Abstract
Chronic biofilm infections are often accompanied by a chronic inflammatory response, leading to impaired healing and increased, irreversible damage to host tissues. Biofilm formation is a major virulence factor for Candida albicans and a challenge for treatment. Most current antifungals have proved ineffective in eradicating infections attributed to biofilms. The biofilm structure protects Candida species against antifungals and provides a way for them to evade host immune systems. This leads to a very distinct inflammatory response compared to that seen in planktonic infections. Previously, we showed the superior efficacy of dl-2-hydroxyisocaproic acid (HICA) against various bacteria and fungi. However, the immunomodulatory properties of HICA have not been studied. Our aim was to investigate the potential anti-inflammatory response to HICA in vivo. We hypothesized that HICA reduces the levels of immune mediators and attenuates the inflammatory response. In a murine model, a robust biofilm was formed for 5 days in a diffusion chamber implanted underneath mouse skin. The biofilm was treated for 12 h with HICA, while caspofungin and phosphate-buffered saline (PBS) were used as controls. The pathophysiology and immunoexpression in the tissues surrounding the chamber were determined by immunohistochemistry. Histopathological examination showed an attenuated inflammatory response together with reduced expression of matrix metalloproteinase 9 (MMP-9) and myeloperoxidase (MPO) compared to those of chambers containing caspofungin and PBS. Interestingly, the expression of developmental endothelial locus 1 (Del-1), an antagonist of neutrophil extravasation, increased after treatment with HICA. Considering its anti-inflammatory and antimicrobial activity, HICA may have enormous therapeutic potential in the treatment of chronic biofilm infections and inflammation, such as those seen with chronic wounds.
INTRODUCTION
Approximately 65% of human infections are biofilm related (1). A residing biofilm infection often causes aggravated inflammation in host tissues, thus leading to a chronic inflammatory status (2). Chronic inflammatory responses complicate healing and cause increased and irreversible damage to host tissues, which is characteristic of chronic wounds and periodontitis (3, 4).
Candida albicans is an opportunistic fungal pathogen that causes superficial and systemic infections in humans (5). Infection arises when the yeast is able to overcome the host immune response, and this interplay is regulated by pro- and anti-inflammatory mediators. The extracellular carbohydrate matrix of biofilms provides a very distinctive and protective niche in which yeast cells can grow within the host, and the cells often show an altered phenotype and antifungal resistance profile compared to those of planktonic counterparts (6, 7). The cells embedded within biofilms are able to evade host immune cells since the cell surface structures are masked (1). Very few studies have assessed the inflammatory response induced by C. albicans biofilm in vivo (8–10).
The management of Candida infections with commonly used antifungals is challenging due to poor efficacy, poor patient compliance, and numerous side effects and interactions (5). The most promising antibiofilm activity has been observed with the echinocandin class of antifungals, which are noncompetitive inhibitors of (1,3)-β-d-glucan synthase, an essential enzyme in fungal cell wall synthesis and integrity (11, 12). Caspofungin is the most extensively used echinocandin, especially in the treatment of invasive candidiasis (13, 14). Recently, more attention has been drawn to the immunopharmacological properties of antifungals, for example, echinocandins, whose mode of action has been shown to be dependent on these properties (15, 16).
The superior antifungal activity of the leucine derivative dl-2-hydroxyisocaproic acid (HICA) against C. albicans biofilms compared with that of caspofungin has been demonstrated (17). The efficacy of HICA against a spectrum of planktonically grown bacteria and fungi has been reported (18, 19). HICA is an α-hydroxy amino acid produced during Lactobacillus fermentation and is also found in human tissues (20, 21). It has been used for muscle recovery by professional athletes and for veterinary purposes, such as in animal feed, thus demonstrating its biocompatibility and safety profile (21, 22). Multiple studies have described the potential anti-inflammatory properties of lactobacilli and their metabolic products (23, 24).
The aim of this study was to determine the potential anti-inflammatory effects of HICA in a murine chamber model of C. albicans biofilm. To elucidate changes in the local inflammatory response, we used immunohistochemistry to detect the expression of immune proteases and other inflammatory mediators belonging to the destructive oxidative tissue cascade, which is known to play a major role in inflammatory diseases such as periodontitis (3). The core of this cascade is characterized by matrix metalloproteinase (MMP) activation by polymorphonuclear neutrophil (PMN)-secreted myeloperoxidase (MPO). Our hypothesis was that HICA attenuates the anti-inflammatory response by altering the expression of tissue proteases and endogenous proinflammatory mediators.
