Abstract
The use of genomics to discover novel targets and biomarkers has placed the field of oncology at the forefront of precision medicine. First-generation epidermal growth factor receptor (EGFR) inhibitors have transformed the therapeutic landscape of EGFR mutant non-small-cell lung carcinoma through the genetic stratification of tumors from patients with this disease. Somatic EGFR mutations in lung adenocarcinoma are now well established as predictive biomarkers of response and resistance to small-molecule EGFR inhibitors. Despite early patient benefit, primary resistance and subsequent tumor progression to first-generation EGFR inhibitors are seen in 10%–30% of patients with EGFR mutant non-small-cell lung carcinoma. Acquired drug resistance is also inevitable, with patients developing disease progression after only 10–13 months of antitumor therapy. This review details strategies pursued in circumventing T790M-mediated drug resistance to EGFR inhibitors, which is the most common mechanism of acquired resistance, and focuses on the clinical development of second-generation EGFR inhibitors, exemplified by afatinib (BIBW2992). We discuss the rationale, mechanism of action, clinical efficacy, and toxicity profile of afatinib, including the LUX-Lung studies. We also discuss the emergence of third-generation irreversible mutant-selective inhibitors of EGFR and envision the future management of EGFR mutant lung adenocarcinoma.
Keywords: afatinib, EGFR, erlotinib, gefitinib, LUX-Lung, NSCLC
Introduction
Our biological understanding of the molecular basis of lung cancer progression has been accelerated following recent advances in the molecular characterization of the cancer genome through the use of high-throughput tumor profiling technologies, such as next-generation sequencing.1 This use of genomics to discover novel targets and biomarkers has put the field of oncology at the forefront of precision medicine.2 In particular, the principle of oncogene addiction, in which specific cancer cells are dependent on a certain pathogenic oncogene that drives malignant progression, has been exploited with great success in the development of targeted therapeutics in lung cancer.3
The epidermal growth factor receptor (EGFR) pathway is one such signaling pathway that has been targeted for the treatment of patients with EGFR mutant non-small-cell lung carcinoma (NSCLC). Somatic EGFR mutations in lung cancers are now well established as analytically validated and clinically qualified predictive biomarkers of response and resistance to small-molecule EGFR tyrosine kinase inhibitors (TKIs). Randomized clinical trials have confirmed significant improvements in both response rates and progression-free survival (PFS) with both erlotinib (OSI Pharmaceuticals/Roche) and gefitinib (AstraZeneca) in advanced EGFR mutated NSCLC when compared with platinum-based chemotherapy, thus providing clear proof of concept for an oncogene addiction strategy in this setting (Table 1).4–7 The approval of these TKIs was a critical milestone for the treatment of NSCLC by presenting a model for targeted therapy development through the genetic stratification of tumors from patients with this disease.
Table 1.
Summary of clinical trials of commercially available EGFR tyrosine kinase inhibitors versus chemotherapy as first-line therapy in non-small-cell lung carcinoma with activating EGFR mutations
| Clinical trial | Epidermal growth factor receptor tyrosine kinase inhibitor | Number of patients | Median progression-free survival in tyrosine kinase inhibitor group (months) | P-value | Hazard ratio |
|---|---|---|---|---|---|
| OPTIMAL52 | Erlotinib | 154 | 13.1 | <0.0001 | 0.16 |
| First Signal53 | Gefitinib | 42 | 8.4 | 0.084 | 0.61 |
| IPASS54 | Gefitinib | 261 | 9.5 | <0.0001 | 0.48 |
| WJTOG340555 | Gefitinib | 177 | 9.2 | <0.001 | 0.48 |
| NEJSG 00256 | Gefitinib | 200 | 10.8 | <0.001 | 0.36 |
| EURTAC57 | Erlotinib | 174 | 9.4 | <0.0001 | 0.42 |
| LUX-Lung 311,12 | Afatinib | 308 | 13.6 | <0.0001 | 0.47 |
Abbreviation: EGFR, epidermal growth factor receptor.
Despite early patient benefit, primary resistance and subsequent tumor progression to these first-generation EGFR inhibitors are seen in 10%–30% of patients with EGFR mutant NSCLC, and acquired drug resistance is ultimately inevitable, with patients developing disease progression after a median of only 10–13 months of antitumor therapy.4–6,8 Such issues have led to increased research on resistance mechanisms to EGFR inhibitors and the search for potential solutions to overcome such challenges. There are a number of potential mechanisms involved in the development of acquired EGFR inhibitor resistance. These include the T790M missense mutation in exon 20 of the EGFR kinase domain, high-grade neuroendocrine (small cell) carcinoma transformation, and compensatory escape mechanisms through other critical genetic drivers, including c-MET/HGF, HER2, PIK3CA, ERK, BRAF, CRKL, and AXL (Figure 1).9
Figure 1.
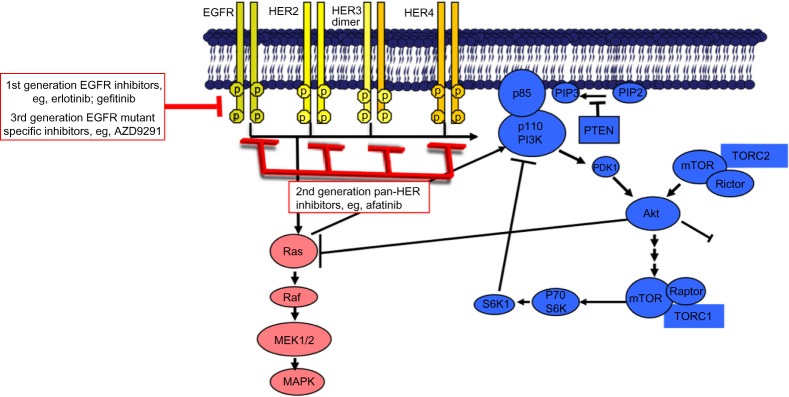
EGFR is part of a family of receptor tyrosine kinases (RTKs) that also includes HER2 (ERBB2), HER3 (ERBB3) and HER4 (ERBB4).
