Abstract
New prevention and treatment strategies are needed for visceral leishmaniasis, particularly ones that can be deployed simply and inexpensively in areas where leishmaniasis is endemic. Synthetic molecules that activate Toll-like receptor 7 and 8 (TLR7/8) pathways have previously been demonstrated to enhance protection against cutaneous leishmaniasis. We initially sought to determine whether the TLR7/8-activating molecule resiquimod might serve as an effective vaccine adjuvant targeting visceral leishmaniasis caused by infection with Leishmania infantum chagasi. Resiquimod was topically applied to the skin of mice either prior to or after systemic infection with L. infantum chagasi, and parasite burdens were assessed. Surprisingly, topical resiquimod application alone, in the absence of vaccination, conferred robust resistance to mice against future intravenous challenge with virulent L. infantum chagasi. This protection against L. infantum chagasi infection persisted as long as 8 weeks after the final topical resiquimod treatment. In addition, in mice with existing infections, therapeutic treatment with topical resiquimod led to significantly lower visceral parasite loads. Resiquimod increased trafficking of leukocytes, including B cells, CD4+ and CD8+ T cells, dendritic cells, macrophages, and granulocytes, in livers and spleens, which are the key target organs of visceralizing infection. We conclude that topical resiquimod leads to systemic immune modulation and confers durable protection against visceralizing L. infantum chagasi infection, in both prophylactic and therapeutic settings. These studies support continued studies of TLR-modulating agents to determine mechanisms of protection and also provide a rationale for translational development of a critically needed, novel class of topical, preventative, and therapeutic agents for these lethal infections.
INTRODUCTION
An estimated 12 million people worldwide are infected by the protozoan parasite Leishmania, with a growing number (350 million) at risk of infection (1, 2). Clinical syndromes associated with leishmaniasis range from skin and mucous-membrane ulcers (cutaneous leishmaniasis [CL] and mucocutaneous leishmaniasis) to systemic, potentially fatal, disease of the organs (visceral leishmaniasis [VL]). First-line chemotherapy for VL includes antimonial compounds, amphotericin B, paromomycin, and miltefosine, but these options are limited by various factors, including cost, toxicity, and drug resistance (3). Although there are currently no effective vaccines for human use, combining immunotherapeutic approaches with conventional chemotherapy might offer several advantages over treating leishmaniasis with chemotherapy alone (4, 5).
Host immunity is a critical factor that influences the outcome of infection with Leishmania species. Toll-like receptors (TLRs) on innate immune cells are critical components of pathways stimulating cellular immunity. TLRs found on antigen-presenting cells (APCs) recognize microbe-associated molecules and trigger APC activation, expression of costimulatory molecules, and cytokine release. Naturally occurring and synthetic TLR ligands have therefore been extensively explored as a means to activate APCs in the context of vaccination. The imidazoquinoline family is comprised of small nucleoside analogs that specifically activate TLR7 and/or TLR8 and stimulate plasmacytoid dendritic cells (pDCs) to mature and produce type I interferons and other cytokines (6, 7). The TLR7 agonist imiquimod is FDA-approved for clinical use against a variety of cutaneous viral infections and neoplasias, including genital warts, actinic keratoses, and superficial basal cell carcinomas (8). Several studies have examined the potential of imiquimod for treating cutaneous Leishmania infections (9). In mice, topical imiquimod application reduced infection levels and ulceration in mouse footpads infected with L. major (10). Human trials examining the therapeutic benefits of imiquimod against both Old World (11–14) and New World (15–18) CL have demonstrated mixed results, with treatment regimens combining imiquimod with antimonial chemotherapy faring better than imiquimod alone.
Resiquimod (also known as R-848 and S-28463) is a related imidazoquinoline that can activate both TLR7 and TLR8 pathways in humans and is 10- to 100-fold more potent in vitro and in vivo than imiquimod (7, 19, 20). Although resiquimod has not been as extensively characterized as imiquimod in infectious disease models, both molecules have been studied as vaccine adjuvants against multiple intracellular pathogens and tumors (21–28). We initially sought to determine whether topically administered resiquimod might serve as a useful vaccine adjuvant to enhance immunity against visceral infection with L. infantum chagasi. We unexpectedly found that topical resiquimod treatment protected mice from systemic VL infection even in the absence of vaccination, suggesting a potency and utility not previously associated with imiquimod. Here we characterize both the prophylactic and therapeutic efficacies of topical resiquimod against VL, as well as the cellular infiltrates in the liver and spleen of resiquimod-treated mice that correlate with reduced parasite burdens.
