Abstract
Purpose
Radical cystectomy and urinary diversion may cause chronic metabolic acidosis, leading to long-term bone loss in patients with bladder cancer. However, the risk of fractures after radical cystectomy has not been defined. We assessed whether radical cystectomy and intestinal urinary diversion are associated with increased risk of fracture.
Patients and Methods
Population-based study using SEER-Medicare–linked data from 2000 through 2007 for patients with stage 0-III bladder cancer. We evaluated the association between radical cystectomy and risk of fracture at any site, controlling for patient and disease characteristics.
Results
The cohort included 50,520 patients, of whom 4,878 had cystectomy and urinary diversion. The incidence of fracture in the cystectomy group was 6.55 fractures per 100 person-years, compared with 6.39 fractures per 100 person-years in those without cystectomy. Cystectomy was associated with a 21% greater risk of fracture (adjusted hazard ratio, 1.21; 95% CI, 1.10 to 1.32) compared with no cystectomy, controlling for patient and disease characteristics. There was no evidence of an interaction between radical cystectomy and age, sex, comorbidity score, or cancer stage.
Conclusion
Patients with bladder cancer who have radical cystectomy and urinary diversion are at increased risk of fracture.
INTRODUCTION
Bladder cancer is the fourth most common cancer and the eighth leading cause of cancer death in men in the United States.1 It is estimated that, in 2013, more than 54,000 men and almost 18,000 women were diagnosed with bladder cancer and more than 15,000 died as a result of this disease.2 Radical cystectomy with urinary diversion and bladder-sparing multimodality therapy are the primary treatment options for patients with nonmetastatic muscle-invasive bladder cancer. After cystectomy, urine is diverted, and the urinary tract reconstructed using an intestinal segment such as the ileum.
Urinary diversions are either incontinent (such as an ileal conduit) or continent (catheterizable pouch or neobladder). Although a variety of intestinal segments may be used, ileum is the most commonly used because of anatomic advantages and the lower rate of metabolic and surgical complications. Intestinal segments are also used for urinary tract reconstruction for other conditions, such as augmentation cystoplasty for neurogenic bladder, ileal ureter for ureteral reconstruction, and urinary diversion after cystectomy for benign conditions.
The long-term metabolic consequences of intestinal urinary diversions are unknown. On exposure to urine, ileum reabsorbs ammonium and chloride and excretes bicarbonate. This impairs acid elimination and results in chronic metabolic acidosis in up to 70% of patients with intestinal urinary diversions.3–5 In response to metabolic acidosis, bone buffers the excess protons and releases calcium, resulting in hypercalciuria, without a concomitant increase in intestinal calcium absorption.6,7 Chronic metabolic acidosis also inhibits osteoblast activity, stimulates osteoclast bone resorption, inhibits renal tubular calcium reabsorption, causes hypophosphatemia through renal phosphate wasting and leads to negative calcium and phosphate balance.7–11 Ileal resection may also compromise calcium absorption given that the majority of the ingested calcium is thought to be absorbed in the ileum.12–14 It is plausible that the combination of these factors might cause osteoporosis and increase the risk of fractures.
However, whether urinary diversion with intestinal segments actually leads to increased risk of fractures has not been well established.15 Prior studies have been limited because of small sample size, cross-sectional study design, limited duration of follow-up, lack of a control group, and use of intermediate outcomes such as serum and urinary electrolytes, bone resorption markers, and bone density rather than use of the occurrence of fractures as an end point. Hence, there is no consensus nor are there guidelines for screening or prophylaxis of osteoporosis for these patients. Our objective was to assess whether radical cystectomy and intestinal urinary diversion are associated with increased risk of fracture in a population-based cohort of patients with bladder cancer.
PATIENTS AND METHODS
Data Source
The primary data source was the National Cancer Institute's SEER-Medicare database, which links information from the SEER cancer registry program with Medicare claims and enrollment records. The SEER program is a consortium of cancer registries in selected states and geographic areas covering approximately 28% of the US population. For all incident cancers, the SEER registries collect information regarding site and extent of disease, clinical and pathologic stage, first course of cancer-directed therapy, and sociodemographic characteristics with active follow-up for date and cause of death.16,17 Medicare is the primary health insurer for 97% of the US population age 65 years and older, covering inpatient hospital care (part A) and outpatient care and physician services (part B). Compared with the general US population age 65 years and older, the SEER-Medicare population has similar age and sex distributions but has a smaller proportion of nonwhites and a higher proportion of patients living in urban and affluent areas.16,17 The SEER-Medicare files were used in accordance with a data-use agreement with the National Cancer Institute, and the study was reviewed and deemed exempt by the institutional review board at Memorial Sloan Kettering Cancer Center.
Study Cohort
We identified all cases of nonmetastatic bladder cancer diagnosed between 2000 and 2007. The cohort was restricted to patients age 66 years or older at diagnosis and a full year of Medicare claims before diagnosis for identifying comorbid conditions and prior fractures. We excluded patients with American Joint Committee on Cancer (AJCC) stage IV cancer (T4, lymph node involvement or metastases) or unknown stage of disease. Patients diagnosed at the time of death were excluded, as were patients who did not have complete Medicare coverage or were enrolled in a Medicare managed care plan at the time of diagnosis.
Outcomes
The primary end point was occurrence of a fracture at any site, identified in Medicare claims by the International Classification of Diseases, ninth revision (ICD-9) diagnosis codes for fracture or pathologic fracture (Appendix Table A1, online only).
Predictors
The predictor of interest was receipt of radical cystectomy with urinary diversion, identified in Medicare claims by ICD-9 procedure codes and Current Procedural Terminology codes any time after diagnosis. Diversion procedures were classified as continent, incontinent, or type unknown. Other bladder cancer treatments ascertained from Medicare claims included intravesical and systemic chemotherapy (Appendix Table A1).
