components and discriminating between lipid core, fibrous cap, intraplaque hemorrhage and calcification, and can also detect macrophage-rick lesions.[26],[27] MRI allows imaging of coronary lumen and plaque non-invasively without using ionizing radiation, with reported negative predictive value of 88%.[28] However, coronary MR angiographic technique remains technically demanding due to limited spatial resolution and suboptimal signal/contrast to noise ratio, thus restricting its widespread acceptance into the clinical practice.
Figure 2. Limitations of invasive coronary angiography to demonstrate plaque components.
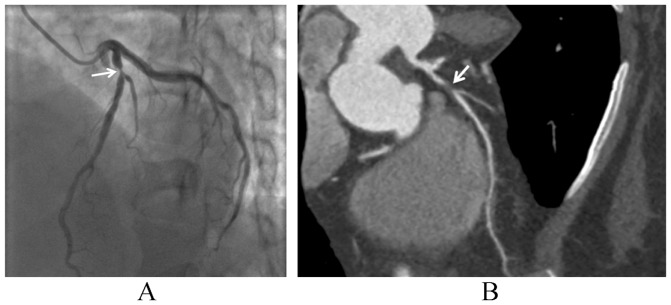
(A): invasive coronary angiography shows significant lumen stenosis (arrow) at the proximal segment of left anterior descending coronary artery, but fails to demonstrate the plaque components; (B): coronary CT angiography clearly shows significant lumen stenosis due to presence of a non-calcified plaque (arrow).
The above-mentioned invasive and non-invasive imaging modalities focus on providing anatomical details of plaque size and composition, however, molecular imaging techniques such as single photon emission computed tomography (SPECT) and positron emission tomography (PET) have the potential to provide functional information on cell biological changes which determine the risk of plaque rupture and furthermore, evaluate important determinants of plaque vulnerability by demonstrating specific cellular or biochemical changes. Although cardiac SPECT and PET have been well regarded as an important diagnostic tool that provides valuable information about myocardial viability, perfusion and function,[29] the diagnostic applications of these techniques in the detection and analysis of coronary plaques are increasingly reported in the literature. This article aims to provide an overview of the clinical value of molecular imaging modalities using PET and SPECT in the detection and characterization of coronary plaques. Diagnostic value of integrated SPECT/CT, PET/CT and PET/MRI is also discussed.
2. Coronary plaque morphology
There is increasing evidence showing that plaque morphology rather than the extent of luminal stenosis determines the susceptibility of an individual to develop an acute coronary event. The culprit lesion (that is ruptured) or vulnerable plaque is defined as a “high-risk” or “thrombosis-prone” plaque. Morphologically, these plaques typically have a lipid-rich necrotic core with a thin fibrous cap (cap thickness of < 65 µm) infiltrated by macrophages. A more clinical relevant definition of a vulnerable plaque is a lesion that places a patient at risk for developing future major adverse cardiac events, including death or myocardial infarction.[30] The identification of such plaques before they become symptomatic would enable prognostic stratification and facilitate primary prevention (e.g., aspirin, statins, and risk factor modification).
The concept of a vulnerable plaque or high-risk plaque originated from data of several studies that analyzed angiograms post-thrombolytic therapy or through analysis of serial angiographic data before and after ST-elevation induced acute myocardial infarction.[31]–[33] These studies showed that most infarctions were caused by lesions that did not have significant coronary stenosis in coronary lumen. It was therefore considered that disruption of mild and moderate coronary plaques resulted in thrombus formation and coronary occlusion, leading to myocardial infarction. Brown, et al.[34] reported that after thrombolytic therapy the actual size of the plaque responsible for the infarction was only moderate, and the remaining luminal narrowing was related to residual thrombus formation. Due to positive (expansive or outward) vascular remodeling, the lumen remains relatively uncompromised until the plaque has grown to a larger volume.[31] Thus, in most cases these plaques are clinically silent before the acute cardiac events occur. This emphasizes the importance of detecting vulnerable coronary plaques.
However, diagnosing a vulnerable plaque which based on imaging assessment is imprecise to some extent, as the criterion of using the thickness of the fibrous cap alone to determine plaque vulnerability is insufficient. Furthermore, if presence of the lipid component indicates a vulnerable plaque, how much lipid-rich necrotic core should it possess, and how much inflammation must be present to make it vulnerable when initially identified?[34] Perhaps the most vulnerable type of coronary plaques might be those that are already disrupted with only small amounts of thrombus formation but when initially identified are not clinically symptomatic (Figure 3).[35],[36]
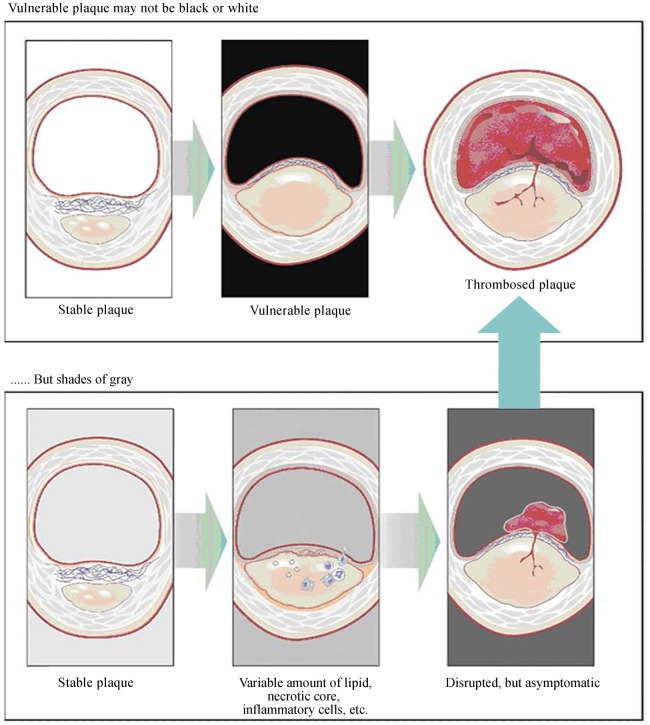
Figure 3. Vulnerable plaque.
In the upper panel, the middle figure shows a presumed vulnerable plaque, a thin capped atheroma with a large/necrotic core, and a thin fibrous cap infiltrated by inflammatory cells, which is thought to be the immediate precursor of symptomatic thrombosed plaque (upper right). However, as shown in the lower panel, a “vulnerable plaque” might not be an easy diagnosis to make with one or more invasive/noninvasive techniques. The true precursor to a symptomatic thrombosed plaque might depend on such factors as the exact cap thickness, size of the lipid/necrotic core, inflammatory cell volume, thrombogenicity of the blood, and others. Reprint with permission from reference [35].