MATERIALS AND METHODS
Animals, parasites, and reagents.
Six- to eight-week-old female BALB/c mice (Jackson Laboratories) were used for all animal experiments. The L. infantum chagasi strain MHOM/BR/00/1669 (previously known as L. chagasi and now considered synonymous with L. infantum) (29), originally isolated from a patient in northeastern Brazil, was used to vaccinate and challenge experimental animals. Parasites were maintained in outbred male golden hamsters, isolated from splenocytes, and passaged in HOMEM medium (30) at 26°C. Cultures enriched for infective metacyclic parasites were obtained by growing freshly subcultured organisms for 5 to 7 days to achieve stationary phase. The imidazoquinoline compounds resiquimod (also known as R-848 or S-28463) and imiquimod were graciously provided by 3M Pharmaceuticals and Graceway Pharmaceuticals (24). 3M Pharmaceuticals prepared the imiquimod in a 5% cream formulation, the resiquimod in 1% cream and 0.2% gel formulations, and control vehicles for both cream and gel. Soluble resiquimod (Sigma-Aldrich) was reconstituted at a concentration of 1 mg/ml in 10% dimethyl sulfoxide (DMSO) and diluted as necessary.
MTT viability assay.
Logarithmic-phase L. infantum chagasi promastigotes were pelleted at 3,500 rpm for 15 min, washed and resuspended in HOMEM, and placed in a sterile 24-well plate at a concentration of 5 × 106/well with various concentrations of resiquimod or a vehicle (0.5% DMSO) control. Promastigotes were incubated at 26°C for 48 h and then treated with 10 μl of 12 mM MTT (Molecular Probes, CA) according to the manufacturer's protocol. The absorbance was read at 540 nm using a Synergy2 multimode microplate reader to determine the amount of formazan production, which correlates with the relative parasite viability.
Intracellular parasite assay.
RAW264.7 mouse macrophages (American Type Culture Collection) growing in complete Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS) were harvested, plated in 12-well plates on top of sterile coverslips at a concentration of 2 × 105/well, and allowed to adhere to coverslips overnight. Cells were infected with 107 L. infantum chagasi/well for 4 h, washed to remove extracellular parasites, and treated with various concentrations of resiquimod. Infection levels were determined after 24 h by microscopic examination of cells, following staining of coverslips using Hema-3 staining reagents (Fisher Scientific).
Prophylactic protection studies.
Mice were vaccinated subcutaneously with 107 L. infantum chagasi promastigotes in the dorsum of the neck, where the parasites remain localized. Resiquimod was applied topically to the area of vaccination on the days described. Mice were euthanized at described time points, and touch preparations of liver sections were made onto glass slides, Giemsa stained, and microscopically analyzed by a blind scorer. Parasites were counted in a minimum of 500 mammalian cells, and the amastigote/cell ratio was multiplied by the liver weight (in mg). As an approximation of the total amastigote load in the liver, this product was multiplied by 2 × 105, a conversion factor that has been previously defined (31).
Therapeutic treatment studies.
An inoculum of 107 stationary-phase virulent L. infantum chagasi promastigotes was intravenously injected into BALB/c mice to establish visceral infection. Starting 3 days after infection, resiquimod (either 1% cream or 0.2% gel) was applied to the shaved backs of mice. Mice were euthanized and organs were isolated for liver touch preps, quantitative PCR (qPCR), or flow cytometric analysis.
qPCR.
To determine parasite loads by qPCR, livers and spleens were mechanically homogenized in phosphate-buffered saline (PBS), and total genomic DNA was harvested using an UltraClean tissue DNA isolation kit (MoBio Laboratories). qPCR assays were then performed using a TaqMan system (Life Technologies), with 200 nM primers/probe and genomic DNA template diluted 1:10 following column elution. L. infantum chagasi parasite DNA and mouse DNA were detected using primers specific for GP63 and RPLP0 (36B4), respectively. GP63 primers and probes were synthesized by Integrated DNA Technologies (Coralville, IA) with the following sequences: GP63 for, 5′-GTA CGG CTG CGA CAC CTT-3′; GP63 rev, 5′-AGC CGA GGT CCT GGA AGA T-3′; and GP63 probe, 5′-/56-FAM-AGC CCG CAC CGC CCT GGT-36-TAMSp-3′ (where FAM is 6-carboxyfluorescein and TAMSp is 6-carboxytetramethylrhodamine). The RPLP0 TaqMan gene expression assay (Mm00725448_s1) was purchased from Life Technologies. GP63 threshold cycle (CT) values below 35 were converted to absolute parasite counts using previously determined standard curves and normalized to tissue DNA amounts by RPLP0 CT values, as previously described (32). Relative parasite loads per cell were calculated by dividing GP63 values by RPLP0 values; relative parasite loads per organ were calculated by multiplying these normalized values by organ weight (in mg). GP63 CT values above 35 were considered to be below the limit of detection, based on negative controls with no Leishmania DNA template.