Several patient and disease characteristics were assessed as possible confounders. Demographic characteristics included age at diagnosis, race, marital status, geographic location, and residence in a metropolitan versus nonmetropolitan county. In the absence of individual-level information, census tract median income was categorized in quartiles and used as a measure of socioeconomic status. Disease characteristics included AJCC stage, grade, and nodal involvement. Comorbidity was estimated using a modification of the Charlson comorbidity index on the basis of inpatient, outpatient, and physician claims in the year before bladder cancer diagnosis.18,19 We similarly identified patients with a history of chronic kidney disease and prior fracture on the basis of Medicare claims in the year before bladder cancer diagnosis.
Statistical Analysis
Unadjusted associations between patient and disease characteristics and receipt of cystectomy were assessed using χ2 tests. The impact of cystectomy and urinary diversion on the risk of fracture was evaluated in a time-to-event framework, in which the time origin was the date of bladder cancer diagnosis and patients who did not have a fracture were censored at the end of 2009 or sooner if they died, enrolled in a health maintenance organization or unenrolled from Medicare part A or B. We also censored patients who developed bone metastases or malignant bone neoplasms, on the basis of the presence of relevant ICD-9 diagnosis codes in Medicare claims (Appendix Table A1). Kaplan-Meier survival functions were estimated, stratified by treatment (cystectomy v no cystectomy) and type of urinary diversion (continent v incontinent). Multivariable Cox proportional hazards regression was used to estimate the impact of cystectomy on fracture risk, controlling for demographic, disease, and health characteristics. To examine the impact of cystectomy on subsequent rather than prior fractures, and because patients varied in the time between diagnosis and cystectomy, receipt of cystectomy was analyzed as a time-dependent variable, with exposure identified at the time of first Medicare claim for surgery. Adjusted hazard ratios (aHRs), 95% CIs, and two-sided P values were estimated for each covariate. To assess the presence of effect modification, we examined terms for the interaction between cystectomy and age, sex, stage, and comorbidity. We also performed a separate analysis of the cystectomy group with known diversion type to estimate the impact of type of diversion on fracture. Statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC).
RESULTS
The study cohort included 50,520 patients with nonmetastatic bladder cancer diagnosed between 2000 and 2007, of whom 4,878 (10%) had cystectomy and urinary diversion. Patients who had a cystectomy were younger, had fewer comorbidities, and were less likely to have a history of chronic kidney disease or fracture (Table 1). They also had more advanced AJCC stage and grade and were more likely to receive systemic chemotherapy. The groups were comparable with respect to sex and race and lived in areas with similar median income.
Table 1.
Characteristics of Cohort by Cystectomy Status
| Characteristic | All Patients | Cystectomy |
No Cystectomy |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | P | ||
| Total | 50,520 | 4,878 | 10 | 45,642 | 90 | |
| Age at diagnosis, years | ||||||
| 66-69 | 7,069 | 1,000 | 21 | 6,069 | 13 | < .001 |
| 70-74 | 11,003 | 1,446 | 30 | 9,557 | 21 | |
| 75-79 | 12,652 | 1,369 | 28 | 11,283 | 25 | |
| ≥ 80 | 19,796 | 1,063 | 22 | 18,733 | 41 | |
| Sex | ||||||
| Male | 37,633 | 3,611 | 74 | 34,022 | 75 | NS |
| Female | 12,887 | 1,267 | 26 | 11,620 | 25 | |
| Race | ||||||
| White | 46,445 | 4,477 | 92 | 41,968 | 92 | NS |
| Black | 1,963 | 178 | 4 | 1,785 | 4 | |
| Hispanic | 572 | 67 | 1 | 505 | 1 | |
| Other | 1,540 | 156 | 3 | 1384 | 3 | |
| Census tract median income | ||||||
| 1st quartile | 11,765 | 1,107 | 23 | 10,658 | 23 | NS |
| 2nd quartile | 11,750 | 1,139 | 23 | 10,611 | 23 | |
| 3rd quartile | 11,751 | 1,112 | 23 | 10,639 | 23 | |
| 4th quartile | 12,110 | 1,218 | 25 | 10,892 | 24 | |
| Unknown | 3144 | 302 | 6 | 2,842 | 6 | |
| Urban-rural residence | ||||||
| Metropolitan | 42,941 | 4,098 | 84 | 38,843 | 85 | .042 |
| Nonmetropolitan | 7,579 | 780 | 16 | 6,799 | 15 | |
