Abstract
Purpose
The use of intensity-modulated radiation therapy (IMRT) in the treatment of soft tissue sarcoma (STS) of the extremity is increasing, but no large-scale direct comparison has been reported between conventional external-beam radiation therapy (EBRT) and IMRT.
Methods
Between January 1996 and December 2010, 319 consecutive adult patients with primary nonmetastatic extremity STS were treated with limb-sparing surgery and adjuvant radiotherapy (RT) at a single institution. Conventional EBRT was used in 154 patients and IMRT in 165 with similar dosing schedules. Median follow-up time for the cohort was 58 months.
Results
Treatment groups were comparable in terms of tumor location, histology, tumor size, depth, and use of chemotherapy. Patients treated with IMRT were older (P = .08), had more high-grade lesions (P = .05), close (< 1 mm) or positive margins (P = .04), preoperative radiation (P < .001), and nerve manipulation (P = .04). Median follow-up was 90 months for patients treated with conventional EBRT and 42 months for patients treated with IMRT. On multivariable analysis adjusting for patient age and tumor size, IMRT retained significance as an independent predictor of reduced LR (hazard ratio = 0.46; 95% CI, 0.24 to 0.89; P = .02).
Conclusion
Despite a preponderance of higher-risk features (especially close/positive margin) in the IMRT group, IMRT was associated with significantly reduced local recurrence compared with conventional EBRT for primary STS of the extremity.
INTRODUCTION
Adjuvant radiation therapy (RT) has been demonstrated to provide improved local control (LC) for soft tissue sarcoma (STS) of the extremity following limb-sparing surgery,1,2 and may be administered via brachytherapy or external-beam RT (EBRT).3,4 In the latter case, many technical options exist, including conventional EBRT, intensity-modulated RT (IMRT),5 and other advanced techniques including proton therapy.6
Based on encouraging dosimetric results comparing IMRT to three-dimensional conformal EBRT (conventional EBRT),5 we began using IMRT in the treatment of primary extremity STS in 2002. Initial clinical data showed favorable morbidity profiles,7 and excellent LC.8 When IMRT was compared with adjuvant brachytherapy, IMRT was shown to be superior in terms of LC.3 With such encouraging results, our policy at Memorial Sloan Kettering Cancer Center (MSKCC) shifted toward increasing use of IMRT over conventional EBRT. The purpose of this study is to compare IMRT-treated patients from 2002 to 2010 with conventional EBRT-treated patients going back to 1996.
METHODS
Patients
Review of the prospective database at our institution from January 1996 to December 2010 identified 395 patients with primary nonmetastatic extremity STS who underwent both limb-sparing surgery and RT at MSKCC. A tumor was considered to be in the upper extremity if it was at or beyond the shoulder and in the lower extremity if it was at or beyond the groin. Patients treated with adjuvant brachytherapy (n = 73) were excluded from this analysis, as their LC compared with IMRT was reported previously.3 Two patients were also excluded due to history of prior RT, and one additional patient due to long interval (> 6 months) between surgery and adjuvant RT. This resulted in 319 consecutive patients (Fig 1); of these patients, 154 (48.3%) were treated with conventional RT techniques and 165 (51.7%) were treated with IMRT. This retrospective analysis was approved by our institutional review board.
Fig 1.
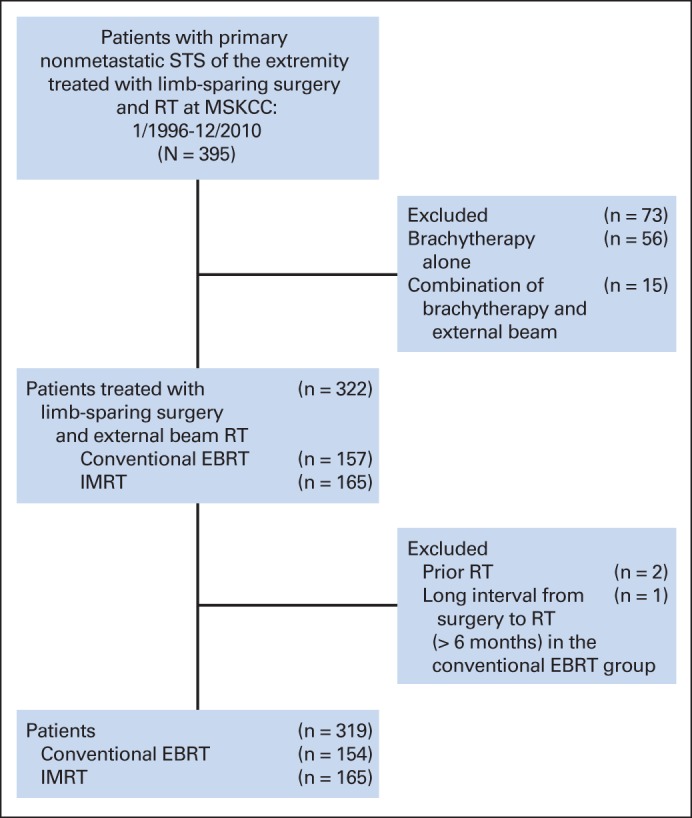
Consort diagram. EBRT, external-beam radiation therapy; IMRT, intensity-modulated radiation therapy; MSKCC, Memorial Sloan Kettering Cancer Center; RT, radtiation therapy; STS, soft tissue sarcoma.
