Abstract
Women with normal glucose tolerance pre-gravid and developing gestational diabetes in late gestation have subclinical metabolic dysfunction prior to conception compared with women with normal glucose tolerance. Because of the 60 % decrease in insulin sensitivity with normal pregnancy, these women develop clinical hyperglycaemia/gestational diabetes in late gestation. The metabolic dysfunction includes impaired insulin response, decreased hepatic suppression of glucose production during insulin infusion and decreased insulin-stimulated glucose uptake in skeletal muscle, i.e. peripheral insulin resistance. The insulin resistance in normal glucose tolerance pregnancy is related to a decrease in the post-receptor insulin signalling cascade, specifically decreased insulin receptor substrate 1 tyrosine phosphorylation. In women with normal glucose tolerance this is reversed post-partum. In contrast, in gestational diabetes, in addition to the decrease in insulin receptor substrate 1 tyrosine phosphorylation, there is an additional decrease in tyrosine phosphorylation of the intracellular portion of the insulin receptor that is not related to the insulin receptor protein content. Post-partum women with gestational diabetes, who had retention of gestational weight gain, had no significant improvement in insulin sensitivity and increased inflammation expressed as increased plasma and skeletal muscle tumour necrosis factor alpha. The increased inflammation or meta-inflammation is a hallmark of obesity and during pregnancy develops in both white adipose tissue and placenta. Last gene array studies of placenta were associated with alterations in gene expression relating primarily to lipid in contrast to glucose metabolic pathways in gestational diabetes compared with Type 1 diabetes. Future studies are directed at decreasing inflammation prior to and during pregnancy using various lifestyle and nutritional interventions.
Introduction
The purpose of this review is to describe the development of the pathophysiology of gestational diabetes and potential treatment options resulting from my collaboration with a number of investigators. I had the great fortune of training in an academic environment during my residency in Obstetrics and Gynecology and fellowship in Maternal Fetal Medicine at the University of Vermont. Although initial attempts at research were directed at fetal physiology using the pregnant ewe model, I was given the opportunity, by my Chair Leon Mann MD and Research Director James Clapp MD, to pursue my interest in the pathophysiology of gestational diabetes with Ethan Allen Sims MD in the Metabolic Unit in the Clinical Research Unit at the University of Vermont. Dr. Sims introduced me to metabolic clinical research in the human using techniques such as body composition, indirect colorimetry, intravenous glucose tolerance tests, oral glucose tolerance tests and hy7perinsulinaemic euglycaemic clamps.
Insulin sensitivity, insulin response, body composition and energy expenditure before and during pregnancy
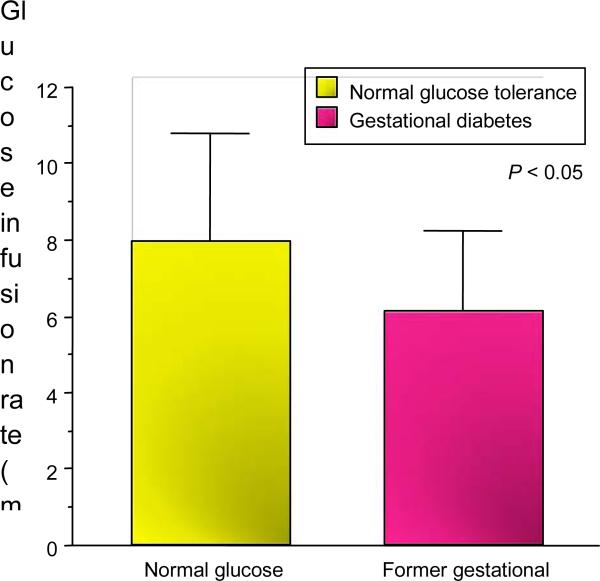
Our initial studies reported that women with a history of gestational diabetes had evidence of decreased insulin sensitivity using the hyperinsulinaemic euglycaemic clamp in comparison with a matched body composition group of women with normal glucose tolerance during pregnancy (Fig. 1) [1]. The hyperinsulinaemic euglycaemic clamp quantifies the amount of glucose required to maintain euglycaemia (for the purposes of our studies 90 mg/dl) during a 2-h steady state period of insulin infusion (40 mU/m2). Insulin sensitivity is expressed as the glucose infusion rate in mg/kg.fat free mass/min. The clamp technique remains the gold standard of estimating insulin resistance [2].
FIGURE 1.
Glucose infusion rate during hyperinsulinaemic– euglycaemic clamp (mg/kg FFM/min) in non-pregnant women with a previous history of gestational diabetes mellitus and normal glucose tolerance during pregnancy [1].