MATERIALS AND METHODS
Ethics statement.
All animals in this study were handled in strict accordance with good animal practice as defined in the United Kingdom Animals (Scientific Procedures) Act. Animal experiments were conducted under the ethically reviewed license authorized by the secretary of state to the University of Manchester, Manchester, United Kingdom (license no. PPL 40/3101).
Murine chamber model.
A previously published chamber model was adapted for this study (25). The biofilm chamber was structurally based on a diffusion chamber kit (Millipore, Watford, United Kingdom) comprising a semipermeable Durapore membrane with a pore size of 0.45 μm fixed to a Plexiglas ring. A nonpermeable silicon sheet was fixed to the opposite side to face the semipermeable membrane and to close the chamber, and the chambers were sterilized prior to use. A total of 24 male CD1 mice weighing 21 to 24 g were used, but one mouse was lost due to bleeding in surgery. The dorsal flank of each mouse was shaved, and a 2-cm incision was made. The diffusion chamber was implanted subcutaneously so that the semipermeable membrane faced the dorsal muscles and the nonpermeable silicon sheet faced the skin. The wound was closed with nonabsorbable braided silk sutures (Ethicon, NJ), and meloxicam (3 mg/kg of body weight daily) was administered intraperitoneally for 3 days postsurgery. One week after surgery, while the mice were under isoflurane anesthesia, the chambers were injected percutaneously with 100 μl of C. albicans strain SC5314 inoculum (106 CFU/mouse). The inoculum was mixed thoroughly before injection, and the inoculum concentration was checked using dilution plating. The mice were left to recover for 5 days, allowing for robust C. albicans biofilms to be formed inside the chambers. Then, 100 μl of 5% (wt/vol) HICA, 10 mg/liter of caspofungin, or phosphate-buffered saline (PBS) was injected percutaneously into the chambers. The mice were euthanized 12 h posttreatment with an overdose of isoflurane. The chambers were collected and the biofilms detached and weighed. Tissues around the chambers were dissected and fixed and stored in 10% formaldehyde until the analyses.
Study design.
A total of 24 mice were used in this study. Diffusion chambers (Millipore, Watford, United Kingdom) with a semipermeable membrane facing the tissues were implanted subcutaneously in the dorsal flank of each mouse, and the animals were allowed to recover for 7 days. The mice were divided into one of two main groups, a biofilm group (n = 15) or a noninfected, nonbiofilm group (n = 8). The chambers in the mice in the biofilm group were infected with C. albicans, and a robust biofilm was established over 5 days. The biofilms were treated for 12 h with HICA (n = 8), and caspofungin (n = 3) or PBS (n = 4) was used as a control treatment. The nonbiofilm chambers were treated similarly with HICA (n = 2), caspofungin (n = 3), or PBS (n = 3). The nonbiofilm HICA group was smaller due to the loss of one mouse in surgery (final n = 23). The mice were euthanized posttreatment, and the chambers and surrounding subcutaneous tissue sections were collected from each mouse. The biofilms were detached from the chambers and weighed. To analyze changes in the cellular and tissue structures and the extent of inflammatory response, tissue sections were stained with hematoxylin and eosin (H&E) and for matrix metalloproteinase-8 (MMP-8) or MMP-9, myeloperoxidase (MPO), neutrophil elastase (NE), tumor necrosis factor alpha (TNF-α), interleukin 1-beta (IL-1β), and developmental endothelial locus 1 (Del-1) using the corresponding antibodies. The stained sections were evaluated by light microscopy, and the staining intensities were semiquantified and graded.
Strain and growth conditions.
C. albicans strain SC5314 was used in this study (26). The strain was stored at −80°C, plated twice on Sabouraud dextrose agar (Melford, Suffolk, United Kingdom), and incubated at 37°C for 48 h before use to check for viability and purity. A colony was suspended in PBS and mixed well, and the cells were washed twice before adjusting the inoculum using a hemocytometer to correspond to 107 CFU/ml. Viable counts were verified by dilution plating. The viabilities of the biofilms were checked by culture after treatment with PBS, caspofungin, or HICA.
Immunohistochemistry.