| Region | ||||||
| Northeast | 13,346 | 1,189 | 24 | 12,157 | 27 | .0055 |
| South | 8,211 | 804 | 16 | 7,407 | 16 | |
| Midwest | 7,612 | 738 | 15 | 6,874 | 15 | |
| West | 21,351 | 2,147 | 44 | 19,204 | 42 | |
| Married | ||||||
| Yes | 30,343 | 3,256 | 67 | 27,087 | 59 | < .001 |
| No | 17,213 | 1,431 | 29 | 15,782 | 35 | |
| Unknown | 2,964 | 191 | 4 | 2,773 | 6 | |
| Grade | ||||||
| 1 | 7,459 | 115 | 2 | 7,344 | 16 | < .001 |
| 2 | 16,821 | 596 | 12 | 16,225 | 36 | |
| 3 | 12,566 | 1,971 | 40 | 10,595 | 23 | |
| 4 | 9,425 | 1,874 | 38 | 7,551 | 17 | |
| Unknown | 4,249 | 322 | 7 | 3,927 | 9 | |
| AJCC stage | ||||||
| 0 | 15,187 | 379 | 8 | 14,808 | 32 | < .001 |
| I | 26,581 | 1,691 | 35 | 24,890 | 55 | |
| II | 5,858 | 1,440 | 30 | 4,418 | 10 | |
| III | 2,894 | 1,368 | 28 | 1,526 | 3 | |
| Lymph node involvement | ||||||
| Negative | 2,797 | 2,079 | 43 | 718 | 2 | < .001 |
| Unknown/not evaluated | 47,723 | 2,799 | 57 | 44,924 | 98 | |
| Chemotherapy | ||||||
| Systemic ± intravesical | 7368 | 949 | 19 | 6,419 | 14 | < .001 |
| Intravesical only | 1,932 | 114 | 2 | 1,818 | 4 | |
| None | 41,220 | 3,815 | 78 | 37,405 | 82 | |
| Charlson comorbidity score | ||||||
| 0 | 27,449 | 3,069 | 63 | 24,380 | 53 | < .001 |
| 1 | 12,717 | 1,133 | 23 | 11,584 | 25 | |
| ≥ 2 | 10,354 | 676 | 14 | 9,678 | 21 | |
| History of chronic kidney disease | ||||||
| Yes | 2,809 | 187 | 4 | 2,622 | 6 | < .001 |
| No | 47,711 | 4,691 | 96 | 43,020 | 94 | |
| History of fracture | ||||||
| Yes | 3,074 | 242 | 5 | 2,832 | 6 | < .001 |
| No | 47,446 | 4636 | 95 | 42,810 | 94 | |
| Urinary diversion | ||||||
| Continent | 721 | 721 | 15 | N/A | ||
| Incontinent | 3,892 | 3,892 | 80 | N/A | ||
| Unknown | 265 | 265 | 5 | N/A | ||
NOTE. P value for unadjusted association between characteristics and receipt of cystectomy. History of chronic kidney disease on the basis of diagnosis codes from inpatient and outpatient claims in year prior to bladder cancer diagnosis. History of fracture on the basis of diagnosis codes from inpatient and outpatient claims in the year prior to bladder cancer diagnosis or cystectomy.
Abbreviations: AJCC, American Joint Committee on Cancer; N/A, not applicable; NS, not significant.
During a median follow-up of 41 months, 10,872 patients (22%) had a fracture, including 792 (16%) of those who had cystectomy and urinary diversion and 10,080 (22%) of those with no cystectomy. The median time to fracture was 16.6 months from cystectomy (interquartile range, 6.2-35.4 months) in the cystectomy group and 21.8 months from cancer diagnosis (interquartile range, 8.3-42.2 months) in the no cystectomy group. The incidence of fracture in the cystectomy group was 6.55 fractures per 100 person-years, compared with 6.39 fractures per 100 person-years in those without cystectomy. The type of fracture did not vary substantially between the two groups (Table 2).
Table 2.
Site of First Fracture by Receipt of Cystectomy
| Fracture Site | Cystectomy (n = 792) |
No Cystectomy (n = 10,080) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Skull | 50 | 6 | 570 | 6 |
| Spine | 224 | 28 | 2,635 | 26 |
| Rib | 109 | 14 | 1,436 | 14 |
| Pelvis | 42 | 5 | 509 | 5 |
| Upper arm | 73 | 9 | 822 | 8 |
| Lower arm | 43 | 5 | 622 | 6 |
| Hand | 30 | 4 | 581 | 6 |
| Femoral neck | 102 | 13 | 1,355 | 13 |
| Other parts of femur | * | < 1 | 60 | 1 |
| Lower leg | 51 | 6 | 614 | 6 |
| Foot | 37 | 5 | 557 | 6 |
| Pathologic fracture, unspecified | * | < 1 | 65 | 1 |
| Other | 23 | 3 | 254 | 3 |
NOTE. Cell counts < 11 masked in accordance with SEER-Medicare Data Use Agreement.
In unadjusted analysis, fracture-free survival was better in patients who received cystectomy compared with those who did not (Fig 1). However, controlling for patient, disease, and health characteristics, radical cystectomy and urinary diversion increased the hazard of fracture by 21% (aHR, 1.21; 95% CI, 1.10 to 1.32; P < .001). The fracture hazard was also significantly greater among older patients, females, whites, those with more advanced disease, and those with a history of chronic kidney disease or fracture. Receipt of chemotherapy was associated with lower hazard of fracture (Table 3).
Fig 1.
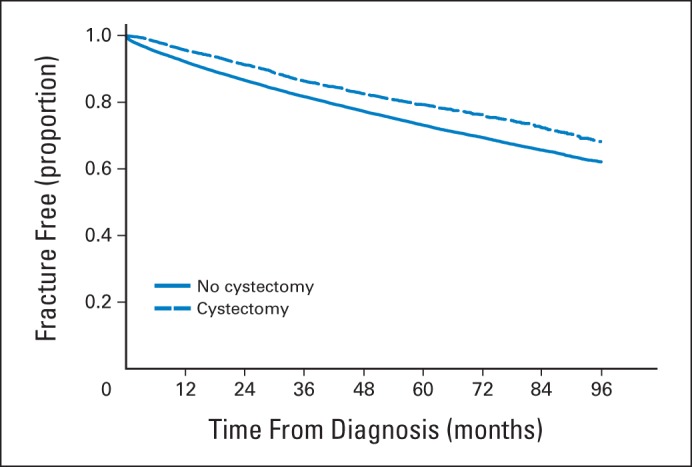
Fracture-free survival by receipt of cystectomy.
Table 3.
