Abstract
This study seeks a route of drug administration that would produce a pharmacokinetic profile for dexamethasone not significantly different from the intravenous route in female rats and would offer reproducible drug input with minimal stress to the animals. The intramuscular (IM) route of drug administration vs intravenous (IV) injection were compared in three female Wistar rats administered 1 mg/kg dexamethasone phosphate. Dexamethasone plasma concentrations were measured by a normal phase HPLC assay for 12 h after drug administration. Dexamethasone exhibited monoexponential behavior after intravenous dosing and was absorbed rapidly after intramuscular dosing (absorption half-life of 14 min) with 86% bioavailability. Dexamethasone had a terminal half-life of 2.3 h after drug administration by either route. The volume of distribution of 0.78 l/kg and the clearance of 0.23 l/h/kg are in good agreement with reported pharmacokinetic parameters in male rats. Intravenous dosing can be replaced by intramuscular dosing without causing any marked difference in dexamethasone pharmacokinetics.
Keywords: pharmacokinetics, dexamethasone, rats, gender, intramuscular
Introduction
Synthetic glucocorticoids are potent anti-inflammatory and immunosuppressive drugs, which produce a variety of genomic and non-genomic effects. The pharmacodynamics of corticosteroids has been extensively studied in the literature [1, 2]. The synthetic fluorinated corticosteroid, dexamethasone, is administered to pregnant women at risk of preterm delivery to induce fetal lung maturation. The pharmacodynamics of dexamethasone as a function of gender and during pregnancy has not been investigated. The characterization of drug pharmacodynamics requires knowledge of the pharmacokinetic profile, which serves as the driving force for drug effects. These pharmacokinetic/pharmacodynamic (PK/PD) studies have traditionally utilized a methodological approach which has been termed the giant rat experiment [3]. In a giant rat study a large group of animals is dosed with the drug and blood/tissue samples are obtained by killing a small group of animals (usually three rats) at each time point.
The pharmacokinetics of dexamethasone has been well studied [4–6], however, all the information has been generated after intravenous dosing. This is usually achieved by administering the drug through an externally implanted cannula, tail vein or the penile vein. Surgery-induced stress from cannulation increases endogenous glucocorticoids [7, 8] and can confound the pharmacodynamic effects produced by the exogenously administered synthetic corticosteroid. Furthermore, there is an appreciable workload in performing surgery on all the animals for cannula placement. The penile vein route is obviously not feasible in female rats. Finally, tail vein injection requires anesthesia or animal restraint and significant technical expertise to ensure reproducible and consistent drug administration. Thus, the objective of this study was to find a parenteral route of drug administration that would produce a pharmacokinetic profile for dexamethasone not significantly different from the intravenous route in female rats and would offer reproducible drug input with minimal stress to the animals. The three parenteral routes considered were the intraperitoneal, subcutaneous and intramuscular injection.
The intraperitoneal route was not chosen because it has the following disadvantages:
Intraperitoneally administered drug enters the systemic circulation via the portal vein and is subject to hepatic first-pass metabolism leading to lower bioavailability in comparison with the intravenous route [9].
Improper injection technique can lead to rupture of abdominal organs and therefore this route of drug administration requires two individuals to ensure correct injection.
The use of this route requires great care when administering drug to pregnant rats in order to prevent injury to the uterine contents. There is also the possibility of drug diffusion from the peritoneal cavity into fetal membranes, which will confound the traditional meaning of maternal/fetal drug exchange through the placenta.
There is also the risk of intestinal adhesion and infections with this injection route [10].
The absorption pattern from the subcutaneous route is very similar to the intramuscular route of drug administration. However, subcutaneous drug administration gives a drug absorption profile that is slower than the intramuscular route [11]. It is also generally thought that the intramuscular route allows more predictable and uniform drug absorption in comparison with the subcutaneous route [12]. The choice of the intramuscular route for drug input is attractive because it is the route used clinically for administration of dexamethasone in threatened preterm labor to induce fetal lung maturation. Thus, the intramuscular route of administration was compared with the intravenous injection for dexamethasone.