Immunohistochemical staining was performed as described previously (27). Briefly, tissue sections were embedded in paraffin. These paraffin-embedded specimens were sectioned, deparaffinized, and pretreated with 0.4% pepsin, and endogenous peroxidase activity was blocked with H2O2-methanol. Staining was performed using either polyclonal Vectastain Elite rabbit or goat avidin-biotin enzyme complex (ABC) kits (Vector Laboratories, Burlingame, CA, USA). The sections were blocked with normal goat or rabbit serum in 2% bovine serum albumin and incubated with the polyclonal antibodies rabbit MMP-8 (Santa-Cruz Biotechnology, Santa-Cruz, CA, USA), goat MMP-9 (R&D Systems, Minneapolis, MN, USA), rabbit MPO (Hycult Biotechnology, Uden, Netherlands), rabbit NE (Calbiochem, San Diego, CA, USA), goat IL-1β (R&D Systems), goat TNF-α (R&D Systems), and rabbit Del-1 (Proteintech, Chicago, IL, USA). Control sections were incubated with nonimmune rabbit or goat serum. The inflammatory markers were visualized using a biotinylated anti-rabbit or anti-goat secondary antibody and avidin-biotin enzyme complex. 3-Amino-9-ethyl-carbazole was used as a chromogen and Mayer's hematoxylin (Histolab Products AB, Frölunda, Sweden) as the counterstain. All sections were also stained with hematoxylin and eosin (H&E) for routine histopathology.
The stained sections were evaluated under an Olympus BX61 light microscope, and representative images were taken using an Olympus DP50 camera and analyzed by AnalySIS v.3.2 software (Soft Imaging System GmbH, Muenster, Germany). The immunoreactivities of the tissue sections surrounding the chamber were semiquantified for each protein antibody and graded based on the staining intensity as no staining (−), mild (+), moderate (++), or strong (+++). The results from the semiquantitative analysis were confirmed blindly by a second evaluator, and a trained pathologist examined the histopathology. The distributions in staining intensity within groups were visualized using GraphPad Prism v.5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Statistical analysis.
The data were analyzed using GraphPad Prism v.5.0 software (GraphPad Software, Inc.). Univariate analysis of variance (ANOVA) was used for comparisons between biofilm weights. P values of <0.05 were considered significant.
RESULTS
C. albicans biofilms.
No significant differences were measured in the biofilm weights after treatment for 12 h with HICA, caspofungin, or PBS (P not significant). The lowest average weight (18.5 ± 7.1 mg) was measured for the biofilms treated with HICA, whereas the highest mean weight (20.8 ± 7.9 mg) was measured for caspofungin-treated biofilms. All the biofilms were viable after treatment with PBS, caspofungin, or HICA.
Histopathology.
Upon histopathological examination (H&E staining), typical components of wound healing and various levels of inflammation were seen in all of the tissue samples. Debris and fibrin together with various amounts of polymorphonuclear neutrophils (PMNs) were present in the tissues that had been resting against the semipermeable membrane of the chamber. The underlying granulation tissue presented various degrees of mixed inflammatory infiltrate. The intensities of the inflammatory responses were different within and between the groups. The degrees of cellular density and edema also varied. When the biofilm and noninfected, nonbiofilm groups were compared, marked differences were seen. In all of the nonbiofilm sections, the granulation tissue was a thin layer and inflammation was mainly composed of lymphocytes, plasma cells, and monocytes. The densities were altogether moderate. Interestingly, the HICA-treated biofilm group showed an inflammatory response similar to that seen in the nonbiofilm control sections, that is, a predominantly mononuclear cellular infiltrate that was moderate in density. However, a PMN infiltrate in the biofilm group was observed superficially compared to observations in sections in the nonbiofilm group. Sections from the two biofilm groups treated with either caspofungin or PBS showed thicker bands of granulation tissue with dense inflammatory cell infiltrates, composed mostly of macrophages and PMNs. In addition, inflammatory foci in muscle and adipose layers and abscess formations that varied in size were frequently observed in the biofilm group, particularly in the controls treated with caspofungin or PBS.
Semiquantitative immunohistochemical analysis.