Predictors of Time to First Fracture
| Characteristic | Adjusted Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Cystectomy | |||
| No | Reference | < .001 | |
| Yes | 1.21 | 1.10 to 1.32 | |
| Age at diagnosis, years | |||
| 66-69 | Reference | < .001 | |
| 70-74 | 1.22 | 1.13 to 1.31 | |
| 75-79 | 1.49 | 1.39 to 1.60 | |
| ≥ 80 | 2.17 | 2.02 to 2.32 | |
| Sex | |||
| Male | Reference | < .001 | |
| Female | 1.61 | 1.54 to 1.68 | |
| Race | |||
| White | Reference | < .001 | |
| Black | 0.48 | 0.42 to 0.55 | |
| Hispanic | 0.78 | 0.64 to 0.95 | |
| Other | 0.85 | 0.75 to 0.95 | |
| Census tract median income | |||
| 1st quartile | Reference | NS | |
| 2nd quartile | 0.99 | 0.93 to 1.05 | |
| 3rd quartile | 1.00 | 0.94 to 1.06 | |
| 4th quartile | 1.04 | 0.98 to 1.10 | |
| Unknown | 1.07 | 0.99 to 1.17 | |
| Urban-rural residence | |||
| Nonmetropolitan | Reference | NS | |
| Metropolitan | 1.06 | 0.99 to 1.12 | |
| Region | |||
| Midwest | Reference | < .001 | |
| South | 1.13 | 1.05 to 1.21 | |
| Northeast | 0.99 | 0.93 to 1.06 | |
| West | 1.06 | 1.00 to 1.12 | |
| Married | |||
| Yes | Reference | < .001 | |
| No | 1.13 | 1.08 to 1.18 | |
| Unknown | 0.98 | 0.90 to 1.06 | |
| Grade | |||
| 1 | Reference | NS | |
| 2 | 0.98 | 0.92 to 1.03 | |
| 3 | 1.02 | 0.95 to 1.08 | |
| 4 | 1.00 | 0.94 to 1.08 | |
| Unknown | 1.03 | 0.95 to 1.12 | |
| AJCC stage | |||
| 0 | Reference | < .001 | |
| I | 1.05 | 0.99 to 1.11 | |
| II | 1.22 | 1.12 to 1.33 | |
| III | 1.29 | 1.15 to 1.46 | |
| Lymph node involvement | |||
| Negative | Reference | < .001 | |
| Unknown/not evaluated | 1.38 | 1.23 to 1.54 | |
| Chemotherapy | |||
| None | Reference | < .001 | |
| Systemic ± intravesical | 0.56 | 0.53 to 0.60 | |
| Intravesical only | 0.49 | 0.43 to 0.55 | |
| Charlson comorbidity score | |||
| 0 | Reference | < .001 | |
| 1 | 1.23 | 1.18 to 1.29 | |
| ≥ 2 | 1.55 | 1.47 to 1.63 | |
| History of chronic kidney disease | |||
| No | Reference | < .001 | |
| Yes | 1.16 | 1.06 to 1.26 | |
| History of fracture | |||
| No | Reference | < .001 | |
| Yes | 2.97 | 2.80 to 3.15 | |
| Year of diagnosis | 1.00 | 0.98 to 1.01 | NS |
NOTE. History of chronic kidney disease on the basis of diagnosis codes from inpatient and outpatient claims in the year prior to bladder cancer diagnosis. History of fracture on the basis of diagnosis codes from inpatient and outpatient claims in the year prior to bladder cancer diagnosis or cystectomy.
Abbreviations: AJCC, American Joint Committee on Cancer; NS, not significant.
We found significant interactions between cystectomy and age and cystectomy and bladder cancer stage. Cystectomy had the greatest impact on fracture risk in the youngest patients (age, 66-69 years), with an aHR of 1.83 (95% CI, 1.47 to 2.29; P < .001; Table 4). Cystectomy also significantly increased the hazard of fracture in those age 75 to 79 years but not in other age groups. aHRs were fairly similar and statistically significant across stages of disease, except in patients with stage II bladder cancer. Interactions between cystectomy and sex and cystectomy and comorbidity were not statistically significant.
Table 4.
Adjusted Impact of Cystectomy on Time to First Fracture by Age and Stage
| Stratum | Impact of Cystectomy |
P | |
|---|---|---|---|
| Adjusted Hazard Ratio* | 95% CI | ||
| Age at diagnosis, years | |||
| 66-69 | 1.83 | 1.47 to 2.29 | < .001 |
| 70-74 | 1.13 | 0.93 to 1.37 | NS |
| 75-79 | 1.29 | 1.08 to 1.53 | .0043 |
| ≥ 80 | 0.97 | 0.82 to 1.15 | NS |
| AJCC stage | |||
| 0 | 1.48 | 1.10 to 1.99 | .0093 |
| I | 1.27 | 1.11 to 1.44 | < .001 |
| II | 1.04 | 0.86 to 1.25 | NS |
| III | 1.39 | 1.09 to 1.77 | .008 |
Abbreviations: AJCC, American Joint Committee on Cancer; NS, not significant.
Hazard ratio for impact of cystectomy on fracture adjusted for all demographic, disease, and health characteristics in Table 3.
In patients who had a cystectomy with known urinary diversion type, in unadjusted analysis, the continent diversion group had better fracture-free survival than patients with incontinent diversion (Fig 2). However, in adjusted analysis there was no difference in fracture hazard by the type of diversion, controlling for demographic, disease, and health characteristics. Compared with patients who had continent diversion, those with incontinent diversion had a similar risk of fracture after radical cystectomy (aHR, 1.15; 95% CI, 0.93 to 1.43; P = .21).
Fig 2.