3. Molecular imaging of coronary plaques
Traditionally, atherosclerotic coronary artery disease was only diagnosed at advanced stages by determining the degree of lumen stenosis or by myocardial perfusion assessment. However, molecular imaging modalities enable assessment of coronary artery disease down to the cellular and molecular level. Plaque inflammation is regarded as a reliable indicator of plaque rupture and thrombosis, while enzymes released by active and apoptotic leukocytes stimulate endothelial injury, cytolysis and plaque disruption with subsequent thrombosis.[37],[38] Apoptosis is considered an important indicator to predict plaque stability, and it has been shown to be closely linked to the development of a vulnerable plaque.[39] Apoptotic smooth muscle cell death contributes to thinning of the fibrous cap, whereas apoptosis of macrophages and accumulation of cellular debris in the necrotic core leads to expansion of the plaque necrotic core and positive vascular remodelling: all these features cause coronary plaques prone to rupture. Therefore, the in vivo detection of apoptosis in atherosclerotic lesions can assist localization of unstable plaques.[40]
Scintigraphic techniques including SPECT and PET have the potential for superior functional and molecular imaging for prediction of the risk of atherosclerotic coronary plaque rupture. Molecular imaging provides diagnostic tools that target specific or nonspecific inflammation in coronary plaques. While such functional or metabolic information is superior to that provided by cardiac CT and MRI, SPECT and PET imaging techniques are limited by poor spatial and temporal resolution. This can be overcome with use of integrated imaging techniques such as SPECT/CT and PET/CT or PET/MRI which are increasingly used in clinical practice.
4. SPECT imaging of coronary plaques
Technetium-99m-labelled (99mTc) radiotracers such as 99mTc-sestamibi and 99mTc-tetrofosmin are widely used in clinical practice for the investigation of myocardial perfusion and viability. New 99mTc-labelled tracers and novel iodine-123 tracers for SPECT myocardial perfusion imaging are being developed recently with results showing high cardiac uptake and improved cardiac image,[41] although further clinical studies are needed to verify their diagnostic value.
99mTc-labelled tracers with SPECT have been used for the specific targeting of vulnerable atherosclerotic lesions by detecting these changes. To evaluate the feasibility of detection of atherosclerotic plaque in the coronary artery in vivo, Johnson, et al.[42] conducted a study in a swine model of atherosclerosis. SPECT imaging with 99mTc-labeled with Annexin A5 was used to image the plaque. Histopathologic analysis showed that the coronary lesions in general were at an early stage and were mainly characterized by smooth muscle cells. Focal uptake of 99mTc Annexin A5 was noted in 13 out of 22 coronary vessels in vivo. A significant correlation was found between the count ratio of the atherosclerotic vessels and that of control vessels. This study showed that it is possible to detect atherosclerotic plaque apoptosis in vivo in the coronary artery with similar dimensions to the human heart.
Although 99mTc SPECT is able to assess atherosclerotic coronary lesions in experimental studies,[39],[41] most of the clinical studies using SPECT for imaging vulnerable plaques focus on the investigation of patients with carotid plaques.[43]–[45] Increased uptake of radioisotopes was reported in culprit coronary lesions when compared to the normal arteries, which did not demonstrate tracer accumulation. Furthermore, 99mTc SPECT has been shown to be able to detect the presence of inflammation in carotid plaques, which plays an important role in promoting plaque rupture.[46],[47] Compared to coronary CT angiography, integrated SPECT/CT technique has been reported to provide a complimentary role in the diagnostic evaluation of patients with suspected coronary artery disease, with improved specificity and positive predictive value without significant change in sensitivity and negative predictive value.[48]–[51] However, most of the current studies using integrated SPECT/CT focus on myocardial perfusion imaging, investigation of the integrated imaging modality for identification of culprit coronary plaque is limited, thus, further studies are warranted.
Despite these promising reports, the important application of nuclear cardiac imaging lies in the utilization of PET in imaging vulnerable plaque as SPECT is limited by its poor spatial resolution in imaging coronary arteries and coronary lesions. Cardiac SPECT imaging has been used for myocardial perfusion and viability for many years and is a well-established and the most commonly used imaging modality in these areas.[28]
5. PET imaging of coronary plaques
5.1. Commonly used radiopharmaceutical-fluoro deoxyglucose PET
Cardiac PET has higher spatial and contrast resolution than SPECT, thus it allows a more conspicuous identification of regional differences in radiotracer uptake and thus increasing the sensitivity for detection of atherosclerotic changes in the coronary arteries. Fluorodeoxyglucose (FDG) with PET is a molecular imaging technique that is highly sensitive to metabolically active processes using glucose as a fuel. After being labeled with 18F, the resultant 18F-FDG is taken up into metabolically active but is not metabolized and thereby accumulates in atherosclerotic plaques. In oncology, 18F-FDG uptake by tumors makes PET the gold standard for the detection of metastatic disease.[52] In cardiology, 18F-FDG PET is routinely performed to estimate myocardial glucose consumption, in compromised myocardium, uptake of FDG indicates viability and likely positive response to myocardial revascularization.[53] In addition to the widespread use of 18F-FDG PET for screening and staging of cancers, 18F-FDG PET is the most sensitive modality for visualizing inflammation, because 18F-FDG shows accumulation in inflammatory cells in which glucose metabolism is activated.[54]
Arterial 18F-FDG PET imaging has been increasingly used as a biomarker to investigate the metabolic activity of atherosclerosis.[39] The first data on 18F-FDG PET imaging in human atherosclerotic plaque inflammation was reported in patients with carotid plaques.[55] The study demonstrated the ability of 18F-FDG PET imaging to identify inflammatory activity in unstable carotid plaques in patients undergoing carotid endarterectomy compared to the contralateral asymptomatic artery. 18F-FDG was taken up by atherosclerotic plaque and selectively accumulated in macrophage-rich areas. Later reports by others confirmed the clinical value of 18F-FDG PET in detecting vulnerable atherosclerotic coronary plaques.[56]–[59]
Dunphy, et al.[56] reported the feasibility of imaging proximal coronary plaques using 18F-FDG PET with results showing greater FDG accumulation in arterial wall due to atherosclerotic plaques (inflammatory changes) than adjacent blood pool activity. Williams and Kolodny in their retrospective study consisting of 60 lymphoma patients found focal FDG uptake along the coronary artery tree in 15 of them.[57] The concordance between focal FDG uptake in coronary arteries and coronary calcification was 55%, and the concordance between coronary artery FDG uptake and history of coronary artery disease was 77%. Their results provided early evidence of the possibility of using 18F-FDG PET to monitor sites of coronary plaque formation, however large and prospective studies are needed to determine the diagnostic value of this technique. Wykrzykowska, et al.[59] evaluated the potential value of 18F-FDG PET to image inflammation in coronary arteries in 32 cancer patients who underwent coronary catheterization. After special dietary intervention suppressing myocardial glucose uptake, 18F-FDG uptake was identified in 15 patients in one or more coronary segments with atherosclerosis confirmed by invasive coronary angiography.