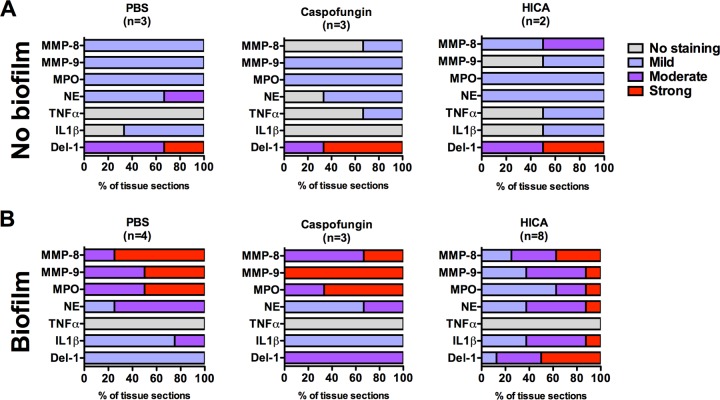
Staining for MMP-8, MMP-9, MPO, NE, IL-1β, and TNF-α was predominantly localized in the inflammatory cells in all groups. In the biofilm group, the staining for MPO, MMP-8, and MMP-9 was less intense after HICA treatment than after caspofungin or PBS treatment (Fig. 1 and 2A and B). The most distinct differences between the treatment groups were observed in the staining intensities of MPO and MMP-9. However, no marked differences were seen in MPO, MMP-8, or MMP-9 staining intensities between the treated and untreated noninfected, nonbiofilm controls (Fig. 1 and 2A and B). In general, staining for MPO, MMP-8, and MMP-9 was stronger in the biofilm group than in the nonbiofilm group. The staining of NE was stronger in the biofilm group than in the nonbiofilm group, although less intense than that for MPO (Fig. 1). However, in contrast to MPO, no marked differences could be seen between the treatments for NE. The expression of IL-1β was also stronger in the biofilm group, with minimal differences between treatments (Fig. 1). In contrast, minimal TNF-α staining was seen in sections from all treatments and groups (Fig. 1).
FIG 1.
Summary of distribution of staining intensities representing protein expression within biofilm (A) or nonbiofilm (B) groups after 12 h of treatment with 5% (wt/vol) HICA or 10 mg/liter caspofungin or PBS. Sections of subcutaneous tissue surrounding the diffusion chamber were stained using polyclonal antibodies against MMP-8, MMP-9, MPO, NE, TNF-α, IL-1β, and Del-1. Intensities of staining were analyzed semiquantitatively by two evaluators and graded as no staining or mild, moderate, or strong staining.
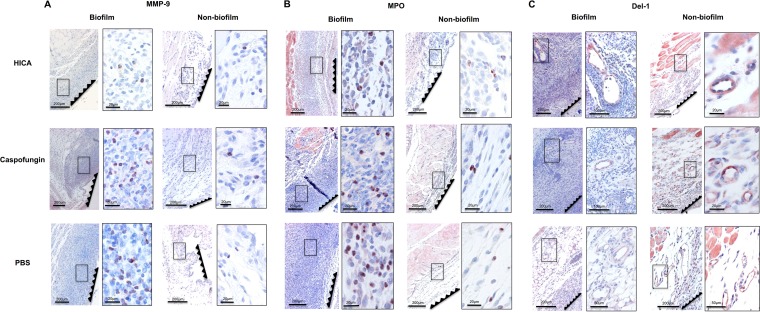
FIG 2.
Representative images of the tissue sections immunostained with polyclonal antibodies for matrix metalloproteinase (MMP-9) (A), myeloperoxidase (MPO) (B), and developmental endothelial locus 1 (Del-1) (C). The serrated lines show the location of the semipermeable membrane of the chamber. Framed panels on the right are magnifications of the areas marked with black rectangles in the left-hand panels. The expression of proinflammatory proteases MMP-9 and MPO localized predominantly in the inflammatory cells. The staining intensities for MMP-9 and MPO were lower in the HICA-treated biofilm group than in controls (treated with caspofungin or PBS), and histopathology was similar to that of nonbiofilm controls but with a thinner and less dense inflammatory cell infiltrate (A and B). Expression of neutrophil extravasation antagonist Del-1 was localized in the endothelium and was stronger in the HICA-treated group (C).
In the nonbiofilm group, Del-1 was strongly expressed by endothelial cells in sections from all treatment groups (Fig. 1 and Fig. 2C). The staining was clearly less intense in the untreated and caspofungin biofilm group sections. However, in sections from the HICA-treated group, moderate to strong staining was seen in the endothelial cells adjacent to the chambers.
DISCUSSION
This is the first study to address the impact of HICA on the inflammatory response to infection in vivo. Less inflammation was observed in tissues surrounding the biofilm-infected diffusion chamber after treatment with HICA than after caspofungin or PBS treatment. Histopathology showed a predominantly mononuclear cell profile and a less prominent and less dense PMN infiltrate. A decrease in MPO expression was observed after HICA treatment. This correlated with the decreased expression of MMP-9 in tissue sections and indicates a reduced oxidative inflammatory burden as a result of the MPO and MMP-9 cascade. Significant differences in the expression of MMP-8 and NE between the HICA and control groups were not seen.