Materials and Methods
Animals
Female Wistar rats (weighing 219–242 g) purchased from Harlan-Sprague-Dawley Inc. (Indianapolis, IN) were used in the study. Animals were housed in our University Laboratory Animal Facility maintained under constant temperature (22°C) and humidity with a controlled 12 h light/dark cycle. A time period of at least 1 week was allowed before they were prepared for surgery. The rats had free access to rat chow and drinking water. This research adheres to the Principles of Laboratory Animal Care (National Institutes of Health publication 85-23, revised 1985) and was approved by the University at Buffalo Institutional Animal Care and Use Committee.
Experimental
One day prior to the study, the rats were subjected to right external jugular vein cannulation under ketamine/xylazine anesthesia. Cannula patency was maintained using heparinized saline (42 U heparin/ml saline). The rats were divided into two groups of three animals each. One group received 1 mg/kg of dexamethasone phosphate (American Regent Laboratories, Inc., Shirley, NY) intravenously via the jugular vein catheter, while rats in the second group were given the same dose by intramuscular injection in the hind leg. The leg opposite to that used for anesthesia during cannulation was used for drug administration. At various times after drug administration, 250 ml of blood was taken though the cannula at 0.17, 0.33, 0.5, 0.75, 1, 1.5, 2, 4 and 8 h. Rats were killed by exsanguniation under ketamine/xylazine anesthesia at 12 h, with blood drained from the abdominal aortic artery. Blood was collected in EDTA containing syringes, centrifuged immediately at 9280 × g for 2 min at 4°C and plasma was quickly harvested and frozen at −20°C until analysed.
Drug assay
Samples were thawed at room temperature and aliquots of rat plasma (0.1–0.5 ml) were extracted with methylene chloride in Pyrex glass culture tubes (Corning GlassWorks, Corning, NY). Tubes were shaken on an Eberbach shaker for 45 min. The methylene chloride phase was then washed with 0.5 ml of 0.1N sodium hydroxide followed by 0.5 ml water and the aqueous phase was discarded. The residue obtained by evaporation of the solvent under purified air was reconstituted with mobile phase and vortex mixed prior to injection into the HPLC system. Concentrations were determined by normal phase HPLC [13] with a lower limit of quantification of 10 ng/ml and a coefficient of variation less than 10% for the assay.
Pharmacokinetic analysis
The intravenous and intramuscular profiles for dexamethasone as a function of time (t) were fitted individually and simultaneously to a one-compartment mammillary model parameterized in terms of Vc (volume of central compartment), CL (clearance), ka (first-order absorption rate constant) and F (bioavailability). The following equations were fit to the data using GraphPad Prism software (GraphPad Software Inc., San Diego, CA).
| (1) |
| (2) |
where C and D stand for concentration and dose and the subscripts IV and IM refer to intravenous and intramuscular routes of drug administration. The choice of the model was based on visual inspection of the fitted curve, weighted sum of squared residuals, Akaike criteria, F test, and confidence of parameter estimates. Graphpad Prism has a built-in capability of calculating 95% confidence and prediction intervals around fitted curves. These intervals are presented in all the fitted curves in this manuscript and the meaning of these intervals are as follows: The confidence intervals demarcate the region (with 95% certainty) where the true best-fit regression line will lie. The prediction intervals demarcate the region where 95% of the new data points are expected to lie if additional experiments are conducted, based upon the fit of the present experimental data.
Additional Data Sources
Additional dexamethasone data after intravenous administration of the phosphate ester prodrug in rats were extracted from the literature [4, 6]. Data for dexamethasone pharmacokinetics in pregnant rats were obtained from one of our own recent publications [14]. Literature data were recaptured by computer digitalization (Sigma Scan, Jandel Scientific, Corte Madera, CA, USA). The characteristics of the data collected from all the studies are listed in Table 1. These data were used for the purpose of model validation. To compare data from this study with the literature data, the literature profiles were normalized using the following equation:
| (3) |
Table 1.