Treatment
Surgery.
The surgical techniques used in limb-sparing surgery for STS of the extremities have been extensively described elsewhere.1 All gross disease is resected en bloc, with previous drain and biopsy sites included in the resection. Goals of surgery included a 1- to 2-cm margin in all directions with compromise made where possible to preserve nerve and vascular integrity. When necessary to accomplish resections with grossly negative margins, vascular resection and neurolysis or nerve resection was performed. For tumors abutting or involving bone, periosteal stripping or cortical bone resection was performed.
RT
Conventional EBRT was used exclusively from 1996 until 2002, at which time IMRT was introduced at our institution. IMRT gradually replaced conventional EBRT for all patients (only 2 patients have been treated with conventional EBRT after 2006). IMRT techniques were as previously described.5,8 Descriptions of RT simulation and planning treatment volumes for IMRT and conventional EBRT are provided in the Appendix. Weekly megavoltage portal beam verification films were checked for both conventional RT and IMRT; no kilovoltage and/or daily image-guidance was used for either technique.
Assessment/Follow-Up
All patients were monitored while on treatment with a minimum of once-weekly status checks by the treating radiation oncologist. Follow-up evaluation for both the IMRT and non-IMRT groups were identical and included assessment with physical examination every 3 to 4 months for the first 2 years and twice yearly thereafter. Imaging of the primary site and chest were performed twice yearly for the first 2 years and yearly thereafter. Local recurrences were confirmed by biopsy. All patient follow-up was performed at MSKCC. Grading of toxicity was based on the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0, with the highest grade of any observed toxicity reported for each patient at the time of follow-up. For the purposes of this study, only ≥ grade 2 toxicities were reported.
Statistics
When comparing the cohorts, median and range are presented for continuous variables while frequency and percentage are given for categorical variables. The Kruskal-Wallis test9 and Fisher's test10 were used to compare groups for continuous and categorical variables, respectively. All outcomes were measured from date of definitive surgery to date of event. An overall survival (OS) event was defined as death from any cause. Patients alive at last follow-up are censored. Competing-risks analysis was used for assessment of local recurrence (LR), with death without LR defined as a competing event for LR. An LR event was defined as disease recurrence at the site of primary presentation irrespective of distant recurrence. In addition, using the postoperative nomogram for LR risk in patients with primary nonmetastatic STS of the extremity treated with surgery alone,11 the risk of LR at 5 years follow-up was calculated for each patient. Patients alive at the date of last follow-up without LR were censored. An overall disease-free survival (DFS) event was defined as any local, regional, or distant failure, or death from any cause. OS and DFS were estimated using the Kaplan-Meier method.12,13 Cumulative incidence, Gray's test, and Fine and Gray regression were used to analyze LR.14,15 95% CIs at specified outcome times were calculated. Variables that were found to have significance on univariable analysis (P ≤ .05) were incorporated into Cox proportional hazards regression models for multivariable analysis.16 P ≤ .05 was considered significant. Statistical analysis was performed using R version 3.0.0 with the cmprsk and survival packages.
RESULTS
Patients
Between January 1996 and December 2010, 319 consecutive eligible patients were identified. Patient characteristics are shown in Table 1. The average patient age was 54 years (range, 17-89 years). Of the 319 patients, 238 (74.6%) presented with sarcomas of the lower extremity, 143 (44.8%) of the lesions were greater than 10 cm in size, 293 (91.8%) of the lesions were deep, 263 (82.4%) of the lesions were high-grade, 146 (45.8%) of the surgical resections had a positive or close (within 1 mm) surgical margin, and 79 (24.8%) patients received chemotherapy as a component of the management of their primary disease. Details regarding surgical management are provided in the Appendix. Histologies included malignant fibrous histiocytoma in 117 (36.7%) patients, liposarcoma in 90 (28.2%) patients, synovial sarcoma in 30 (9.4%) patients, leiomyosarcoma in 15 (4.7%) patients, and other sarcoma histologies in 67 (21%) patients. Malignant fibrous histiocytoma is no longer a recognized designation but was used at the time the tissue was collected. Of the 319 patients, 39 (12.2%) were treated with preoperative RT to a median dose of 50 Gy (range, 48-50.4 Gy) and 280 (87.8%) were treated with postoperative RT to a median dose of 63 Gy (range, 18-70.2 Gy).
Table 1.
