Abstract
Objective
Recent randomized clinical trials have shown the efficacy of a restrictive transfusion strategy in critically ill children. The impact of these trials on pediatric transfusion practice is unknown. Additionally, long-term trends in pediatric transfusion practice in the intensive care unit have not been described. We assessed transfusion practice over time, including the effect of clinical trial publication.
Design
Single-center, retrospective observational study.
Setting
A 10-bed pediatric intensive care unit (PICU) in an urban academic medical center.
Patients
Critically ill, non-bleeding children between the ages of 3 days and 14 years old, admitted to the University of Maryland Medical Center PICU between January 1, 1998 and December 31, 2009, excluding those with congenital heart disease, hemolytic anemia, and hemoglobinopathies.
Interventions
None.
Measurements and Main Results
During the time period studied, 5327 patients met inclusion criteria. Of these, 335 received at least one red cell transfusion while in the PICU. The overall proportion transfused declined from 10.5% in 1998 to 6.8% in 2009 (p = 0.007). Adjusted for acuity, the likelihood of transfusion decreased by calendar year of admission. In transfused patients, the pre-transfusion hemoglobin level declined, from 10.5 g/dL to 9.3 g/dL, though these changes failed to meet statistical significance (p=0.09). Neonatal age, respiratory failure, shock, multi-organ dysfunction, and acidosis were associated with an increased likelihood of transfusion in both univariate and multivariable models.
Conclusions
The overall proportion of patients transfused between 1998 and 2009 decreased significantly. The magnitude of the decrease varied over time, and no additional change in transfusion practice occurred after the publication of a major pediatric clinical trial in 2007. Greater illness acuity and younger patient age were associated with an increased likelihood of transfusion.
Keywords: pediatrics, pediatric intensive care units, physicians practice patterns, erythrocyte transfusion, blood component transfusion, evidence-based practice
Introduction
Allogeneic red cell (RBC) transfusion is common in the pediatric intensive care unit (PICU) for the treatment of anemia[1]. Over the past two decades, pediatric providers—general pediatricians, hematologists, intensivists, surgeons, and anesthesiologists—have increasingly advocated a restrictive strategy in providing RBCs to ill children (Table 1). In 1999, the Transfusion Requirements in Critical Care (TRICC) trial demonstrated that the restrictive use of RBCs, defined as transfusing at a threshold of hemoglobin (Hgb) of 7g/dL, reduced the number of transfusions received by euvolemic, non-hemorrhaging critically ill adults, without worsening outcome[2]. In 2007, the Transfusion Strategies for Patients in Pediatric Intensive Care Units (TRIPICU) trial showed that this Hgb threshold of 7g/dL could be safely applied in stable, critically ill children[3]. The Premature Infants in Need of Transfusion (PINT) trial (2006) demonstrated similar findings in the neonatal population [4]. These three, multicenter, randomized clinical trials provided consistent evidence that transfusing RBCs more conservatively is at least equivalent, and possibly superior to maintaining higher Hgb levels by liberal administration of donor blood.
Table 1.
Selected studies and guidelines
| Year | Publication | Comments |
|---|---|---|
| 1992 | Crosby, E.T. [44] | In “young, healthy patients” a perioperative Hgb of 8–9 g/dL is recommended |
| 1997 | Luchtman-Jones, et. al.[45] | Ideal hematocrit for an ill infant unknown, but the “general guideline” is to maintain severely ill patients at a hematocrit of 30–40% |
| 1998 | Kevy, et. al. [46] | “It is common practice in intensive care units to use transfusions to maintain a hematocrit of about 30% in children and one of 40% in neonates with cardiorespiratory compromise” |
| 1998 | Davis, et. al. [47] | Maintaining a Hgb concentration of at least 7–8 g/dL is “reasonable” for adults and children, and infants > 3 months old. Infants < 3 months old “probably” require a Hgb level of 13 or more |
| 1999 | Lanzowski, P. [48] | In patients undergoing an oncologic emergency, “packed red cell transfusion are indicated for patients whose Hgb…is less than 8 g/dL or those whose Hgb is > 8 g/dL but who are cardiovascularly unstable or bleeding” |
| 2002 | Camboulives, J. [49] | “RBC transfusions are almost always required when the Hgb level is less than 6 g/dL and are rarely indicated when the Hgb levels are more than 10 g/dL. There is no single Hgb trigger” |
| 2004 | Strauss, R.G. [50] |
Children and Adolescents: Transfusion indicated for Hgb < 8 g/dL in perioperative period, “symptomatic chronic anemia,” and marrow failure. For patients with“severe” cardiopulmonary disease, transfusion indicated at a Hgb < 13 g/dL Infants within first 4 months of life: Transfusion indicated at Hgb < 13 g/dL in “severe” pulmonary or cardiac disease; < 10 g/dL in “moderate” pulmonary disease or major surgery; < 8 g/dL in “symptomatic” anemia |
| 2007 | Lacroix, et. al. [3] | “In stable, critically ill children, a Hgb threshold of 7 g/dL for red cell transfusion can decrease transfusion requirements without increasing adverse outcomes” |
Hgb, hemoglobin
Studies evaluating transfusion practice in adults over shorter time periods indicate partial implementation of such a strategy[5–7]. An approach evaluating a prolonged time course both before and after TRICC’s publication found that physician behavior changed most rapidly after TRICC publication, but that the rate of transfusion continued to decrease over time [8]. To our knowledge, no similar studies have been conducted in the pediatric literature, evaluating physician behavior over an extended time period and assessing the impact of the TRIPICU study.