High expression levels of MMP-8, MMP-9, MPO, and NE have been detected in chronic inflammatory diseases and linked to the loss of soft and hard tissues (3, 28). However, numerous studies have described the anti-inflammatory effect of MMP-8 and have shown its role in wound healing (27, 29, 30). NE, secreted by PMNs, plays an important role in wound healing, but prolonged secretion and excessive levels can impair the healing process, as observed in chronic wounds (31). This underlines the importance of homeostasis in the expression of immune mediators.
Tissue sections from the caspofungin and PBS biofilm groups showed characteristics of chronic inflammation, since abscess formation and inflammation in deeper tissue layers were frequently observed. This correlated with the staining pattern representing the expression (Fig. 1). In vivo studies have shown that caspofungin exerts its immunomodulatory effects through the morphological changes in the fungal cell wall structure as a result of increasing β-glucan exposure, which leads to an increased inflammatory response (32, 33).
In humans, HICA is a by-product of ketoisocaproic acid (KICA) in the leucine pathway (34). Multiple studies have investigated the immunomodulatory role of leucine, and anticatabolic and anti-inflammatory activities have been observed (35, 36). A study using a combination of herbs and leucine for the treatment of articular diseases showed an induction of IL-1β and strong downregulation of MMP-9 (37), similar to the effects seen in our study. To further support our hypothesis, multiple studies have shown the potential anti-inflammatory effect of Lactobacillus metabolites (23, 24). This is relevant because the antimicrobial activity of HICA was discovered in a mixture of fermentation products from Lactobacillus plantarum (38, 39).
Interestingly, endothelial cell-secreted protein Del-1 showed stronger expression in the HICA-treated biofilm group than in those treated with either caspofungin or PBS. The staining profile was similar to that of sections from nonbiofilm controls (Fig. 1 and 2C). Del-1 has been linked to inflammatory diseases such as periodontitis and Sjögren syndrome (40, 41). In addition to its role as an inhibitory agent against intercellular adhesion molecule 1 (ICAM-1)-dependent neutrophil adherence to lymphocyte function-associated antigen 1 (LFA-1)-integrin and extravasation, a recent study described a Del-1 inhibitory action against ICAM-1-dependent chemokine release from neutrophils, thus potentiating its regulatory role and further extending it to inflammatory circuitry (42). Our observations support the results of others and provide evidence of a potential anti-inflammatory shield induced by HICA.
A single dose of HICA or caspofungin induced no major inhibitory activity against C. albicans biofilms in a 12-h incubation. This is in line with the results of previous studies where no major antifungal activity against fully mature biofilms was observed in caspofungin lock therapy in vivo when similarly short treatment times were used (43). In our model, biofilms were left to form for 5 days before treatment. The properties and structures of such biofilms have been shown to correlate with mature (24- or 48-h) in vitro biofilm (44). In our in vitro study, HICA was highly active against mature biofilms after 24 h of treatment (17). The different inflammatory responses observed after HICA treatment may have been affected by its deteriorating effect on the biofilm ultrastructure and fungal cells.
Ours is one of the few studies presenting the inflammatory response against fully mature C. albicans biofilms in vivo. An in vitro coculture study with C. albicans biofilms and mononuclear cells showed pro- and anti-inflammatory cytokine profiles strikingly different from those of planktonic cells (45). IL-1β was significantly upregulated and, in contrast, TNF-α was significantly downregulated. Multiple cell culture and in vivo studies have shown similar downregulation of TNF-α in bacterial biofilm infections (46–48). Our findings are in line with those of previous studies and further support the view that biofilms induce a distinct immune response (Fig. 1). Interestingly, a recent study showed that neutrophils can modulate the inflammatory response by inhibiting the expression of TNF-α and IL-1β (49).
HICA can increase protein synthesis and improve muscle recovery after immobilization-induced atrophy (50). The induction of protein synthesis was interpreted to occur through the activation of mammalian target of rapamycin (mTOR) signaling. Interestingly, innate inflammatory responses induced by bacteria, fungi, parasites, and viruses have also been shown to be regulated by the mTOR pathway (51, 52). In addition, protection against mucosal damage during C. albicans infection is mediated through mTOR activation (53). These findings provide evidence for the potential action of HICA to exert its anti-inflammatory and protective effects and should be addressed in future studies.
Biofilm infections are challenging to manage, especially in patients with a compromised immune system. In addition to the efficacy of antimicrobial agents against microbial pathogens, attention should be aimed at their immunomodulatory activities. Considering its antimicrobial efficacy and anti-inflammatory activities, HICA may provide a huge therapeutic potential in the treatment of chronic biofilm infections and inflammation, such as those seen with chronic wounds.