Preparation of lymph nodes, spleens, and liver leukocytes and flow cytometry.
Spleens and livers were isolated and pressed through a nylon filter, and suspensions were centrifuged. Pellets were resuspended in 1× Pharmlyse (BD Biosciences) and incubated at room temperature for 5 min; PBS was then added, and the samples were centrifuged and resuspended in PBS. Aliquots of 106 cells from each organ sample were prepared in staining buffer (PBS, 3% FBS, 0.1% sodium azide) with Fc block, incubated with the appropriate antibodies for 15 to 30 min at 4°C, washed and resuspended in staining buffer, and analyzed by flow cytometry on a BD LSR II cytometer. CD45+ leukocytes were gated for further analysis. Total leukocytes in both liver and spleen were counted and multiplied by percentages of each gated subset to quantitate. All antibodies were purchased from BD Pharmingen.
Statistics.
Student t test was used for all statistical analyses. P values < 0.05 were considered significant.
Ethics statement.
All animal procedures were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals and the LA BioMed IACUC.
RESULTS
Topical resiquimod prophylactically protects mice against visceral leishmaniasis in the absence of antigen-specific vaccination.
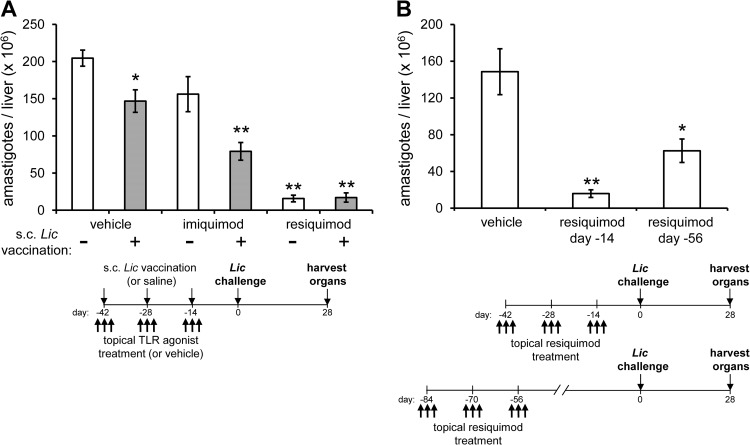
We initially set out to investigate the vaccine adjuvant properties of resiquimod, given the previously reported ability of the related molecule imiquimod to enhance both cellular and humoral immune responses, particular TH1 responses (33). We used an established method of antileishmanial vaccination (34), by subcutaneously injecting a high inoculum (107) of live L. infantum chagasi as an antigen source into the dorsum of the neck (these parasites remain localized and do not disseminate). Topical formulations of either imiquimod (included as a control reference) or resiquimod were applied to shaved areas of skin in conjunction with subcutaneous inoculation of the live leishmanial vaccine. A total of three subcutaneous vaccinating doses were delivered, each 2 weeks apart, and each accompanied by three TLR agonist applications (on days −1, 0, and 1 relative to the vaccine injection). As observed by other investigators (34), subcutaneous vaccination with L. infantum chagasi alone (with vehicle) led to a reduction in parasite burden (∼25%) compared to control (saline-injected) mice at 4 weeks after challenge (Fig. 1A). Vaccination accompanied by imiquimod treatment significantly enhanced protection (∼65%), whereas vaccination plus resiquimod conferred even higher levels of protection (>90%). Surprisingly, topical administration of resiquimod to unvaccinated mice also conferred high levels (>90%) of protection. These results demonstrate a potent efficacy of resiquimod compared to imiquimod against intravenous challenge with L. infantum chagasi and suggest that the full protective effects of resiquimod do not require prior or concurrent antigen-specific priming.
FIG 1.