Notes: These RTKs comprise a ligand-binding extracellular domain, a transmembrane link and an intracellular catalytic domain. Binding of growth factors to the extracellular domain leads to homo- or hetero-dimerization of the respective receptor, with subsequent activation of RTK activity and regulation of multiple key intracellular signaling substrates as shown in the Figure.
Abbreviations: EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor.
This review details strategies pursued in circumventing T790M-mediated drug resistance to EGFR inhibitors, which is the most common mechanism of acquired resistance, and focuses on the clinical development of second-generation EGFR inhibitors, and in particular afatinib (BIBW2992; Boehringer Ingelheim). We detail the rationale, mechanism of action, clinical efficacy, and toxicity profile of afatinib, including the recent LUX-Lung studies.10,11–15 We also briefly discuss the recent development of third-generation mutant-selective inhibitors of EGFR and look ahead to the future management of EGFR mutant lung adenocarcinoma. A detailed discussion on the other mechanisms of resistance to EGFR inhibitors is beyond the scope of this review, but the reader is directed to several excellent articles.9–17
Circumventing resistance due to EGFR T790M mutation
One of the most critical mechanisms for acquired resistance is the gatekeeper EGFR T790M missense mutation, which is found in approximately 49%–63% of patients who have developed resistance to EGFR inhibitors.18,19 Preliminary studies also indicate that the T790M mutation may play a crucial role in primary resistance to first-generation EGFR inhibitors because of clonal evolution in tumor cells with preexisting T790M mutations.20 Different strategies have been pursued in the management of progressive disease after treatment with first-generation EGFR TKIs, including monotherapies such as dasatinib21 and neratinib,22 as well as the rational combinations of cetuximab plus erlotinib23 and of erlotinib/gefitinib plus everolimus.24 To date, the results of these clinical trials have, however, been generally disappointing.
A different approach has been the discovery and development of the second-generation pan-human epidermal growth factor receptor (HER) kinase inhibitors afatinib and dacomitinib (PF-00299804; Pfizer; Tables 2 and 3). Both compounds are irreversible TKIs with antitumor activity in lung cancer cell lines with both sensitive and resistant EGFR mutations, including the critical T790M mutation.
Table 2.
Summary of clinical trials of EGFR tyrosine kinase inhibitors in development in NSCLC with EGFR mutations
| EGFR tyrosine kinase inhibitor | Phase | Trial registration | Targets | Trial design |
|---|---|---|---|---|
| Lapatinib | II | NCT00528281 | EGFR, HER2 | Single-arm study with pemetrexed |
| Neratinib | II | NCT00266877 | EGFR, HER2 | Three-group study |
| Icotinib (BPI-2009H) | II, III |
NCT01690390 NCT01707329 NCT01516983 NCT01719536 |
EGFR | Monotherapy and with chemotherapy, radiation or other targeted therapies |
| Afatinib | II, III | Multiple studies | Pan-HER family | |
| Dacomitinib (PF00299804) | III |
NCT01774721 NCT01360554 |
Pan-HER family | Monotherapy versus gefitinib or erlotinib |
| Poziotinib (HM781-36B) | II |
NCT01819428 NCT01718847 |
Pan-HER and TEC family | First- and second-line monotherapy |
| AZD9291 | I | NCT01802632 | EGFR mutation specific | Monotherapy in previously treated EGFR mutant NSCLC |
| CO-1686 | I, II | NCT01526928 | EGFR mutation specific | Monotherapy in previously treated EGFR mutant NSCLC |
| HM61713 | I | NCT01588145 | EGFR mutation specific | Monotherapy in previously treated EGFR mutant NSCLC |
| AP26113 | I, II | NCT01449461 | Dual ALK and EGFR inhibitor | Monotherapy in NSCLC with ALK gene rearrangement or mutant EGFR |
Abbreviations: EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; NSCLC, non-small-cell lung carcinoma.
Table 3.