The goals of this study were to assess changes in the proportion of patients transfused and pre-transfusion Hgb over time, and evaluate patient characteristics associated with transfusion. We hypothesized that changes in transfusion practice have occurred over time in the PICU, and that the publication of TRIPICU was associated with a more restrictive transfusion strategy. We also hypothesized that a greater likelihood of transfusion would be associated with a greater severity of illness and co-morbidity.
Materials and Methods
We conducted a single-center, retrospective, observational study of transfusion practice in the University of Maryland Medical Center (UMMC) PICU, a 10-bed ICU in an urban, academic medical center. Pediatric intensivists provide care for all patients admitted, with multiple subspecialties consulting as part of a modified closed-model unit. During the 12 years studied, the PICU was staffed by 12 attending intensivists, fellows and residents, and nurse practitioners. No ICU transfusion protocols were initiated during this time.
Patients were selected using the UMMC Clinical Data Repository (CDR), a validated administrative database. Previously, random sampling of 10% of patients found the positive and negative predictive value of CDR data to be greater than 99% [9], and that the accuracy of ventilator data was 94.1%, based on random sampling of 2062 patient days [10]. We evaluated patients meeting inclusion criteria, hospitalized between January 1, 1998 and December 31, 2009. Enrollment reflected the patient population enrolled in TRIPICU[3], including non-bleeding children between the ages of 3 days to 14 years old and excluding patients with acute blood loss or hemolysis, or congenital heart disease. Patients with hemoglobinopathies were also excluded. Patient selection was performed using ICD-9CM codes. To maintain independence of observations, only first and single PICU admissions were evaluated. Transfusion data, including pre-transfusion (last recorded) Hgb prior to the first discrete transfusion event in transfused patients, were obtained from a separate collection of data in the hospital’s electronic blood bank record system.
Patient demographic and clinical characteristics were extracted from the CDR’s collection of administrative data [11]. Clinical variables of interest were chosen a priori, based on a review of the literature and biological plausibility, and reflected increased severity of illness and mortality in the PICU[12–22]. We assessed the association of age (neonate vs. non-neonate, infant vs. non-infant), Multiple Organ Dysfunction Syndrome (MODS), respiratory failure, shock and/or acidosis and the likelihood of transfusion. Age categorization was per the Pediatric Logistic Organ Dysfunction (PELOD) and Pediatric Risk of Mortality III (PRISM III) scoring systems, with neonatal age being defined as less than 1 month old, and infant age defined as under 12 months old [20, 21]. The likelihood of transfusion was evaluated as a dichotomous outcome.
Statistical analyses were performed using SAS Version 9.1.3 (SAS Institute, Cary, NC). Baseline characteristics in transfused and non-transfused patients were compared using two sample t-tests for continuous variables [23] and comparison of proportions (Chi-square tests) for categorical variables [24]. Changes in the proportion of patients transfused, as well as changes in the mean pre-transfusion Hgb by year were assessed using linear regression. A spline was used to assess the effect of TRIPICU publication on the slope of change in the proportion transfused[25]. The associations between selected baseline clinical variables and the likelihood of transfusion were assessed in univariate and multivariable models. In selecting potential confounders for the multivariable logistic regression, variables meeting a significance test of 0.20 in univariate testing were included[26]. We applied the traditional definition of a p value of 0.05 or less for statistical significance[27]. The Institutional Review Board of the University of Maryland Baltimore reviewed and approved this study with waiver of consent.
Results
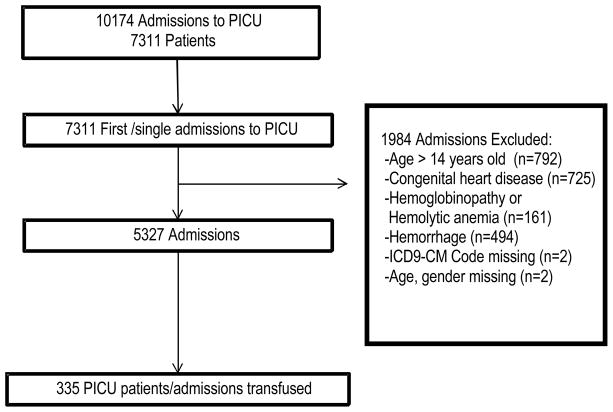
Of the 10,174 patients admitted to the PICU during the time period studied, 7311 were first and single-admissions. Of these, 5327 patients met inclusion criteria (Figure 1). A random sample of 419 patients meeting inclusion found a 97.6% concordance in enrollment criteria between our study patients and with those in TRIPICU. One or more discrete RBC transfusion events occurred in 335 (6.3%) of the patients studied. At baseline, transfused patients were younger, and during their ICU course were more likely to be suffering from respiratory failure, shock, MODS, and acidosis (Table 2). Patients over one year of age were transfused at a lower mean Hgb threshold than younger patients (9.6 g/dL vs. 8.7 g/dL, p=0.001).