Patient Characteristics
| Characteristic | Conventional External Beam Radiation Therapy |
Intensity-Modulated Radiation Therapy |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| ≤ 50 | 65 | 42 | 54 | 33 | .08 |
| > 50 | 89 | 58 | 111 | 67 | |
| Time period | |||||
| Prior to 2002 | 97 | 100 | 0 | 0 | < .001 |
| 2002-2006 | 55 | 42 | 75 | 58 | |
| After 2006 | 2 | 2 | 90 | 98 | |
| Extremity location | |||||
| Upper | 39 | 25 | 42 | 25 | 1.0 |
| Lower | 115 | 75 | 123 | 75 | |
| Depth | |||||
| Superficial | 11 | 7 | 15 | 9 | .55 |
| Deep | 143 | 93 | 150 | 91 | |
| Tumor size | |||||
| ≤ 10 cm | 84 | 55 | 92 | 56 | .91 |
| > 10 cm | 70 | 45 | 73 | 44 | |
| Positive/close margin | |||||
| No | 93 | 60 | 80 | 48 | .04 |
| Yes | 61 | 40 | 85 | 52 | |
| Grade | |||||
| Low | 34 | 22 | 22 | 13 | .05 |
| High | 120 | 78 | 143 | 87 | |
| Histology | |||||
| Malignant fibrous histiocytoma* | 60 | 39 | 57 | 35 | .60 |
| Liposarcoma | 39 | 25 | 51 | 31 | |
| Synovial | 16 | 10 | 14 | 8 | |
| Leiomyosarcoma | 9 | 6 | 6 | 4 | |
| Other | 30 | 19 | 37 | 22 | |
| Radiation sequence | |||||
| Pre | 5 | 3 | 34 | 21 | < .001 |
| Post | 149 | 97 | 131 | 79 | |
| Adjuvant chemotherapy | |||||
| No | 120 | 78 | 120 | 73 | .30 |
| Yes | 34 | 22 | 45 | 27 | |
NOTE. The Kruskal-Wallis test and Fisher's test were used for continuous and categorical variables, respectively.
Includes myxofibrosarcoma.
Conventional EBRT techniques were used in 154 (48.3%) patients and IMRT techniques in 165 (51.7%). In the conventional RT and IMRT groups, patients were comparable in terms of location of tumor, size, depth, and histology. The IMRT cohort had a significantly larger proportion of positive/close margins (51.5% v 39.6%; P = .04) and more patients with high-grade histology (86.7% v 77.9%; P = .05) and age greater than 50 years (67.3% v 57.8%; P = .08). In addition, there were more patients treated with preoperative RT in the IMRT group (21.2% v 3.2%; P < .001).
During the time period 2002 to 2006, patients may have been treated with either IMRT (n = 75) or conventional EBRT (n = 55); therefore a subset analysis was performed on the distribution of prognostic factors. No significant differences were noted between the two groups of patients with the exception of significantly more tumors larger than 10 cm in the IMRT group (49% v 31%; P = .047). (Supplement 3).
The median preoperative radiation dose was 50 Gy (range, 48-50 Gy) in the IMRT group and 50.4 Gy (range, 50-50.4 Gy) in the conventional EBRT group. The median postoperative doses were equivalent: 63 Gy (range, 27-66.6 Gy) in the IMRT group and 63 Gy (range, 18-70.2 Gy) in the conventional EBRT group. For patients treated postoperatively, three patients received less than 50 Gy: in the IMRT cohort, one patient developed a brisk skin reaction and discontinued treatment against medical advice; in the conventional cohort, one patient developed cellulitis and a decision was made to discontinue RT, and one patient developed a LR on treatment, requiring reoperation and salvage brachytherapy.
Of the 79 patients who received chemotherapy as part of their primary treatment, 34 (22.1%) patients were in the conventional RT group and 45 (27.3%) patients were in the IMRT group (P = .30). Chemotherapy was doxorubicin based in 76 (23.8%) patients.
The median follow-up time for the cohort was 58 months; 90 months (range, 3-187 months) for patients treated with conventional EBRT and 42 months (range, 3-129 months) for patients treated with IMRT, P < .01). The median follow-up for all patients still alive was 84.7 months; 103 months (range, 3-187 months) for patients treated with conventional EBRT and 55 months (range, 3-129 months) for patients treated with IMRT, P < .01).