Summary of clinical trials of afatinib in advanced non-small-cell lung carcinoma (LUX-Lung clinical trial program)
| LUX-Lung trial | Phase | EGFR mutation mandated | Line of treatment | Design | Primary endpoint | Efficacy results | Toxicity results |
|---|---|---|---|---|---|---|---|
| LUX-Lung 1, NCT0065613613,14 | IIb/III | N/R | Third-/fourth-line after patient-based chemotherapy and first-generation EGFR TKI | Afatinib + BSC versus placebo + BSC | Overall survival | Primary endpoint of overall survival not met (median, 10.8 months versus 12.0 months; P=0.74); PFS, 3.3 months in the treatment group versus 1.1 months in the placebo group (P<0.0001); 29 (7%) patients achieved a partial response versus one patient in the placebo group | Diarrhea in 339 (87%) of 390 patients (17% grade 3) and acneiform rash in 305 (78%) patients (14% grade 3) |
| LUX-Lung 2, NCT0052514815 | II | Yes | First-/second-line after chemotherapy only; no prior EGFR TKI | Afatinib monotherapy | Objective response (complete response or partial response) | 70 (66%) of 106 patients harboring the two common activating EGFR mutations (deletion 19 or L858R) had objective antitumor responses, whereas 9 (39%) of 23 patients with less common mutations also had objective responses | The two most common toxicities were diarrhea and rash, with grade 3 events more common in patients receiving 50 mg afatinib daily compared with 40 mg daily |
| LUX-Lung 3, NCT0094965011,12 | III | Yes | First-line | Afatinib versus cisplatin/pemetrexed | PFS | PFS of 11.1 months versus 6.9 months (P=0.001); patients with common EGFR mutations had PFS of 13.6 months versus 6.9 months (P=0.001); response rate, 56% versus 23% (P=0.001) | Diarrhea in 95.2% of patients, (G3 14.4%); rash in 89.1% of patients (G3 16.2%); mucositis in 72.1% (G3 in 8.7%; G4 in 0.4%) and paronychia in 56.8% (G3 in 11.4%) of patients; four deaths in the afatinib group in this study |
| LUX-Lung 4, NCT0071159410 | I/II | N/R | Third-/fourth-line after patient-based chemotherapy and first-generation EGFR TKI | Afatinib monotherapy | Phase I: safety, dose-limiting toxicity; Phase II: objective response (complete response or partial response) | 8.2% had a confirmed partial response. PFS was 4.4 months, whereas median overall survival was 19 months; two patients with acquired T790M mutations had SD for 9 months and 1 month, respectively | The most common afatinib-related toxicities were diarrhea in all patients and rash/acne in 91.9% of patients |
| LUX-Lung 5, NCT0108513638 | III | N/R | Third-/fourth-line after chemotherapy ± first-generation EGFR TKI | Part A: afatinib monotherapy Part B: Afatinib + weekly paclitaxel versus investigators’ choice of chemotherapy | PFS | PFS for afatinib was 3.3 months; 8% overall response rate, with 56% SD at 6 weeks; PFS for EGFR-mutated patients was 4.2 months versus 2.6 months in non-EGFR-mutated patients (n=35) | – |
| LUX-Lung 6, NCT01121393 | III | Yes | First-line | Afatinib versus cisplatin/gemcitabine | PFS | PFS 11.0 versus 5.6 months; hazard ratio, 0.28 (P<0.0001); overall response rate, 66.9% versus 23.0% (P<0.0001); and DCR, 92.6% versus 76.2% (P<0.0001) | ≥G3 drug-related toxicities in 36.0% versus 60.2% of patients in each group; the most common toxicities included rash/acne (14.6%), diarrhea (5.4%); stomatitis/mucositis (5.4%) with afatinib; and neutropenia (17.7%), vomiting (15.9%), and leukopenia (13.3%) with gemcitabine-cisplatin |
| LUX-Lung 7, NCT01466660 | IIb | Yes | First-line | Afatinib versus gefitinib | PFS; DCR | – | – |
| LUX-Lung 8, NCT01523587 | III | N/R | Second-line after patient-based chemotherapy; squamous cell histology | Afatinib versus erlotinib | PFS | – | – |
Abbreviations: BSC, best supportive care; DCR, disease control rate; G3, grade 3; N/R, not recorded; PFS, progression-free survival; EGFR, epidermal growth factor receptor; SD, stable disease; TKI, tyrosine kinase inhibitor.
Second-generation irreversible pan-HER kinase inhibitors
Dacomitinib
Although similar to reversible EGFR inhibitors in competing with adenosine triphosphate (ATP) in the kinase domain, dacomitinib also covalently binds at the ATP binding cleft on Cys773 of EGFR, leading to irreversible blockade of ATP.25 Dacomitinib was found to be more potent than reversible EGFR inhibitors in cell-based assays and had a low off-rate compared with reversible inhibitors. Importantly, dacomitinib is a pan-HER inhibitor that targets both sensitizing EGFR mutants and the secondary EGFR T790M mutant.
In clinical studies, dacomitinib was shown to be safe and generally well-tolerated in Phase I trials, with dose-limiting stomatitis, diarrhea, and skin toxicities observed. The maximum tolerated dose was established at 45 mg daily. However, two recent Phase III NSCLC trials failed to meet their primary objectives.26 Both Phase III trials assessed dacomitinib as second- or third-line therapy in molecularly unselected patients with advanced NSCLC who had received prior chemotherapy. A Study of Dacomitinib (PF-00299804) vs Erlotinib in the Treatment of Advanced Non-Small Cell Lung Cancer (ARCHER 1009) failed to meet its objective of PFS compared with its erlotinib control group, whereas the NCIC CTG BR.26 study, in which patients with advanced NSCLC had previously received both chemotherapy and an EGFR inhibitor, did not meet its primary objective of overall survival versus placebo.26,27 A third Phase III study, ARCHER-1050: A Study of Dacomitinib vs Gefitinib in 1st-Line Treatment of Advanced NSCLC (ARCHER 1050), of treatment-naive EGFR mutant NSCLC is currently ongoing and comparing dacomitinib to gefitinib, with results due in 2015.26
Afatinib
Rationale and preclinical data
Afatinib is an ATP-competitive inhibitor initially found to potently and irreversibly inhibit cysteine residues within the catalytic domains of EGFR (Cys797) and HER2 (Cys805), with half maximal effective concentration values of 0.5 and 14 nM, respectively.28 In contrast to first-generation reversible EGFR inhibitors, afatinib binds to both EGFR and HER2 through covalent bonds, resulting in their irreversible blockade and leading to more sustained target modulation. By inhibiting HER2 at low nanomolar potency, afatinib effectively blocks HER2, the preferred dimerization partner of EGFR, and subsequently prevents the formation of HER2 dimers that contribute to RTK activity and downstream pathway signaling. This combined targeting of both EGFR and HER2 may be important in overcoming drug resistance after treatment with the more specific first-generation EGFR inhibitors. Recent preclinical data have now indicated that afatinib also irreversibly and potently suppresses the enzymatic activity of HER4 (half maximal effective concentration, 1 nM) in addition to EGFR and HER2.28 Importantly, preclinical studies suggest that afatinib is more active than first-generation EGFR inhibitors in NSCLC cell lines harboring T790M mutations,29 whereas in vivo studies have shown the antitumor efficacy of afatinib in EGFR L858R/T790M double-mutant cancers.29
Afatinib-related toxicities
Several Phase I studies of afatinib have been pursued, assessing different dosing schedules in patients with advanced solid tumors. In the Phase I clinical trial of afatinib that assessed continuous daily dosing, dose-limiting toxicities were observed at dose levels of 30, 40, and 50 mg of afatinib daily and comprised grade 3 reversible pneumonitis (n=1) and acneiform rash (n=2).30 Overall, an assessment of safety data from four afatinib Phase I trials, including this study, led to the establishment of the recommended Phase II dose of 50 mg afatinib daily.30–32 These afatinib-related toxicities mainly included rash, fatigue, and gastrointestinal symptoms including diarrhea, stomatitis, mucositis, and nausea.