Figure 1.
Enrollment of study participants. Excluded patients may have met more than one exclusion criterion. PICU, pediatric intensive care unit
Table 2.
Patient characteristics by transfusion exposure
| Not transfused, n = 4992 | Transfused, n= 335 | P value | |
|---|---|---|---|
| Gender, male | 2099 (42.1%) | 156 (46.6%) | 0.11 |
| Age, yrs | 4.0 ± 4.2 | 1.5 ± 2.3 | <0.001 |
| Neonate, age<1 month old | 360 (7.2%) | 41 (12.2%) | <0.001 |
| Age for non-neonate, yrs | 4.4 ± 4.5 | 2.1 ± 3.8 | <0.001 |
| Respiratory failure | 472 (9.5%) | 175 (52.2%) | <0.001 |
| Shock | 84 (1.7%) | 74 (22.1%) | <0.001 |
| MODS | 841 (16.9%) | 237 (70.8%) | <0.001 |
| Acidosis | 276 (5.5%) | 86 (25.7%) | <0.001 |
MODS, multiple organ dysfunction syndrome
The percentage of patients transfused (Figure 2) decreased from 10.5% in 1998–1999 to 6.6% in 2009–2010 (p=0.007). From 1998 to 2002, the proportion of patients transfused declined steadily from 10.5% to 3.8%. After an increase in 2003 to 6.3%, the lowest proportion transfused was in 2004, at 2.5%. In 2005, the proportion transfused rose to 3.4%, and then stabilized at 6.0–6.8% from 2006–2009. Spline testing using the TRIPICU date of publication as the knot point found no significant change in the rate of transfusion before and after publication (p = 0.78).
Figure 2.
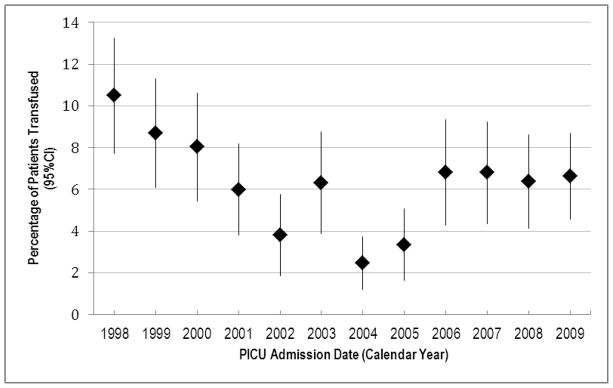
Percentage of patients transfused, by interval. The proportion of patients transfused decreased from 10.5% in 1998–1999 to 6.6% in 2009–2010 (linear regression, p=0.007).
Analysis of the mean pre-transfusion Hgb over time period found a decrease in the transfusion threshold for the first discrete RBC transfusion event, from 10.5 mg/dL in 1998–1999 to 9.3 g/dL in 2009–2010, though this did not meet the traditional definition of significance (p=0.09) (Figure 3). In 2009, 70.3% of patients were transfused at a Hgb threshold above 7.0 g/dL.
Figure 3.
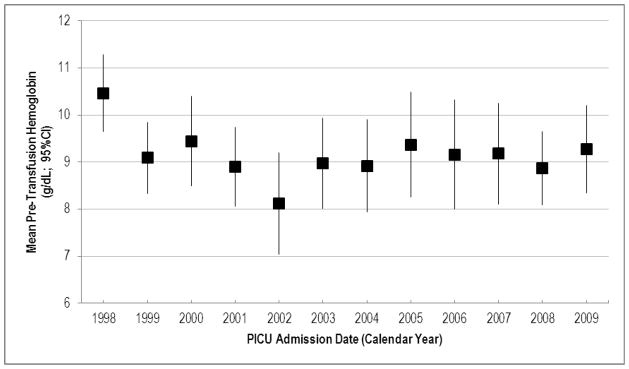
Mean pre-transfusion hemoglobin (g/dL) for the first red cell transfusion event, by admission year. Mean hemoglobin decreased from 10.5 mg/dL in 1998–1999 to 9.3 mg/dL in 2009–2010 (linear regression, p=0.09).