In addition to detecting the above changes associated with atherosclerotic plaques, 18F-FDG PET has been shown to monitor response to therapeutic interventions, according to several recently published studies.[60]–[65] Inflammation plays a key role in the progression and destabilization of atherosclerotic plaque. 18F-FDG PET has been confirmed to be a promising tool for visualizing inflammation of atherosclerotic plaque, thus, anti-inflammatory action by medications could be demonstrated through modification of vascular FDG uptake. The authors of a prospective study assessed effects of a 3-month statin supplementation on vascular FDG uptake in 43 patients with high baseline FDG uptake in aorta and carotid arteries.[64] Half of the patients received simvastatin (5−20 mg/day) while the other half was dietary group receiving dietary management only. The mean standardized uptake value of FDG at the end of the study period was found to decrease by 10% in the simvastatin-treated group when compared to the baseline. Changes that were demonstrated on FDG-PET represented decreased inflammation in the atherosclerotic plaque.[64] These early findings are confirmed by a recent multicenter study using FDG-PET to monitor the statin therapy.[65] Tawakol, et al.[65] investigated whether high-dose statin treatment resulted in greater reductions in plaque inflammation than low-dose statins using FDG-PET. Sixty-eight patients were randomized to atorvastatin 10 vs. 80 mg and followed up at 4 and 12 weeks with FDG-PET imaging of the aorta and carotid arteries. Results showed that reduction in plaque activity was apparent as early as 4 weeks, although inflammation (FDG uptake) in these arteries was significantly reduced from baseline with atovastatin 80 mg at 12 weeks, but not with atorvastatin 10 mg. This first multicenter trial further confirms the ability of PET imaging as a tool to detect changes in vascular inflammation early in the course of treatment.
5.2. New radiopharmaceuticals in cardiac PET
Although 18F-FDG is a convenient and widely used radiopharmaceutical, investigation of more specific targeting agents for the detection of plaque inflammation and identification of plaque rupture has been increasingly reported in the literature.[66]–[68] 18F-labeled mannose (2-deoxy-2-[18F] fluoro-D-mannose, 18F-FDM) represents a subset of macrophages and it may offer a convenient target for imaging of vascular inflammation using appropriately labeled 18F-FDM. Tahara, et al.[69] in their recent report compared the uptake of 18F-FDM with 18F-FDG in animal experiments, and their results showed comparably high specific uptake of both tracers in the abdominal aortas and aortic arches of atherosclerotic animals, according to both in vivo and ex vivo images. Furthermore, increased expression of mannose receptor-bearing macrophages in unstable plaques and specific binding of FDM to the mannose receptor was observed in the study, which indicates the potential value of use of 18F-FDM in high-risk plaques, although clinical studies are required to confirm these findings.
Quantifying the amount of coronary artery calcium with cardiac CT scan has been widely accepted as a reliable non-invasive technique for screening risk of future cardiac events.[17],[70] Despite clinical value for predicting future cardiac events, cardiac CT does not accurately assess plaque inflammatory state and the degree of molecular calcification which reflects plaque stability.[71],[72] 18F-sodium fluoride (18F-NaF) is a new radiopharmaceutical which has been proposed to assess the accuracy in detecting the process of molecular calcification in the vasculature.[73] Preliminary studies have demonstrated high correlation between the cardiovascular risk factor and the degree of molecular calcification in the heart and aorta,[73],[74] as well as additional information about plaque physiology with use of 18F-NaF, although more prospective studies are required to verify these findings.
In addition to the above-mentioned radiopharmaceuticals, many other tracers have been investigated and tested for imaging of atherosclerotic plaques including 11C-choline,[75] 18F-galacto-RGD,[76] 11C-acetate,[77] and 11C-PK11195.[78] Since 11C-PK11195 specifically binds to macrophages through translocator proteins that are highly expressed on activated macrophages, it may be more specific for imaging of atherosclerosis than 18F-FDG.[79] Despite promising results reported, clinical trials on patients are needed to prove their clinical efficacy of imaging atherosclerosis.
In summary, PET imaging of atherosclerosis contributes significantly to identify individuals at high-risk earlier in the disease processes by detecting the changes at the early stage of disease. It may also prove to be useful in determining the biological responses to certain therapeutic treatment and intervention. The principal strength of PET imaging lies in its excellent sensitivity, good depth of penetration, and quantitative capabilities.[79] However, there are some issues to be resolved in PET imaging of coronary lesions: the small size of atherosclerotic lesions and their closeness with blood, and the continuous respiratory and cardiac movements during the acquisition of the images.[80]–[82] Furthermore, there is a lack of correlation between PET imaging of atherosclerotic plaques and pathological findings and clinical confirmation of adverse cardiac events.
6. Integrated PET/CT imaging of coronary plaques
The integration of nuclear medicine with multislice CT such as PET/CT and SPECT/CT provides a unique opportunity to delineate cardiovascular abnormalities and their physiological consequences in a single setting. For the assessment of the patient with known or suspected coronary artery disease, it allows detection and quantitative analysis of the plaque burden, vascular reactivity and endothelial health, identification of flow-limiting coronary lesions, and assessment of myocardial perfusion and viability. Thus, by revealing the burden of anatomic coronary artery disease and its physiologic significance, integrated imaging can provide unique information for risk assessment and guidance of management of coronary artery disease.[83] As discussed in previous sections, SPECT/CT mainly deals with myocardial perfusion imaging in the current literature,[48]–[51] thus, this section will focus on PET/CT in imaging analysis of coronary plaques.
The feasibility of using PET/CT in the detection of soft plaque in the coronary artery was demonstrated in a case report.[58] Fused PET/CT images identified and localized areas of increased FDG uptake in the proximal segments of the left coronary artery with noncalcified plaque, which was related to inflamed atherosclerotic lesions. The increased clinical availability of PET/CT scanners enables non-invasive detection of high-risk atherosclerotic plaques. Menezes, et al.[84] retrospectively reviewed PET/CT images in 50 patients who had at least 4 examinations over a period of five years. Results showed that areas of focal arterial 18F-FDG uptake are transient, and there is no relationship between arterial 18F-FDG uptake and calcification. As there is considerable evidence indicating that 18F-FDG is a biomarker for plaque instability,[55],[85],[86] their results provide insight into the natural history of arterial lesions, which could translate into important clinical implications.
Although 18F-FDG is useful as a marker of vascular inflammation, 18F-FDG PET may not be able to demonstrate the plaque rupture due to complex recruitment process of multiple cell types that are involved in the high-risk plaque. Therefore, there remains necessity for development of other tracers that can selectively target inflammation in plaques and that is able to identify an atherosclerotic plaque at risk for rupture, thus enabling earlier intervention and possibly improving outcomes. 18F-NaF-PET/CT imaging is considered a useful tool to detect molecular calcification at earlier stages of the atherosclerotic process as microcalcification has been implicated in plaque rupture.[73],[74],[87] The authors of a study published in 2014 elegantly demonstrated that compared to 18F-FDG, 18F-NaF-PET/CT represents the first noninvasive imaging modality to identify and localize ruptured and high-risk coronary plaques.[88] In this prospective clinical trial, 40 patients with myocardial infarction and 40 patients with stable angina underwent 18F-FDG and 18F-NaF-PET/CT, and invasive coronary angiography. 18F-NaF activity in the culprit coronary plaques was 34% higher than the myocardium activity (maximum tissue-to-background ratio 1.66 (1.40-2.25) vs. 1.24 (1.06-1.38), P < 0.001) (Figure 4). In contrast, coronary 18F-FDG uptake was essentially indistinguishable from patch myocardial uptake in 22 patients and increased uptake was observed in the culprit vessels of 6 patients.[88] This study shows for the first time that ruptured or high-risk plaque of rupture can be identified by noninvasive imaging modality, thus 18F-NaF-PET/CT has the potential to alter management of patients with stable and unstable coronary artery disease.