Proof of concept in EGFR inhibitor-naïve patients: LUX-Lung 2 study
This Phase II study evaluated response evaluation criteria in solid tumors (RECIST) response rates using two different doses (50 or 40 mg daily) of afatinib in EGFR inhibitor-naïve patients with EGFR mutant NSCLC in both first- and second-line settings (Table 3).33 Of 129 patients treated, 99 received 50 mg afatinib daily, whereas 30 received 40 mg of the drug daily. Seventy (66%) of 106 patients harboring the two common sensitizing EGFR mutations (exon 19 deletion or L858R) achieved objective antitumor responses, whereas nine (39%) of 23 patients with less-common mutations also responded. Overall, afatinib-related toxicities appeared less common in patients receiving 40 mg daily of afatinib, although there did not appear to be significant differences in the efficacy between both doses tested. In view of this better risk-to-benefit ratio, the 40 mg dose of afatinib was selected for testing in Phase III studies in the early line setting.
First-line afatinib versus chemotherapy in EGFR mutant NSCLC: LUX-Lung 3 and 6 studies
The LUX-Lung 3 and 6 studies are randomized Phase III trials of afatinib versus up to 6 cycles of standard platinum-doublet chemotherapy in patients with EGFR mutated lung adenocarcinoma in the first-line treatment setting (Table 3). Although the LUX-Lung 3 study recruited globally and randomized both Asian and non-Asian patients, the LUX-Lung 6 Phase III trial was hosted solely in East Asia.11,12,34 The latter trial was conducted because pemetrexed was not routinely available in certain countries at the time, including the People’s Republic of China, India, and Korea.34 Although LUX-Lung 3 compared afatinib with cisplatin/pemetrexed in 345 patients, LUX-Lung 6 compared afatinib with gemcitabine/cisplatin in 364 patients. The trials were otherwise identical in design. Primary analyses demonstrated an improved PFS with afatinib versus chemotherapy in the overall EGFR mutant-positive population (LUX-Lung 3: hazard ratio [HR], 0.58; LUX-Lung 6: HR, 0.28) and specifically benefits in patients with common EGFR genotypes (Del19 or L858R: LUX-Lung 3: HR, 0.47; LUX-Lung 6: HR, 0.25). The US Food and Drug Administration and European Medicines Agency have since approved afatinib for the first-line treatment of patients with advanced NSCLC harboring EGFR mutations.
Although all of the large randomized Phase III trials of EGFR TKI versus platinum-doublet chemotherapy have demonstrated a marked improvement in PFS for EGFR-RKI compared with chemotherapy, none had demonstrated an overall survival (OS) advantage because of significant crossover between trial groups. Most recently, a pooled analysis of mature OS data from the LUX-Lung 3 and 6 trials was presented.35 This analysis included 631 of 709 patients randomized into both trials with common EGFR mutations (Del19, n=355; L858R, n=276). A total of 419 patients received afatinib and 212 received chemotherapy. OS was significantly improved with afatinib versus chemotherapy (median, 27.3 versus 24.3 months; HR, 0.81 [95% confidence interval [CI], 0.66–0.99; P=0.037]). Individual HRs for OS in both studies were consistent with the pooled analysis. Among patients with EGFR Del19 mutations, HR was 0.59 (95% CI, 0.45–0.77; P<0.001), with a median OS of 31.7 months, and in those with EGFR L858R mutations, the HR was 1.25 (95% CI, 0.92–1.71; P=0.160). Updated PFS and safety data were consistent with earlier primary reports. Overall, this pooled analysis confirmed that first-line afatinib significantly improved OS in patients with advanced NSCLC harboring common EGFR mutations (Del19 and L858R) compared with standard chemotherapy. This was the first EGFR TKI to demonstrate this. Afatinib is the only TKI in which such a pooled analysis has been undertaken. Moreover, the OS pooled analysis indicated the differential activity for afatinib by a specific EGFR mutant allele, indicating that for future analyses, outcomes of exon 19 deletion patients and L858R in studies of EGFR TKIs should not be pooled.
First-line afatinib versus gefitinib: LUX-Lung 7 study
The LUX-Lung 7 study is an ongoing randomized Phase IIb global study directly comparing afatinib with gefitinib in patients with EGFR mutated lung adenocarcinoma in the front-line setting (NCT01466660; Table 3). The co-primary endpoints include PFS, time to treatment failure, and OS. Given that there are three currently licensed EGFR TKIs in Europe (gefitinib, erlotinib, and afatinib), each of which has demonstrated a PFS advantage over chemotherapy, this is an important study for the future development and registration strategy for afatinib and will assist in assessing the optimal EGFR TKI to use in the first-line EGFR mutant setting. The final data collection date for primary outcome measures is estimated in late 2014.
Overcoming acquired resistance: LUX-Lung 1 study
This Phase IIb/III LUX-Lung 1 trial compared the administration of afatinib versus placebo in a 2:1 ratio in patients with advanced lung adenocarcinoma who were previously treated with first-line chemotherapy and who had subsequently acquired drug resistance to either erlotinib or gefitinib after initial benefit (Table 3).36 This study was designed and started before routine EGFR genotyping, and thus patients were not mandated to harbor EGFR mutations; nevertheless, the clinical demographics of patients suggested the likelihood of an enriched population for EGFR mutations.