Neonatal or infant age, respiratory failure, shock, MODS, and acidosis were associated with increased odds of transfusion in the multivariable model (Table 3). Adjusted for patient characteristics and severity of illness, calendar year of admission was associated with a linear decrease in transfusion likelihood over time (Odds Ratio (OR) 0.90, 95% Confidence Interval (CI): 0.86 to 0.93, p < 0.001). The case mix was assessed by calendar year (Table 4). Comparing 1998 to 2009, all markers of severity (respiratory failure, shock, MODS and acidosis) increased in proportion (p = 0.004); gender and mean age were unchanged, and the proportion of neonates decreased (from 7.9% to 4.7%).
Table 3.
Multivariable model of likelihood of transfusion
| Variable | OR of transfusion (95% CI) | P value |
|---|---|---|
| Neonate vs. non-neonate | 2.45 (1.62 to 3.72) | <0.001 |
| Age, less than one year | 1.14 (1.10 to 1.19) | <0.001 |
| Respiratory failure | 2.28 (1.59 to 3.26) | <0.001 |
| Shock | 6.26 (4.08 to 9.60) | <0.001 |
| MODS | 5.71 (3.98 to 8.19) | <0.001 |
| Acidosis | 2.45 (1.73 to 3.47) | <0.001 |
| Calendar year | 0.90 (0.86 to 0.93) | <0.001 |
OR, odds ratio; MODS, multiple organ dysfunction syndrome
Table 4.
Patient characteristics by year.
| 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Chi-square/ANOVA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age (years) | 3.9 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.2 | 3.4 ± 0.2 | 4.4 ± 0.2 | 4.0 ± 0.2 | 4.4 ± 0.2 | 4.1 ± 0.2 | 4.0 ± 0.2 | 4.0 ± 0.2 | 4.2 ± 0.2 | 0.059 |
| Gender, male | 199 (42.6%) | 193 (43.0%) | 181 (42.8%) | 186 (41.2%) | 158 (43.1%) | 168 (44.2%) | 239 (42.2%) | 167 (40.0%) | 154 (40.4%) | 171 (41.5%) | 188 (41.3%) | 251 (45.0%) | 0.96 |
| Neonate | 37 (7.9%) | 42 (9.4%) | 40 (9.5%) | 41 (9.1%) | 36 (9.8%) | 19 (5.0%) | 59 (10.4%) | 30 (7.2%) | 20 (5.3%) | 19 (4.6%) | 32 (7.0%) | 26 (4.7%) | 0.0004 |
| Resp. Failure | 32 (6.9%) | 44 (9.8%) | 40 (9.5%) | 44 (9.8%) | 27 (7.4%) | 54 (14.2%) | 52 (9.2%) | 55 (13.2%) | 44 (11.6%) | 67 (16.3%) | 80 (17.6%) | 108 (19.4%) | <0.0001 |
| Shock | 3 (0.6%) | 4 (0.9%) | 9 (2.1%) | 9 (2.0%) | 5 (1.4%) | 15 (4.0%) | 10 (1.8%) | 14 (3.4%) | 14 (3.7%) | 20 (4.9%) | 28 (6.2%) | 27 (4.8%) | <0.0001 |
| MODS | 110 (23.6%) | 89 (19.8%) | 97 (22.9%) | 89 (19.7%) | 54 (14.7%) | 93 (24.5%) | 83 (14.7%) | 72 (17.2%) | 68 (17.9%) | 87 (21.1%) | 102 (22.4%) | 134 (24.0%) | <0.0001 |
| Acidosis | 18 (3.9%) | 18 (4.0%) | 30 (7.1%) | 25 (5.5%) | 18 (4.9%) | 22 (5.8%) | 25 (4.4%) | 20 (4.8%) | 29 (7.6%) | 55 (13.4%) | 42 (9.2%) | 60 (10.8%) | <0.0001 |
MODS, multiple organ dysfunction syndrome; ANOVA, analysis of variance
Approximately one third (128/335) of patients transfused underwent more than one transfusion event. The mean pre-transfusion Hgb for subsequent transfusion events decreased over time, from 11.8 g/dL in 1998 to 9.2 g/dL in 2010 (p<0.001) (Figure 4). Spline testing did not find a change in slope at the TRIPICU publication date for these repeat transfusions. A mixed effect model adjusting for intra-subject correlation between pre-transfusion Hgb measurements found that repeat transfusion events occurred at a higher mean Hgb threshold (10.1 g/dL, standard error=0.17, vs. 9.3 g/dL, standard error=0.13, p value<0.001).
Figure 4.