Outcomes
The median time to LR was 18 months (range, 2-69 months) for conventional EBRT and 18 months (range, 9-33 months) for IMRT. For patients treated with IMRT, 5-year LR was 7.6% (95% CI, 3.4% to 11.8%) versus 15.1% (95% CI, 9.2% to 20.9%) for those treated with conventional EBRT (P = .05; Fig 2). Factors significantly associated with LR on univariable analysis included lesion size (5-year LR, 7.8% for lesions ≤ 10 cm and 16% for lesions > 10 cm; P = .05) and age (5-year LR, 7% for age < 50 years and 13.9% for age ≥ 50 years; P = .05). Tumor histology, grade, depth, margin status, the sequencing of RT (preoperative v postoperative), and the use of chemotherapy were not significantly associated with LR (Table 2). On multivariable analysis, IMRT retained significance as an independent predictor of reduced LR (hazard ratio [HR] = 0.458; 95% CI, 0.235 to 0.891; P = .02). The other independent predictors of reduced LR were age younger than 50 years (HR = 0.437; 95% CI, 0.197 to 0.967; P = .04) and tumor size ≤ 10 cm (HR = 0.530; 95% CI, 0.278 to 1.010; P = .05) When variables regardless of significance on univariable analysis were included in the multivariable model, the independent predictors remained the same (use of IMRT [HR = 0.469; P = .029], age younger than 50 years [HR = 0.448; P = .048], and tumor size ≤ 10 cm [HR = 0.514; P = .05]).
Fig 2.
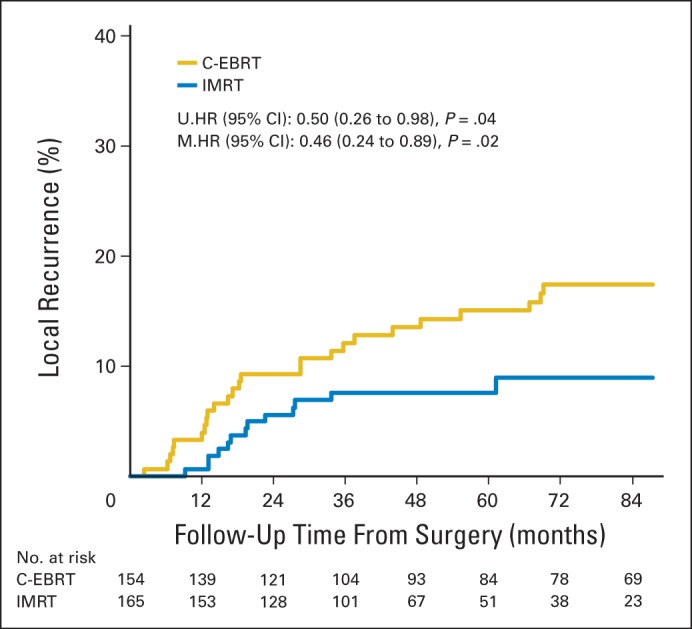
Cumulative incidence curve for local recurrence by radiation treatment group. C-EBRT, conventional external-beam radiation therapy; HR, hazard ratio; IMRT, intensity-modulated radiation therapy; M, multivariable; U, univariable.
Table 2.
Five-Year Local Recurrence Based on Patient Prognostic Factors and Univariable Analysis
| Characteristic | No. | % | LR at 5 Years (%) | 95% CI (%) |
P* | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Treatment | ||||||
| Conventional EBRT | 154 | 48 | 15.1 | 9.2 | 20.9 | .05 |
| IMRT | 165 | 52 | 7.6 | 3.4 | 11.8 | |
| Time period | .09 | |||||
| Prior to 2002 | 97 | 80 | 71.2 | 88.8 | ||
| 2002-2006 | 130 | 91.1 | 85.7 | 96.5 | ||
| After 2006 | 92 | 91.4 | 85 | 97.8 | ||
| Age, years | ||||||
| ≤ 50 | 119 | 37 | 7.0 | 2.3 | 11.7 | .05 |
| > 50 | 200 | 63 | 13.9 | 8.9 | 18.9 | |
| Extremity location | ||||||
| Upper | 81 | 25 | 10.7 | 3.6 | 17.8 | .97 |
| Lower | 238 | 75 | 11.7 | 7.4 | 16.0 | |
| Depth | ||||||
| Superficial | 26 | 8 | 10.4 | 0.0 | 20.7 | .54 |
| Deep | 293 | 92 | 11.8 | 7.9 | 15.6 | |
| Tumor size | ||||||
| ≤ 10 cm | 176 | 55 | 7.8 | 3.5 | 12.0 | .05 |
| > 10 cm | 143 | 45 | 16.0 | 9.8 | 22.1 | |
| Positive/close margin | ||||||
| No | 173 | 54 | 8.7 | 4.3 | 13.0 | .17 |
| Yes | 146 | 46 | 14.8 | 8.7 | 20.9 | |
| Grade | ||||||
| Low | 56 | 18 | 7.9 | 0.3 | 15.5 | .37 |
| High | 263 | 82 | 12.2 | 8.0 | 16.3 | |
| Histology | ||||||
| Malignant fibrous histiocytoma† | 117 | 37 | 15 | 8.3 | 21.6 | .17 |
| Liposarcoma | 90 | 28 | 7 | 1.6 | 12.4 | |
| Synovial | 30 | 9 | 4 | 0 | 11.4 | |
| Leiomyosarcoma | 15 | 5 | 21 | 0 | 42.8 | |
| Other | 67 | 21 | 13 | 3.6 | 22.7 | |
| Radiation sequence | ||||||
| Pre | 40 | 13 | 10.8 | 0.5 | 21.0 | .74 |
| Post | 279 | 87 | 11.5 | 7.6 | 15.4 | |
| Adjuvant chemotherapy | ||||||
| No | 240 | 75 | 10.5 | 6.4 | 14.5 | .51 |
| Yes | 79 | 25 | 14.5 | 6.4 | 22.5 | |
Abbreviations: EBRT, external beam radiation therapy; IMRT, intensity-modulated radiation therapy; LR, local recurrence.
