Abstract
OBJECTIVE
To study the pharmacokinetics of different betamethasone doses and preparations used to enhance fetal lung maturation in the maternal and fetal circulation of sheep and the adverse effects on fetal blood pressure.
METHODS
Doses of 170 (n = 6) and 110 µg/kg (n = 6) betamethasone phosphate equivalent to 12 or 8mg, respectively, administered to a 70kg pregnant woman or 170 µg/kg (n = 6) of a depot formulation (50% betamethasone phosphate and 50% betamethasone acetate) were injected intramuscularly to chronically instrumented pregnant sheep.
RESULTS
Both betamethasone preparations produced highest maternal concentrations after 15 min followed by an exponential decline with a t1/2 of about 3 hours. The drug fell below the limit of detection at 8 to 12 hours. Betamethasone was first detectable in the fetal circulation at 1 hour, peaked at 3 hours, and decreased below the limit of detection at 8 hours independently of the dose or preparation. Maternal and fetal betamethasone concentrations achieved with the phosphate and acetate formulation were one half of those obtained with betamethasone phosphate, suggesting that very little betamethasone is released from the acetate within the first 8 hours when the effect on lung maturation is needed. Betamethasone led to a maximal increase of mean fetal blood pressure from 42 ± 1 to 51 ± 1 mm Hg (P<.05) and did not differ between the doses and preparations, although plasma concentrations showed a clear dose–concentration relationship.
CONCLUSION
The doses of betamethasone used in obstetrics are supramaximal in terms of cardiovascular effects in sheep. Risk-benefit studies are needed to find the effective steroid dose with the least adverse effects.
Administration of synthetic glucocorticoids to women in premature labor has a clearly proven clinical benefit in decreasing the incidence of respiratory distress syndrome and intraventricular hemorrhage in the offspring if they are born prematurely.1 On the other hand, this treatment exposes the fetus to glucocorticoid concentrations higher than those that would normally be present in fetal blood at that stage of development. Fetal exposure at critical developmental stages to glucocorticoid concentrations that are inappropriate for that stage has been shown to have acute adverse effects on the central nervous2–6 and cardiovascular systems in the fetal sheep and baboon.7–11 In the cardiovascular system of sheep and nonhuman primates, synthetic glucocorticoids increase peripheral7–9,11 and cerebral vascular resistance,10 leading to increased mean fetal arterial blood pressure and decreased cerebral blood flow. Although the increased blood pressure may have harmful effects, the increase of cerebral vascular resistance may protect the brain from postasphyxic blood pressure peaks and intraventricular hemorrhage (Schwab M, Muller T, Loehle M, Coksaygan T, Antonow-Schlorke I, Wood CE, et al. Glucocorticoid induced maturation of cerebral autoregulation may explain the decreased incidence of intraventricular hemorrhage and increased risk of periventricular leukomalacia [abstract]. J Soc Gynecol Investig 2004;11 suppl: 355).
Although it has been shown in sheep that repeated treatments may be of benefit for the lung,12 to our knowledge there has never been a study designed to examine a broad range of doses at the clinical level to determine the dose of maternal glucocorticoid that is optimal for the effects on the fetus in rodents, sheep, nonhuman primates or humans (PubMed, January 1966–January 2006; no language restrictions; search terms: antenatal or prenatal; glucocorticoids, steroids or betamethasone; dose or pharmacokinetics; lung or blood pressure). Recommendations1 for dose and timing of treatments are mainly based on the study of Liggins and Howie13 who chose the dose for clinical trials partly from the doses used in experiments with sheep. Retrospective data collected on mothers who received only a partial course of steroids, however, suggests that any exposure to an appropriate corticosteroid has beneficial effects on postnatal outcome.14 The present study was designed to estimate the time course of maternal and fetal betamethasone concentrations after maternal exposure to two doses and two different betamethasone preparations frequently used clinically in women in premature labor. Secondly, we evaluated the fetal arterial blood pressure responses to the different betamethasone doses and preparations as an index of cardiovascular adverse effects. Fetal arterial blood pressure changes in their time course are a sensitive measure of the biological activity of glucocorticoids.7–11 We hypothesized that the clinical doses of betamethasone produce supramaximal effects on the fetal cardiovascular system as an indicator that the doses used clinically may also need evaluation in relation to lung maturation.
MATERIALS AND METHODS
Experimental procedures were approved by the Cornell University Animal Use and Care Committee and were performed in facilities approved by the American Association for the Accreditation of Laboratory Animal Care. Rambouillet-Colombia ewes bred on a single occasion and of known gestational age were acclimated to the animal facilities for at least 5 days before surgery and kept in rooms with controlled light and dark cycles (14 hours light and 10 hours dark: lights off at 2100 hours and lights on at 0700 hours). After food withdrawal for 24 hours, surgery was performed under isoflurane general anesthesia at 112 ± 1 days gestational age. Following 1g of ketamine (Ketaflo, Abbott, North Chicago, IL) intramuscular, anesthesia was induced by 4% isoflurane (Isoflo, Abbott) using a face mask. Ewes were intubated and anesthesia was maintained with 1.0–1.5% isoflurane in 100% oxygen. Briefly, ewes were instrumented with catheters inserted into the carotid artery for blood sampling and into the jugular vein for postoperative administration of antibiotics. Fetuses were instrumented with polyvinyl catheters (Tygon, Norton Performance Plastics; 0.1 mm inside diameter, 0.18 mm outside diameter) inserted into the left carotid artery and jugular vein. An additional catheter was placed in the amniotic cavity to record the amniotic pressure to permit correction of fetal arterial blood pressure. Stainless steel wire electrodes were implanted into the uterine body to record myometrial activity. All ewes and fetuses received 0.5 g ampicillin (AMP-Equine; SmithKline Beecham, West Chester, PA) intravenously and into the amniotic sac, respectively, twice a day during the first three postoperative days. One gram of phenylbutazone (Equiphene paste, Luitpold Pharmaceuticals, Shirley, NY) was administered orally to the ewe twice daily as an analgesic for at least three days. All catheters were maintained patent via a continuous infusion of heparin at 15 IU/mL in 0.9 % NaCl solution delivered at 0.5 mL/h.