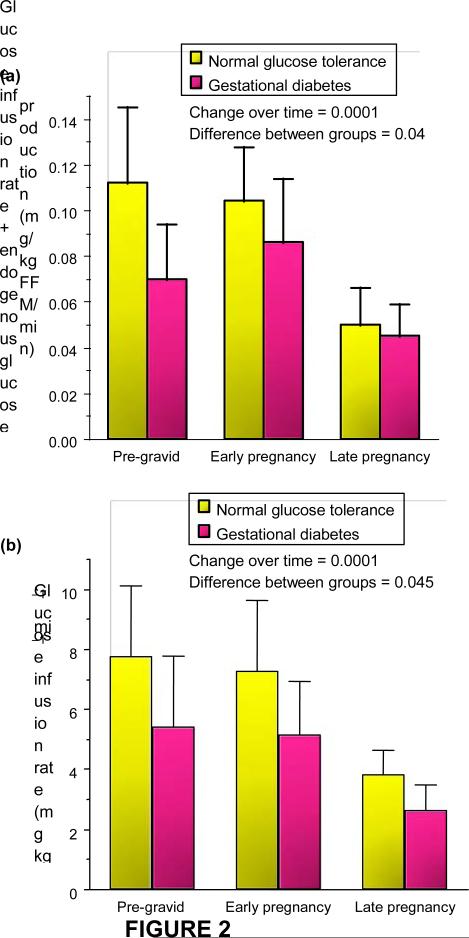
Based on these results, we developed a series of studies to estimate the longitudinal changes in peripheral insulin sensitivity (primarily skeletal muscle) in lean and obese women with normal glucose tolerance and gestational diabetes prior to a planned pregnancy, then in early (12– 14 weeks’ gestation) and late gestation (34–36 weeks) [3,4]. These metabolic research techniques are safe and reproducible in pregnant women. Our results showed that during pregnancy there is a uniform 50–60% decrease in insulin sensitivity with advancing gestation in both normal glucose tolerance and gestational diabetes. The significant decreases in insulin sensitivity in late gestation observed in women with gestational diabetes in comparison with a matched control group are a reflection of the decreased insulin sensitivity that exists prior to pregnancy (Fig. 2). While the changes in insulin sensitivity in late pregnancy are uniform and predictable, there can either be increases or decreases in insulin sensitivity in early pregnancy in comparison with pre-gravid measures. Only in later gestation are there the constant decreases in insulin sensitivity.
FIGURE 2.
Longitudinal changes in peripheral insulin sensitivity in ( a ) lean women and (b) obese women as indicated by infusion of glucose required to maintain euglycaemia (90 mg/dl) + endogenous glucose production during insulin infusion (mean SD). Reproduced from (a) Catalano et al. (1993) [3], with permission from the American Physiological Society, and (b) Catalano et al. (1999) [4], with permission from Elsevier.
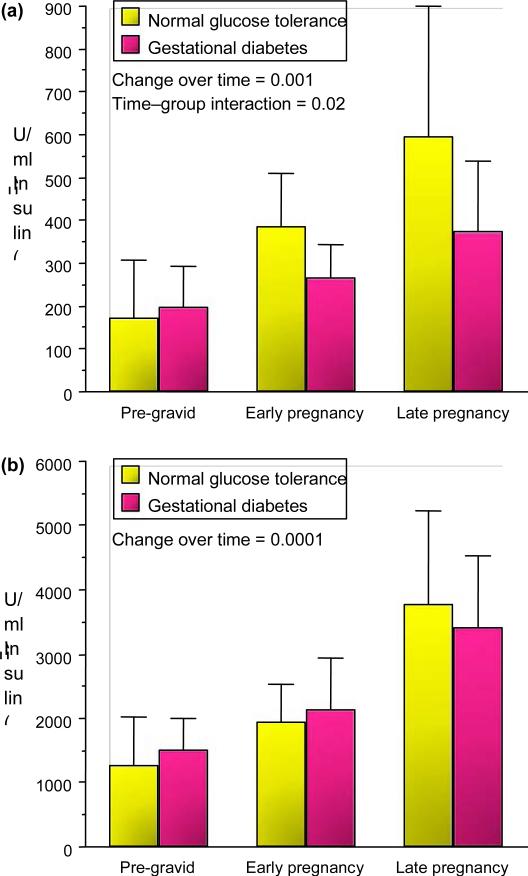
The changes in insulin sensitivity from baseline/pre-gravid through early pregnancy in lean women are inversely related to changes in maternal fat mass [5]. The mechanisms, however, are not yet well defined. Another interesting feature of early pregnancy metabolism is the significant increases in insulin response as estimated during an intravenous glucose tolerance test in all subjects. The increases in insulin response occur regardless of the changes in insulin sensitivity (Figs 3 and 4) [3,4]. Again the mechanisms are not yet well characterized but may account for decreases in insulin requirements observed in some women with well-controlled pre-existing Type 2 diabetes. In lean women there is a significant increase in insulin response throughout pregnancy, but a significant interaction in first-phase insulin response in between normal glucose tolerance and gestational diabetes (Fig. 3). In obese women, while there is a significant increase in first- and second-phase insulin response over time in both groups, women with gestational diabetes have a significant increase in second insulin response in comparison with normal glucose tolerance (Fig. 4). This relates to the severe β-cell stress in obese gestational diabetes placing them at increased risk for later b- cell dysfunction and Type 2 diabetes.
FIGURE 3.
Longitudinal changes in (a) first-phase and (b) second-phase insulin response during intravenous glucose tolerance test in lean normal glucose tolerance and gestational diabetes (mean SD). (b) Reproduced from Catalano et al. (1993) [3], with permission from Elsevier.
FIGURE 4.
Longitudinal changes in (a) first-phase insulin response during intravenous glucose tolerance test in obese normal glucose tolerance and gestational diabetes and (b) second-phase insulin response during intravenous glucose tolerance test (mean SD). (b) Reproduced from Catalano et al. (1999) [4] with permission from Elsevier.