ACKNOWLEDGMENTS
This work was financially supported by the Biotechnology and Biological Sciences Research Council, the Engineering and Physical Sciences Research Council, EU Framework 7, the Finnish Dental Society Apollonia, the Fondo Nacional de Desarrollo Científico y Tecnológico, Gilead Sciences, GlaxoSmithKline, the Helsinki University Central Hospital Research Foundation, the National Aspergillosis Centre United Kingdom, the Orion Research Foundation, the Yrjö Jahnsson Foundation, and the Wellcome Trust.
We thank Taina Tervahartiala for valuable advice regarding the technical and analytical issues and Marjatta Kivekäs for skillful technical assistance.
T.S. is one of the inventors of European patent EP0871438B1, “Use of alpha-hydroxy acids in the manufacture of a medicament for the treatment of inflammation” (39), but has not received any royalties regarding this patent. P.W. is the chief scientific officer, the director, and a shareholder of Euprotec, Ltd., which is a contract research company, and provides discovery services to multiple companies that develop treatments and vaccines for infectious diseases.
Footnotes
Published ahead of print 2 July 2014
REFERENCES
- 1.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193. 10.1128/CMR.15.2.167-193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. 10.1126/science.284.5418.1318 [DOI] [PubMed] [Google Scholar]
- 3.Hajishengallis G. 2014. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 35:3–11. 10.1016/j.it.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trostrup H, Thomsen K, Christophersen LJ, Hougen HP, Bjarnsholt T, Jensen PO, Kirkby N, Calum H, Hoiby N, Moser C. 2013. Pseudomonas aeruginosa biofilm aggravates skin inflammatory response in BALB/c mice in a novel chronic wound model. Wound Repair Regen. 21:292–299. 10.1111/wrr.12016 [DOI] [PubMed] [Google Scholar]
- 5.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci. Transl. Med. 4:165rv113. 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 6.Anderson GG, O'Toole GA. 2008. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 322:85–105. 10.1007/978-3-540-75418-3_5 [DOI] [PubMed] [Google Scholar]
- 7.Ramage G, Robertson SN, Williams C. 2014. Strength in numbers: antifungal strategies against fungal biofilms. Int. J. Antimicrob. Agents 43:114–120. 10.1016/j.ijantimicag.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 8.Xu H, Sobue T, Thompson A, Xie Z, Poon K, Ricker A, Cervantes J, Diaz PI, Dongari-Bagtzoglou A. 2013. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol. 10.1111/cmi.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nett JE, Marchillo K, Spiegel CA, Andes DR. 2010. Development and validation of an in vivo Candida albicans biofilm denture model. Infect. Immun. 78:3650–3659. 10.1128/IAI.00480-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson CC, Yu A, Lee H, Fidel PL, Jr, Noverr MC. 2012. Development of a contemporary animal model of Candida albicans-associated denture stomatitis using a novel intraoral denture system. Infect. Immun. 80:1736–1743. 10.1128/IAI.00019-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin. Microbiol. Infect. 18(Suppl 7):S19–S37. 10.1111/1469-0691.12039 [DOI] [PubMed] [Google Scholar]
- 12.Deresinski SC, Stevens DA. 2003. Caspofungin. Clin. Infect. Dis. 36:1445–1457. 10.1086/375080 [DOI] [PubMed] [Google Scholar]
- 13.Pfaller M, Boyken L, Hollis R, Kroeger J, Messer S, Tendolkar S, Diekema D. 2011. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Candida species to anidulafungin, caspofungin, and micafungin. J. Clin. Microbiol. 49:624–629. 10.1128/JCM.02120-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S, Diekema DJ. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44:760–763. 10.1128/JCM.44.3.760-763.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Ami R, Lewis RE, Kontoyiannis DP. 2008. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin. Infect. Dis. 47:226–235. 10.1086/589290 [DOI] [PubMed] [Google Scholar]