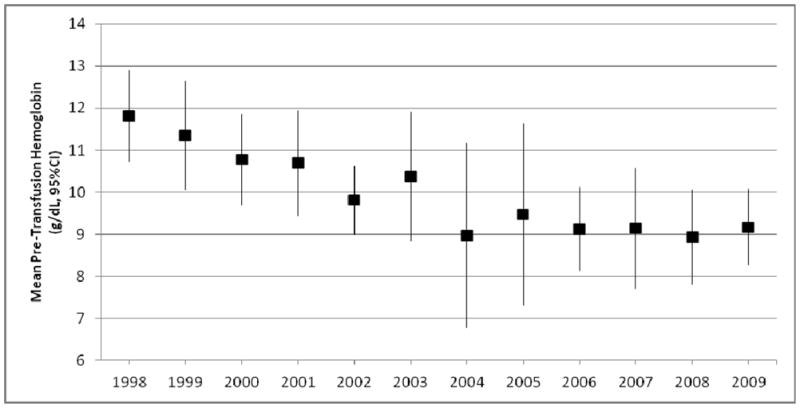
Mean pre-transfusion hemoglobin (g/dL) for repeat red cell transfusion events, by admission year. Mean hemoglobin decreased from 11.8 mg/dL in 1998–1999 to 9.2 mg/dL in 2009–2010 (linear regression, p<0.001)
Discussion
We found that from 1998 to 2009, the proportion of patients transfused decreased and remained lower as compared to that in 1998. There was a steady decline in the proportion transfused between 1998 and 2001–2002, and after some fluctuations of unclear significance, the proportion transfused between 2006 and 2009 remained steady at 6.0–6.8%. The mean pre-transfusion Hgb prior to the first transfusion event also decreased, in a pattern similar to that in the proportion transfused, though shy of statistical significance (p=0.09). Neonatal and infant age, respiratory failure, shock, MODS, and acidosis were all associated with an increased likelihood of transfusion. The threshold for repeat transfusion events decreased over time as well. The changes in transfusion behavior appear to be independent of case mix; in the multivariable model, calendar year of admission was independently associated with a linear decrease in the likelihood of transfusion, as the severity of illness increased over time.
While the case rate of transfusion decreased from 1998 to 2009, the publication of TRIPICU in 2007 [3] was not associated with additional changes in transfusion behavior. Additionally, post-hoc spline analysis using the PINT publication date as a knot point similarly demonstrated no corresponding trend towards a more restrictive practice. Instead, the greatest decline in the proportion of patients transfused occurred between 1998 and 2002. In this observational study, we are not able to elucidate mechanism, but some historical observations may be made. In 1999, the TRICC trial was published, advocating a restrictive transfusion practice in critically ill adults[2]. Pediatric intensivists must often extrapolate best practice strategies from adult data[28], and the publication of the TRICC study, validating a conservative approach to adult transfusion, may have influenced PICU physician behavior. This change in behavior, based on adult data, may have led to the greater magnitude of decline from 1998–2002 and preempted further changes in behavior after TRIPICU publication.
Studies of transfusion practice in adults have shown incomplete adoption of a restrictive strategy. A study evaluating practice after TRICC in a large, multidisciplinary ICU in the United Kingdom found that the median pre-transfusion Hgb was 7.8 g/dL [5]. Post-TRICC publication, a survey of 284 adult medical and/or surgical ICUs in the United States found that 44% of patients received one or more transfusions during their admission [6]. The mean pre-transfusion hemoglobin was found to be 8.6 g/dL, and the majority (90%) of transfusions were triggered by Hgb levels alone. In 2009, among adult patients with acute lung injury, 47% received RBCs for reasons other than acute blood loss or cardiac disease. The mean pre-transfusion Hgb prior to those patients’ first transfusion was 7.7 g/dL [7]. Our study shows, for the first time in the pediatric population, that while transfusion prevalence and likelihood—and, possibly, transfusion threshold—decreased over a prolonged period, transfusion behavior does not reflect complete adoption of the randomized control trial data. In this respect, our results are similar to long-term trends in behavior in the adult ICU population, in which gradual but incomplete implementation of a restrictive transfusion strategy was found [8].
Hospital and ICU type and provider specialty may influence transfusion practice. For example, in acute lung injury patients, surgical ICUs are more likely to transfuse than medical ICUs[7]. During this time period, the proportion of surgical patients transfused in Maryland has increased[29]. Evaluating statewide data, while adjusted transfusion rates have declined between 1994 and 2007 in higher ICU volume hospitals, in lower volume hospitals, the inverse is true[30]. Given the array of technique available to influence physician transfusion behavior [31, 32], it may be necessary to tailor interventions by setting type. By evaluating trends in the PICU, our study adds to our understanding of transfusion practice by suggesting that pediatric intensivists have followed adult literature in advance of pediatric data. While “early adopters” changed practice at the time of TRICC publication, neither the publication of pediatric data (i.e. TRIPICU), nor neonatal data (i.e. PINT), appeared to prompt a more restrictive transfusion strategy.
Our study validates existing literature by identifying important patient characteristics associated with transfusion. Our results found associations between neonatal and infant age, presence of shock, acidosis, respiratory failure, and MODS – all validated predictors of illness severity and poor outcome [12–22]—with increased transfusion likelihood. These data are consistent with prior findings that young age, presence of shock upon admission, admission diagnosis (e.g. malignancy, cardiac illnesses), and the occurrence of invasive procedures around the time of arrival in the PICU are predictive of transfusion [1, 33, 34]. In 2002, Laverdiere, et. al. surveyed 163 pediatric intensivists and presented several theoretical clinical scenarios. They found that the mean Hgb at which practitioners recommended transfusion varied according to factors such as presenting illness, age, and lactate levels[35].