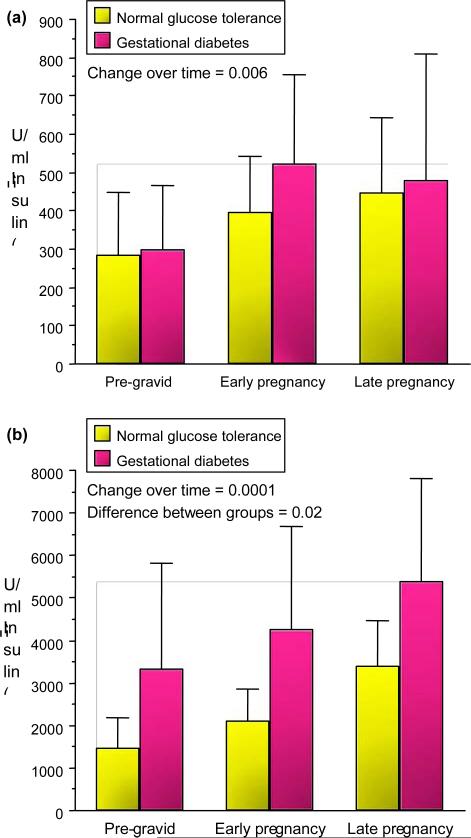
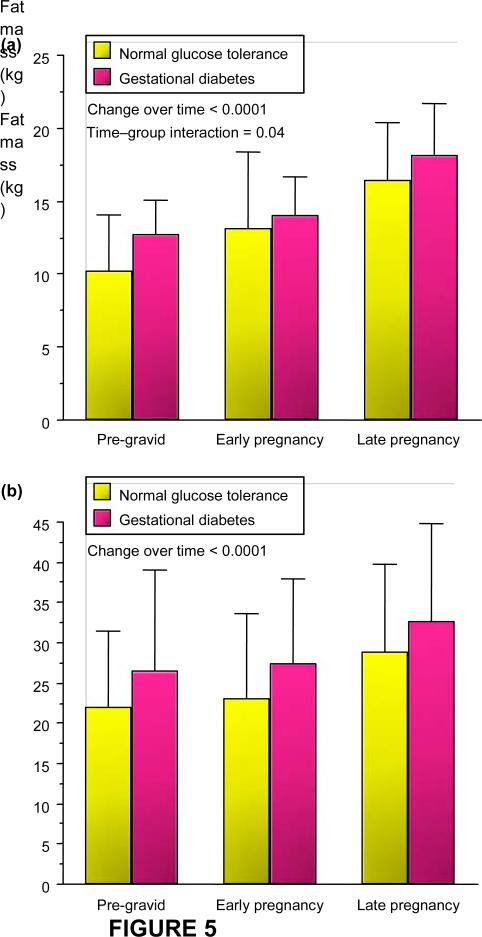
Maternal body composition during these studies was estimated using hydrodensitometry (underwater weighing) with correction for residual lung volume [6,7]. There was a significant increase in fat mass in both lean and obese normal glucose tolerance and gestational diabetes over time (Fig. 5). The increases in fat mass in early pregnancy were more apparent in lean women with normal glucose tolerance as compared with those developing gestational diabetes, most likely related to the significant decrease in insulin sensitivity in early pregnancy in these women. There was a wide distribution of fat mass accretion in obese subjects, ranging from 2.0 to 13.1 kg.
FIGURE 5.
Longitudinal changes in fat mass in (a) lean and (b) obese normal glucose tolerance and gestational diabetes pre-gravid, in early pregnancy and in late pregnancy (mean SD). Reproduced from (a) Catalano et al. (1998) [5] and (b) Okereke et al. (2004) [8] with permission from Elsevier and the American Physiological Society.
Indirect calorimetry was used to assess basal or resting energy expenditure, carbohydrate and lipid metabolism (oxidation rates) in women with normal glucose tolerance and gestational diabetes [5,8]. There was a significant 30% increase in resting energy expenditure (kcal/day) during pregnancy in normal glucose tolerance and gestational diabetes. Basal non-protein respiratory quotient increased over time in both groups from 0.84 pre-gravid to 0.88 in late gestation, but this did not reach statistical significance. There was a significant 55–80% increase in basal carbohydrate oxidation in lean gestational diabetes and normal glucose tolerance, respectively, over the duration of pregnancy, but no significant difference in either group in fat oxidation over time.
In obese women there was also a significant 30% increase in resting energy expenditure (kcal/day) during gestation. In contrast to the lean normal glucose tolerance and gestational diabetes, there was no significant increase in basal carbohydrate oxidation over time in obese women; there was, however, a significant 52–81% increase in basal fat oxidation with advancing gestation.
Endogenous glucose production and suppression during pregnancy
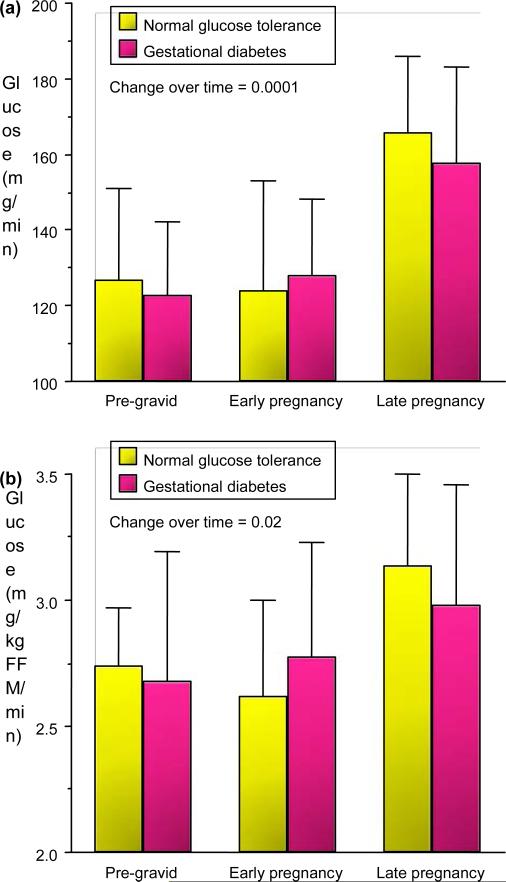
In collaboration with Dr Satish Kalhan MD, we conducted a series of experiments using stable isotopes of glucose (6,6-2H2 glucose) to estimate endogenous, primarily hepatic glucose production and suppression during insulin infusion during our clamp studies [3,4]. In lean women there was a significant 30% increase in hepatic glucose production (mg/min) by late pregnancy in women with normal glucose tolerance and gestational diabetes, but no significant differences between groups (Fig. 6). When expressed in mg kg fat-free mass/min there remained a significant 15% increase in hepatic glucose production in both normal glucose tolerance and gestational diabetes, but again no difference between groups. During the insulin infusion with the clamp procedure there was less suppression (80%) of hepatic glucose production in the gestational diabetes compared with the normal glucose tolerance (95%) in late gestation.