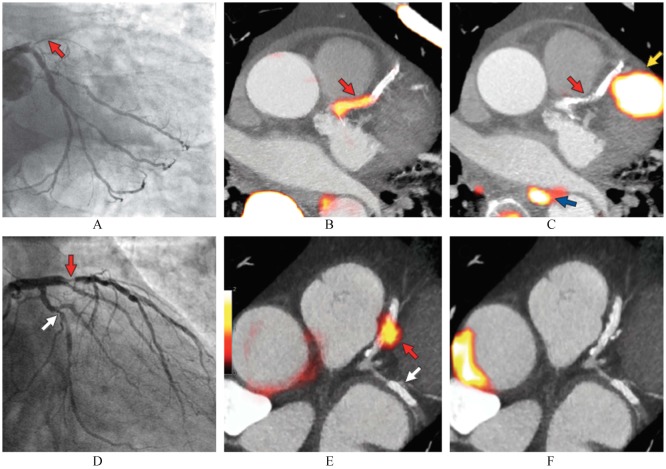
Figure 4. Focal 18F-fluoride and 18F-fluorodeoxyglucose uptake in patients with myocardial infarction and stable angina.
Patient with acute ST-segment elevation myocardial infarction with (A): proximal occlusion (red arrow) of the left anterior descending artery on invasive coronary angiography and (B): intense focal 18F-fluoride [18F-NaF, tissue-to-background ratios, culprit 2.27 vs. reference segment 1.09 (108% increase)] uptake (yellow-red) at the site of the culprit plaque (red arrow) on the combined positron emission and computed tomogram (PET-CT). (C): Corresponding 18F-fluorodeoxyglucose PET-CT image showing no uptake at the site of the culprit plaque (18F-FDG, tissue-to-background ratios, 1.63 versus reference segment 1.91 (15% decrease)). Note the significant myocardial uptake overlapping with the coronary artery (yellow arrow) and uptake within the oesophagus (blue arrow). (D): Patient with anterior non-ST-segment elevation myocardial infarction with culprit (red arrow; left anterior descending artery) and bystander non-culprit (white arrow; circumflex artery) lesions on invasive coronary angiography that were both stented during the index admission. (E): Only the culprit lesion had increased 18F-NaF uptake [18F-NaF, tissue-to-background ratios, culprit 2.03 vs. reference segment 1.08 (88% increase)] on PET-CT after percutaneous coronary intervention. Corresponding 18F-fluorodeoxyglucose (18F-FDG), PET-CT showing no uptake either at the culprit [18F-FDG, tissue-to-background ratios, culprit 1.62 vs. reference segment 1.49 (9% increase)] or the bystander stented lesion. Note intense uptake within the ascending aorta (F). Reprint with permission from reference [88].
Nahrendorf, et al.[89] presented their preclinical data on imaging vascular cell adhesion molecule (VCAM)-1 by integrated PET/CT imaging in murine atherosclerosis. The expression of VCAM-1 is increased at lesion-prone sites and in atherosclerotic plaques, which is expressed by endothelial as well as smooth muscle cells and macrophages.[89],[90] To validate in vivo PET/CT data, the authors correlated 18F-4V (4V, a synthetic peptide, was labeled with 18F for PET imaging of VCAM-1) uptake to expression of VCAM-1 in atherosclerotic mice receiving a high-cholesterol diet as well as wild type murine models of myocardial infarction and heart transplant rejection. The authors reported that 18F-4V accumulated in atherosclerotic plaques, mostly in the endothelium overlying aortic plaques, which is detected by in vivo and ex vivo PET/CT imaging (Figure 5). Furthermore, PET/CT showed increased uptake of 18F-4V in myocardium after myocardial infarction and during transplant rejection. Their results demonstrate an important advance in molecular imaging of vascular inflammation that brings a step closer to the reality of PET/CT imaging in humans.[89]
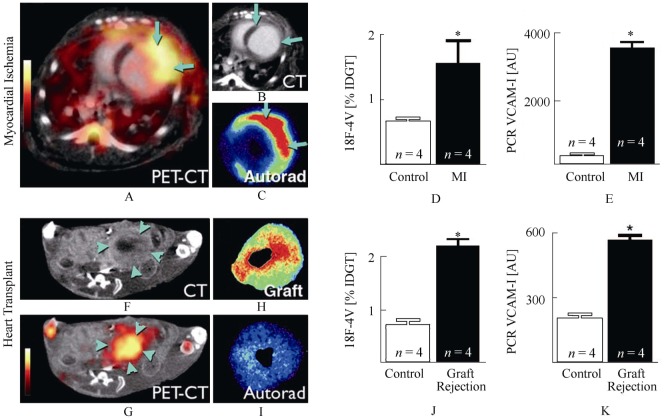
Figure 5. 18F-4V imaging in myocardial infarction and transplant rejection.
(A): PET-CT shows strong signal in the infarcted left ventricular wall. (B): Infarcted myocardium shows delayed CT hyperenhancement after iodine (arrows). (C): Autoradiography of myocardial ring. (D): %IDGT in the infarct. (E): VCAM-1 mRNA in infarct tissue. (F, G): PET-CT of a heart transplanted heterotopically into the abdominal cavity. The rejected allograft (arrowheads) shows high uptake of 18F-4V. (H, I): Autoradiography of graft and orthotopic recipient heart. (J): Uptake of 18F-4V in rejected allografts. (K): VCAM-1 mRNA levels in control heart tissue and rejected cardiac allografts. *P < 0.05. IDGT: injected dose/gram tissue; MI: myocardial infarction; VCAM: vascular cell adhesion muscle. Reprint with permission from reference [89].
7. Integrated PET/MRI imaging of coronary plaques
The availability of PET/MRI systems may dramatically facilitate the translation of promising PET imaging into the clinic for atherosclerotic plaque imaging owing to the superior soft tissue contrast of MRI imaging.[91],[92] Millon, et al.[93] in their recent experimental study showed the feasibility of using combined PET/MRI for assessment of changes in the inflammation of atherosclerotic plaques. 18F-FDG PET seems to be more sensitive than contrast-enhanced MRI to detect early changes in plaque inflammation.