Characteristics of the dexamethasone pharmacokinetic data collected from the literature
| Species | Study design | Dexamethasone phosphate dose |
Vss (l/kg) |
CL (l/h/kg) |
Reference |
|---|---|---|---|---|---|
| Male Sprague-Dawley rat | Serial sampling | 1 and 3 mg/kg | 1.1 | 0.19 | [4] |
| Male adrenalectomized Wistar rat | Giant rat study | 0.1 mg/kg | 1.2 | 0.16 | [6] |
| Female Wistar rat | Serial sampling | 1 mg/kg | 0.78 | 0.23 | Current study |
| Pregnant Sprague-Dawley rat | Giant rat study | 1.9 mg/kg | 0.87 | 0.19 | [14] |
| Human | Serial sampling | 4.8 mg (4 mg dexamethasone) | 1.2 | 0.18 | [1] |
Results and Discussion
The time course of dexamethasone plasma concentrations with fitted curves and confidence and prediction bands is shown in Figures 1 and 2. Rats were dosed with the phosphate ester prodrug of dexamethasone via the intravenous and intramuscular route. The phosphate ester corticosteroid prodrugs release the active steroid with a half-life of less than 10 min in humans [15] after intravenous administration. Based on allometric scaling principles, metabolic activation of the prodrug is expected to occur much faster in rats. The formation of dexamethasone after intravenous administration of the prodrug is barely visible in the pharmacokinetic profile despite the early sampling time points (10, 20 and 30 min). The extremely rapid activation of the prodrug allows the assumption of an instantaneous input of dexamethasone for pharmacokinetic analysis of the intravenous data. Dexamethasone was absorbed rapidly after intramuscular dosing, reaching Cmax (concentration maximum) within 45 min. Dexamethasone exhibited a mild biexponential character after intravenous drug administration in two of the three rats. The distribution phase was extremely rapid and lasted for about 20 min in these two animals. Attempts to fit the data to a two-compartment mammillary model gave higher CV% for the parameter estimates and the modeling criteria (F test and Akaike criteria) favored a one- over a two-compartment scheme for simultaneous and individual fittings. Since there was no compelling evidence supporting a two-compartment model the rule of parsimony was followed and a one-compartment model was selected for the data. Parameter values and the CV% of parameter estimates from the individual and simultaneous fitting procedures are listed in Table 2. Simultaneous curve fitting indicated that the absorption half-life after intramuscular injection was only 14 min and 86% of the dose was absorbed intramuscularly compared with the intravenous dose. The 95% confidence interval for the bioavailability estimate reported by GraphPad Prism was 0.69–1.03. The interval contained a value of 1 and hence the bioavailability can be considered as nearly complete. The difference between the fitted value of 0.86 and the expected value of 1 for bioavailability can be attributed to the variability observed in the data. The simultaneously fitted terminal slope indicates that dexamethasone has a terminal half-life of 2.3 h after drug administration by either route. The Vc value of 0.78 l/kg and the CL value of 0.23 l/h/kg are in good agreement with reported pharmacokinetic parameters in male and pregnant rats (Table 1) indicating that dose, gender and pregnancy do not affect dexamethasone pharmacokinetics. The lack of gender effect on the clearance parameter is surprising considering the fact that rats exhibit sex-specific microsomal metabolism for dexamethasone where female rat liver microsomes produce minimal amounts of dexamethasone metabolites compared with those from male rats [16]. Another property of dexamethasone that has not been previously recognized is that principal pharmacokinetic parameters (CL and Vss) for dexamethasone are very similar between humans and rats (Table 1). This is in contrast to the more rapid clearance mechanism that has been observed for synthetic corticosteroids such as methylprednisolone in rats [17].
Figure 1.
Data points represent dexamethasone plasma concentrations from three animals (filled circles, open circles and open triangles) after intramuscular (A) and intravenous (B) injection of 1 mg/kg dexamethasone phosphate. Solid lines represent individual fitting of a one-compartment mammillary model to the data. Dotted and dashed lines represent 95% confidence and prediction intervals around the fitted lines
Figure 2.