After at least 3 days of postoperative recovery, ewes were allocated randomly at 117 ± 1 days gestational age to receive betamethasone phosphate, a fast releasing sodium salt (betamethasone phosphate; Celestan solubile, Essex, Munich, Germany), intramuscular at a dose of 170 µg/kg (n = 6) and 110 µg/kg (n = 6) equivalent to 12 and 8 mg betamethasone administered to a 70-kg woman. A third group of six animals received 170 µg/kg of a slow-release betamethasone preparation, consisting of 50% betamethasone phosphate and 50% betamethasone acetate (betamethasone phosphate acetate, Celestan Depot, Essex). Maternal and fetal betamethasone plasma concentrations were measured at 15 and 30 minutes and 1, 2, 3, 4, 6, 8, 12, and 24 hours after injection of 170 µg/kg betamethasone phosphate and betamethasone phosphate and acetate. Because fetal betamethasone plasma concentrations were expected to be close to or below the lower limit of assay detection, only peak concentrations were analyzed 2 and 4 hours after injection of 110 µg/kg betamethasone phosphate. The fetal arterial blood pressure and amniotic pressure were measured continuously using calibrated pressure transducers (Cobe, Lakewood, CO) connected to the fetal carotid artery and amniotic catheters. Myometrial activity was recorded to recognize pressure artifacts during contractures. Pressures and uterine electromyograms were amplified using an amplification system previously described in detail.15 All biophysical parameters monitored were digitized using a 16-channel analog-to-digital board (Windaq, DATAQ Instruments, Akron, OH) at a sample rate of 64 Hz and continuously stored on a hard disc of a personal computer.
To check fetal health, fetal and maternal arterial blood samples were taken after surgery daily at 0900 hours for measurement of blood gases, hemoglobin concentration, and oxygen saturation using a blood gas analyzer (ABL600, Radiometer, Copenhagen, Denmark; measurements corrected to 39°C) and a Hemoximeter (OSM2, Radiometer).
Betamethasone was measured by adapting a normal phase high-performance liquid chromatography (HPLC) method that has been routinely used for measuring a variety of corticosteroids in rat, rabbit, and human plasma and serum samples.16 The HPLC assay used prednisolone as the internal standard and had a lower limit of quantification of 5 ng/mL.17 Samples of one of the betamethasone phosphate acetate–treated animals could not be used due to interfering peaks, and samples from another animal of this group were incorrectly stored and not available for processing.
Noncompartmental analysis was performed based on linear pharmacokinetic concepts described by Gibaldi and Perrier18 and Gabrielsson and Weiner19 on the maternal betamethasone data from the 170 µg/kg betamethasone phosphate and betamethasone phosphate acetate dose using the Win-Nonlin pharmacokinetic software package (Pharsight Corporation, Mountain View, CA). The highest concentration observed for the two formulations were designated as Cmax. Terminal half-life was estimated as 0.693/λ, where λ is the slope associated with the terminal log-linear portion of the plasma concentration profile. The betamethasone area under the curve (AUC) was estimated by linear trapezoidal rule with extrapolation to time infinity using Clast/λ, where Clast is the last measurable concentration. The apparent volume of distribution (V/F) was estimated using the formula Dose/(AUC×λ) and the apparent clearance (CL/F) was computed from the ratio of dose/AUC. The clearance and volume are designated as apparent for several reasons: 1) The drug is administered as a prodrug by the intramuscular route, and the pharmacokinetic parameters are confounded by the degree of prodrug conversion to active betamethasone and the extent of drug absorption from the intramuscular site. 2) The AUC is overestimated due to the instability of prodrug in the early plasma samples, which leads to the formation of betamethasone after sample collection. This problem was investigated recently by our group. It was found that for the 170 µg/kg intramuscular betamethasone phosphate formulation, the AUC is overestimated by 7%. 3) The traditional noncompartmental pharmacokinetic formulas used for V/F and CL/F in this report assume no fetal elimination of betamethasone. This assumption may not be true for betamethasone, and hence the estimates obtained for V/F and CL/F are apparent approximations. Finally, the relative bioavailability of betamethasone (Fbetamethasone phosphate/acetate/Fbetamethasone phosphate) from the betamethasone phosphate acetate formulation versus that of the betamethasone phosphate formulation was computed as AUCbetamethasone phosphate/acetate/AUCbetamethasone phosphate.
Betamethasone plasma concentrations after administration of 170 µg/kg and 110 µg/kg betamethasone phosphate were compared using the Mann Withney U test. Fetal arterial blood pressure was averaged over 1-hour blocks throughout the study period. For statistical analysis with repeated measures analysis of variance and the Student-Newman-Keuls post hoc test, fetal arterial blood pressure was averaged in 3-hour blocks and transformed logarithmically to be independent of the data distribution.20 Each block of the baseline period was compared with its corresponding time during the treatment period and corresponding time points were compared between treatment groups. Baseline blood gas values of the different treatment groups were compared using the Mann Whitney U test and Bonferroni correction. All results are given as mean plus or minus standard error of the mean. P<.05 was considered to be significant.
RESULTS
Fetal and maternal blood gases were within the physiological range (Table 1).
Table 1.
Physiological Parameters Before Betamethasone Administration
| pH (7.28–7.38) |
Pco2 (mmHg) (45–55) |
Po2 (mmHg) (20–30) |
Hb (mg/dL) (7.0–9.0) |
O2% (55–85) |
||
|---|---|---|---|---|---|---|
| 170 µg/kg BMp | Maternal | 7.47±0.01 | 32.5±0.9 | 122.2±1.8 | 9.1±0.7 | 98.6±0.4 |
| Fetal | 7.34±0.01 | 47.5±1.0 | 25.8±1.4 | 8.4±0.5 | 81.3±3.7 | |
| 110 µg/kg BMp | Maternal | 7.46±0.02 | 37.2±0.9 | 112.4±3.0 | 9.3±0.5 | 98.4±0.8 |
| Fetal | 7.36±0.01 | 46.2±1.3 | 26.1±1.2 | 7.5±0.3 | 81.4±1.5 | |
| 170 µg/kg BMp/a | Maternal | 7.44±0.03 | 39.4±3.9 | 111.3±4.4 | 8.9±0.3 | 98.8±0.6 |
| Fetal | 7.34±0.02 | 50.2±1.6 | 27.7±2.9 | 8.5±0.6 | 78.9±5.6 |
BMp, betamethasone phosphate; BMp/a, betamethasone phosphate acetate.
Maternal and fetal blood gases were measured in the carotid artery. The physiologic ranges of the fetal values are in parentheses. Data are expressed as mean±standard error of the mean.