Although CT can provide high-resolution anatomical images during PET/CT scanners, misalignments between PET and CT data are common since the two imaging modalities are not acquired simultaneously. In the recently developed PET/MRI scanners, PET and MR data are acquired simultaneously so that information obtained with MRI can be used to improve analysis of information content of PET images. Motion corrected PET/MRI which was proposed in a recent study solved the issue of misalignments encountered in PET/CT since all PET data are reconstructed within the same reference phase, which allows accurate spatial registration between PET and MR data.[94] This technique has been shown to improve visualization and detection of coronary plaques. Multimodal imaging of plaques such as PET/MRI could be an ideal application since combination of the imaging methods works synergistically to maximize diagnostic potential of each imaging modality and enables the detection and quantification of the burden of the extent of calcified and non-calcified plaques, quantification of vascular reactivity and endothelial health, identification of flow-limiting coronary stenosis, and potential identification of high-risk plaques in coronary arteries.[95] Currently, PET/MRI is limited to preclinical cardiac imaging with focus on animal experiments, thus, more studies with further technical advances are expected to prove its clinical value in cardiac imaging.
8. Nanoparticle-targeted imaging of coronary plaques
The advancements of nanotechnology have led to several inventions of novel nanoparticle-targeted contrast agents for various imaging modalities. This achievement may also significantly contribute towards detecting and characterizing the vulnerable atherosclerotic plaques. Indeed, several studies have shown that nanoparticle-targeted imaging enables to increase the sensitivity value as compared with those ‘traditional’ invasive procedures.[96]–[98] A number of nanoparticle contrast agents have been comprehensively studied for molecular imaging of atherosclerotic-related biomarkers, and most of the works have involved iron oxide nanoparticle which has been assessed by MRI.[99]–[101]
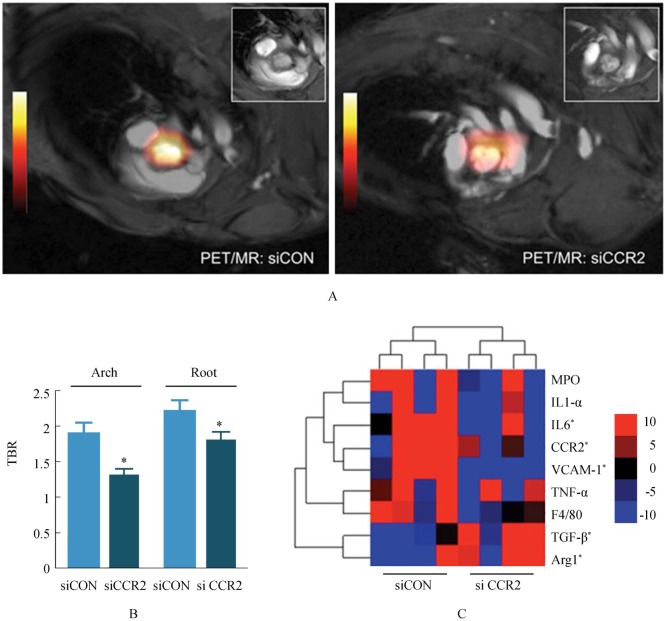
Iron oxide nanoparticle is a biocompatible superparamagnetic material that has been widely used as MRI contrast agent for the identification of atherosclerotic plaques in clinical setting.[102] However, to be a clinically approved MRI contrast agent, iron oxide nanoparticle shall be coated with dextran (known as dextran-coated iron oxide; DIO) which is biocompatible and biodegradable, making it suitable for clinical application.[103] This DIO nanoparticle is commonly applied for contrast-enhanced T1 and T2-weighted MRI scans. Nevertheless, due to the difficulty in fabricating a uniform dimension of DIO nanoparticle, Tu, et al.[104] introduced a new synthetic method by sulfating DIO with sulfur trioxide pyridine complex, and produced the sulfated DIO (SDIO) nanoparticle. Their preclinical T2-weighted MRI (with gradient echo sequence) data showed that at 4-hour post injection, SDIO provides a significantly higher contrast ratio than those of DIO. This finding suggests that SDIO has superior contrast enhancing quality because it is more preferably to be accumulated at the site of atherosclerotic plaque. Majmudar, et al.[105] in their recent study used polymetric nanoparticles consisting of zirconium-89 radiolabelled dextra nanoparticles (DNP) in integrated PET/MRI imaging of atherosclerotic plaques. PET/MRI has been shown to be able to detect inflammatory cells in atheroma with radioisotope-labelled DNP, and track response in the arterial intima after anti-inflammatory therapy (Figure 6). Clinical translation of this molecular imaging technique could assist identification of vulnerable plaques at high risk for complications and assessment of the treatment outcomes in atherosclerotic plaques.
Figure 6. Positron emission tomography/magnetic resonance image (PET/MRI) and gene expression analysis of siRNA treated ApoE−/− mice.
(A): Representative PET/MRI of control siRNA (siCON) and siCCR2-treated ApoE−/− mice. Inset: MRI frame at the level of the aortic valve. (B): PET target-to-background ratio in ApoE−/− mice treated with control siRNA (siCON) vs. siCCR2 (n = 5 per group). Data are presented as mean ± SE, *P < 0.05. (C): Heat map of genes in aortic roots (n = 4 per group). Each row of the heat map represents a gene, whereas each column represents an experimental treatment group (labeled at the bottom). The color scale represents the level of gene expression, with red indicating an increase in gene expression and blue indicating a decrease in gene expression. ApoE: apolipoprotein; Arg1: arginase 1; CCR2: C-C chemokine receptor type 2; IL: interleukin; MPO: myeloperoxidase; PET/MRI: Positron emission tomography/magnetic resonance image; siCON: si control; siRNA: short-interfering RNA; TBR: target-to-background ratio; TGF: transforming growth factor; TNF: tumor necrosis factor; VCAM: vascular cell adhesion molecule 1. Reprint with permission from reference [105].
Finally, with the advent of integrated imaging modality, Nahrendorf et al have successfully developed a novel trimodality nanoparticle contrast agent that can directly detect the high level of macrophages in atherosclerotic plaques.[106] This fascinating discovery would be of great value for being utilized in PET/CT and PET/MR scans, and therefore numerous molecular imaging-based outcomes on early detection of vulnerable atherosclerotic plaques are expected to be revealed in the near future.
9. Summary and concluding remarks
Imaging research of coronary artery disease has mainly focused on myocardial perfusion imaging and morphological analysis of coronary lumen stenosis or obstruction, however, the great challenge at present is to differentiate stable plaques from vulnerable ones, thus, identifying patients at high risk for developing acute cardiac events before clinical syndromes appear, therefore, achieving the goal of reducing cardiac mortality. Molecular imaging with use of radiopharmaceuticals by SPECT and PET techniques has the potential to identify vulnerable or high-risk plaques. This review provides an overview of the diagnostic applications of cardiac SPECT and PET in the detection of coronary plaques. In particular, cardiac PET including integrated PET/CT and PET/MRI has been shown to identify atherosclerotic changes at the early stage of coronary artery disease by accurately determining the plaque activity. With more research being conducted in the near future, it is expected that research findings will improve risk stratification of patients with coronary artery disease. More studies are needed to investigate whether increased uptake of radioisotopes correlates with a future risk of cardiac events or plaque rupture, or whether molecular imaging can be used to guide patient therapy and management.