The point transfusion case rate in the PICU has been described previously as between 14% and 50%[1, 33, 36, 37]. Studies limiting patients to those with a length of stay greater than 48 hours have found higher rates[1]. Transfusion rates in our ICU are lower than prior studies; e.g. in 2000, the proportion transfused was 8%, compared to 14% in the study by Armano et al [33]. This may be due to differences in patient population; as these studies did not exclude patients with hemorrhage or congenital heart disease[1, 33]. The overall proportion of patients transfused in our studied population was lower than previously reported. This may reflect the patient population after selecting out first and single-admissions, and after applying our inclusion and exclusion criteria. The overall transfusion incidence among all 10,174 patients admitted to the PICU (not limited to the study population) between 1998 and 2009 was 8.7%, closer to prior studies. It is unclear what degree of variation may be attributable to either case mix or physician practice.
While the overall slope of the line between 1998–2010 shows a linear decrease in overall transfusion rates and pre-transfusion Hgb, variation did occur during some intervals in the time studied. As an observational study, we are not able to discern whether this variation reflects statistical variation occurring by chance or another process influencing practice. One possibility for this variation was the publication of a study in 2001 by Rivers et al [38] that included a goal hematocrit of 30% in adults with severe sepsis. A post-hoc spline analysis using this publication date showed a change in slope (p=0.01).
In assessing our findings, some limitations should be considered. The study patient population was selected to reflect those enrolled in TRIPICU, which excluded patients with hemodynamic instability. The use of administrative data did not allow the determination of this clinical condition. We excluded all patients with a diagnosis code for congenital heart disease, differing from TRIPICU, which excluded only congenital heart disease patients under 28 days of age when surgery occurred and/or those with cyanotic heart disease[39]. Because the administrative data did not allow the accurate identification of these patients, we excluded all patients suffering from congenital heart defects. However, in validating our patient selection process we found a high fidelity (97.6% concordance) to TRIPICU inclusion and exclusion criteria as a whole, with 99.6% concordance in excluding patients with congenital cardiopathies. While we utilized multiple, validated risk factors for death to adjust for severity of illness and potential changes in case mix, a standardized scoring system such as the Pediatric Risk of Mortality (PRISM) score would have been useful in comparing our patient population to others.
No transfusion protocols or guidelines existed in this ICU during the time period studied, nor was formal review or training given regarding the findings of the TRIPICU study. As an observational study, the mechanisms underlying the adoption of this more restrictive transfusion strategy are unclear. As a teaching hospital, the practice changes seen may also reflect changes in house officer education and training over time. Analyzing data from a variety of PICU settings, including nonteaching institutions, may potentially help elucidate potential mechanisms. While we did not find that TRIPICU impacted the proportion transfused, with two years of post-publication data, the possibility exists that insufficient time was assessed to observe a delayed or extremely slow knowledge transfer.
Our findings suggest that a restrictive transfusion approach, as advocated by TRIPICU, was not fully implemented over the course of time studied. In critically ill children, multiple studies have found that transfusion results in immunomodulation and microvascular damage [40], is associated with worsened outcomes [41, 42], and greater resource utilization [43]. This gap between best evidence and physician transfusion practice merits attention.
Conclusions
This study is the first long-term assessment of trends in RBC transfusion practice in a tertiary care PICU. Since 1998, physicians have adopted a more restrictive transfusion strategy in critically ill children. This change in practice was sustained throughout the time period studied. The rate of change was greatest between 1999 and 2002, and no difference was found in the rate of change after publication of the TRIPICU study in 2007. Consistent with previous studies, greater acuity of illness and decreased age were associated with increased likelihood of transfusion. Despite these changes, a large proportion of patients continue to be transfused above a threshold Hgb of 7.0 g/dL, indicating that pediatric intensivists have not fully adopted a restrictive transfusion strategy. Further room for improvement in blood utilization practice exists in the PICU.
Acknowledgments
Supported, in part, by a Clinical Research Career Development Award from the National Institutes of Health (NIH), Bethesda, MD (5K12RR023250-03 to Dr. Netzer) and by a Midcareer Investigator Grant from the NIH (1K24AI079040-01A1 to Dr. Harris). We thank Colleen Riley and Jingkun Zhu for their assistance in database maintenance and abstraction. Helpful criticism was received from Carl Shanholtz, MD.
Footnotes
The authors declare that they have no conflicts of interest relevant to this manuscript submitted to Pediatric Critical Care Medicine.