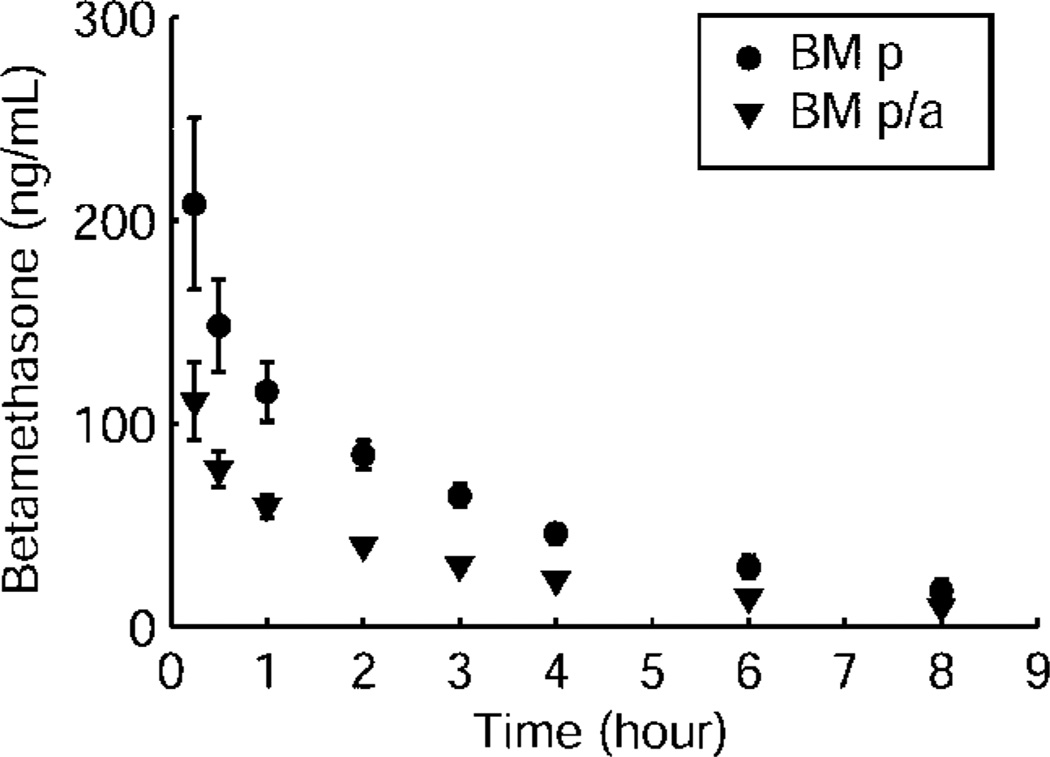
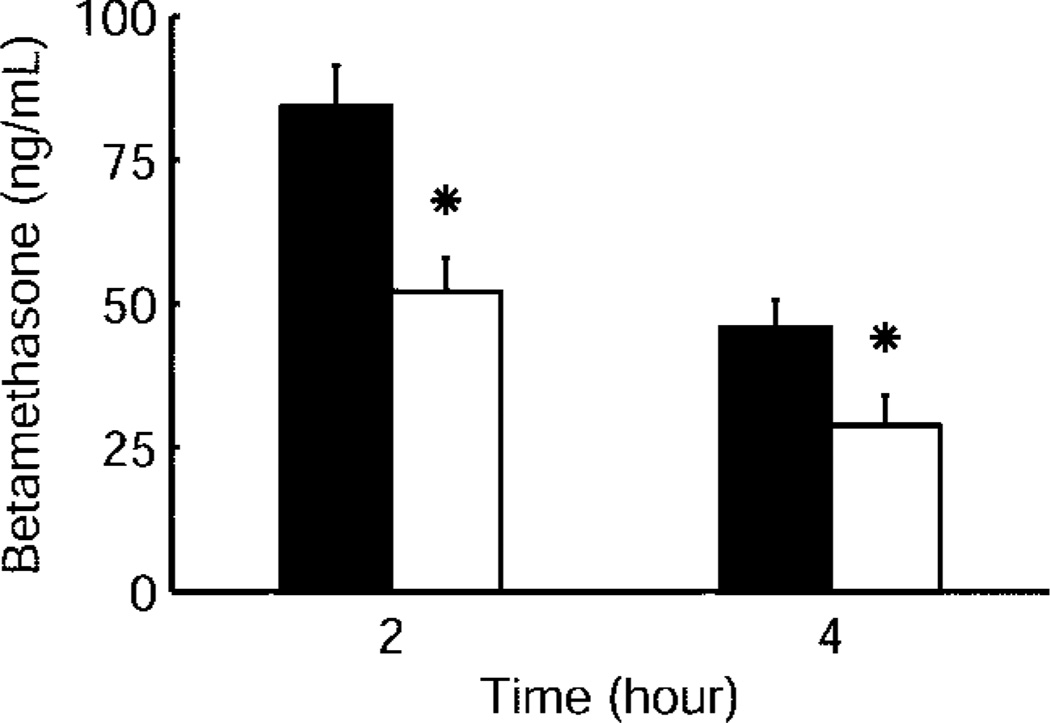
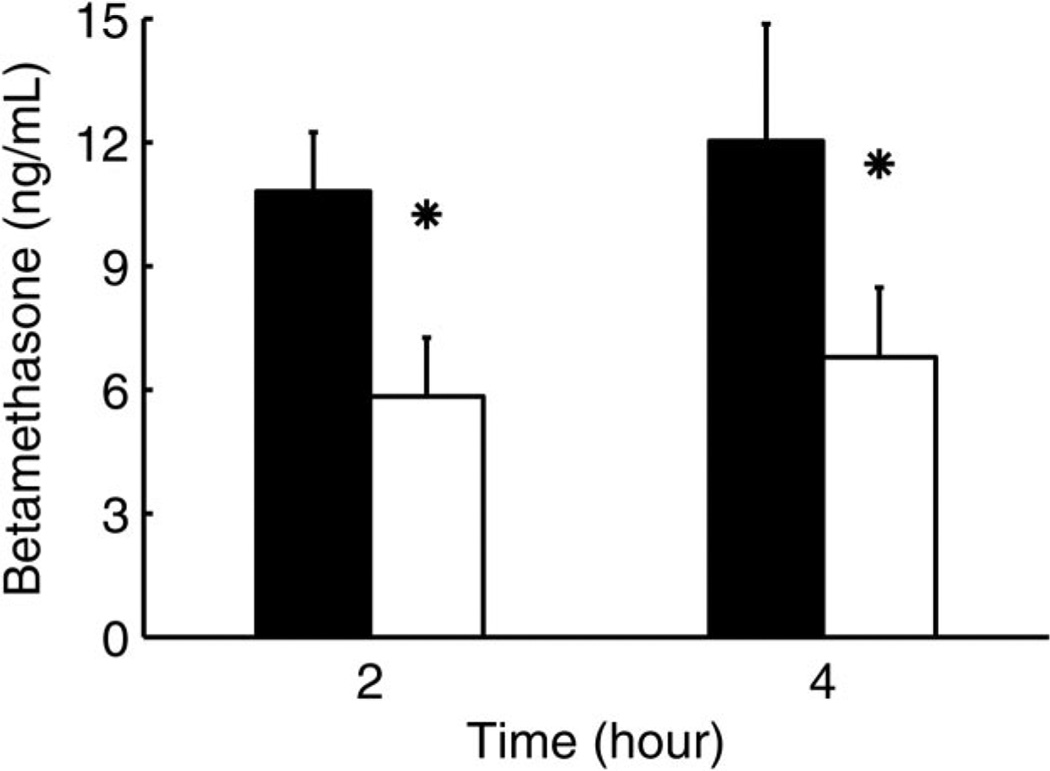
Betamethasone phosphate was released to the maternal plasma from both the betamethasone phosphate and betamethasone phosphate and acetate mixture within 15 minutes after intramuscular injection of 170 µg/kg corresponding to 12 mg used under clinical conditions (Fig. 1). Maternal betamethasone plasma concentration reached its peak 15 minutes after injection, independently of the betamethasone formulation used (Fig. 1). Betamethasone cleared exponentially from maternal plasma with a half-life of 2.75–3.04 hours and decreased below the limit of assay detection between 8 hours and 12 hours after injection of both betamethasone phosphate and betamethasone phosphate and acetate. At 12 hours after injection, betamethasone plasma concentration was below limit of assay detection in five of six betamethasone phosphate and two of four betamethasone phosphate acetate treated animals. Betamethasone phosphate acetate produced mean maternal betamethasone plasma concentrations that were 47–55% of those after injection of betamethasone phosphate during the entire time of measurement; for example, at 2 hours they were 47%, at 4 hours 50%, and at 8 hours 55% (P<.05, Fig. 1). When 110 µg/kg betamethasone phosphate corresponding to the lower clinical dose of 8 mg used by some obstetricians was administered to the mother, values at 2 hours were 62 % and at 4 hours 63 % of the values obtained with 170 µg/kg indicating that plasma values are proportional to administered dose (Fig. 2). Maternal pharmacokinetic parameters for the two formulations are reported in Table 2.