A total of 697 patients were identified, with 585 patients randomized to receive afatinib (n=390) or placebo (n=195). The primary endpoint of OS (median, 10.8 months in the afatinib group versus 12.0 months in the placebo group; P=0.74) was not met. This could be because of the unexpected median OS observed in the placebo control group of 12 months, which could be a result of most patients in this study receiving a number of antitumor treatments after trial discontinuation (79% in the control group versus 68% in the afatinib group). A post hoc analysis of patients who did not receive any subsequent antitumor therapies suggested an improved OS benefit with afatinib compared with the placebo control group (5.8 versus 4.6 months; HR, 0.65). However, the median PFS was 3.3 months in the afatinib group compared with 1.1 months in the placebo group (P<0.0001), and 29 (7%) patients achieved a Response Evaluation Criteria In Solid Tumors (RECIST) partial response in the afatinib group compared with just one patient in the placebo group, providing clear evidence of afatinib antitumor activity in this EGFR inhibitor-resistant population. Furthermore, when limiting the PFS analysis to those who met Jackman criteria for acquired resistance (36.6% of the trial population),37 the PFS difference was 4.5 months versus 1.0 months in favor of the afatinib group, suggesting a greater effect of afatinib in subgroups with a high likelihood of EGFR mutations.36 Overall, the significant improvements in PFS, as well as patient-reported symptoms and health-related quality of life observed in this clinical study were clinically important findings. There were, however, no translational studies reported from this trial to validate the activity of afatinib in patients harboring the EGFR T790M allele.
Afatinib beyond progression: LUX-Lung 5 study
The LUX-Lung 5 study, a randomized Phase III trial, assessed the role of continuing EGFR inhibition alongside chemotherapy in likely EGFR mutant NSCLC, after progression on afatinib, by assessing combination chemotherapy-afatinib versus mono-chemotherapy alone in patients failing afatinib monotherapy (Table 3).38 The primary endpoint was PFS. The population of patients recruited to this study was similar to that in the LUX-Lung 1 trial: patients with metastatic NSCLC who had received prior chemotherapy and gefitinib/erlotinib, with initial clinical benefit. All patients initially received 50 mg afatinib daily until disease progression; on progression, those who received at least 12 weeks of afatinib were randomized 2:1 to afatinib plus weekly paclitaxel (40 mg/day; 80 mg/m2 per week) or investigator’s choice of mono-chemotherapy alone.
A total of 202 patients were randomized, including 134 patients to afatinib and paclitaxel and 68 to chemotherapy alone.38 A statistically significant improvement in PFS was observed with combination treatment versus chemotherapy alone (median, 5.6 versus 2.8 months; HR, 0.60; 95% CI, 0.43–0.85; P=0.003). The overall response rate was also higher in the combination group versus chemotherapy alone (32.1% versus 13.2%; P=0.005). OS was, however, similar in both groups (12.2 versus 12.2 months; HR, 1.00; 95% CI, 0.70–1.43; P=0.994). The most commonly observed drug-related toxicities in the combination versus chemotherapy-alone group included diarrhea (53.8% versus 6.7%), alopecia (32.6% versus 15.0%), and asthenia (27.3% versus 28.3%). Overall, this study demonstrated that continued HER family inhibition with afatinib with the addition of paclitaxel significantly improved both PFS and response rates versus chemotherapy alone in heavily pretreated NSCLC patients with acquired resistance to first-generation EGFR inhibitor after disease progression on afatinib monotherapy.
Afatinib activity in the Japanese population: LUX-Lung 4 study
A Phase II study was undertaken in Japanese patients with advanced lung adenocarcinoma after progression after at least 12 weeks of prior erlotinib and/or gefitinib (Table 3).10 Sixty-two patients were treated, including 45 (72.6%) with EGFR mutant NSCLC and 51 (82.3%) who had acquired resistance to first-generation EGFR inhibitors. There were five (8.1%) patients who had RECIST partial response, and two patients with secondary T790M mutations (L858R T790M, and deletion in exon 19 T790M) achieved disease stabilization for 9 months and 1 month, respectively.
Afatinib in squamous NSCLC: LUX-Lung 8 study
In view of the antitumor activity observed in EGFR-wild-type patients with first-generation EGFR inhibitors, coupled with evidence of benefit with other irreversible pan-HER inhibitors, this study was initiated to assess the activity of afatinib versus erlotinib in squamous subtype NSCLC after the failure of at least one prior platinum-based chemotherapy (NCT01523587; Table 3). A total of 800 patients are expected to be enrolled in this study, with PFS as the primary endpoint. This trial has not completed recruitment.