Data points represent dexamethasone plasma concentrations from three animals (filled circles, open circles and open triangles) after intramuscular (A) and intravenous (B) injection of 1 mg/kg dexamethasone phosphate. Solid lines represent simultaneous fitting of a one-compartment mammillary model to the data. Dotted and dashed lines represent 95% confidence and prediction intervals around the fitted lines
Table 2.
Dexamethasone pharmacokinetic parameters in female rats after intramuscular and intravenous administration of 1 mg/kg dexamethasone phosphate. Values in parenthesis represents CV% of the estimate and is not reflective of inter-animal variability
| Parameter | Source | Individual fit |
Simultaneous fit |
|---|---|---|---|
| Parameter estimate (CV%) |
Parameter estimate (CV%) |
||
| CL (l/h/kg) | IV | 0.26 (7.8) | 0.23 (14) |
| Vc (l/kg) | IV | 0.75 (3.4) | 0.78 (6.4) |
| F | N/A | N/A | 0.86 (10) |
| ka (h−1) | IM | 3.8 (47) | 2.9 (29) |
| CL/F (l/h/kg) | IM | 0.22 (31) | N/A |
| Vc/F (l/kg) | IM | 1.0 (17) | N/A |
The intramuscular data were more variable than the intravenous data, which led to individual fitting of the intramuscular data producing higher CV% for the parameter estimates and wide confidence and prediction intervals around the fitted curve (Figure 1A). The individual fitting has the disadvantage of producing two sets of pharmacokinetic parameters (apparent and true) for the intramuscular and intravenous data. Simultaneous fitting on the other hand produces a single set of true pharmacokinetic parameters and allows estimation of the bioavailability of the drug from the intramuscular site. The simultaneously fitted parameters are also estimated with a greater degree of precision (CV% is below 30% for all the parameters). An interesting observation can be made by examining Figures 1 and 2, which exemplify the effect of simultaneous and individual fitting on the reliability of the fitted curves. The confidence bands are a measure of the reliability of the fitted curves because there is 95% certainty that the true best fitted profile lies within the two curved confidence boundaries. Individual fittings produce wider confidence bands around the fitted intramuscular curve, which are narrowed upon simultaneous fitting. In contrast the confidence bands around the fitted intravenous curve widen when the fitting procedure is changed from the individual to the simultaneous method. Thus the higher degree of certainty associated with the intravenous data helps fit the intramuscular data more precisely. However, the higher degree of variability associated with the intramuscular data leads to a trade-off in the form of wider confidence bands and a lower certainty about the fitted curve when fitting the intravenous data. Despite the trade-off the simultaneous fitting is the superior method of fitting the data because it provides global pharmacokinetic parameters, allows estimation of the intramuscular bioavailability, and generates parameter estimates with a higher degree of precision.