Fig. 1.
Maternal betamethasone (BM) plasma concentrations after maternal intramuscular injection of 170 µg/kg betamethasone phosphate (BM p, n = 6) and betamethasone phosphate acetate (BM p/a, n = 4). Data are given as mean plus or minus standard error of the mean.
Schwab. Pharmacokinetics of Antenatal Glucocorticoids. Obstet Gynecol 2006.
Fig. 2.
Comparison of maternal betamethasone plasma concentrations 2 and 4 hours after maternal intramuscular injection of 170 µg/kg (black bars) (n = 6) and 110 µg/kg betamethasone phosphate (white bars) (n = 6). Data are given as mean plus standard error of the mean. * P<.05 for 110 compared with 170 µg/kg betamethasone phosphate.
Schwab. Pharmacokinetics of Antenatal Glucocorticoids. Obstet Gynecol 2006.
Table 2.
Betamethasone Pharmacokinetic Parameters
| 170 µg/kg BM Phosphate |
170 µg/kg BM Phosphate/Acetate |
|
|---|---|---|
| Average body weight (kg) | 54.00 | 53.75 |
| Cmax (ng/mL) | 208.2 | 111.0 |
| AUC (ng·h/mL) | 559.5 | 287.7 |
| Terminal t1/2 (h) | 2.75 | 3.04 |
| V/FBMp (L/kg) | 1.20 | 2.46 |
| CL/FBMp (L·h−1·kg−1) | 0.30 | 0.56 |
| Relative bioavailability (FBMp/a/FBMp) | 51% |
Cmax, highest concentration observed; AUC, area under the curve; V/F, apparent volume of distribution; CL/F, apparent clearance; BMp, betamethasone phosphate; BMp/a, betamethasone phosphate acetate.
Values are obtained from the average maternal plasma concentration after 170 µg/kg betamethasone phosphate (n=6) and betamethasone phosphate acetate (n=4).
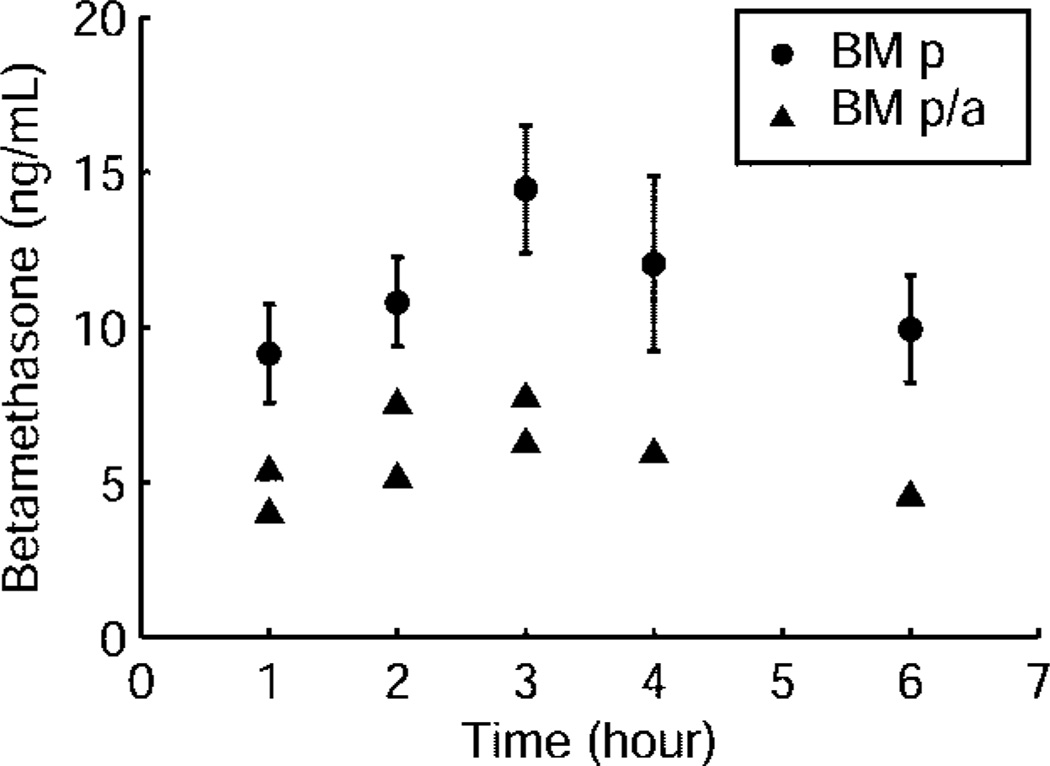
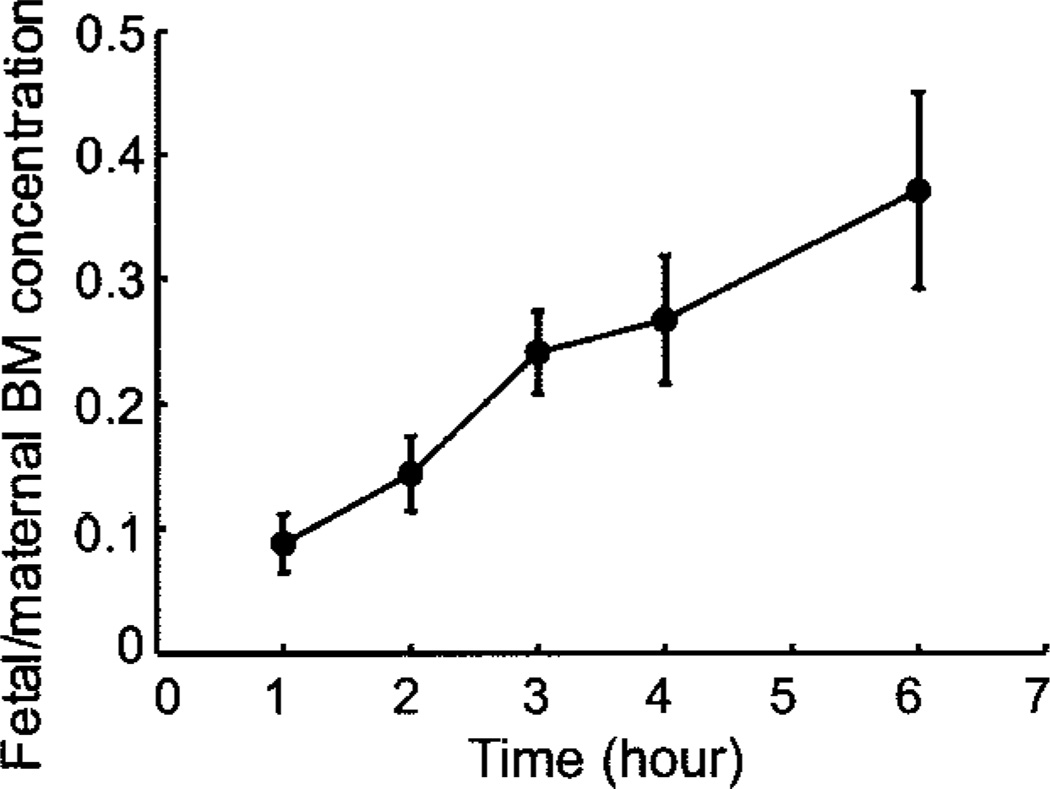
Fetal betamethasone plasma concentrations were first detectable at 1 hour after both betamethasone phosphate and betamethasone phosphate acetate administration and peaked at 3 hours (Fig. 3). Fetal betamethasone plasma concentrations decreased below the limit of assay detection in five of six animals at 8 hours after betamethasone phosphate. Half-life could not be calculated for both preparations because it is difficult to observe a clear terminal phase in the PK profiles (Fig. 3). In the betamethasone phosphate acetate group, betamethasone was below limit of assay detection for most of the fetal samples and decreased in all fetuses at 8 hours below the limit of assay detection (Fig. 3). After injection of betamethasone phosphate acetate, fetal betamethasone plasma concentrations ranged roughly between 46% and 58% of those observed