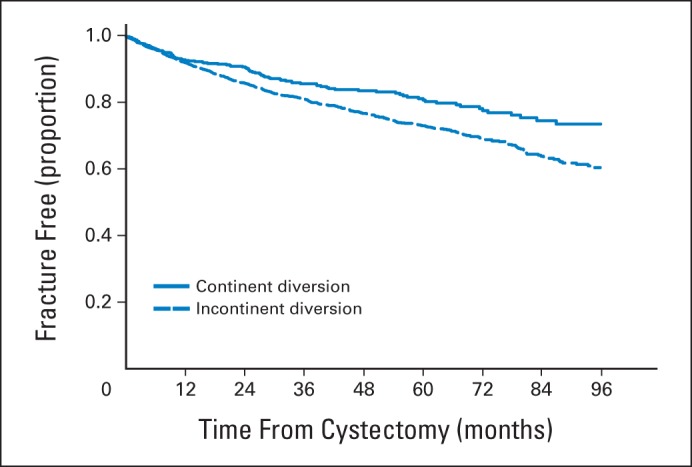
Fracture-free survival by type of urinary diversion in patients who received cystectomy.
DISCUSSION
In this population-based cohort of older patients with bladder cancer, we found that radical cystectomy and urinary diversion were associated with an increased risk of fracture. Interestingly, patients who had cystectomy seemed to have fewer risk factors for fracture at the time of cancer diagnosis, given that they were generally younger, had fewer comorbidities, were less likely to have chronic kidney disease, and had a lower incidence of fractures in the year before diagnosis. Despite these favorable characteristics, after cystectomy their risk of fracture was increased by 21%, compared with their peers who did not have cystectomy.
These results have strong biologic plausibility, given that cystectomy and urinary diversion lead to chronic metabolic acidosis, which is associated with bone loss through bone resorption and urinary calcium loss. Prior studies that evaluated the association between urinary diversion and bone health were case series or cross-sectional studies with small sample sizes, poorly defined entry criteria, and poorly matched controls.15 Moreover, none evaluated fracture as an end point, instead examining intermediate end points and finding conflicting results. Some studies showed decreased bone mineral density after cystectomy, and others did not.15,20–30 Two longitudinal, prospective studies31,32 found no decrease in bone density in the 2 or 3 years after cystectomy, but both had sample sizes of fewer than 50 patients. Similarly, there are conflicting results from studies of pediatric patients who have had augmentation cystoplasty or urinary diversion. Some of these studies reported decreased bone density and linear growth after augmentation cystoplasty or urinary diversion,33–39 whereas others have not found such associations.40–42
In addition to bone loss, metabolic acidosis also increases protein catabolism and induces a negative nitrogen balance by increasing amino acid and protein degradation and decreasing albumin synthesis.43–45 Treatment of acidosis has been shown to reverse these effects, decrease protein degradation, improve nitrogen balance, and increase serum albumin.45–47 Therefore, it is plausible that the chronic metabolic acidosis after urinary diversion may lead to loss of muscle mass and strength, which may make these patients frail and more susceptible to falls and thus more likely to develop fractures.
Our findings have important implications for the management of patients with bladder cancer after radical cystectomy and urinary diversion. Currently, there are no guidelines for the detection and management of osteoporosis in these patients. Periodic monitoring of bone density and early initiation of treatment for osteoporosis may reduce the risk of fracture after cystectomy. Other strategies that may improve bone health and decrease fracture risk include prophylactic treatment of metabolic acidosis, oral supplementation of calcium and vitamin D3, and prophylactic treatment with oral bicarbonate.11,48
Our results may have broader implications for bone health beyond patients with intestinal urinary diversions. Endogenous acid production from dietary precursors has been hypothesized to lead to a state of chronic acidosis and cause age-related osteoporosis.49 Diets rich in animal protein lead to a high acid load and cause metabolic acidosis even in healthy persons. High dietary acid load has been associated with increased bone resorption markers and decreased bone density in women and with lower bone cortical area and decreased bone mineral content in healthy children.50–53 Although chronic metabolic acidosis is thought to lead to long-term bone loss, studies so far have not demonstrated an association between acidosis and increased risk of fractures. This may be the result of the lack of a large cohort of patients with chronic metabolic acidosis with long-term follow-up. Hence intestinal urinary diversion might be a suitable model to study the bone effects of chronic metabolic acidosis.
Patients who received chemotherapy appeared to have a lower risk of developing fractures. It is possible that this reflects selection bias if healthier patients were more likely to receive chemotherapy. Despite controlling for several patient characteristics, including comorbidity, there may have been residual confounding by other, unmeasured characteristics associated with overall health status and fracture risk. Other factors such as age, sex, race, comorbidities, chronic kidney disease, prior history of fracture, and region of the county were associated with fracture risk. These are all known risk factors for development of a fracture.54–56
Several limitations of our study should be noted. Although we controlled for numerous demographic, disease, and health characteristics, it is possible that the patients in our cohort who had cystectomy also had more aggressive cancers and hence were more susceptible to bone metastases and consequent pathologic fractures. To minimize confounding from bone metastases, we excluded patients with stage IV disease and with lymph node involvement, adjusted for disease stage and grade, and censored patients at the time of diagnosis of bone metastases or malignant bone neoplasm. We also found that cystectomy increased the risk of fracture across all cancer stages. Although we included pathologic fractures of unspecified site in the end point, these fractures constituted a very small proportion (1% or less) of all fractures among patients who had cystectomy and those who did not. Hence, our findings are unlikely to be explained primarily by pathologic fractures from bone metastases.
Other potential confounders that were not available in our data set included bone mineral density measures and use of medications for osteoporosis. Although these characteristics would likely be associated with fracture risk, it is not clear whether they would be differently distributed between the patients who received cystectomy and those who did not. Similarly, we had no information regarding patient preferences and physician recommendations for cystectomy.