Acknowledgments
This work has been supported by research grant from Natural Science Foundation of China (grant 81271542), grant for Excellent Talents from Beijing City (2011D003034000030), and grant for High Levels of Health Technical Personnel in Beijing City Health System (2013-3-011).
References
- 1.Schmid M, Pflederer T, Jang JK, et al. Relationship between degree of remodeling and CT attenuation of plaque in coronary atherosclerotic lesions: an in-vivo analysis by multi-detector computed tomography. Atherosclerosis. 2008;197:457–464. doi: 10.1016/j.atherosclerosis.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Hausleiter J, Meyer T, Hadamitzky M, et al. Prevalence of noncalcified coronary plaques by 64-Slice CT in patients with an intermediate risk for significant coronary artery disease. J Am Coll Cardiol. 2006;48:312–318. doi: 10.1016/j.jacc.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa T, Yamamoto H, Horiguchi J, et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging. 2009;2:153–160, 2009. doi: 10.1016/j.jcmg.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Sun Z, Choo GH, Ng KH. Coronary CT angiography: current status and continuing challenges. Br J Radiol. 2012;85:495–510. doi: 10.1259/bjr/15296170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Z, Jiang W. Diagnostic value of multislice CT angiography in coronary artery disease: a meta-analysis. Eur J Radiol. 2006;60:279–286. doi: 10.1016/j.ejrad.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Hammer-Hansen S, Kofoed KF, Kelbaek H, et al. Volumetric evaluation of coronary plaque in patients presenting with acute myocardial infarction or stable angina pectoris—a multislice computerized tomography study. Am Heart J. 2009;157:481–487. doi: 10.1016/j.ahj.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Kashiwagi M, Tanaka A, Kitabata H, et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging. 2009;2:1412–1419. doi: 10.1016/j.jcmg.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Sato A, Ohigashi H, Nozato T, et al. Coronary artery spatial distribution, morphology, and composition of nonculprit coronary plaques by 64-slice computed tomographic angiography in patients with acute myocardial infarction. Am J Cardiol. 2010;105:930–935. doi: 10.1016/j.amjcard.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging. 2011;4:537–548. doi: 10.1016/j.jcmg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Cao Y. Multislice CT angiography assessment of left coronary artery: correlation between bifurcation angle and dimensions and development of coronary artery disease. Eur J Radiol. 2011;79:e90–e95. doi: 10.1016/j.ejrad.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, Dimpudus FJ, Nugroho J, et al. CT virtual intravascular endoscopy assessment of coronary artery plaques: A preliminary study. Eur J Radiol. 2010;75:e112–e119. doi: 10.1016/j.ejrad.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Korosoglou G, Lehrke S, Mueller D, et al. Determinants of troponin release in patients with stable coronary artery disease: insights from CT angiography characteristics of atherosclerotic plaque. Heart. 2011;97:823–831. doi: 10.1136/hrt.2010.193201. [DOI] [PubMed] [Google Scholar]
- 13.Petranovic M, Soni A, Bezzera H, et al. Assessment of nonstenotic coronary lesions by 64-slice multidetector computed tomography in comparison to intravascular ultrasound: evaluation of nonculprit coronary lesions. J Cardiovasc Comput Tomogr. 2009;3:24–31. doi: 10.1016/j.jcct.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Gao D, Ning N, Guo Y, et al. Computed tomography for detecting coronary artery plaques: a meta-analysis. Atherosclerosis. 2011;219:603–609. doi: 10.1016/j.atherosclerosis.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Sun Z. Coronary CT angiography in coronary artery disease: correlation between virtual intravascular endoscopic appearances and left bifurcation angulation and coronary plaques. Biomed Res Int. 2013;2013:1–14. doi: 10.1155/2013/732059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Z, Wan YL, Hsieh IC, et al. Coronary CT angiography in the diagnosis of coronary artery disease. Curr Med Imaging Rev. 2013;9:184–193. [Google Scholar]
- 17.Sun Z, Cao Y, Liu H. Multislice CT angiography in the diagnosis of coronary artery disease. J Geriatr Cardiol. 2011;8:104–113. doi: 10.3724/SP.J.1263.2011.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motoyama S, Kondo T, Sarai M, et al. Multislice computer tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 20.Ambrose J. Angiographic correlations of advanced coronary lesions in acute coronary syndromes. In: Fuster V, editor. Syndromes of atherosclerosis: correlations of clinical imaging and pathology. Armonk, NY, USA: Futura Publishing Co, Inc; 1996. pp. 105–122. [Google Scholar]
- 21.Nadir A, Kuban BD, Tuzcu EM, et al. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 22.Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 23.Van Herck J, De Meyer G, Ennekens G, et al. Validation of in vivo plaque characterisation by virtual histology in a rabbit model of atherosclerosis. EuroIntervention. 2009;5:149–156. doi: 10.4244/eijv5i1a23. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues-Granillo GA, Garcia-Garcia HM, McFadden EP, et al. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005;46:2038–2042. doi: 10.1016/j.jacc.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 25.Calvert PA, Obaid DR, O'Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease. JACC Cardiovasc Imaging. 2011;4:894–901. doi: 10.1016/j.jcmg.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Davies JR, Rudd JH, Weissberg PL. Molecular and metabolic imaging of atherosclerosis. J Nucl Med. 2004;45:1898–1907. [PubMed] [Google Scholar]
- 27.Lee J. Quantitative analysis in cardiovascular imaging: current status. Curr Med Imaging Rev. 2013;9:214–222. [Google Scholar]
- 28.Kato S, Kitagawa KM, Ishida N, et al. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J Am Coll Cardiol. 2010;56:983–91. doi: 10.1016/j.jacc.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 29.Sun Z, Azizi A, Md Yusof AK. Cardiac nuclear imaging: current status and future directions. Curr Med Imaging Rev. 2013;9:170–183. [Google Scholar]
- 30.Stone GW, Maehara A, Mintz GS. The reality of vulnerable plaque detection. JACC Cardiovasc Imaging. 2011;4:902–904. doi: 10.1016/j.jcmg.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Ambrose JA, Tannenbaum MA, Alexopoulos B, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:6–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 32.Little WC, Constaintinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild to moderate coronary artery disease? Circulation. 1988;78:1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 33.Yamagishi M, Terashima M, Awano K, et al. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol. 2000;35:106–111. doi: 10.1016/s0735-1097(99)00533-1. [DOI] [PubMed] [Google Scholar]
- 34.Brown BG, Gallery CA, Badger RS, et al. Incomplete lysis of thrombus in the moderate underlying atherosclerosis lesion during intracoronary infusion of streptokinase for acute myocardial infarction. Circulation. 1986;73:653–661. doi: 10.1161/01.cir.73.4.653. [DOI] [PubMed] [Google Scholar]