after injection of betamethasone phosphate. Placental transfer of betamethasone determined by the concentration ratio of maternal and fetal arterial plasma increased from 0.09 at 1 hour to 0.37 at 6 hours after injection for the betamethasone phosphate group (Fig. 4). Administration of 110 µg/kg betamethasone phosphate led to betamethasone plasma concentrations of 59% and 64% of those after injection of 170 µg/kg betamethasone phosphate at 2 hours and 4 hours after administration (Fig. 5), indicating that not only maternal but also fetal plasma values are proportional to the administered dose at this age.
Fig. 3.
Fetal betamethasone plasma concentrations after maternal intramuscular injection of 170 µg/kg betamethasone phosphate (BM p, n = 6) and betamethasone phosphate acetate (BM p/a, n = 4). Data for the betamethasone phosphate group are given as mean plus or minus standard error of the mean. Betamethasone was below the limit of detection for most of the fetal samples in the betamethasone phosphate acetate group, and therefore only the raw data for eight samples where concentrations could be measured have been reported for this group.
Schwab. Pharmacokinetics of Antenatal Glucocorticoids. Obstet Gynecol 2006.
Fig. 4.
Fetal and maternal betamethasone (BM) concentration ratios against time after maternal intramuscular injection of 170 µg/kg betamethasone phosphate (n = 6). Data are given as mean plus or minus standard error of the mean.
Schwab. Pharmacokinetics of Antenatal Glucocorticoids. Obstet Gynecol 2006.
Fig. 5.
Comparison of fetal betamethasone plasma concentrations 2 hours and 4 hours after maternal intramuscular injection of 170 µg/kg (black bars) (n = 6) and 110 µg/kg betamethasone phosphate (white bars) (n = 6). Data are given as mean plus or minus standard error of the mean. * P<.05 for 110 compared with 170 µg/kg betamethasone phosphate.
Schwab. Pharmacokinetics of Antenatal Glucocorticoids. Obstet Gynecol 2006.
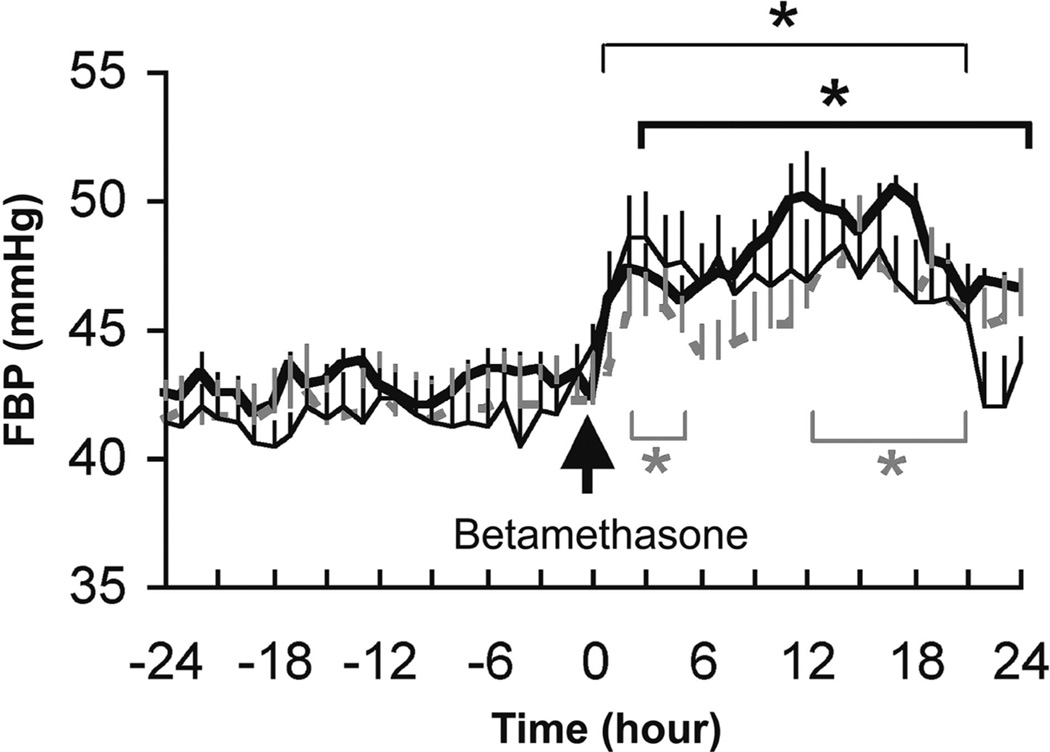
There were no differences in baseline fetal arterial blood pressure among the three groups or in the maximal values after administration of 170 µg/kg betamethasone phosphate and betamethasone phosphate acetate or 110 µg/kg betamethasone phosphate (Fig. 6). Betamethasone injection of all three doses led to a marked increase of fetal arterial blood pressure within 3 hours and remained elevated for 18 hours (P<.05, Fig. 6).