P value determined by Gray's test.
Includes myxofibrosarcoma.
The estimated risk of recurrence at 5 years was calculated using the postoperative nomogram for LR risk in patients with primary sarcoma of the extremity treated with surgery alone.11 The calculated median risk for LR in the IMRT group was significantly higher than the conventional EBRT group (23.3% v 20.5%; P = .005).
Details of salvage therapy for local failure are provided in the Appendix. In terms of 5-year disease-free survival (DFS), overall it was 56.8% (95% CI, 51.4% to 62.8%); 57.2% (95% CI, 49% to 65.4%) in the IMRT group and 56.3% (95% CI, 48.1% to 64.5%) in the non-IMRT group. The overall 5-year OS was 71.7% (95% CI, 66.6% to 77.2%); 68.1% (95% CI, 60.6% to 76.4%) in the IMRT group and 75.6% (95% CI, 68.9% to 83%) in the non-IMRT group.
Toxicity
Data for ≥ grade 2 toxicities from conventional EBRT and IMRT are provided in Table 3. In terms of acute toxicities, there were 127 instances of ≥ grade 2 radiation dermatitis (39.8%): 75 (48.7%) in the conventional EBRT group compared with 52 (31.5%) in the IMRT group (P = .002). Median elapsed treatment time in the IMRT cohort was 48 days (range, 28-72 days), compared with 51 days (range, 12-87 days) in the conventional cohort. Accounting for weekends and holidays, the difference between planned treatment time and actual treatment time was defined as the treatment interruption. The mean duration of treatment interruption in the IMRT cohort was 0.8 days (range, 0-16 days), compared with 2.2 days in the conventional cohort (range, 0-23 days) (P < .001). Noninfected wound complications ≥ grade 2 were seen in 28 patients (8.8%): 14 (9.1%) in the conventional EBRT group and 14 (8.5%) in the IMRT group (P = 1.0). Infected wound complications ≥ grade 2 occurred in 30 patients (9.4%): 13 (8.4%) in the conventional EBRT group and 17 (10.3%) in the IMRT group (P = .70).
Table 3.
Grade ≥ 2 Toxicity Comparison Between Conventional RT and IMRT
| Toxicity | Overall |
Conventional RT |
IMRT |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Wound complications | |||||||
| Noninfected | 28 | 8.8 | 14 | 9.1 | 14 | 8.5 | 1.0 |
| Infected | 30 | 9.4 | 13 | 8.4 | 17 | 10.3 | .70 |
| Radiation dermatitis | 127 | 39.8 | 75 | 48.7 | 52 | 31.5 | .002 |
| Fracture | 22 | 6.9 | 14 | 9.1 | 8 | 4.8 | .18 |
| Nerve damage* | 7 | 2.6 | 2 | 1.6 | 5 | 3.5 | .45 |
| Joint stiffness | 41 | 12.9 | 17 | 11 | 24 | 14.5 | .40 |
| Edema | 36 | 11.3 | 23 | 14.9 | 13 | 7.9 | .05 |
Abbreviations: IMRT, intensity-modulated radiation therapy; RT, radiation therapy.
Assessment of nerve damage was limited to patients who did not undergo nerve resection as part of their limb-sparing surgery (n = 269).
Chronic ≥ grade 2 toxicities observed included nerve damage, fractures, joint stiffness, and edema. Assessment of nerve damage was limited to patients who did not undergo nerve resection as part of their limb-sparing surgery (n = 269). There were seven ≥ grade 2 nerve injuries observed (2.6%): two (1.6% of patients at risk) in the conventional EBRT group and five (3.5% of patients at risk) in the IMRT group (P = .45). There were 22 ≥ grade 2 fractures observed (6.9%): 14 (9.1%) in the conventional EBRT group and eight (4.8%) in the IMRT group (P = .18). Joint stiffness ≥ grade 2 was observed in 41 patients (12.9%); 17 (11%) in the conventional EBRT group and 24 (14.5%) in the IMRT group (P = .40). There were 36 patients with ≥ grade 2 edema (11.3%); 23 in the conventional EBRT group (14.9%) and 13 (7.9%) in the IMRT group (P = .05).