- 16.Lewis RE, Liao G, Young K, Douglas C, Kontoyiannis DP. 2014. Macrophage reporter cell assay for screening immunopharmacological activity of cell wall-active antifungals. Antimicrob. Agents Chemother. 58:1738–1743. 10.1128/AAC.02408-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieminen MT, Novak-Frazer L, Rautemaa V, Rajendran R, Sorsa T, Ramage G, Bowyer P, Rautemaa R. 2014. A novel antifungal is active against Candida albicans biofilms and inhibits mutagenic acetaldehyde production in vitro. PLoS One 9:e97864. 10.1371/journal.pone.0097864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakko M, Moore C, Novak-Frazer L, Rautemaa V, Sorsa T, Hietala P, Jarvinen A, Bowyer P, Tjaderhane L, Rautemaa R. 2014. 2-Hydroxyisocaproic acid is fungicidal for Candida and Aspergillus species. Mycoses 57:214–221. 10.1111/myc.12145 [DOI] [PubMed] [Google Scholar]
- 19.Sakko M, Tjaderhane L, Sorsa T, Hietala P, Jarvinen A, Bowyer P, Rautemaa R. 2012. 2-Hydroxyisocaproic acid (HICA): a new potential topical antibacterial agent. Int. J. Antimicrob. Agents 39:539–540. 10.1016/j.ijantimicag.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Brosnan B, Furey A, Arendt E, Murphy P, Coffey A. 2012. Antifungal activity of Lactobacillus against Microsporum canis, Microsporum gypseum and Epidermophyton floccosum. Bioeng. Bugs 3:104–113. 10.4161/bbug.19624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mero AA, Ojala T, Hulmi JJ, Puurtinen R, Karila TA, Seppala T. 2010. Effects of alfa-hydroxy-isocaproic acid on body composition, DOMS and performance in athletes. J. Int. Soc. Sports Nutr. 7:1. 10.1186/1550-2783-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boebel KP, Baker DH. 1982. Comparative utilization of the alpha-keto and d- and l-alpha-hydroxy analogs of leucine, isoleucine and valine by chicks and rats. J. Nutr. 112:1929–1939 [DOI] [PubMed] [Google Scholar]
- 23.Jones SE, Versalovic J. 2009. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 9:35. 10.1186/1471-2180-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos AN, Gobbato N, Rachid M, Gonzalez L, Yantorno O, Valdez JC. 2010. Effect of Lactobacillus plantarum and Pseudomonas aeruginosa culture supernatants on polymorphonuclear damage and inflammatory response. Int. Immunopharmacol. 10:247–251. 10.1016/j.intimp.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 25.Rajendran R, Mowat E, McCulloch E, Lappin DF, Jones B, Lang S, Majithiya JB, Warn P, Williams C, Ramage G. 2011. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob. Agents Chemother. 55:2092–2097. 10.1128/AAC.01189-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179–182. 10.1007/BF00328721 [DOI] [PubMed] [Google Scholar]
- 27.Kuula H, Salo T, Pirila E, Tuomainen AM, Jauhiainen M, Uitto VJ, Tjaderhane L, Pussinen PJ, Sorsa T. 2009. Local and systemic responses in matrix metalloproteinase 8-deficient mice during Porphyromonas gingivalis-induced periodontitis. Infect. Immun. 77:850–859. 10.1128/IAI.00873-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorsa T, Tjaderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mantyla P. 2006. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann. Med. 38:306–321. 10.1080/07853890600800103 [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noel A, Werb Z, Krane SM, Lopez-Otin C, Puente XS. 2007. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J. 21:2580–2591. 10.1096/fj.06-7860com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirila E, Korpi JT, Korkiamaki T, Jahkola T, Gutierrez-Fernandez A, Lopez-Otin C, Saarialho-Kere U, Salo T, Sorsa T. 2007. Collagenase-2 (MMP-8) and matrilysin-2 (MMP-26) expression in human wounds of different etiologies. Wound Repair Regen. 15:47–57. 10.1111/j.1524-475X.2006.00184.x [DOI] [PubMed] [Google Scholar]
- 31.McDaniel JC, Roy S, Wilgus TA. 2013. Neutrophil activity in chronic venous leg ulcers—a target for therapy? Wound Repair Regen. 21:339–351. 10.1111/wrr.12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamaris GA, Lewis RE, Chamilos G, May GS, Safdar A, Walsh TJ, Raad II, Kontoyiannis DP. 2008. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J. Infect. Dis. 198:186–192. 10.1086/589305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler RT, Kombe D, Agarwala SD, Fink GR. 2008. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 4:e1000227. 10.1371/journal.ppat.1000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffer LJ, Taveroff A, Robitaille L, Mamer OA, Reimer ML. 1993. Alpha-keto and alpha-hydroxy branched-chain acid interrelationships in normal humans. J. Nutr. 123:1513–1521 [DOI] [PubMed] [Google Scholar]