Fig. 6.
Mean fetal arterial blood pressure (FBP) changes after maternal intramuscular injection of 170 µg/kg betamethasone phosphate (bold) (n = 6), 110 µg/kg betamethasone phosphate (fine) (n = 6) and betamethasone phosphate acetate (gray) (n = 4). Data are given as mean plus standard error of the mean. * P<.05 in comparison with baseline for 170 µg/kg and 110 µg/kg betamethasone phosphate (bold and fine) and betamethasone phosphate acetate (gray).
Schwab. Pharmacokinetics of Antenatal Glucocorticoids. Obstet Gynecol 2006.
DISCUSSION
This study compared two different doses and preparations of betamethasone that are used clinically to enhance fetal lung maturation in women in premature labor. We evaluated the time course of maternal and fetal betamethasone plasma concentrations and the accompanying induced fetal arterial blood pressure changes as an indication of extent of unwanted adverse effects. The maximal fetal arterial blood pressure responses did not differ between betamethasone phosphate and betamethasone phosphate acetate. The fetal arterial blood pressure response was the same with both the full and two-thirds doses, although maternal and fetal betamethasone plasma concentrations showed a clear dose–concentration relationship.
The betamethasone plasma concentration after administration of 170 µg/kg betamethasone phosphate acetate was approximately 50% of those observed after injection of a similar dose of betamethasone phosphate. The betamethasone phosphate acetate contains 50% betamethasone phosphate and the relative bioavailability of this formulation in comparison to betamethasone phosphate was 51% by the AUC ratio method (Table 2). These data therefore suggests that very little or none of the betamethasone is released from betamethasone acetate and the depot formulation provides poor bioavailability of betamethasone within the first 8 hours after administration when the betamethasone effect on lung maturation is needed. These data may also imply that pregnant women who are administered 12 mg of the fast-acting formulation compared with those who receive 12 mg of the depot formulation are receiving twice as much exposure to betamethasone. The poor bioavailability of the depot formulation has been previously observed in parturient women,21 and our data confirm these previous observations in humans. The pharmacokinetic comparison shows that depot formulation primarily releases the fast-acting betamethasone phosphate and offers very little betamethasone release from the slow-acting betamethasone acetate. We have recently evaluated the in vivo betamethasone release from the acetate formulation in nonpregnant sheep after intramuscular administration using a highly sensitive assay involving liquid chromatography and mass spectrometry.22 The betamethasone release occurs for several days after betamethasone acetate administration, albeit at extremely low concentrations. This recent work also suggests (through mathematical simulations) that these low concentrations may have very little effect on fetal function because they translate into minimal to negligible fetal exposures. However, these low concentrations may have implications for the maternal health. Recent meta-analysis indicate that women threatened with preterm labor and injected the dual formulation have a higher incidence of infections.23 This is thought to occur because of the suppression of their immune system by the prolonged betamethasone exposure from the acetate formulation.
Administration of betamethasone phosphate and betamethasone phosphate acetate led to maternal peak betamethasone plasma concentrations within 15 minutes after intramuscular injection independent of the preparation used. The peak time is similar to that found previously in sheep24 and in clinical studies25,26 with 12 mg betamethasone phosphate acetate intramuscularly to the mother. In those studies, maximal maternal betamethasone plasma concentrations were found at the first sampling time at 30 minutes and 1 hour, respectively. The peak maternal betamethasone plasma concentration of 105 ng/mL reached in the clinical studies25 compares well to the peak value of 111 ± 19 ng/mL−1 found in our study.
The low bioavailability of the betamethasone phosphate acetate formulation caused most of the fetal samples from the 170 µg/kg betamethasone phosphate acetate group to fall below the limit of assay detection of the betamethasone assay. All the samples from the betamethasone phosphate acetate group which could be measured with the betamethasone assay exhibited concentrations that were approximately 50% of the concentrations observed in the 170 µg/kg betamethasone phosphate group (Fig. 3). Thus, the lower bioavailability of the depot formulation led to decreased exposure of the fetus to betamethasone.
There was a delay of betamethasone appearance in the fetal circulation. Betamethasone could be detected first at 1 hour after injection and reached peak concentrations at 3 hours. The delay was independent of the betamethasone preparation injected. In humans, distribution of betamethasone in the fetal circulation seems to be similar. Betamethasone could be detected in cord serum of premature infants 1 hour after maternal intramuscular injection of 12 mg betamethasone phosphate acetate and peaked at 2 hours after injection.25,26 Peak human fetal plasma concentrations after 12 mg betamethasone phosphate acetate were about 20 ng/mL compared to 7 ng/mL in fetal sheep arterial plasma in our study.25,26 The delayed appearance of betamethasone in the fetal circulation is probably due to the initial very low placental transfer of betamethasone caused by placental retention or metabolism of betamethasone. Although in vitro studies in the human placenta have shown that placental metabolism27 and placental retention28 of betamethasone or dexamethasone (a synthetic glucocorticoid that is very close in structure and pharmacokinetics to betamethasone) is generally low, placental retention and metabolism of betamethasone in vivo is probably high enough to delay the peak fetal concentration of betamethasone by more than 2 hours as we have observed. Thus, greater metabolism of betamethasone has been shown in the perfused human placenta.29 A study in pregnant sheep has also shown that placental transfer of dexamethasone is limited.30 Another poorly understood process that could possibly explain the limited placental transfer of dexamethasone and betamethasone is the removal of these steroids by placental transporters that protect the fetus by removing toxic endogenous compounds and xenobiotics from the developing conceptus.31 One such transporter that is expressed in placental tissue and could restrict access of betamethasone to the fetal circulation is P-glycoprotein.