- 35.Ambrose JA, Srikanth S. Vulnerable plaques and patients: improving prediction of future coronary events. Am J Med. 2010;123:10–16. doi: 10.1016/j.amjmed.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Ambrose JA. In search of the “vulnerable plaque”. Can it be localized and will focal regional therapy even be an option for cardiac prevention? J Am Coll Cardiol. 2008;51:1539–1542. doi: 10.1016/j.jacc.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 37.Fuster V, Lewis A. Conner Memorial Lecture. Mechanisms leading to myocardial infarction: insights from studies of vascular biology. Circulation. 1994;90:2126–2146. doi: 10.1161/01.cir.90.4.2126. [DOI] [PubMed] [Google Scholar]
- 38.Marques AS, Pinto FJ. The vulnerable plaque: current concepts and future perspectives on coronary morphology, composition and wall stress imaging. Rev Port Cardiol. 2014;33:101–110. doi: 10.1016/j.repc.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Laufer EM, Winkens MHM, Corsten MF, et al. PET and SPECT imaging of apoptosis in vulnerable atherosclerotic plaques with radiolabeled Annexin A5. QJ Nucl Med Mol Imaging. 2009;53:26–34. [PubMed] [Google Scholar]
- 40.Sogbein OO, Pelletier-Galarneau M, Schindler TH, et al. New SPECT and PET radiopharmaceuticals for imaging cardiovascular disease. Biomed Res Int. 2014:942–960. doi: 10.1155/2014/942960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schillaci O, Danielo R, Padovano, et al. Molecular imaging of atherosclerotic plaque with nuclear medicine techniques (review) Int J Mol Med. 2008;22:3–7. [PubMed] [Google Scholar]
- 42.Johnson LL, Schofield L, Donahay T, et al. 99mTc-annexin V imaging for in vivo detection of atherosclerotic lesions in coronary arteries. J Nucl Med. 2005;46:1186–1193. [PubMed] [Google Scholar]
- 43.Kietselaer BL, Hofstra L, Dumont EA, et al. The role of labelled Annexin A5 in imaging of programmed cell death. From animal to clinical imaging. Q J Nucl Med Mol Imaging. 2003;47:349–361. [PubMed] [Google Scholar]
- 44.Iuliano L, Signore A, Vallabajosula S, et al. Preparation and biodistribution of 99m-technetium labelled oxidized LDL in man. Atherosclerosis. 1996;126:131–141. doi: 10.1016/0021-9150(96)05888-1. [DOI] [PubMed] [Google Scholar]
- 45.Schafers M, Riemann B, Kopka K, et al. Scintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivo. Circulation. 2004;109:2554–2559. doi: 10.1161/01.CIR.0000129088.49276.83. [DOI] [PubMed] [Google Scholar]
- 46.Signore A, Chianelli M, D'Alessandria C, et al. Receptor targeting agents for imaging inflammation/infection: where are we now? Q J Nucl Med Mol Imaging. 2006;50:236–242. [PubMed] [Google Scholar]
- 47.Annovazzi A, D'Alessandria C, Bonanno E, et al. Synthesis of 99mTc-HYNIC-interleukin-12, a new specific radiopharmaceutical for imaging T lymphocytes. Eur J Nucl Med Mol Imaging. 2006;33:474–482. doi: 10.1007/s00259-005-0001-6. [DOI] [PubMed] [Google Scholar]
- 48.Rispler S, Keidar Z, Ghersin E, et al. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49:1059–1067. doi: 10.1016/j.jacc.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 49.Santana CA, Garcia EV, Faber TL, et al. Diagnostic performance of fusion of myocardial perfusion imaging (MPI) and computed tomography coronary angiography. J Nucl Cardiol. 2009;16:201–211. doi: 10.1007/s12350-008-9019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato A, Nozato T, Hikita H, et al. Incremental value of combining 64-slice computed tomography angiography with stress nuclear myocardial perfusion imaging to improve noninvasive detection of coronary artery disease. J Nucl Cardiol. 2010;17:19–26. doi: 10.1007/s12350-009-9150-5. [DOI] [PubMed] [Google Scholar]
- 51.Slomka PJ, Cheng VY, Dey D, et al. Quantitative analysis of myocardial perfusion SPECT anatomically guided by coregistered 64-slice coronary CT angiography. J Nucl Med. 2009;50:1621–1630. doi: 10.2967/jnumed.109.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 53.Le Guludec D, Lautamaki R, Knuuti J, et al. Present and future of clinical cardiovascular PET imaging in Europe—a position statement by the European Council of Nuclear Cardiology (ECNC) Eur J Nucl Med Mol Imaging. 2008;35:1709–1724. doi: 10.1007/s00259-008-0859-1. [DOI] [PubMed] [Google Scholar]
- 54.Kai H. Novel non-invasive approach for visualizing inflamed atherosclerotic plaques using fluorodeoxyglucose-positron emission tomography. Geriatr Gerontol Int. 2010;10:1–8. doi: 10.1111/j.1447-0594.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 55.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 56.Dunphy MP, Freiman A, Larson SM, et al. Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med. 2005;46:1278–1284. [PubMed] [Google Scholar]
- 57.Williams G, Kolodny GM. Retrospective study of coronary uptake of 18Ffluorodeoxyglucose in association with calcification and coronary artery disease: a preliminary study. Nucl Med Commun. 2009;30:287–291. doi: 10.1097/MNM.0b013e328328bfc3. [DOI] [PubMed] [Google Scholar]
- 58.Alexanderson E, Slomka P, Cheng V, et al. Fusion of positron emission tomography and coronary computed tomographic angiography identifies fluorine 18 fluorodeoxyglucose uptake in the left main coronary artery soft plaque. J Nucl Cardiol. 2008;15:841–843. doi: 10.1007/BF03007367. [DOI] [PubMed] [Google Scholar]
- 59.Wykrzykowska J, Lehman S, Williams G, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009;50:563–568. doi: 10.2967/jnumed.108.055616. [DOI] [PubMed] [Google Scholar]
- 60.Wasselius J, Larsson S, Sundin A, et al. Assessment of inactive, active and mixed atherosclerotic plaques by 18F-FDG–PET; an age group based correlation with cardiovascular risk factors. Int J Cardiovasc Imaging. 2009;25:133–140. doi: 10.1007/s10554-008-9366-5. [DOI] [PubMed] [Google Scholar]
- 61.Ogawa M, Magata Y, Kato T, et al. Application of 18F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med. 2006;47:1845–1850. [PubMed] [Google Scholar]
- 62.Potter K, Lenzo N, Eikelboom JW, et al. Effect of long-term homocysteine reduction with B vitamins on arterial wall inflammation assessed by fluorodeoxyglucose positron emission tomography: a randomised double-blind, placebo-controlled trial. Cerebrovasc Dis. 2009;27:259–265. doi: 10.1159/000199463. [DOI] [PubMed] [Google Scholar]
- 63.Lee SJ, On YK, Lee EJ, et al. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008;49:1277–1282. doi: 10.2967/jnumed.108.052233. [DOI] [PubMed] [Google Scholar]
- 64.Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 65.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–917. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 66.Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 67.Hartung D, Petrov A, Haider N, et al. Radiolabeled monocyte chemotactic protein for the detection of inflammation in experimental atherosclerosis. J Nucl Med. 2007;48:1816–1821. doi: 10.2967/jnumed.107.043463. [DOI] [PubMed] [Google Scholar]
- 68.Tsimikas S. Noninvasive imaging of oxidized low-density lipoprotein in atherosclerotic plaques with tagged oxidation-specific antibodies. Am J Cardiol. 2002;90:22L–27L. doi: 10.1016/s0002-9149(02)02958-2. [DOI] [PubMed] [Google Scholar]