FIGURE 6.
(a) Longitudinal changes in total basal endogenous glucose production (mean SD) in lean normal glucose tolerance and gestational diabetes. (b) Longitudinal changes in total basal endogenous glucose production expressed in mg/kg fat-free mass1 min1 (mean SD). (b) Reproduced from Catalano et al. (1993) [3] with permission from Elsevier.
In obese subjects, there was a similar significant 30 % increase in basal hepatic glucose production in normal glucose tolerance and gestational diabetes by late gestation. During the clamp, however, there was a significant decrease in suppression of hepatic glucose production in both obese groups, but less suppression in the group with gestational diabetes (85%) than in the group with normal glucose tolerance (93%). These data indicate evidence of decreased basal hepatic insulin sensitivity in both normal glucose tolerance and gestational diabetes with advancing gestation. During insulin infusion there is less suppression (insulin resistance) in obese normal glucose tolerance compared with lean normal glucose tolerance, and a further decrease in suppression in obese gestational diabetes in contrast to obese normal glucose tolerance.
Post-receptor insulin signalling defects in normal glucose tolerance and gestational diabetes in late pregnancy and 1 year post-partum
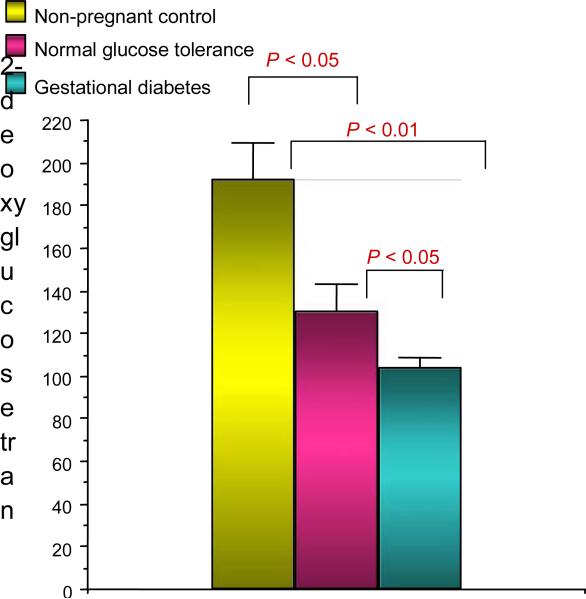
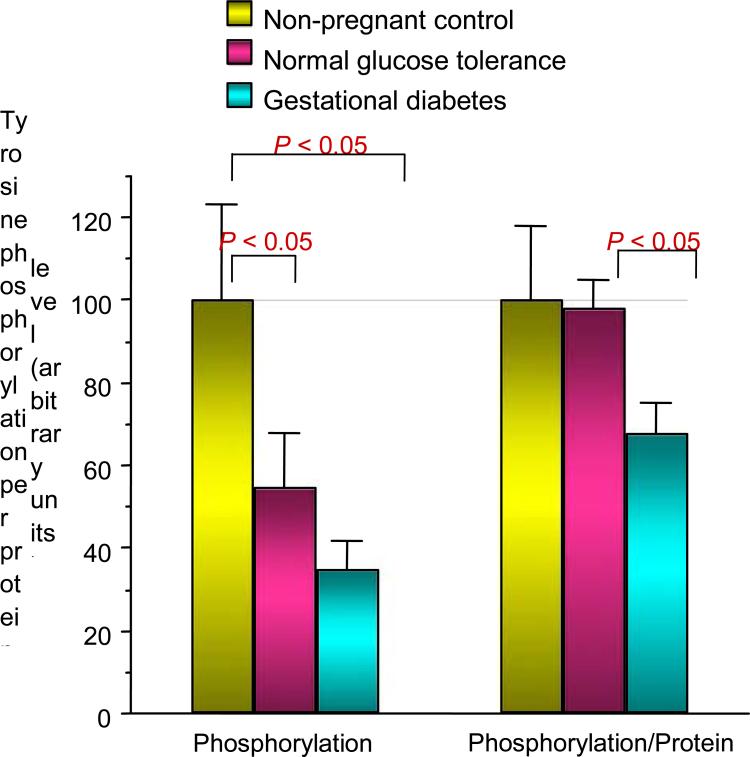
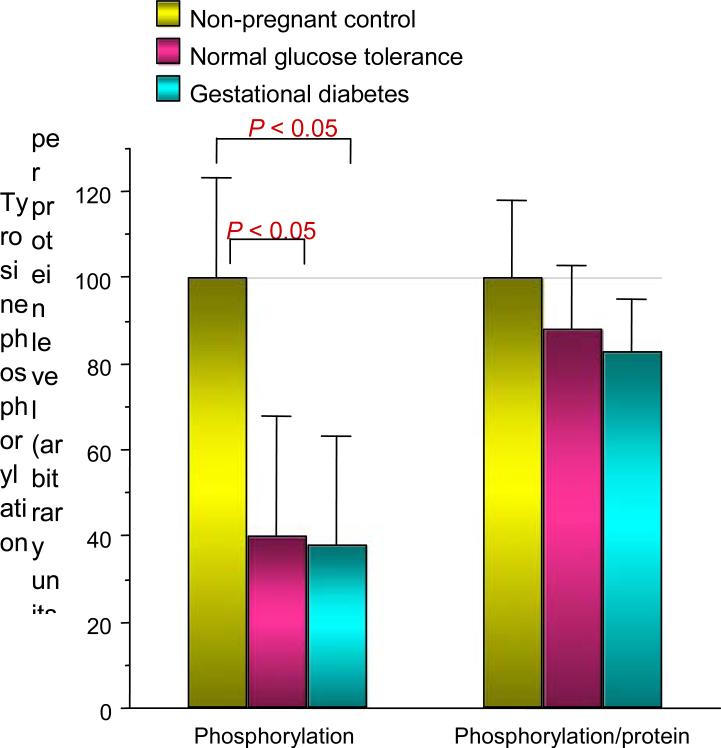
In an effort to understand the mechanisms relating to decreased skeletal muscle glucose uptake in pregnancy, we conducted a series of experiments in collaboration with Jacob Friedman PhD [9]. At the time of scheduled Caesarean delivery, rectus abdominis muscle biopsies were obtained from women with normal glucose tolerance and gestational diabetes. The subjects were matched for age and obesity. In vitro glucose uptake in skeletal muscle was estimated using 2-deoxyglucose uptake [3H-2-deoxyglucose). The rate of maximal insulin (10–7 mol/l) stimulated 2-deoxyglucose transport was significantly reduced by 32% in the normal glucose tolerance pregnant women compared with nonpregnant control subjects and even further (54%) in women with gestational diabetes comparison with normal glucose tolerance (Fig. 7). The maximal effect of insulin on tyrosine phosphorylation on the insulin receptor was significantly 37% lower in gestational diabetes compared with normal glucose tolerance. This was not related to changes in abundance of the insulin receptor (Fig. 8). Compared with non-pregnant control subjects, maximal insulin-stimulated insulin receptor substrate 1 (IRS-1) tyrosine phosphorylation was significantly lower by a mean 59% in normal glucose tolerance and 62% in gestational diabetes. This was related to a 23% and 44% decrease in IRS-1 protein content in normal glucose tolerance and gestational diabetes, respectively (Fig. 9). Both normal glucose tolerance and gestational diabetes had a significant 1.5- to 2.0-fold increase in IRS-2 and the p85α regulatory subunit of the phosphatidylinositol (PI) 3-kinase, despite decreased in vitro glucose transport. In summary, insulin resistance to glucose skeletal muscle transport in normal glucose tolerance is associated with a decrease in IRS-1 tyrosine phosphorylation, primarily because of a decreased expression of IRS-1 protein. In contrast, in women with gestational diabetes there is an additional decrease in tyrosine phosphorylation of the insulin receptor, which is associated with a further decrease in in vitro glucose transport.