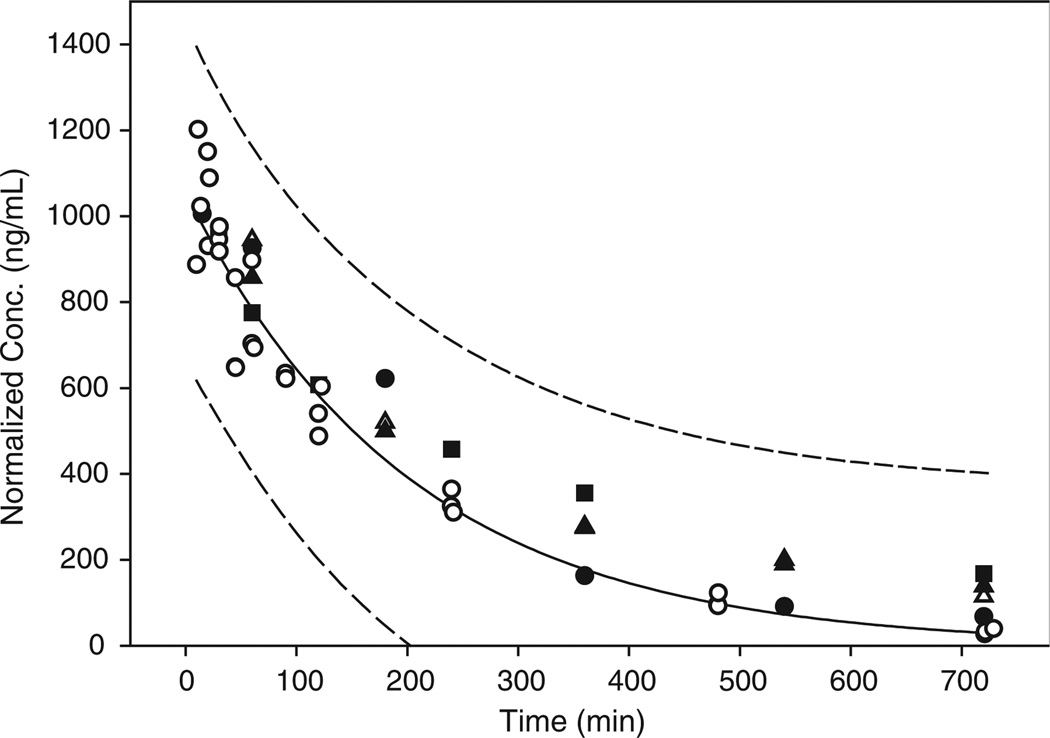
The ability of the intravenous data to produce tighter prediction bands around the intramuscular profile helps to narrow the area where future data points from intramuscular dexamethasone rat PK/PD studies are expected to lie. This is possible because the 95% prediction bands indicated in Figures 1 and 2 demark the area in which 95% of all data points are expected to fall. The validation of this concept in presented in Figure 3, where additional literature data on intravenous dexamethasone kinetics are plotted after dose normalization. Despite the fact that the data in Figure 3 came from studies involving different study designs all the data points fall within the 95% prediction band. Thus, although intramuscular drug administration gave more variable data, the knowledge of the 95% prediction interval for the intramuscular route and the availability of curve fitting techniques such as Bayesian analysis (which would incorporate knowledge already gained regarding dexamethasone pharmacokinetics in the curve fitting process) may allow dexamethasone dosing by the intramuscular route without sacrificing the quality of the pharmacokinetic profile driving the drug pharmacodynamics. Finally, since intramuscular dosing of dexamethasone allowed rapid drug input with almost complete bioavailability, it can be concluded that intravenous dosing can be replaced by intramuscular dosing without causing any marked difference in dexamethasone pharmacokinetics during rat pharmacodynamic studies.
Figure 3.
Comparison of the current intravenous data with other rat pharmacokinetic profiles reported for dexamethasone. Solid and dashed lines represent the fitted curve and 95% prediction bands for the data generated in the current study. Open circles are the intravenous data from this study. Filled and open triangles represent normalized concentrations from reference [4] for the 1 and 3 mg/kg dose. Filled squares represent normalized concentrations from reference [6] for the 0.1 mg/kg dose. Filled circles represent normalized concentrations from reference [14] for the 1.9 mg/kg dose
Acknowledgements
The authors would like to thank Ms Nancy Pyszczynski and Ms Suzette Mis for providing valuable support in this study. This study was supported by Grant GM 24211 from the National Institutes of Health and a predoctoral fellowship for MNS from Merck.