Third-generation irreversible mutant-selective EGFR inhibitors
In view of the inevitable development of drug resistance despite initial antitumor benefit with first-generation EGFR inhibitors, coupled with drug-related toxicities with both first-and second-generation EGFR inhibitors, more effective and less toxic strategies are needed, including those that target the acquired EGFR T790M mutation. Although second-generation irreversible pan-HER inhibitors such as afatinib are also effective in treating EGFR mutant lung cancers, their ability to overcome T790M-mediated resistance in the clinic is likely to be limited because the concentrations at which these drugs overcome T790M activity preclinically are not achievable in humans as a result of dose-limiting toxicities related to the nonselective inhibition of wild-type EGFR.39 These second-generation EGFR inhibitors have also been shown to drive drug resistance through the acquisition of T790M in both preclinical and clinical settings, suggesting low potency against T790M.40,41 As a potential solution, afatinib was combined with the EGFR antibody cetuximab in a Phase IIB study, which demonstrated an impressive response rate of 32% in patients with EGFR mutant lung cancers resistant to first-generation EGFR inhibitors. This combination regimen was, however, associated with grade 3 (G3) or worse skin rash in 18% of patients.42
In view of these factors, it is clear that pursuing a more potent and selective approach to targeting the EGFR mutant lung cancers is a critical need. This has led to the development of third-generation EGFR inhibitors that specifically target both activating and resistant EGFR mutations, including the T790M aberration but not wild-type EGFR. These selective EGFR inhibitors include WZ4002, which was the first in class to be published but has not proceeded to clinical trials, as well as the oral inhibitors AZD9291 (AstraZeneca),43 CO-1686 (Clovis),44 and HM61713 (Hanmi Pharmaceutical Company Ltd),45 which are currently in clinical trial testing (Table 2). The Phase I studies of these latter three drugs were recently presented at the 2014 Annual Meeting of the American Society of Clinical Oncology.43–45
AZD9291
The Phase I AZD9291 First Time in Patients Ascending Dose Study (AURA) involved EGFR mutant NSCLC patients who had developed progressive disease to first- and second-generation EGFR inhibitors.43 Dose levels explored ranged from 20 to 240 mg once a day of AZD9291. Although the T790M mutation was not mandated prospectively during dose escalation (n=31), the maximum tolerated dose expansion required T790M testing with central laboratory confirmation (n=201). Pharmacokinetic studies showed a terminal half-life of 55 hours. No dose-limiting toxicities were observed during the study, and a maximum tolerated dose was not established. There was an increase in grade 2 adverse events as doses approached 240 mg of AZD9291 once daily, including diarrhea, rash, and nausea, suggesting some inhibition of wild-type EGFR at higher doses of the drug. The overall response rate observed with AZD9291 was 53%; an overall response rate of 64% was observed in EGFR T790M-positive patients versus 22% in EGFR T790M-negative patients. This was an impressive response rate considering all patients had developed prior disease progression to EGFR inhibitors. The recommended Phase II dose was established at 80 mg once daily, based on the clinical activity observed in T790M mutant patients and an increase in grade 2 toxicities at higher doses of AZD9291.
CO-1686
The Phase I study was recently completed in patients with EGFR mutant advanced NSCLC previously treated with EGFR inhibitors who had a tumor biopsy during screening for central EGFR genotyping.44 Eighty-eight patients were treated, including 63% who were positive for the EGFR T790M mutation. The dose-limiting toxicity rate at all doses was less than 33%. Related toxicities included nausea, fatigue, and impaired glucose tolerance/hyperglycemia. Hyperglycemia was well managed with oral hypoglycemics and/or dose reduction. A Phase II dose of 750 mg twice daily was recommended. Forty T790M-positive patients treated with doses above 625 mg twice daily were evaluable for response, with a 58% partial response rate observed.
HM61713
The Phase I study of HM61713 was conducted in seven centers in Korea and involved 118 patients who were pretreated with an EGFR inhibitor who had EGFR mutant advanced NSCLC.45 Patients received 75–800 mg of the drug daily, with mainly G1–G2 toxicities observed. Two cases of dose-limiting toxicities were observed of a G3 drug-induced idiosyncratic reaction composed of skin rash and dyspnea, as well as G3 raised amylase and G4 lipase. The maximum tolerated dose has not been reached, and recruitment to the 800 mg daily cohort is ongoing. Of 83 patients treated at the 300 mg daily cohort expansion, 15 confirmed partial responses were observed, including those with T790M mutations. There were no obvious differences in those who had progressed on prior EGFR inhibitors within 4 weeks or after 4 weeks or more.
Future perspectives and conclusion
The first-generation EGFR inhibitors gefitinib and erlotinib have transformed the treatment landscape of EGFR mutant NSCLC and clearly demonstrate the promise of precision medicine in molecularly defined tumors through the concept of oncogene addiction. However, the emergence of both resistant clones, leading to inevitable disease progression, and drug-related toxicities resulting from the inhibition of wild-type EGFR has limited their effectiveness in this population of patients.
The second-generation pan-HER inhibitors, exemplified by afatinib, have shown great promise in patients with EGFR mutant NSCLC. A pooled analysis showed that afatinib significantly improved OS in patients with advanced NSCLC harboring common EGFR mutations (Del 19 and L858R) when compared with standard chemotherapy in the first-line setting. Importantly, this was the first analysis to show that genotype-directed therapy for patients with EGFR mutant NSCLC can improve survival. This survival advantage was mostly driven by improved OS for the deletion 19 allele, a first in the field of EGFR TKI therapy, defining afatinib as current standard of care for this specific genotype. Nevertheless, this was only observed in a pooled analysis, and not in the respective individual clinical trials. With at least two other EGFR TKIs demonstrating a PFS benefit over chemotherapy and with the pending head-to-head comparisons of first- versus second-generation TKIs (ARCHER 1050 and LUX-Lung 7 studies), the optimal TKI to choose for EGFR mutant NSCLC remains to be established. For example, the LUX-Lung 7 randomized trial comparing afatinib and gefitinib in patients with EGFR mutated lung adenocarcinoma in the first-line setting will yield important comparative data to aid decision-making and allow both a direct comparison of efficacy as well as drug-related toxicities, which may ultimately limit utility.
Another strategy pursued has been the rational combination of afatinib with other selective molecular therapeutics. For example, the combination trial of cetuximab and afatinib has shown promising results, with an overall response rate of 29% and a median duration of response of 5.7 months in patients with advanced lung adenocarcinoma and acquired resistance to erlotinib/gefitinib.46 Importantly, this combination conferred robust and durable clinical responses irrespective of T790M status (T790M-positive, 32%, versus T790M-negative, 25%; P=0.341), as well as an acceptable safety profile.