We were also unable to identify metabolic acidosis as the cause of the increased risk of fractures for these patients. It is plausible that other factors associated with cystectomy such as frailty after major surgery may play a role. Finally, the results of our study, although generalizable to adults age 66 years and older, may not be applicable to younger patients with bladder cancer.
In summary, we found that radical cystectomy and urinary diversion were associated with an increased risk of fracture in older patients with bladder cancer. These findings emphasize the need to monitor bone health and to conduct trials of prophylactic therapies that may reduce the risk of fracture in these patients.
Glossary Terms
- American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging:
a cancer staging system that describes the extent of cancer in a patient's body. “T” describes the size of the tumor and whether it has invaded nearby tissue; “N” describes regional lymph nodes that are involved; “M” describes distant metastasis (spread of cancer from one body part to another). The TNM Classification of Malignant Tumours was developed and maintained by the UICC to achieve consensus on one globally recognized standard for classifying the extent of spread of cancer. The TNM classification was also used by the AJCC. In 1987, the UICC and AJCC staging systems were unified into a single staging system. Prognosis of a patient is defined by TNM classification.
- Cox proportional hazards regression model:
a statistical model for regression analysis of censored survival data, examining the relationship of censored survival distribution to one or more covariates. This model produces a baseline survival curve, covariate coefficient estimates with their standard errors, risk ratios, 95% CIs, and significance levels.
- osteoclast:
a cell that breaks down bone and is responsible for bone resorption. Osteoclasts are large multinucleate cells that differentiate from macrophages.
- Surveillance, Epidemiology, and End Results (SEER):
a national cancer registry that collects information from all incident malignancies in multiple geographic areas of the United States.
Appendix
Table A1.
Codes Used to Identify Diagnoses and Procedures
| Diagnosis or Procedure | ICD-9 | CPT/HCPCS |
|---|---|---|
| Radical cystectomy | 57.7, 57.71, 57.79, 68.8 | 51570, 51575, 51580, 51585, 51590, 51595, 51596 |
| Urinary diversion | ||
| Continent | 57.87 | 51596 |
| Incontinent | 56.51 | 50815, 50820, 51590, 51595 |
| Chemotherapy | 51720, 90586, 96400-96549, J0640, J8510, J8520, J8521, J8530, J8600, J8610, J8999, J9000-J9999, Q0083-Q0085 | |
| Intravesical therapy | 51720, 90586, J9031 | |
| Fracture | 733.1X (pathologic), 800-829 | |
| Skull | 800-804 | |
| Spine | 733.13, 805-806 | |
| Rib | 807 | |
| Pelvis | 808 | |
| Upper arm | 733.11, 810-812 | |
| Lower arm | 733.12, 813 | |
| Hand | 814-817 | |
| Femoral neck | 733.14, 820 | |
| Other parts of femur | 733.15, 821 | |
| Lower leg | 733.16, 822-824 | |
| Foot | 825-826 | |
| Pathologic fracture, unspecified | 733.1, 733.10 | |
| Other | 733.19, 809, 818-819, 827-829 | |
| Bone metastases or bone neoplasm | 198.5, 170.X | |
| Chronic kidney disease | 585.X |
Abbreviations: CPT, current procedural terminology; HCPCS, Healthcare Common Procedure Coding System; ICD-9, International Classification of Diseases, ninth revision.
Footnotes
Listen to the podcast by Dr Saylor at www.jco.org/podcasts
Supported by National Cancer Institute Career Development Award (K07 CA118189; E.B.E.).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Amit Gupta, Behfar Ehdaie, Shahrokh F. Shariat, Farhang Rabbani, Harry W. Herr, Bernard H. Bochner, Elena B. Elkin
Administrative support: Elena B. Elkin
Collection and assembly of data: Amit Gupta, Coral L. Atoria, Elena Elkin
Data analysis and interpretation: Amit Gupta, Coral L. Atoria, Behfar Ehdaie, Elena B. Elkin
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual report to the nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDougal WS. Metabolic complications of urinary intestinal diversion. J Urol. 1992;147:1199–1208. doi: 10.1016/s0022-5347(17)37517-1. [DOI] [PubMed] [Google Scholar]
- 4.Castro JE, Ram MD. Electrolyte imbalance following ileal urinary diversion. Br J Urol. 1970;42:29–32. doi: 10.1111/j.1464-410x.1970.tb11903.x. [DOI] [PubMed] [Google Scholar]
- 5.Malek RS, Burke EC, Deweerd JH. Ileal conduit urinary diversion in children. J Urol. 1971;105:892–900. doi: 10.1016/s0022-5347(17)61655-0. [DOI] [PubMed] [Google Scholar]
- 6.Lemann J, Jr, Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: Further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966;45:1608–1614. doi: 10.1172/JCI105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemann J, Litzow JR, Lennon EJ. Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest. 1967;46:1318–1328. doi: 10.1172/JCI105624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnett T. Regulation of bone cell function by acid-base balance. Proc Nutr Soc. 2003;62:511–520. doi: 10.1079/pns2003268. [DOI] [PubMed] [Google Scholar]