- 35.Bruckbauer A, Biggerstaff J, Zemel MB. 2012. Leucine and calcitriol modulation of human airway inflammation and hyper-reactivity. FASEB J. 26(Suppl):1012.2. 10.1096/fj.1530-6860 [DOI] [Google Scholar]
- 36.Zanchi NE, Nicastro H, Lancha AH. 2008. Potential antiproteolytic effects of l-leucine: observations of in vitro and in vivo studies. Nutr. Metab. (Lond.) 5:20. 10.1186/1743-7075-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhtar N, Miller MJ, Haqqi TM. 2011. Effect of a herbal-leucine mix on the IL-1beta-induced cartilage degradation and inflammatory gene expression in human chondrocytes. BMC Complement. Altern. Med. 11:66. 10.1186/1472-6882-11-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hietala PK, Westermarck HW, Jaarma M. 1979. Identification of antimicrobial alpha-hydroxyacids in Lactobacillus plantarum-fermented animal protein. Nutr. Metab. 23:227–234. 10.1159/000176260 [DOI] [PubMed] [Google Scholar]
- 39.Westermarck HW, Hietala P, Jaarma M, Sorsa T, Vaara M. January 1997. Use of alpha-hydroxy acids in the manufacture of a medicament for the treatment of inflammation. European patent EP0871438B1
- 40.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. 2012. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 13:465–473. 10.1038/ni.2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baban B, Liu JY, Abdelsayed R, Mozaffari MS. 2013. Reciprocal relation between GADD153 and Del-1 in regulation of salivary gland inflammation in Sjogren syndrome. Exp. Mol. Pathol. 95:288–297. 10.1016/j.yexmp.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 42.Shin J, Hosur KB, Pyaram K, Jotwani R, Liang S, Chavakis T, Hajishengallis G. 2013. Expression and function of the homeostatic molecule Del-1 in endothelial cells and the periodontal tissue. Clin. Dev. Immunol. 2013:617809. 10.1155/2013/617809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walraven CJ, Lee SA. 2013. Antifungal lock therapy. Antimicrob. Agents Chemother. 57:1–8. 10.1128/AAC.01351-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricicova M, Kucharikova S, Tournu H, Hendrix J, Bujdakova H, Van Eldere J, Lagrou K, Van Dijck P. 2010. Candida albicans biofilm formation in a new in vivo rat model. Microbiology 156:909–919. 10.1099/mic.0.033530-0 [DOI] [PubMed] [Google Scholar]
- 45.Chandra J, McCormick TS, Imamura Y, Mukherjee PK, Ghannoum MA. 2007. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect. Immun. 75:2612–2620. 10.1128/IAI.01841-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 70:6339–6345. 10.1128/IAI.70.11.6339-6345.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seth AK, Geringer MR, Hong SJ, Leung KP, Galiano RD, Mustoe TA. 2012. Comparative analysis of single-species and polybacterial wound biofilms using a quantitative, in vivo, rabbit ear model. PLoS One 7:e42897. 10.1371/journal.pone.0042897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 186:6585–6596. 10.4049/jimmunol.1002794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gresnigt MS, Joosten LA, Verschueren I, van der Meer JW, Netea MG, Dinarello CA, van de Veerdonk FL. 2012. Neutrophil-mediated inhibition of proinflammatory cytokine responses. J. Immunol. 189:4806–4815. 10.4049/jimmunol.1103551 [DOI] [PubMed] [Google Scholar]
- 50.Lang CH, Pruznak A, Navaratnarajah M, Rankine KA, Deiter G, Magne H, Offord EA, Breuille D. 2013. Chronic alpha-hydroxyisocaproic acid treatment improves muscle recovery after immobilization-induced atrophy. Am. J. Physiol. Endocrinol. Metab. 305:E416–E428. 10.1152/ajpendo.00618.2012 [DOI] [PubMed] [Google Scholar]
- 51.Shertz CA, Cardenas ME. 2011. Exploiting and subverting Tor signaling in the pathogenesis of fungi, parasites, and viruses. PLoS Pathog. 7:e1002269. 10.1371/journal.ppat.1002269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, Muller M, Saemann MD. 2008. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29:565–577. 10.1016/j.immuni.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 53.Moyes DL, Shen C, Murciano C, Runglall M, Richardson JP, Arno M, Aldecoa-Otalora E, Naglik JR. 2014. Protection against epithelial damage during Candida albicans infection is mediated by PI3K/Akt and mammalian target of rapamycin signaling. J. Infect. Dis. 10.1093/infdis/jit824 [DOI] [PMC free article] [PubMed] [Google Scholar]