More recently, third-generation EGFR mutation-specific inhibitors have shown potential in circumventing toxicities related to wild-type EGFR and overcoming resistance resulting from the acquired T790M mutation. Although large cohort expansions embedded within Phase I studies have only just been completed with these inhibitors, high response rates exceeding 50% in patients who have progressed on first- and second-generation EGFR inhibitors have demonstrated their promise as effective and well-tolerated drugs.43
There have also been studies that indicate that T790M clones preexist in a proportion of EGFR mutant cancers before EGFR inhibitor therapy, although the actual prevalence remains unclear because of varying sensitivities of the assays used.47 These data have important implications for determining which space these third-generation EGFR inhibitors should occupy in the increasingly busy EGFR mutant NSCLC therapeutic landscape. These third-generation EGFR inhibitors have been shown to potently block single activating mutant forms of EGFR in cell line xenograft and human EGFR transgenic mouse models.39,48 It will be important to assess whether these preclinical data will translate into clinical benefit in treatment-naïve patients and whether there will be a delay in the time to the development of acquired resistance observed with first-generation EGFR inhibitors, thus suggesting the potential to improve PFS in such patients. Ultimately, these EGFR mutation-specific third-generation inhibitors should be assessed in the first-line setting in patients with EGFR mutant NSCLC, as they potently inhibit sensitizing EGFR mutations similarly to the T790M allele, suggesting the potential for increased survival benefit. There are also in vitro data that suggest the potential to inhibit the kinase activity of both HER2 and HER4.39
In view of the highly selective nature of these third-generation EGFR inhibitors, it is likely that drug resistance will develop through potential compensatory mechanisms that will need to be identified. Preclinical studies have suggested that a direct mutation of cysteine 797, which results in acquired resistance to such irreversible inhibitors, may play an important role.39 Other key signaling networks may also provide escape pathways through non-EGFR-related resistance mechanisms, such as the insulin-like growth factor receptor, mitogen-activated protein kinase, and phosphatidylinositol-3-kinase–AKT pathways.49,50 In view of this potential for different compensatory resistance mechanisms, coupled with their mild safety profiles, these inhibitors may ultimately represent an ideal partner for combination regimens with chemotherapy, targeted therapy, and immunotherapies.
These are exciting times in the treatment of EGFR mutant lung cancer, with an armamentarium of antitumor agents now available for use. In the future, the optimal sequencing of these different EGFR inhibitors will need to be determined for maximal benefit to patients. In addition, strategies to identify potential escape mechanisms will be critical to direct the sequential application of drugs, depending on the underlying resistance mechanism. This may involve the molecular characterization of sequential tumor biopsies or surrogate tissue, such as circulating plasma DNA or circulating tumor cells.51 Only through such modern approaches will we truly achieve precision and individualized medicine for patients with EGFR mutant NSCLC.
Acknowledgments
SP and TAY acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre.
Footnotes
Disclosure
TAY is funded by the National Institute for Health Research and the Academy of Medical Sciences. SP is a noncompensated consultant to AstraZeneca, Boehringer Ingelheim, Clovis, Lilly, and Roche.
References
- 1.Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11(10):685–696. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467(7315):543–549. doi: 10.1038/nature09339. [DOI] [PubMed] [Google Scholar]
- 3.Yap TA, Sandhu SK, Workman P, de Bono JS. Envisioning the future of early anticancer drug development. Nat Rev Cancer. 2010;10(7):514–523. doi: 10.1038/nrc2870. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Morita S, Yatabe Y, et al. West Japan Oncology Group Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 6.Maemondo M, Inoue A, Kobayashi K, et al. North-East Japan Study Group Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 9.Sacher AG, Jänne PA, Oxnard GR. Management of acquired resistance to epidermal growth factor receptor kinase inhibitors in patients with advanced non-small cell lung cancer. Cancer. 2014;120(15):2289–2298. doi: 10.1002/cncr.28723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katakami N, Atagi S, Goto K, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31(27):3335–3341. doi: 10.1200/JCO.2012.45.0981. [DOI] [PubMed] [Google Scholar]
- 11.Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3342–3350. doi: 10.1200/JCO.2012.46.1764. [DOI] [PubMed] [Google Scholar]
- 12.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 13.Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13(5):528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 14.Hirsh V, Cadranel J, Cong XJ, et al. Symptom and quality of life benefit of afatinib in advanced non-small-cell lung cancer patients previously treated with erlotinib or gefitinib: results of a randomized phase IIb/III trial (LUX-Lung 1) J Thorac Oncol. 2013;8(2):229–237. doi: 10.1097/JTO.0b013e3182773fce. [DOI] [PubMed] [Google Scholar]
- 15.Schuler MH, Planchard D, Yang JCH, et al. Interim analysis of afatinib monotherapy in patients with metastatic NSCLC progressing after chemotherapy and erlotinib/gefitinib (E/G) in a trial of afatinib plus paclitaxel versus investigator’s choice chemotherapy following progression on afatinib monotherapy; Presented at: 2012 Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2012. [Google Scholar]
- 16.Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17(17):5530–5537. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10(11):760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17(5):1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson ML, Riely GJ, Rizvi NA, et al. Phase II trial of dasatinib for patients with acquired resistance to treatment with the epidermal growth factor receptor tyrosine kinase inhibitors erlotinib or gefitinib. J Thorac Oncol. 2011;6(6):1128–1131. doi: 10.1097/JTO.0b013e3182161508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(18):3076–3083. doi: 10.1200/JCO.2009.27.9414. [DOI] [PubMed] [Google Scholar]
- 23.Janjigian YY, Azzoli CG, Krug LM, et al. Phase I/II trial of cetuximab and erlotinib in patients with lung adenocarcinoma and acquired resistance to erlotinib. Clin Cancer Res. 2011;17(8):2521–2527. doi: 10.1158/1078-0432.CCR-10-2662. [DOI] [PubMed] [Google Scholar]
- 24.Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13(17):5150–5155. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter RL, Lo HW. Dacomitinib, an emerging HER-targeted therapy for non-small cell lung cancer. J Thorac Dis. 2012;4(6):639–642. doi: 10.3978/j.issn.2072-1439.2012.10.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfizer Inc Pfizer announces top-line results from two phase 3 trials of dacomitinib in patients with refractory advanced non-small cell lung cancer. [Accessed: July 1, 2014]. Available from: http://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_top_line_results_from_two_phase_3_trials_of_dacomitinib_in_patients_with_refractory_advanced_non_small_cell_lung_cancer.