FIGURE 7.
Insulin effect on in vitro glucose uptake in rectus abdominus skeletal muscle. Reproduced from Friedman et al. (1999) [9], with permission from the American Diabetes Association.
FIGURE 8.
Insulin effect on tyrosine phosphorylation of insulin receptor is decreased in normal glucose tolerance and gestational diabetes. When adjusted for protein content, the tyrosine phosphorylation was significantly decreased in gestational diabetes. Reproduced from Friedman et al. (1999) [9], with permission from the American Diabetes Association.
FIGURE 9.
Tyrosine phosphorylation of insulin receptor substrate 1 (IRS-1) is decreased in normal glucose tolerance and gestational diabetes but not after normalizing for IRS-1 protein content. Reproduced from Friedman et al. (1999) [9], with permission from the American Diabetes Association.
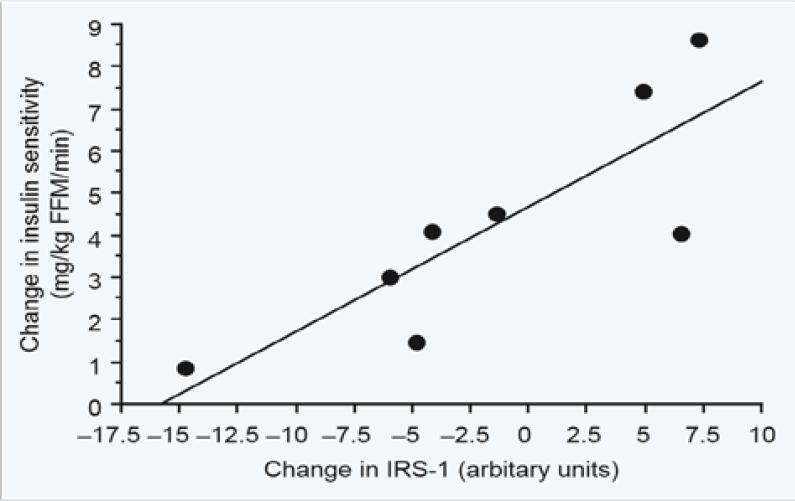
We next conducted a series of longitudinal studies to determine the alterations in insulin resistance and post-receptor insulin signalling in women with normal glucose tolerance and gestational diabetes in the late pregnancy and 1 year post-delivery. In addition to Jacob Friedman, John Kirwan PhD, a former faculty member in our department was the lead collaborator on these studies. The studies were conducted in non-obese normal glucose tolerance in late pregnancy (30–36 weeks’ gestation) and approximately 1 year post-partum [10]. Insulin sensitivity was estimated using the hyperinsulinaemic euglycaemic clamp as described previously. Punch biopsies of the vastus lateralis muscle were obtained in the basal state using a Bergstrom needle biopsy procedure prior to each clamp. Insulin sensitivity improved 74% from late pregnancy to 1 year post-partum. Skeletal muscle insulin receptor significantly increased 42%. However, in vitro studies showed that, when adjusted for insulin receptor concentration, maximal insulin receptor tyrosine phosphorylation and insulin receptor tyrosine kinase activity were unchanged. There was a 69% increase in IRS-1 protein expression and 55% decrease in the p85α regulatory subunit of the phosphatidylinositol (PI) 3-kinase. The change in insulin sensitivity from late pregnancy to 1 year post-partum was significantly correlated with the corresponding change in IRS-1 protein (Fig. 10). These data suggest that the reversal or insulin resistance in lean normal glucose tolerance is associated with an increase in IRS-1 and down-regulation of the p85 α regulatory subunit of the phosphatidylinositol (PI) 3-kinase and not insulin receptor tyrosine kinase activity. A similar study was conducted in gestational diabetes using the identical protocol as described previously [11]. There was no significant improvement in insulin sensitivity in the gestational diabetes. Body weight, fat mass, fasting glucose and plasma tumour necrosis