- 9.Bushinsky DA. Acid-base imbalance and the skeleton. Eur J Nutr. 2001;40:238–244. doi: 10.1007/s394-001-8351-5. [DOI] [PubMed] [Google Scholar]
- 10.Wiederkehr M, Krapf R. Metabolic and endocrine effects of metabolic acidosis in humans. Swiss Med Wkly. 2001;131:127–132. doi: 10.4414/smw.2001.09666. [DOI] [PubMed] [Google Scholar]
- 11.Sebastian A, Harris ST, Ottaway JH, et al. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med. 1994;330:1776–1781. doi: 10.1056/NEJM199406233302502. [DOI] [PubMed] [Google Scholar]
- 12.Cramer CF, Copp DH. Progress and rate of absorption of radiostrontium through intestinal tracts of rats. Proc Soc Exp Biol Med. 1959;102:514–517. doi: 10.3181/00379727-102-25301. [DOI] [PubMed] [Google Scholar]
- 13.Marcus CS, Lengemann FW. Absorption of Ca45 and Sr85 from solid and liquid food at various levels of the alimentary tract of the rat. J Nutr. 1962;77:155–160. doi: 10.1093/jn/77.2.155. [DOI] [PubMed] [Google Scholar]
- 14.Cramer CF. Sites of calcium absorption and the calcium concentration of gut contents in the dog. Can J Physiol Pharmacol. 1965;43:75–78. doi: 10.1139/y65-009. [DOI] [PubMed] [Google Scholar]
- 15.Roosen A, Gerharz EW, Roth S, et al. Bladder, bowel and bones: Skeletal changes after intestinal urinary diversion. World J Urol. 2004;22:200–209. doi: 10.1007/s00345-004-0434-8. [DOI] [PubMed] [Google Scholar]
- 16.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 17.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV-55-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.Campanello A, Herlitz H, Lindstedt G, et al. Bone mineral and related biochemical variables in patients with Kock ileal reservoir or Bricker conduit for urinary diversion. J Urol. 1996;155:1209–1213. [PubMed] [Google Scholar]
- 21.Davidsson T, Lindergård B, Obrant K, et al. Long-term metabolic effects of urinary diversion on skeletal bone: Histomorphometric and mineralogic analysis. Urology. 1995;46:328–333. doi: 10.1016/s0090-4295(99)80215-5. [DOI] [PubMed] [Google Scholar]
- 22.Fujisawa M, Nakamura I, Yamanaka N, et al. Changes in calcium metabolism and bone demineralization after orthotopic intestinal neobladder creation. J Urol. 2000;163:1108–1111. quiz 1295. [PubMed] [Google Scholar]
- 23.Giannini S, Nobile M, Sartori L, et al. Bone density and skeletal metabolism in patients with orthotopic ileal neobladder. J Am Soc Nephrol. 1997;8:1553–1559. doi: 10.1681/ASN.V8101553. [DOI] [PubMed] [Google Scholar]
- 24.Kawakita M, Arai Y, Shigeno C, et al. Bone demineralization following urinary intestinal diversion assessed by urinary pyridinium cross-links and dual energy x-ray absorptiometry. J Urol. 1996;156:355–359. doi: 10.1097/00005392-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Minervini R, Felipetto R, Cecchi M, et al. Densitometric and biochemical alterations in bony tissue induced by ureterosigmoidostomy. Urol Int. 1995;54:217–219. doi: 10.1159/000282727. [DOI] [PubMed] [Google Scholar]
- 26.Mundy AR, Nurse DE. Calcium balance, growth and skeletal mineralisation in patients with cystoplasties. Br J Urol. 1992;69:257–259. doi: 10.1111/j.1464-410x.1992.tb15524.x. [DOI] [PubMed] [Google Scholar]
- 27.Pfitzenmaier J, Lotz J, Faldum A, et al. Metabolic evaluation of 94 patients 5 to 16 years after ileocecal pouch (Mainz pouch 1) continent urinary diversion. J Urol. 2003;170:1884–1887. doi: 10.1097/01.ju.0000091900.57347.ee. [DOI] [PubMed] [Google Scholar]
- 28.Sevin G, Koşar A, Perk H, et al. Bone mineral content and related biochemical variables in patients with ileal bladder substitution and colonic Indiana pouch. Eur Urol. 2002;41:655–659. doi: 10.1016/s0302-2838(02)00176-8. [DOI] [PubMed] [Google Scholar]
- 29.Stein R, Fisch M, Andreas J, et al. Whole-body potassium and bone mineral density up to 30 years after urinary diversion. Br J Urol. 1998;82:798–803. doi: 10.1046/j.1464-410x.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 30.Tschopp AB, Lippuner K, Jaeger P, et al. No evidence of osteopenia 5 to 8 years after ileal orthotopic bladder substitution. J Urol. 1996;155:71–75. doi: 10.1097/00005392-199601000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Campanello M, Herlitz H, Lindstedt G, et al. Determinants of bone loss in patients with Kock ileal urinary reservoir. Scand J Urol Nephrol. 1999;33:312–316. doi: 10.1080/003655999750017383. [DOI] [PubMed] [Google Scholar]
- 32.Poulsen AL, Overgaard K, Steven K. Bone metabolism following bladder substitution with the ileal urethral Kock reservoir. Br J Urol. 1997;79:339–347. doi: 10.1046/j.1464-410x.1997.00376.x. [DOI] [PubMed] [Google Scholar]
- 33.Feng AH, Kaar S, Elder JS. Influence of enterocystoplasty on linear growth in children with exstrophy. J Urol. 2002;167:2552–2555. discussion 2555. [PubMed] [Google Scholar]
- 34.Gros DA, Dodson JL, Lopatin UA, et al. Decreased linear growth associated with intestinal bladder augmentation in children with bladder exstrophy. J Urol. 2000;164:917–920. doi: 10.1097/00005392-200009020-00001. [DOI] [PubMed] [Google Scholar]