References
- 1.Bateman ST, Lacroix J, Boven J, et al. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26–33. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- 2.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 3.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 4.Kirpalani H, Whyte RK, Anderson C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Chohan SS, McArdle F, McClelland DBL, et al. Red cell transfusion practice following the transfusion requirements in critical care (TRICC) study: prospective observational cohort study in a large UK intensive care unit. Vox Sang. 2003;84:211–218. doi: 10.1046/j.1423-0410.2003.00284.x. [DOI] [PubMed] [Google Scholar]
- 6.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: Anemia and blood transfusion in the critically ill--current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 7.Murphy DJ, Howard D, Muriithi A, et al. Red blood cell transfusion practices in acute lung injury: what do patient factors contribute? Crit Care Med. 2009;37:1935–1940. doi: 10.1097/CCM.0b013e3181a0022d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netzer G, Liu X, Harris AD, et al. Transfusion practice in the intensive care unit: a 10-year analysis. Transfusion. 2010;50:2125–2134. doi: 10.1111/j.1537-2995.2010.02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris AD, Carmeli Y, Samore MH, et al. Impact of severity of illness bias and control group misclassification bias in case-control studies of antimicrobial-resistant organisms. Infect Control Hosp Epidemiol. 2005;26:342–345. doi: 10.1086/502549. [DOI] [PubMed] [Google Scholar]
- 10.Parker AM, Liu X, Harris AD, et al. Respiratory Therapy Organizational Changes Are Associated With Increased Respiratory Care Utilization. Respir Care. 2012 doi: 10.4187/respcare.01562. [DOI] [PubMed] [Google Scholar]
- 11.Johnston JA, Yi M, Britto MT, et al. Importance of organ dysfunction in determining hospital outcomes in children. J Pediatr. 2004;144:595–601. doi: 10.1016/j.jpeds.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 12.Ben Abraham R, Torin A, Ono N, et al. Predictors of outcome in the pediatric intensive care units of children with malignancies. J Pediatr Hematol Oncol. 2002;24:23–26. doi: 10.1097/00043426-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Abraham R, Efrati O, Mishali D, et al. Predictors for mortality after prolonged mechanical ventilation after cardiac surgery in children. J Crit Care. 2002;17:235–239. doi: 10.1053/jcrc.2002.36760. [DOI] [PubMed] [Google Scholar]
- 14.Castellanos-Ortega A, Delgado-Rodriguez M, Llorca J, et al. A new prognostic scoring system for meningococcal septic shock in children. Comparison with three other scoring systems. Intensive Care Med. 2002;28:341–351. doi: 10.1007/s00134-001-1196-z. [DOI] [PubMed] [Google Scholar]
- 15.Duke TD, Butt W, South M. Predictors of mortality and multiple organ failure in children with sepsis. Intensive Care Med. 1997;23:684–692. doi: 10.1007/s001340050394. [DOI] [PubMed] [Google Scholar]
- 16.Dursun O, Hazar V, Karasu GT, et al. Prognostic factors in pediatric cancer patients admitted to the pediatric intensive care unit. J Pediatr Hematol Oncol. 2009;31:481–484. doi: 10.1097/MPH.0b013e3181a330ef. [DOI] [PubMed] [Google Scholar]
- 17.Graciano AL, Balko JA, Rahn DS, et al. The Pediatric Multiple Organ Dysfunction Score (P-MODS): development and validation of an objective scale to measure the severity of multiple organ dysfunction in critically ill children. Crit Care Med. 2005;33:1484–1491. doi: 10.1097/01.ccm.0000170943.23633.47. [DOI] [PubMed] [Google Scholar]
- 18.Jung J, Eo E, Ahn K, et al. Initial base deficit as predictors for mortality and transfusion requirement in the severe pediatric trauma except brain injury. Pediatr Emerg Care. 2009;25:579–581. doi: 10.1097/PEC.0b013e3181b9b38a. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix J, Cotting J. Severity of illness and organ dysfunction scoring in children. Pediatr Crit Care Med. 2005;6:S126–S134. doi: 10.1097/01.PCC.0000161287.61028.D4. [DOI] [PubMed] [Google Scholar]
- 20.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Proulx F, Joyal JS, Mariscalo M, et al. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10:12–22. doi: 10.1097/PCC.0b013e31819370a9. [DOI] [PubMed] [Google Scholar]
- 22.Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172:206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 23.Rosner B. Fundamentals of Biostatistics. 4. Belmont (CA): Duxbury Press; 1995. [Google Scholar]
- 24.Fleiss JL. Statistical Methods for Rates and Proportions. 2. New York: Wiley; 1981. [Google Scholar]
- 25.Greene W. Econometric Analysis. Upper Saddle River (NJ): Prentice Hall; 2003. [Google Scholar]
- 26.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 27.Fisher R. The Design of Experiments. 6. Edinburgh, Oliver and Boyd; 1953. [Google Scholar]