factor alpha (TNF-α remained more elevated 1 year post-partum than was seen in the subjects with normal glucose tolerance. Skeletal muscle TNF-α mRNA was elevated 5- to 6-fold in gestational diabetes and remained higher 1 year post-partum. The levels of insulin receptor, IRS-1 and p85α improved post-partum. Insulin-stimulated insulin receptor tyrosine kinase phosphorylation and receptor tyrosine kinase activity did not significantly improve post-partum in the gestational diabetes. The levels of 312SER-IRS-1 also did not improve and correlated with TNF-α mRNA, consistent with meta-inflammation in the skeletal muscle. In summary, these series of experiments suggest that the chronic insulin resistance in gestational diabetes may be related to inflammation affecting the post-receptor insulin signalling cascade. Our group had previously reported that maternal TNF-α was the factor most strongly correlated with the decrease in insulin sensitivity during pregnancy [12]. Our data are consistent with what has been previously reported in the non-pregnancy literature [13]. These data also highlight the importance of retention of gestational weight post-partum as a risk factor for later metabolic dysfunction.
Figure 10.
Correlation between the change in insulin sensitivity and insulin receptor substrate 1 (IRS-1) protein in skeletal muscle from late pregnancy to 1 year post-partum in lean normal glucose tolerance, r = 0.84, P < 0.007. Reproduced from Kirwan et al. [10], with permission from the Endocrine Society.
Meta-inflammation in adipose tissue and placenta
The next series of studies was performed examining the role of inflammation in white adipose tissue and placenta as primary sites of inflammation during pregnancy resulting in decreased insulin sensitivity. Sylvie Hauguel-de Mouzon PhD was the lead investigator in these studies. During the last decade, white adipose tissue has been recognized as more than simply a storage depot for lipids. White adipose tissue is an active endocrine organ secreting adipokines and cytokines such as leptin, adiponectin, TNF-α, interleukin 6 (IL-6) and many others. With the exception of adiponectin, a marker of increased insulin sensitivity, the placenta has a similar secretory profile of cytokine gene expression and proteins as white adipose tissue [14,15]. In a series of studies, it was determined that the number of CD68+ and CD14+ macrophages were increased 2- to 3-fold in the placenta of obese compared with lean women [16]. The macrophage population was characterized by increased expression of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1. The local inflammatory changes were associated with higher plasma concentrations of C-reactive protein and IL-6 in obese compared with lean women. Of interest, the source of the CD14+ macrophages in placenta was confirmed to be of maternal and not fetal origin [17].
In summary, maternal obesity as commonly associated with gestational diabetes in the USA is related to increased meta-inflammation in maternal white adipose tissue, plasma and placenta. The inflammation may be the primary mechanism relating to the increased insulin resistance observed in obese gestational diabetes.