- 35.Hafez AT, McLorie G, Gilday D, et al. Long-term evaluation of metabolic profile and bone mineral density after ileocystoplasty in children. J Urol. 2003;170:1639–1641. doi: 10.1097/01.ju.0000083887.58315.7e. discussion 1641-1642. [DOI] [PubMed] [Google Scholar]
- 36.Taskinen S, Fagerholm R, Mäkitie O. Skeletal health after intestinal bladder augmentation: Findings in 54 patients. BJU Int. 2007;100:906–910. doi: 10.1111/j.1464-410X.2007.07085.x. [DOI] [PubMed] [Google Scholar]
- 37.Taskinen S, Rintala R, Mäkitie O. Bone health in patients with cloacal exstrophy and persistent cloaca after bladder augmentation. J Pediatr Surg. 2008;43:700–704. doi: 10.1016/j.jpedsurg.2007.12.062. [DOI] [PubMed] [Google Scholar]
- 38.Vajda P, Pinter AB, Harangi F, et al. Metabolic findings after colocystoplasty in children. Urology. 2003;62:542–546. doi: 10.1016/s0090-4295(03)00580-6. discussion 546. [DOI] [PubMed] [Google Scholar]
- 39.Koch MO, McDougal WS, Hall MC, et al. Long-term metabolic effects of urinary diversion: A comparison of myelomeningocele patients managed by clean intermittent catheterization and urinary diversion. J Urol. 1992;147:1343–1347. doi: 10.1016/s0022-5347(17)37560-2. [DOI] [PubMed] [Google Scholar]
- 40.Clementson Kockum C, Helin I, Malmberg L, et al. Pediatric urinary tract reconstruction using intestine. Scand J Urol Nephrol. 1999;33:53–56. doi: 10.1080/003655999750016285. [DOI] [PubMed] [Google Scholar]
- 41.Gerharz EW, Preece M, Duffy PG, et al. Enterocystoplasty in childhood: A second look at the effect on growth. BJU Int. 2003;91:79–83. doi: 10.1046/j.1464-410x.2003.04012.x. [DOI] [PubMed] [Google Scholar]
- 42.Mingin GC, Nguyen HT, Mathias RS, et al. Growth and metabolic consequences of bladder augmentation in children with myelomeningocele and bladder exstrophy. Pediatrics. 2002;110:1193–1198. doi: 10.1542/peds.110.6.1193. [DOI] [PubMed] [Google Scholar]
- 43.Reaich D, Channon SM, Scrimgeour CM, et al. Ammonium chloride-induced acidosis increases protein breakdown and amino acid oxidation in humans. Am J Physiol. 1992;263:E735–E739. doi: 10.1152/ajpendo.1992.263.4.E735. [DOI] [PubMed] [Google Scholar]
- 44.Ballmer PE, McNurlan MA, Hulter HN, et al. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest. 1995;95:39–45. doi: 10.1172/JCI117668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitch WE. Metabolic and clinical consequences of metabolic acidosis. J Nephrol. 2006;19(suppl 9):S70–S75. [PubMed] [Google Scholar]
- 46.Movilli E, Zani R, Carli O, et al. Correction of metabolic acidosis increases serum albumin concentrations and decreases kinetically evaluated protein intake in haemodialysis patients: A prospective study. Nephrol Dial Transplant. 1998;13:1719–1722. doi: 10.1093/ndt/13.7.1719. [DOI] [PubMed] [Google Scholar]
- 47.Williams B, Hattersley J, Layward E, et al. Metabolic acidosis and skeletal muscle adaptation to low protein diets in chronic uremia. Kidney Int. 1991;40:779–786. doi: 10.1038/ki.1991.275. [DOI] [PubMed] [Google Scholar]
- 48.Jonsson O, Herlitz H, Lindholm E, et al. Prophylaxis against bone loss in Kock reservoir patients with reduced glomerular filtration rate. Scand J Urol Nephrol. 2005;39:200–205. doi: 10.1080/00365590510007829. [DOI] [PubMed] [Google Scholar]
- 49.Wachman A, Bernstein DS. Diet and osteoporosis. Lancet. 1968;1:958–959. doi: 10.1016/s0140-6736(68)90908-2. [DOI] [PubMed] [Google Scholar]
- 50.Welch AA, Bingham SA, Reeve J, et al. More acidic dietary acid-base load is associated with reduced calcaneal broadband ultrasound attenuation in women but not in men: Results from the EPIC-Norfolk cohort study. Am J Clin Nutr. 2007;85:1134–1141. doi: 10.1093/ajcn/85.4.1134. [DOI] [PubMed] [Google Scholar]
- 51.Macdonald HM, New SA, Fraser WD, et al. Low dietary potassium intakes and high dietary estimates of net endogenous acid production are associated with low bone mineral density in premenopausal women and increased markers of bone resorption in postmenopausal women. Am J Clin Nutr. 2005;81:923–933. doi: 10.1093/ajcn/81.4.923. [DOI] [PubMed] [Google Scholar]
- 52.New SA, MacDonald HM, Campbell MK, et al. Lower estimates of net endogenous non-carbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am J Clin Nutr. 2004;79:131–138. doi: 10.1093/ajcn/79.1.131. [DOI] [PubMed] [Google Scholar]
- 53.Alexy U, Remer T, Manz F, et al. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr. 2005;82:1107–1114. doi: 10.1093/ajcn/82.5.1107. [DOI] [PubMed] [Google Scholar]
- 54.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 55.Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: Prospective open cohort study. BMJ. 2012;344:e3427. doi: 10.1136/bmj.e3427. [DOI] [PubMed] [Google Scholar]
- 56.Jacobsen SJ, Goldberg J, Miles TP, et al. Regional variation in the incidence of hip fracture: US white women aged 65 years and older. JAMA. 1990;264:500–502. [PubMed] [Google Scholar]