- 28.Randolph AG, Lacroix J. Randomized clinical trials in pediatric critical care: Rarely done but desperately needed. Pediatr Crit Care Med. 2002;3:102–106. doi: 10.1097/00130478-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Pham JC, Catlett CL, Berenholtz SM, et al. Change in use of allogeneic red blood cell transfusions among surgical patients. J Am Coll Surg. 2008;207:352–359. doi: 10.1016/j.jamcollsurg.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy DJ, Needham DM, Berenholtz S, et al. red blood cell transfusions for critically ill patients: Has evidence changed practice? Am J Respir Crit Care Med. 2011;183:A2492. doi: 10.1097/CCM.0b013e31828e9a49. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez Perez ER, Winters JL, Gajic O. The addition of decision support into computerized physician order entry reduces red blood cell transfusion resource utilization in the intensive care unit. Am J Hematol. 2007;82:631–633. doi: 10.1002/ajh.20888. [DOI] [PubMed] [Google Scholar]
- 32.Tinmouth A, Macdougall L, Fergusson D, et al. Reducing the amount of blood transfused: a systematic review of behavioral interventions to change physicians’ transfusion practices. Arch Intern Med. 2005;165:845–852. doi: 10.1001/archinte.165.8.845. [DOI] [PubMed] [Google Scholar]
- 33.Armano R, Gauvin F, Ducruet T, et al. Determinants of red blood cell transfusions in a pediatric critical care unit: a prospective, descriptive epidemiological study. Crit Care Med. 2005;33:2637–2644. doi: 10.1097/01.ccm.0000185645.84802.73. [DOI] [PubMed] [Google Scholar]
- 34.Slonim AD, Joseph JG, Turenne WM, et al. Blood transfusions in children: a multi-institutional analysis of practices and complications. Transfusion. 2008;48:73–80. doi: 10.1111/j.1537-2995.2007.01484.x. [DOI] [PubMed] [Google Scholar]
- 35.Laverdiere C, Gauvin F, Hebert PL, et al. Survey on transfusion practices of pediatric intensivists. Pediatr Crit Care Med. 2002;3:335–340. doi: 10.1097/00130478-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Desmet L, Lacroix J. Transfusion in pediatrics. Crit Care Clin. 2004;20:299–311. doi: 10.1016/S0749-0704(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 37.Istaphanous GK, Wheeler DS, Lisco SJ, et al. Red blood cell transfusion in critically ill children: a narrative review. Pediatr Crit Care Med. 2011;12:174–183. doi: 10.1097/PCC.0b013e3181e30d09. [DOI] [PubMed] [Google Scholar]
- 38.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 39.Willems A, Harrington K, Lacroix J, et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med. 2010;38(2):649–56. doi: 10.1097/CCM.0b013e3181bc816c. [DOI] [PubMed] [Google Scholar]
- 40.Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest. 2005;127:295–307. doi: 10.1378/chest.127.1.295. [DOI] [PubMed] [Google Scholar]
- 41.Church GD, Matthay M, Liu K, et al. Blood product transfusions and clinical outcomes in pediatric patients with acute lung injury. Pediatr Crit Care Med. 2009;10:297–302. doi: 10.1097/PCC.0b013e3181988952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kneyber MC, Hersi MI, Twisk JW, et al. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007;33:1414–1422. doi: 10.1007/s00134-007-0741-9. [DOI] [PubMed] [Google Scholar]
- 43.Goodman AM, Pollack MM, Patel KM, et al. Pediatric red blood cell transfusions increase resource use. J Pediatr. 2003;142:123–127. doi: 10.1067/mpd.2003.14. [DOI] [PubMed] [Google Scholar]
- 44.Crosby ET. Perioperative haemotherapy: I. Indications for blood component transfusion. Can J Anaesth. 1992;39:695–707. doi: 10.1007/BF03008233. [DOI] [PubMed] [Google Scholar]
- 45.Luchtman-Jones L, Schwartz AL. Blood. In: Oldham KT, Colombani PM, Foglia RD, editors. Surgery of Infants and Children: Scientific Principles and Practice. Philadelphia: Lippincott-Raven; 1997. p. 316. [Google Scholar]
- 46.Kevy SV, Gorlin JB. Red cell transfusion. In: Nathan DG, Orkin SH, editors. Nathan and Oski’s Hematology of Infancy and Childhood. Philadelphia: WB Saunders; 1998. pp. 1786–1787. [Google Scholar]
- 47.Davis PJ, Sarner JB. Organ system considerations that affect anesthetic management. In: Fuhrman BP, Zimmermann JJ, editors. Pediatric Critical Care. 2. St Louis: Mosby; 1998. p. 1326. [Google Scholar]
- 48.Lanzowski PE. Manual of Pediatric Hematology and Oncology. San Diego: Academic Press; 1999. Supportive Care Management of Oncologic Emergencies; p. 692. [Google Scholar]
- 49.Camboulives J. Fluid, Transfusion, and Blood Sparing Techniques. In: Bisonette B, Dalens BJ, editors. Pediatric Anesthesia: Prinicples and Practice. USA: McGraw Hill; 2002. p. 588. [Google Scholar]
- 50.Strauss RG. Red Blood Cell Transfusions and Erythropoietin Therapy. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson Textbook of Pediatrics. 17. Philadelphia: Saunders; 2004. p. 1647. [Google Scholar]