References
- 1.Jusko WJ, Ludwig EA. Corticosteroids. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied Pharmacokinetics. Vancouver: Applied Therapeutics Inc; 1992. pp. 1–34. [Google Scholar]
- 2.Mollmann H, Balbach S, Hochhaus G, Barth J, Derendorf H. Pharmacokinetic-pharmacodynamic correlations of corticosteroids. In: Derendorf H, Hochhaus G, editors. Handbook of Pharmacokinetic/Pharmacodynamic Correlation. Boca Raton: CRC Press Inc; 1995. pp. 323–361. [Google Scholar]
- 3.Jin JY, DuBois DC, Almon RR, Jusko WJ. Receptor/gene-mediated pharmacodynamic effects of methylprednisolone on phosphoenolpyruvate carboxykinase regulation in rat liver. J Pharmacol Exp Ther. 2004;309:328–339. doi: 10.1124/jpet.103.061515. [DOI] [PubMed] [Google Scholar]
- 4.Varma DR, Mulay S. Anti-inflammatory and ulcerogenic effects and pharmacokinetics of dexamethasone in protein-deficient rats. J Pharmacol Exp Ther. 1980;214:197–202. [PubMed] [Google Scholar]
- 5.Varma DR, Yue TL. Influence of protein-calorie malnutrition on the pharmacokinetics, placental transfer and tissue localization of dexamethasone in rats. Br J Pharmacol. 1984;83:131–137. doi: 10.1111/j.1476-5381.1984.tb10127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mager DE, Pyszczynski NA, Jusko WJ. Integrated QSPR-pharmacodynamic model of genomic effects of several corticosteroids. J Pharm Sci. 2003;92:881–889. doi: 10.1002/jps.10343. [DOI] [PubMed] [Google Scholar]
- 7.Lestage P, Vitte PA, Rolinat JP, Minot R, Broussolle E, Bobillier P. A chronic arterial and venous cannulation method for freely moving rats. J Neurosci Methods. 1985;13:213–222. doi: 10.1016/0165-0270(85)90069-x. [DOI] [PubMed] [Google Scholar]
- 8.Shakhar G, Blumenfeld B. Glucocorticoid involvement in suppression of NK activity following surgery in rats. J Neuroimmunol. 2003;138:83–91. doi: 10.1016/s0165-5728(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 9.Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther. 1971;178:562–564. [PubMed] [Google Scholar]
- 10.Seeman P, Kalant H. Drug solubility, absorption, and movement across body membranes. In: Kalant H, Roschlau WHE, editors. Principles of Medical Pharmacology. New York: Oxford University Press; 1998. pp. 11–27. [Google Scholar]
- 11.Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Applications. Baltimore: Williams and Wilkins; 1995. pp. 126–127. [Google Scholar]
- 12.Franklin MR. Drug absorption, action, and disposition. In: Gennaro AR, editor. Remington’s Pharmaceutical Sciences. Easton, PA: Mack Publishing Company; 1995. pp. 697–723. [Google Scholar]
- 13.Haughey DB, Jusko WJ. Analysis of methylprednisolone, methylprednisone and corticosterone for assessment of methylprednisolone disposition in the rat. J Chromatogr. 1988;430:241–248. doi: 10.1016/s0378-4347(00)83159-x. [DOI] [PubMed] [Google Scholar]
- 14.Samtani MN, Schwab M, Nathanielsz PW, Jusko WJ. Area/moment and compartmental modeling of pharmacokinetics during pregnancy: Applications to maternal/fetal exposures to corticosteroids in sheep and rats. Pharm Res. 2004;21:2279–2292. doi: 10.1007/s11095-004-7681-7. [DOI] [PubMed] [Google Scholar]
- 15.Samtani MN, Schwab M, Nathanielsz PW, Jusko WJ. Stabilization and HPLC analysis of betamethasone sodium phosphate in plasma. J Pharm Sci. 2004;93:726–732. doi: 10.1002/jps.10577. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson ES, Maggs JL, Park BK, Back DJ. Dexamethasone metabolism in vitro: species differences. J Steroid Biochem Mol Biol. 1997;62:345–352. doi: 10.1016/s0960-0760(97)00038-1. [DOI] [PubMed] [Google Scholar]
- 17.Kong AN, Jusko WJ. Disposition of methylprednisolone and its sodium succinate prodrug in vivo and in perfused liver of rats: nonlinear and sequential first-pass elimination. J Pharm Sci. 1991;80:409–415. doi: 10.1002/jps.2600800502. [DOI] [PubMed] [Google Scholar]