DISCUSSION
The use of IMRT in the treatment of extremity STS is limited, but appears to be increasing, with surveys showing less than 5% use in 200217 rising to 17% use in 2004.18 In the current study, IMRT was shown to be an independent predictor of reduced LR (HR = 0.46; 95% CI, 0.24 to 0.89; P = .02) in a group of patients with primary nonmetastatic STS of the extremity treated with surgery and adjuvant RT at a single institution. In a recent phase II study, O'Sullivan et al19 reported on 59 patients treated with preoperative IMRT. With a median follow-up of 49 months, the crude LR rate was 6.8% (4 of 59). In that study, 34% of patients had tumors larger than 10 cm.19
One likely reason for improved local tumor control is the manner in which IMRT may be used to deliver conformal and uniform doses over the entire tumor volume. Dose conformality is especially important for large tumors such as sarcoma, providing adequate coverage of the periphery, and homogeneous coverage ensures that all tumor cells within the clinical volume receive adequate doses. Improved conformality with IMRT was described by Stewart et al20 in patients treated with postoperative RT for primary-extremity STS, with mean Radiation Therapy Oncology Group (RTOG) conformity indices of 1.33-1.59 for IMRT versus 1.76 for conventional EBRT; mean heterogeneity indices were 1.036-1.045 for IMRT versus 1.052 for conventional EBRT. Griffin et al21 also reported significantly improved conformality indices in patients treated for lower extremity sarcoma, 1.27 for IMRT versus 1.76-2.34 for non-IMRT plans.
The toxicities observed in the current study highlight some of the potential added benefits of IMRT in sparing soft tissues. The rate of ≥ grade 2 dermatitis was 48.7% with conventional EBRT compared with 31.5% with IMRT (P = .002). In addition, treatment interruptions were significantly less in the IMRT group (P < .001). The rate of identified ≥ grade 2 edema was also less in the IMRT group compared with conventional EBRT (7.9% v 14.9%, P = .05) further highlighting the high degree of dose conformity obtained with IMRT. The incidence of bone fracture, a potential limb-threatening complication, was reduced by almost 50% in patients treated with IMRT compared with conventional EBRT, although this did not reach statistical significance.
The current study shows significant reduction in LR with IMRT compared with conventional EBRT; however, this should only be considered an association, rather than proof of its superiority. This can only be demonstrated with a well-designed prospective randomized trial, for which we strongly advocate. The IMRT group had significantly more positive/close margins (51.5% v 39.6%; P = .04), as well as more tumors with high-grade histology (86.7% v 77.9%; P = .05). To further assess whether the observed difference in LR between IMRT and conventional EBRT may have been due to factors other than radiation technique, an estimate of the risk of recurrence at 5 years was calculated using the postoperative nomogram for LR risk in patients with primary sarcoma of the extremity treated with surgery alone.11 The calculated median risk for LR in the IMRT group was significantly higher than the conventional EBRT group (23.3% v 20.5%; P = .005), indicating that the IMRT group was actually at a significantly higher baseline risk of LR.
One could argue that the improvement in LC in patients treated more recently could be attributed to improved imaging techniques such as magnetic resonance imaging or positron emission tomography/computed tomography or refinement in surgical techniques such as robotic laparoscopic surgery. We did attempt to minimize the influence of potential bias from incremental improvements in imaging and surgical technique by limiting the study to include only patients treated starting in 1996. However, when it comes to primary STS of the extremity, neither imaging nor surgical techniques have significantly changed over the study period. For the most part such patients are imaged using magnetic resonance imaging for the primary and computed tomography scan of the chest; positron emission tomography/computed tomography has not been shown to improve staging or treatment planning in adult sarcomas,22,23 and increasing intensity of imaging follow-up has been studied prospectively, with no evidence of benefit.24 In the current study comparing conventional EBRT to IMRT, image guidance was not used. Therefore, it is unclear whether the superiority of IMRT in terms of LC would be sustained in a comparison with image-guided three-dimensional conformal RT.
In conclusion, based on this study, LR was only 7.6% at 5 years using IMRT in the management of primary extremity sarcoma, despite a preponderance of adverse features, including significantly more patients with close or positive surgical margins and high-grade disease. The morbidity profile was favorable compared with conventional RT, associated with a significant reduction in radiation dermatitis, treatment breaks and chronic lymphedema.
Acknowledgment
The authors thank Lawrence A. Herman, who provided editorial assistance in the preparation of the final version of this article.
Glossary Terms
- conformal radiation therapy:
an irradiation technique developed to limit the highest radiation dose to volumes at risk for tumors while sparing surrounding normal tissues. Treatment planning is based on three-dimensional reconstructions of individual patient anatomy.
- image-guided radiation therapy:
a technique of radiation therapy delivery in which the location of the tumor is monitored by imaging on a daily basis to ensure the precise delivery of the radiation therapy dose to the predefined volume of interest.