- 27.Ellis PM, Liu G, Millward M, et al. NCIC CTG BR.26: A phase III randomized, double blind, placebo controlled trial of dacomitinib versus placebo in patients with advanced/metastatic non-small cell lung cancer (NSCLC) who received prior chemotherapy and an EGFR TKI; Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2014. [Google Scholar]
- 28.Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342–350. doi: 10.1124/jpet.112.197756. [DOI] [PubMed] [Google Scholar]
- 29.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28(25):3965–3972. doi: 10.1200/JCO.2009.26.7278. [DOI] [PubMed] [Google Scholar]
- 31.Eskens FA, Mom CH, Planting AS, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer. 2008;98(1):80–85. doi: 10.1038/sj.bjc.6604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap TA, Popat S. The role of afatinib in the management of non-small cell lung carcinoma. Expert Opin Drug Metab Toxicol. 2013;9(11):1529–1539. doi: 10.1517/17425255.2013.832755. [DOI] [PubMed] [Google Scholar]
- 33.Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol. 2012;13(5):539–548. doi: 10.1016/S1470-2045(12)70086-4. [DOI] [PubMed] [Google Scholar]
- 34.Wu YL, Zhou C, Hu CP, et al. LUX-Lung 6: A randomized, open-label, phase III study of afatinib (A) versus gemcitabine/cisplatin (GC) as first-line treatment for Asian patients (pts) with EGFR mutation-positive (EGFR M) advanced adenocarcinoma of the lung; Presented at: 2013 Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2013. [Google Scholar]
- 35.Yang JC, Sequist LV, Schuler MH, et al. Overall survival (OS) in patients (pts) with advanced non-small cell lung cancer (NSCLC) harboring common (Del19/L858R) epidermal growth factor receptor mutations (EGFR mut): Pooled analysis of two large open-label phase III studies (LUX-Lung 3 [LL3] and LUX-Lung 6 [LL6]) comparing afatinib with chemotherapy (CT); Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2014. [Google Scholar]
- 36.Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13(5):528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 37.Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28(2):357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuler MH, Yang CH, Park K, et al. Continuation of afatinib beyond progression: Results of a randomized, open-label, phase III trial of afatanib plus paclitaxel (P) versus investigator’s choice chemotherapy (CT) in patients (pts) with metastatic non-small cell lung cancer (NSCLC) progressed on erlotinib/gefitinib (E/G) and afatanib – LUX-Lung 5 (LL5); Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2014. [Google Scholar]
- 39.Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014 Jun 3; doi: 10.1158/2159-8290.CD-14-0337. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, Ko J, Cui Z, et al. The EGFR T790M mutation in acquired resistance to an irreversible second-generation EGFR inhibitor. Mol Cancer Ther. 2012;11(3):784–791. doi: 10.1158/1535-7163.MCT-11-0750. [DOI] [PubMed] [Google Scholar]
- 42.Janjigian YY, Groen HJ, Horn L, et al. Activity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib or gefitinib; Presented at: 2011 Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2011. [Google Scholar]
- 43.Janne PA, Ramalingam SS, Yang JC, et al. Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients (pts) with EGFR inhibitor–resistant non-small cell lung cancer (NSCLC); Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2014. [Google Scholar]
- 44.Sequist LV, Soria JC, Gadgeel SM, et al. First-in-human evaluation of CO-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations of EGFR (activating and T790M); Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2014. [Google Scholar]
- 45.Kim DW, Lee DH, Kang JH, et al. Clinical activity and safety of HM61713, an EGFR-mutant selective inhibitor, in advanced non-small cell lung cancer (NSCLC) patients (pts) with EGFR mutations who had received EGFR tyrosine kinase inhibitors (TKIs); Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2014. [Google Scholar]
- 46.Janjigian YY, Smit EF, Groen HJ, et al. Dual Inhibition of EGFR with Afatinib and Cetuximab in Kinase Inhibitor-Resistant EGFR-Mutant Lung Cancer with and without T790M Mutations. Cancer Discov. 2014 Jul 29; doi: 10.1158/2159-8290.CD-14-0326. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye X, Zhu ZZ, Zhong L, et al. High T790M detection rate in TKI-naive NSCLC with EGFR sensitive mutation: truth or artifact? J Thorac Oncol. 2013;8(9):1118–1120. doi: 10.1097/JTO.0b013e31829f691f. [DOI] [PubMed] [Google Scholar]
- 48.Walter AO, Sjin RT, Haringsma HJ, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 2013;3(12):1404–1415. doi: 10.1158/2159-8290.CD-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortot AB, Repellin CE, Shimamura T, et al. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res. 2013;73(2):834–843. doi: 10.1158/0008-5472.CAN-12-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ercan D, Xu C, Yanagita M, et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2012;2(10):934–947. doi: 10.1158/2159-8290.CD-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res. 2014;20(10):2553–2568. doi: 10.1158/1078-0432.CCR-13-2664. [DOI] [PubMed] [Google Scholar]
- 52.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 53.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 54.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 55.Mitsudomi T, Morita S, Yatabe Y, et al. West Japan Oncology Group Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 56.Maemondo M, Inoue A, Kobayashi K, et al. North-East Japan Study Group Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 57.Rosell R, Carcereny E, Gervais R, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]