The increasing concentration ratio of fetal to maternal arterial plasma with time in our study compares well with the increasing betamethasone and dexamethasone concentration ratio shown previously.8,32 Comparison of several clinical studies using different dosages of betamethasone and dexamethasone has shown similar maternal and fetal plasma concentrations calculated per milligram steroid injected.25 Bennet et al8 used a radioimmunoassay that is more sensitive than HPLC and were thus able to detect maternal and fetal dexamethasone plasma concentrations in sheep up to 24 hours after injection. The dexamethasone concentration ratio seems to increase further to almost 1.0 at 12–24 hours, reflecting slow maternal–fetal equilibration. Placental transfer of betamethasone in humans has been demonstrated, and concentration ratios of maternal venous and fetal cord plasma averaged 0.3726 or 0.4033 after maternal intramuscular injection of 12 mg betamethasone phosphate acetate and 0.28 after maternal intramuscular injection of 8 mg betamethasone phosphate.34 Because cord plasma samples were taken during delivery, sample times varied between 1.5 and 78 hours, 1 and 61, and 2 and 7 hours after treatment, and gestational ages ranged between 25 and 32, 27 and 34, and 31 and 38 weeks, respectively. Generally, these values are comparable to those that we observed in sheep.
No differences were observed in the maternal plasma disappearance between the two betamethasone preparations in the first 12 hours after injection. Although we did not measure betamethasone before 15 minutes following injection, it is highly likely that the maternal peak plasma betamethasone concentration was not much earlier because of the latency necessary for diffusion from muscle. The maternal plasma half-life of betamethasone determined in our study (circa 3 hours) is similar to that of dexamethasone (circa 2.5 hours) observed by Bennet and colleagues,8 but shorter than the 4.8 hours after betamethasone phosphate acetate determined by Moss et al33 in sheep. Maternal plasma half-life found in our study is also a little bit shorter than that of 3.6 hours for dexamethasone25 and of 5–6 hours25 or approximately 9 hours33 for betamethasone estimated from clinical studies. These differences are likely due to the different methodologies used, because placental transfer of betamethasone is similar in humans and sheep (see above).
The fetal arterial blood pressure response was much more prolonged than the fetal betamethasone disappearance curve, suggesting that the biologic effect outlasted the exposure to betamethasone. In designing an optimal treatment regimen, clearly, the time course of the biologic effect is more important than the plasma disposition rate. The time course of the biologic effect is longer than that of betamethasone in plasma because it depends on rate of dissociation of betamethasone from receptors in target cells and the turnover times of induced second messenger cascades, mRNA, and proteins.25 Attempts have been made to estimate the duration of the biologic effect of glucocorticoids on the basis of pituitary–adrenal function by determining suppression of plasma glucocorticoid concentrations.35 These authors concluded from their measurements in human pregnancy that half-life of the biologic effect was from one and one half to two times longer than half-time for clearance of dexamethasone from plasma.
Our results show that the doses of 8 mg or 12 mg betamethasone phosphate daily, more commonly used in Europe, or 12 mg betamethasone phosphate acetate daily as commonly used in the United States are supramaximal in respect to the cardiovascular effects. Clearly, the potential of lowering the dose depends on the beneficial multisystem effects of glucocorticoid therapy. These multisystem effects of steroids complicate urgently needed dose–response studies. On one side, dose–response studies need to consider potential beneficial effects of premature activation of those systems required for adaptation to birth, improvement in lung function, and decreased risk of cystic periventricular leukomalacia. On the other side, potential adverse effects are concealed in the same systems, especially in the cardiovascular7–11 and central nervous system.2–6
In conclusion, our study extends findings from previous investigations of the pharmacokinetics of clinical doses of betamethasone phosphate acetate34 and dexamethasone phosphate8 in pregnant sheep. The pregnant sheep seems to be an adequate model for pharmacokinetics of betamethasone in human pregnancy. The pharmacokinetics of betamethasone show a clear dose–fetal plasma concentration relationship. The studies reported here clearly demonstrate that 6 mg betamethasone phosphate (released from the betamethasone phosphate acetate formulation) has maximal effects with respect to the acute cardiovascular effects. Therefore, studies examining the effects of lower doses of betamethasone are urgently required. We hypothesize that the clinical dose will also prove to be supramaximal for accelerating fetal lung maturation. In-depth physiological costbenefit studies are needed to evaluate the optimal dose for antenatal glucocorticoid treatment.
Acknowledgments
Supported by grants from the Max Kade Foundation and Grants No. GM 24211 and HD 21350 from the National Institutes of Health.
The authors thank Xiu Ying Ding for surgical assistance and Sue Jenkins for statistical analysis.