- intensity-modulated radiation therapy:
radiation treatment using beams with nonuniform fluence profiles that shape the dose distribution in the target volume and adjacent normal structures. Beam modulation is typically achieved via multileaf collimators or custom-milled compensators to achieve the appropriate fluence profiles calculated by inverse optimization algorithms. The radiation beam is divided into beamlets of varying intensity such that the sum from multiple beams via inverse planning results in improved tumor targeting and normal tissue sparing. A technique of radiation therapy delivery in which the intensity of each beamlet of radiation coming from a specific angle can be adjusted to provide a desired dose distribution when the doses delivered from all beamlets are added from a single angle and from all dose delivery angles. An advanced type of high-precision radiotherapy, which aims to improve the coverage of the radiotherapy target and/or minimize radiation dose to surrounding normal tissue.
- magnetic resonance imaging:
a procedure in which radio waves and a powerful magnet linked to a computer are used to create detailed pictures of areas inside the body. These pictures can show the difference between normal and diseased tissue.
Appendix
Radiation Therapy Simulation and Treatment Volume Description
Patients are simulated and treated with immobilization using customized molds (alpha-cradles); simulation was performed using axial computed tomography (CT) with the limb to be treated immobilized in a neutral anatomic position. For conventional external-beam radiation therapy (EBRT) patients, they were immobilized using an alpha cradle, and were treated using computerized treatment plans. Treated volumes were similar to those using intensity-modulated radiation therapy (IMRT) except that no modifications for minimizing bone overlap were made.
In the preoperative setting, the clinical target volume (CTV) was defined as the gross tumor volume (GTV) defined on CT and magnetic resonance imaging, with a longitudinal expansion of 3 to 4 cm in the superior and inferior direction and 1 to 1.5 cm in the axial direction, excluding nearby bone. RT dose was generally 50 Gy in 2 Gy fractions for preoperative treatment and was prescribed to the planning target volume (PTV), a uniform expansion off the CTV of 1 cm in all directions. In the postoperative setting, the CTV was defined in a similar fashion to preoperative IMRT by substituting GTV with the reconstructed surgical bed (generally delineated by surgical clips placed at the time of resection, as visualized on CT), with a longitudinal expansion of 3 to 4 cm in the superior and inferior direction. The postoperative PTV was then generated by expanding the postoperative CTV uniformly in all directions by 1 cm. In the postoperative setting, the PTV is treated to 45 Gy in 1.8 Gy fractions, and then the PTV volume is reduced by 3 cm longitudinally in the superior and inferior direction and treated to an additional 18 to 21.6 Gy in 1.8 Gy fractions to a total dose of 63 to 66.6 Gy.
Details Regarding Surgical Management
At the time of definitive surgery, 82 (25.7%) patients had bone manipulation, including periosteal stripping (n = 60; 18.8%) or cortical bone resection (n = 22; 6.9%), and 137 (42.9%) had nerve manipulation, including neurolysis (n = 87; 27.3%) or nerve resection (n = 50; 15.7%). There was no significant difference in the frequency of surgical bone manipulation in the conventional and IMRT groups (22.7% v 28.5%, respectively; P = .25), but the IMRT group had significantly more surgical nerve manipulation (46.7% v 39%; P = .04).
Details of Salvage Therapy for Local Failure
For those patients who developed local recurrence, the median time to failure was the same; 18 months (range, 2-69 months) for conventional EBRT and 18 months (range, 9-33 months) for IMRT. Of 319 treated patients, there were 38 local failures, for a crude local failure rate of 11.9%: 25 failures in the conventional group and 13 in the IMRT group. Salvage consisted of chemotherapy in six patients (2 in the IMRT cohort, 4 in the conventional cohort); repeat excision alone in nine patients (4 in the IMRT cohort, 5 in the conventional cohort); and repeat excision with chemotherapy and/or RT in 10 patients (3 in the IMRT cohort, 7 in the conventional cohort). There were a total of eight amputations (3 in the IMRT cohort and 5 in the conventional cohort), and five patients did not receive any salvage treatment (1 in the IMRT cohort and 4 in the conventional cohort).
Table A1.
Distribution of Risk Factors Over 2002-2006 Study Time Period
| Risk Factor | 2002-2006, EBRT |
2002-2006, IMRT |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age > 50 years | 37 | 67.3 | 52 | 69.3 | .850 |
| High grade | 44 | 80 | 63 | 84 | .644 |
| Tumor size > 10 cm | 17 | 31 | 37 | 49 | .047 |
| Deep | 53 | 96.4 | 69 | 92 | .466 |
| Close/positive margin | 25 | 45.5 | 38 | 51 | .597 |
Abbreviations: EBRT, conventional external-beam radiation therapy; IMRT, intensity-modulated radiation therapy.