Gestational diabetes a disorder of glucose alone or glucose and lipid metabolism
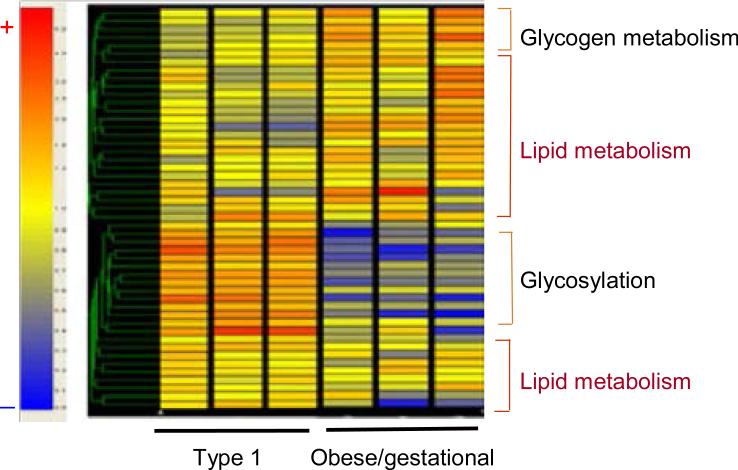
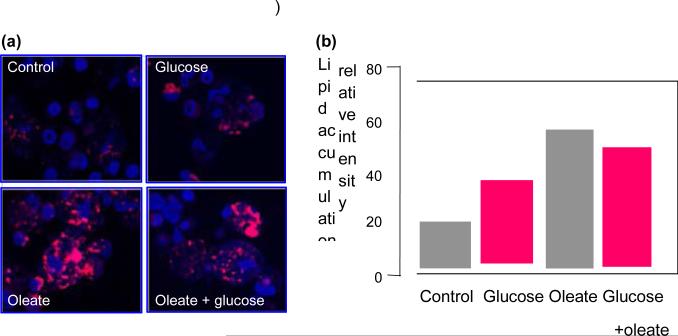
Jorgen Pedersen in the 1950s is generally given credit for the hyperglycaemia–hyperinsulinaemia hypothesis. The hypothesis as stated by Pedersen is as follows ‘Maternal hyperglycaemia results in fetal hyperglycaemia and, hence, in hypertrophy of fetal islet tissue with insulin hypersecretion. This again means a greater utilization of glucose. This phenomenon will explain several abnormal changes found in the newborn’. The most common structural change associated with the Pedersen hypothesis is fetal overgrowth or macrosomia. When Pedersen was caring for women with diabetes in pregnancy, the vast majority of his patients had Type 1 diabetes and were not obese. Over the last two decades there has been a significant increase in obesity not only in developed countries but in the developing world as well. This increase in obesity is associated with an increased risk of pre-existing Type 2 diabetes and gestational diabetes. As obesity is associated not only with hyperglycaemia but hyperlipidaemia, our group began examining the potential role of placental lipids in women with diabetes [18]. Dr Hauguel-de Mouzon initiated a series of studies examining gene expression in the placenta of women with Type 1 diabetes and gestational diabetes [19]. Diabetes was associated with alterations in gene expression in key steps in placental energy metabolism, with 67% of alterations related to lipid pathways and 9% to glucose pathways (Fig. 11). Preferential activation of lipid genes was observed in gestational diabetes pregnancies, whereas Type 1 diabetes induced fewer lipid modifications but enhanced expression of glycosylation and acylation pathways. Long-chain saturated palmitic acid was three times more efficient than glucose to enhance expression of genes for fatty acid esterification and formation of lipid droplets in cultured placental cells (Fig. 12). These data suggest that, in obese gestational diabetes, lipids may provide additional substrates for fetal lipid synthesis. These observations correlate well with a secondary analysis of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) data showing an independent effect of maternal obesity and glucose on excessive fetal growth [20].
FIGURE 11.
Unsupervised hierarchical clustering shows expression data for the metabolic genes that are modified in pregnancy with gestational diabetes mellitus and Type 1 diabetes mellitus. The prominent clusters that are shown on the right are based on the putative functional characteristics of the genes. The colour tag and intensity represent the expression level from lowest (blue) to highest (red). Data are shown from six representative arrays that were run in duplicate. Reproduced from Radaelli et al. (2009) [19], with permission from Elsevier.
FIGURE 12.
(a) A visulization of lipid droplets in primary term placental cells. Freshly isolated trophoblast cells were cultured for 48 h with no addition (control) or the addition of glucose (10 mmol/l), oleate (400 nmol/l) or the combination of both. (b) The quantification of lipid accumulation by scanning densitometry. Results are given as mean SE of 4–6 independent experiments with duplicate culture wells. *Probability value of < 0.0001. Reproduced from Radaelli et al. (2009) [19], with permission from Elsevier.
Future directions
Based on our previous research, we hypothesize that the metabolic dysfunction in obese women and those at risk for gestational diabetes began not in the third trimester when the clinical diagnosis of gestational diabetes is usually made, but the pathophysiology is initiated in the first trimester. The decreased insulin resistance and accompanying increased insulin response in obese women and those developing gestational diabetes is already present in the first trimester. Based on work by Gernot Desoye PhD and others there is evidence that the placenta in early pregnancy expresses insulin receptors [21–23]. Preliminary in vitro work by Dr Hauguel-de Mouzon suggests that there is evidence of insulin effecting placental gene expression of lipid metabolism/ transport and cytokines as early as 8–12 weeks’ gestation. Hence, efforts to mitigate the adverse effects of maternal metabolism on feto-placental function and growth would by necessity need to begin in early gestation. Our group is currently in the process of developing a lifestyle intervention trial in overweight and obese women to improve maternal metabolism before pregnancy in order to decrease maternal and neonatal short- and possibly long-term complications. Emilio Herrera PhD has collaborated with our group over the years in understanding the role of various lipids affecting maternal metabolism. As discussed previously, the increased insulin resistance in pregnancy is associated with cytokine disrupting the insulin signalling cascade. Saturated fatty acids in addition to cytokines bind to the Toll-like receptor 4 (TLR4) activating nuclear factor kappa B (NFjB), resulting in increased cytokine production [24]. In contrast, polyunsaturated fatty acids, as found in fish oil, are excellent anti-inflammatory lipids, which decrease inflammation and increasing adiponectin. Based on these findings, we have just completed a pilot randomized controlled trial examining the role omega x-3 poly-unsaturated fatty acid supplementation in overweight and obese women to improve inflammation and insulin sensitivity and decrease fetal adiposity.
Summary
Although gestational diabetes is most often diagnosed in late gestation, the seeds of metabolic dysfunction are planted well before conception and possibly, based on the Barker hypothesis, when the women herself was developing in utero. Maternal insulin resistance, as seen in many women developing gestational diabetes in the USA, is related to the metabolic syndrome of obesity, inflammation, insulin resistance resulting hyperglycaemia and hyperinsulinaemia. Because of the 60% decrease in insulin sensitivity during gestation, the predisposing baseline insulin resistance is further exacerbated and, when associated with β-cell dysfunction, results in mild hyperglycaemia, which we refer to as gestational diabetes. This is the clinical diagnosis.