REFERENCES
- 1.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 2.Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol. 2003;547:117–123. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonow-Schlorke I, Kuhn B, Muller T, Schubert H, Sliwka U, Nathanielsz PW, Schwab M. Antenatal betamethasone treatment reduces synaptophysin immunoreactivity in presynaptic terminals in the fetal sheep brain. Neurosci Lett. 2001;297:147–150. doi: 10.1016/s0304-3940(00)01605-0. [DOI] [PubMed] [Google Scholar]
- 4.Edwards HE, Burnham WM. The impact of corticosteroids on the developing animal. Pediatr Res. 2001;50:433–440. doi: 10.1203/00006450-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Schwab M, Antonow-Schlorke I, Kuhn B, Muller T, Schubert H, Walter B, et al. Effect of antenatal betamethasone treatment on microtubule-associated proteins MAP1B and MAP2 in fetal sheep. J Physiol. 2001;530:497–506. doi: 10.1111/j.1469-7793.2001.0497k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwab M, Schmidt K, Roedel M, Mueller T, Schubert H, Anwar MA, et al. Non-linear changes of electrocortical activity after antenatal betamethasone treatment in fetal sheep. J Physiol. 2001;531:535–543. doi: 10.1111/j.1469-7793.2001.0535i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher AJ, McGarrigle HH, Edwards CM, Fowden AL, Giussani DA. Effects of low dose dexamethasone treatment on basal cardiovascular and endocrine function in fetal sheep during late gestation. J Physiol. 2002;545:649–660. doi: 10.1113/jphysiol.2001.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennet L, Kozuma S, McGarrigle HH, Hanson MA. Temporal changes in fetal cardiovascular, behavioural, metabolic and endocrine responses to maternally administered dexamethasone in the late gestation fetal sheep. Br J Obstet Gynaecol. 1999;106:331–339. doi: 10.1111/j.1471-0528.1999.tb08270.x. [DOI] [PubMed] [Google Scholar]
- 9.Derks JB, Giussani DA, Jenkins SL, Wentworth RA, Visser GH, Padbury JF, et al. A comparative study of cardiovascular, endocrine and behavioural effects of betamethasone and dexamethasone administration to fetal sheep. J Physiol. 1997;499:217–226. doi: 10.1113/jphysiol.1997.sp021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwab M, Roedel M, Anwar MA, Muller T, Schubert H, Buchwalder LF, et al. Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow. J Physiol. 2000;528:619–632. doi: 10.1111/j.1469-7793.2000.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenen SV, Mecenas CA, Smith GS, Jenkins S, Nathanielsz PW. Effects of maternal betamethasone administration on fetal and maternal blood pressure and heart rate in the baboon at 0.7 of gestation. Am J Obstet Gynecol. 2002;186:812–817. doi: 10.1067/mob.2002.121654. [DOI] [PubMed] [Google Scholar]
- 12.Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med. 1997;156:178–184. doi: 10.1164/ajrccm.156.1.9612036. [DOI] [PubMed] [Google Scholar]
- 13.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 14.White A, Marcucci G, Andrews E, Edwards K, Long W. Antenatal steroids and neonatal outcomes in controlled clinical trials of surfactant replacement. The American Exosurf Neonatal Study Group I and The Canadian Exosurf Neonatal Study Group. Am J Obstet Gynecol. 1995;173:286–290. doi: 10.1016/0002-9378(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 15.Unno N, Wong CH, Jenkins SL, Wentworth RA, Ding XY, Li C, et al. Blood pressure and heart rate in the ovine fetus: ontogenic changes and the effects of fetal adrenalectomy. Am J Physiol. 1999;276:H248–H256. doi: 10.1152/ajpheart.1999.276.1.H248. [DOI] [PubMed] [Google Scholar]
- 16.Jusko WJ, Pyszczynski NA, Bushway MS, D’Ambrosio R, Mis SM. Fifteen years of operation of a high-performance liquid chromatographic assay for prednisolone, cortisol and prednisone in plasma. J Chromatogr B Biomed Appl. 1994;658:47–54. doi: 10.1016/0378-4347(94)00218-5. [DOI] [PubMed] [Google Scholar]
- 17.Samtani MN, Schwab M, Nathanielsz PW, Jusko WJ. Stabilization and HPLC analysis of betamethasone sodium phosphate in plasma. J Pharm Sci. 2004;93:726–732. doi: 10.1002/jps.10577. [DOI] [PubMed] [Google Scholar]
- 18.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York (NY): Marcel Dekker Inc; 1982. [Google Scholar]
- 19.Gabrielsson J, Weiner D. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. 2nd ed. Stockholm (Sweden): Apotekarsocieteten; 1997. [Google Scholar]
- 20.Bland JM, Altman DG. Transforming data. BMJ. 1996;312:770. doi: 10.1136/bmj.312.7033.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen MC, Ashley JJ, McBride WG, Nation RL. Disposition of betamethasone in parturient women after intramuscular administration. Br J Clin Pharmacol. 1984;18:383–392. doi: 10.1111/j.1365-2125.1984.tb02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samtani MN, Lohle M, Grant A, Nathanielsz PW, Jusko WJ. Betamethasone pharmacokinetics after two prodrug formulations in sheep: implications for antenatal corticosteroid use. Drug Metab Dispos. 2005;33:1124–1130. doi: 10.1124/dmd.105.004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. Am J Obstet Gynecol. 2004;190:878–881. doi: 10.1016/j.ajog.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 24.Moss TJ, Doherty DA, Nitsos I, Harding R, Newnham JP. Pharmacokinetics of betamethasone after maternal or fetal intramuscular administration. Am J Obstet Gynecol. 2003;189:1751–1757. doi: 10.1016/s0002-9378(03)00825-1. [DOI] [PubMed] [Google Scholar]
- 25.Ballard PL. Hormones and lung maturation. Monogr Endocrinol. 1986;28:1–354. [PubMed] [Google Scholar]
- 26.Ballard PL, Granberg P, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J Clin Invest. 1975;56:1548–1554. doi: 10.1172/JCI108236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanford AT, Murphy BE. In vitro metabolism of prednisolone, dexamethasone, betamethasone, and cortisol by the human placenta. Am J Obstet Gynecol. 1977;127:264–267. doi: 10.1016/0002-9378(77)90466-5. [DOI] [PubMed] [Google Scholar]
- 28.Dancis J, Jansen V, Levitz M. Placental transfer of steroids: effect of binding to serum albumin and to placenta. Am J Physiol. 1980;238:208–213. doi: 10.1152/ajpendo.1980.238.3.E208. [DOI] [PubMed] [Google Scholar]
- 29.Levitz M, Jansen V, Dancis J. The transfer and metabolism of corticosteroids in the perfused human placenta. Am J Obstet Gynecol. 1978;132:363–366. doi: 10.1016/0002-9378(78)90768-8. [DOI] [PubMed] [Google Scholar]
- 30.Anderson DF, Stock MK, Rankin JH. Placental transfer of dexamethasone in near-term sheep. J Dev Physiol. 1979;1:431–436. [PubMed] [Google Scholar]
- 31.Young AM, Allen CE, Audus KL. Efflux transporters of the human placenta. Adv Drug Deliv Rev. 2003;55:125–132. doi: 10.1016/s0169-409x(02)00174-6. [DOI] [PubMed] [Google Scholar]
- 32.Moss TJ, Doherty DA, Nitsos I, Harding R, Newnham JP. Pharmacokinetics of betamethasone after maternal or fetal intramuscular administration. Am J Obstet Gynecol. 2003;189:1751–1757. doi: 10.1016/s0002-9378(03)00825-1. [DOI] [PubMed] [Google Scholar]
- 33.Ballabh P, Lo ES, Kumari J, Cooper TB, Zervoudakis I, Auld PA, et al. Pharmacokinetics of betamethasone in twin and singleton pregnancy. Clin Pharmacol Ther. 2002;71:39–45. doi: 10.1067/mcp.2002.120250. [DOI] [PubMed] [Google Scholar]
- 34.Petersen MC, Nation RL, Ashley JJ, McBride WG. The placental transfer of betamethasone. Eur J Clin Pharmacol. 1980;18:245–247. doi: 10.1007/BF00563006. [DOI] [PubMed] [Google Scholar]
- 35.Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids: effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977;63:200–207. doi: 10.1016/0002-9343(77)90233-9. [DOI] [PubMed] [Google Scholar]