Footnotes
This work was supported by the National Cancer Institute, National Institutes of Health SPORE in Soft Tissue Sarcoma (P50 CA140146, S.S. and L.Q.).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Murray F. Brennan, ZIOPHARM Oncology (U) Consultant or Advisory Role: None Stock Ownership: Murray F. Brennan, ZIOPHARM Oncology Honoraria: None Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Michael R. Folkert, Samuel Singer, Murray F. Brennan, Kaled M. Alektiar
Administrative support: Samuel Singer, Murray F. Brennan, Kaled M. Alektiar
Provision of study materials or patients: Samuel Singer, Murray F. Brennan, Aimee M. Crago, Kaled M. Alektiar
Collection and assembly of data: Michael R. Folkert, Samuel Singer, Deborah Kuk, Wendy K. Kobayashi, Aimee M. Crago, Kaled M. Alektiar
Data analysis and interpretation: Michael R. Folkert, Samuel Singer, Murray F. Brennan, Deborah Kuk, Li-Xuan Qin, Kaled M. Alektiar
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 2.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 3.Alektiar KM, Brennan MF, Singer S. Local control comparison of adjuvant brachytherapy to intensity-modulated radiotherapy in primary high-grade sarcoma of the extremity. Cancer. 2011;117:3229–3234. doi: 10.1002/cncr.25882. [DOI] [PubMed] [Google Scholar]
- 4.Alektiar KM, Velasco J, Zelefsky MJ, et al. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2000;48:1051–1058. doi: 10.1016/s0360-3016(00)00753-7. [DOI] [PubMed] [Google Scholar]
- 5.Hong L, Alektiar KM, Hunt M, et al. Intensity-modulated radiotherapy for soft tissue sarcoma of the thigh. Int J Radiat Oncol Biol Phys. 2004;59:752–759. doi: 10.1016/j.ijrobp.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Swanson EL, Indelicato DJ, Louis D, et al. Comparison of three-dimensional (3D) conformal proton radiotherapy (RT), 3D conformal photon RT, and intensity-modulated RT for retroperitoneal and intra-abdominal sarcomas. Int J Radiat Oncol Biol Phys. 2012;83:1549–1557. doi: 10.1016/j.ijrobp.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Alektiar KM, Brennan MF, Healey JH, et al. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol. 2008;26:3440–3444. doi: 10.1200/JCO.2008.16.6249. [DOI] [PubMed] [Google Scholar]
- 8.Alektiar KM, Hong L, Brennan MF, et al. Intensity modulated radiation therapy for primary soft tissue sarcoma of the extremity: Preliminary results. Int J Radiat Oncol Biol Phys. 2007;68:458–464. doi: 10.1016/j.ijrobp.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 9.Hollander M, Douglas A, Wolfe D. Nonparametric Statistical Methods. New York, NY: John Wiley & Sons; 1973. pp. 115–120. [Google Scholar]
- 10.Agresti A. Categorical Data Analysis. New York, NY: Wiley; 1990. pp. 59–66. [Google Scholar]
- 11.Cahlon O, Brennan MF, Jia X, et al. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. 2012;255:343–347. doi: 10.1097/SLA.0b013e3182367aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 13.Mantel N. Evaluation of survival data and two new ranks order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 14.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94:496–509. [Google Scholar]
- 15.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 16.Cox D. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 17.Mell LK, Roeske JC, Mundt AJ. A survey of intensity-modulated radiation therapy use in the United States. Cancer. 2003;98:204–211. doi: 10.1002/cncr.11489. [DOI] [PubMed] [Google Scholar]
- 18.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the US, 2004. Cancer. 2005;104:1296–1303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 19.O'Sullivan B, Griffin AM, Dickie CI, et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer. 2013;119:1878–1884. doi: 10.1002/cncr.27951. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AJ, Lee YK, Saran FH. Comparison of conventional radiotherapy and intensity-modulated radiotherapy for post-operative radiotherapy for primary extremity soft tissue sarcoma. Radiother Oncol. 2009;93:125–130. doi: 10.1016/j.radonc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Griffin AM, Euler CI, Sharpe MB, et al. Radiation planning comparison for superficial tissue avoidance in radiotherapy for soft tissue sarcoma of the lower extremity. Int J Radiat Oncol Biol Phys. 2007;67:847–856. doi: 10.1016/j.ijrobp.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 22.Roberge D, Hickeson M, Charest M, Turcotte RE. Initial McGill experience with fluorodeoxyglucose pet/ct staging of soft-tissue sarcoma. Curr Oncol. 2010;17:18–22. doi: 10.3747/co.v17i6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karam I, Devic S, Hickeson M, et al. PET/CT for radiotherapy treatment planning in patients with soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2009;75:817–821. doi: 10.1016/j.ijrobp.2008.11.055. [DOI] [PubMed] [Google Scholar]
- 24.Puri A, Gulia A, Hawaldar R, et al. Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. Clin Orthop Relat Res. 2014;472:1568–1575. doi: 10.1007/s11999-013-3385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