But understanding the pathophysiology involved, we now recognize that hyperlipidaemia is a contributing factor not only for fetal overgrowth/adiposity, but also depending on the type of dietary fat, saturated fatty acids or polyunsaturated fatty acids diet, may have a potential detrimental effect through enhancing cytokine expression and resulting insulin resistance. Various dietary recommendations, avoidance of excessive gestational weight gain and a healthy lifestyle of diet and exercise may be beneficial to overall health during pregnancy in women at risk for gestational diabetes. However, many of these practices are often initiated well after the first trimester, when the placenta and other maternal tissues may already be programmed. Just as the women with pre-existing diabetes and high glucose concentrations increases her risk of having a fetus with structural congenital anomalies, so too does the overweight/obese women with subclinical metabolic dysfunction at risk for gestational diabetes. As coined by Freinkel, the term ‘fuel-mediated teratogenesis’ of pregnancy in overweight/obese women may be more subtle on the developing fetus, but the effects of metabolic dysfunction may last a shortened lifetime with increased morbidity [25]. Therefore, achieving optimal metabolic health before a planned pregnancy may offer the best, and not necessarily the easiest, option to achieve a health pregnancy outcome short term, and possibly long term as well.
Acknowledgments
Funding sources
The Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research; and HD 22965-19 (PMC) and does not necessarily represent the official views of the NICHD or NIH.
Footnotes
This manuscript is a review based on my presentation of the Jorgen Pedersen Lecture at the 43rd Diabetes in Pregnancy Group (DPSG) of the European Association for the Study of Diabetes (EASD) held on 22–25 September 2011 at Queens’ College, Cambridge University.
Competing interests None declared.
References
- 1.Catalano PM, Bernstein IM, Wolfe RR, Srikanta S, Tyzbir E, Sims EAH. Subclinical abnormalities of glucose metabolism in subjects with previous gestational diabetes. Am J Obstet Gynecol. 1986;155:1255–1262. doi: 10.1016/0002-9378(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo R, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264:E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 4.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obest Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 5.Catalano PM, Roman-Drago NM, Amini SB, Sims EAH. Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy. Am J Obstet Gynecol. 1998;179:156–165. doi: 10.1016/s0002-9378(98)70267-4. [DOI] [PubMed] [Google Scholar]
- 6.Goldman RF, Buskirk ER. A method for underwater weighing and the determination of body density. In: Brozek J, Herschel A, editors. Techniques for Measuring Body Composition. National Academy of Science; Washington: 1961. pp. 78–79. [Google Scholar]
- 7.Keys A, Brozek J. Body fat in adult man. Physiol Rev. 1953;33:245–325. doi: 10.1152/physrev.1953.33.3.245. [DOI] [PubMed] [Google Scholar]
- 8.Okereke NC, Huston-Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2004;287:E472–E479. doi: 10.1152/ajpendo.00589.2003. [DOI] [PubMed] [Google Scholar]
- 9.Friedman JE, Ishizuka T, Shao J, Huston L, Highman T, Catalano P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes. 1999;48:1807–1814. doi: 10.2337/diabetes.48.9.1807. [DOI] [PubMed] [Google Scholar]
- 10.Kirwan JP, Varastehpour A, Jing M, Presley L, Shao J, Friedman JE, et al. Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J Clin Endocrinol Metab. 2004;89:4678–4684. doi: 10.1210/jc.2004-0749. [DOI] [PubMed] [Google Scholar]
- 11.Friedman JE, Kirwan JP, Jing M, Presley L, Catalano PM. Increased skeletal muscle tumor necrosis factor-α and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes. 2008;57:606–613. doi: 10.2337/db07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirwan JP, Hauguel-de Mouzon S, Lepercq J, Challier J-C, Huston-Presley L, Friedman JB, et al. TNF-α is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1 mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 14.Radaelli T, Varastehpour A, Catalano P. Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52:2951–2958. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 15.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27:794–798. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;2:274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu S, Leahy P, Challier J-C, Minium J, Catalano P. Hauguel-de Mouzon S. Molecular phenotype of monocytes at the maternal-fetal interface. Am J Obstet Gynecol. 2011;205(265):e1–e8. doi: 10.1016/j.ajog.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catalano PM. Hauguel-de Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–487. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radaelli T, Leqercq J, Vasrastehpour A, Basu S, Catalano PM, Hauguel-de Mouzon S. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am J Obstet Gynecol. 2009;201:209, e1–e10. doi: 10.1016/j.ajog.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The Hyperglycemia and Adverse Pregnancy Outcome study. Diabetes Care. 2012;35:780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones CJ, Hartmann M, Blaschitz A, Desoye G. Ultrastructural localization of insulin receptors in human placenta. Am J Reprod Immunol. 1993;30:136–145. doi: 10.1111/j.1600-0897.1993.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 22.Desoye G, Hartmann M, Jones CJ, Wolf HJ, Kohnen G, Kosanke G, et al. Location of insulin receptors in the placenta and its progenitor tissues. Microsc Res Tech. 1997;38:36–75. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<63::AID-JEMT8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 23.Posner BI. Insulin receptors in human and animal placental tissue. Diabetes. 1974;23:209–217. doi: 10.2337/diab.23.3.209. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Basu S, Minium J, Catalano P, de-Mouzon Hauguel. Saturated but not unsaturated fatty acids induce TLR4 dependent placental inflammation.. American Diabetes Association 73rd Scientific Sessions; Chicago, IL. 21–25 June 2013. [Google Scholar]
- 25.Freinkel N. Banting lecture: of pregnancy and progeny. Diabetes. 1980;29:1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]