- 69.Tahara N, Mukherjee J, de Haas H, et al. 2-deoxy-2-[18F] fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20:215–219. doi: 10.1038/nm.3437. [DOI] [PubMed] [Google Scholar]
- 70.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Circulation. 2007;115:402–426. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 71.Doherty TM, Asotra K, Fitzpatrick LA, et al. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci USA. 2003;100:11201–11206. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van der Wal AC, Becker AE, van der Loos CM, et al. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 73.Beheshti M, Saboury B, Mehta NN, et al. Detection and global quantification of cardiovascular molecular calcification by fluoro-18-fluoride positron emission tomography/computed tomography-A novel concept. Hell J Nucl Med. 2011;14:114–120. [PubMed] [Google Scholar]
- 74.Derlin T, Richter U, Bannas P, et al. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med. 2010;51:862–865. doi: 10.2967/jnumed.110.076471. [DOI] [PubMed] [Google Scholar]
- 75.Kato K, Schober O, Ikeda M, et al. Evaluation and comparison of 11C-choline uptake and calcification in aortic and common carotid arterial walls with combined PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:1622–1628. doi: 10.1007/s00259-009-1152-7. [DOI] [PubMed] [Google Scholar]
- 76.Beer AJ, Haubner R, Wolf I, et al. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging alpha v beta3 expression. J Nucl Med. 2006;47:763–769. [PubMed] [Google Scholar]
- 77.Derlin T, Habermann CR, Lengyel Z, et al. Feasibility of 11C-acetate PET/CT for imaging of fatty acid synthesis in the atherosclerotic vessel wall. J Nucl Med. 2011;52:1848–1854. doi: 10.2967/jnumed.111.095869. [DOI] [PubMed] [Google Scholar]
- 78.Gaemperli O, Shalhoub J, Owen DR, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J. 2012;33:1902–1910. doi: 10.1093/eurheartj/ehr367. [DOI] [PubMed] [Google Scholar]
- 79.Orbay H, Hong H, Zhang Y, et al. Positron emission tomography imaging of atherosclerosis. Theranostics. 2013;3:894–902. doi: 10.7150/thno.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leuschner F, Nahrendorf M. Molecular imaging of coronary atherosclerosis and myocardial infarction: considerations for the bench and perspectives for the clinic. Cir Res. 2011;108:593–606. doi: 10.1161/CIRCRESAHA.110.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lucignani G, Schafers M. PET, CT and MRI characterisation of the atherosclerotic plaque. Eur J Nucl Med Mol Imaging. 2010;37:2398–2404. doi: 10.1007/s00259-010-1634-7. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Vidan E, Bergman GW. Cardiac motion of coronary arteries: variability in the rest period and implications for coronary MR angiography. Radiology. 1999;213:751–758. doi: 10.1148/radiology.213.3.r99dc41751. [DOI] [PubMed] [Google Scholar]
- 83.Loeffelbein DJ, Souvatzoglou M, Wankerl V, et al. PET-MRI fusion in head-and-neck oncology: current status and implications for hybrid PET/MRI. J Oral Maxillofac Surg. 2012;70:473–483. doi: 10.1016/j.joms.2011.02.120. [DOI] [PubMed] [Google Scholar]
- 84.Menezes LJ, Kayani I, Ben-Haim S, et al. What is the natural history of 18F-FDG uptake in arterial atheroma on PET/CT? Implications for imaging the vulnerable plaque. Atherosclerosis. 2010;211:136–140. doi: 10.1016/j.atherosclerosis.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 85.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1822. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 86.Davies JR, Rudd JH, Fryer TD, et al. Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron emission tomography and high-resolution magnetic resonance imaging. Stroke. 2005;36:2642–2647. doi: 10.1161/01.STR.0000190896.67743.b1. [DOI] [PubMed] [Google Scholar]
- 87.Sun Z, Xu L. Coronary CT angiography in the quantitative assessment of coronary plaques. Biomed Res Int. 2014;2014:346–380. doi: 10.1155/2014/346380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 89.Nahrendorf M, Keliher E, Panizzi P, et al. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2:1213–1222. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iiyama K, Hajra L, Iiyama M, et al. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Cir Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 91.Tang TY, Howarth SP, Miller SR, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) study: evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 92.Zaidi H, Del Guerra A. An outlook on future design of hybrid PET/MRI systems. Med Phys. 2011;38:5667–5689. doi: 10.1118/1.3633909. [DOI] [PubMed] [Google Scholar]
- 93.Millon A, Dickson SD, Klink A, et al. Monitoring plaque inflammation in atherosclerotic rabbits with an iron oxide (P904) and 18F-FDG using a combined PET/MRI scanner. Atherosclerosis. 2013;228:339–345. doi: 10.1016/j.atherosclerosis.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petibon Y, El Fakhri G, Nezafat R, et al. Towards coronary plaque imaging using simultaneous PET-MR: a simulation study. Phys Med Biol. 2014;59:1203–1222. doi: 10.1088/0031-9155/59/5/1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jarrett BR, Correa C, Ma KL, et al. In vivo mapping of vascular inflammation using multimodal imaging. Plos One. 2010;5:e13254. doi: 10.1371/journal.pone.0013254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frias JC, Ma Y, Williams KJ, et al. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006;6:2220–2224. doi: 10.1021/nl061498r. [DOI] [PubMed] [Google Scholar]
- 97.Winter PM, Morawski AM, Caruthers SD, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha (v) beta3-integrintargeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 98.Winter PM, Neubauer AM, Caruthers SD, et al. Endothelial alpha (v) beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 99.Yu SS, Ortega RA, Reagan BW, et al. Emerging applications of nanotechnology for the diagnosis and management of vulnerable atherosclerotic plaques. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:620–646. doi: 10.1002/wnan.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 101.Nahrendorf M, Sosnovik DE, French BA, et al. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging. 2009;2:56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qiao RR, Yang CH, Gao MY. Superparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applications. J Mater Chem. 2009;19:6274–6293. [Google Scholar]
- 103.Thorek DL, Chen AK, Czupryna J, et al. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng. 2006;34:23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- 104.Tu C, Ng TS, Sohi HK, et al. Receptor-targeted iron oxide nanoparticles for molecular MR imaging of inflamed atherosclerotic plaques. Biomaterials. 2011;32:7209–7216. doi: 10.1016/j.biomaterials.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Majmudar MD, Yoo J, Keliher EJ, et al. Polymetric nanoparticle PET/MRI imaging allows macrophage detection in atherosclerotic plaques. Cir Res. 2013;122:755–761. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]