Topical resiquimod treatment prophylactically and durably protects mice against experimental visceral leishmaniasis. (A) Mice were injected subcutaneously with three doses of either saline (no antigen; white bars) or a live L. infantum chagasi vaccine (gray bars), each administered 2 weeks apart. Concurrently, either imiquimod (5% cream), resiquimod (1% cream), or a vehicle cream was applied topically to the shaved dorsal area of mice on the day before, the same day, and the day after vaccination injections. At 14 days after the final vaccination, the mice were intravenously challenged with virulent L. infantum chagasi and euthanized 4 weeks later to assess average parasite burdens in the liver (n = 8 mice per group. (B) Vehicle cream or resiquimod (1% cream) was applied to mice in the absence of vaccination, in three 3-day sets, as before. Mice were challenged with L. infantum chagasi either 14 days or 56 days after the last treatment and then euthanized 4 weeks later (n = 4 to 5 mice/group for each resiquimod group, n = 7 mice/group for vehicle). All results are representative of at least two independent experiments. Error bars denote the standard errors of the means (SEM); all comparisons are to vehicle with no vaccination. *, P < 0.05; **, P < 0.005.
Short-term induction of cytokine and chemokine expression by resiquimod, as previously shown, could result in protection from infection. To determine whether resiquimod-induced protection was due to short-lived immune responses or persisted over time as a form of durable immunity, we again treated mice over the course of 4 weeks with topical resiquimod alone. We then challenged the mice intravenously with virulent L. infantum chagasi either two or 8 weeks after the last resiquimod application. Although protection after 8 weeks was somewhat diminished compared to the protection observed after 2 weeks, the parasite burden remained significantly lower in resiquimod pretreated mice compared to untreated mice in both cases (Fig. 1B), indicating a long-lasting, vaccine-independent protective effect of resiquimod alone against systemic infection.
Topical resiquimod is effective therapy against established experimental murine visceral leishmaniasis.
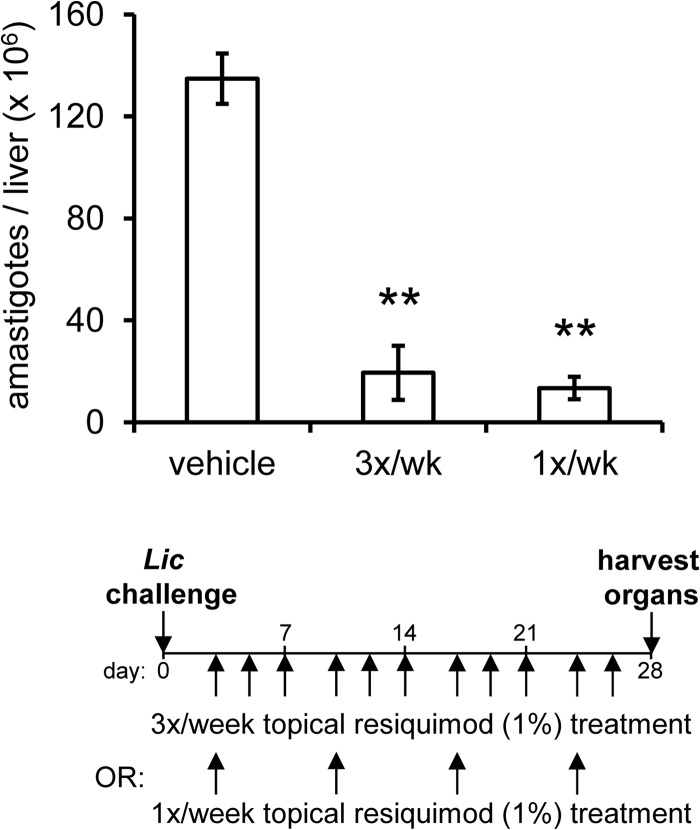
The ability of resiquimod to prophylactically protect mice against subsequent parasite challenge raised the possibility that resiquimod might also have therapeutic efficacy against existing infections. To test whether topical resiquimod treatment would decrease the parasite burden of mice with established VL infections, we injected naive BALB/c mice intravenously with virulent L. infantum chagasi. Four days later, we began topical applications of resiquimod either once or three times per week to the shaved healthy skin of infected animals. After 4 weeks of treatment, the animals were euthanized and parasite burdens were quantitated. Mice treated with resiquimod had a significant reduction in liver parasite burdens compared to untreated mice (Fig. 2). Resiquimod treatment once per week displayed equivalent protection levels as treatment three times per week.
FIG 2.
Topical resiquimod treatment leads to reduction of established visceral Leishmania infection. (A) Mice were intravenously infected with L. infantum chagasi. After 4 days, vehicle cream or resiquimod (1% cream) was applied to shaved dorsal areas either once or three times per week, for 4 weeks. Mice were euthanized 28 days after infection to assess parasite burden in the liver (n = 5 mice per group). The results are representative of two independent experiments. Error bars denote the SEM. **, P < 0.005.
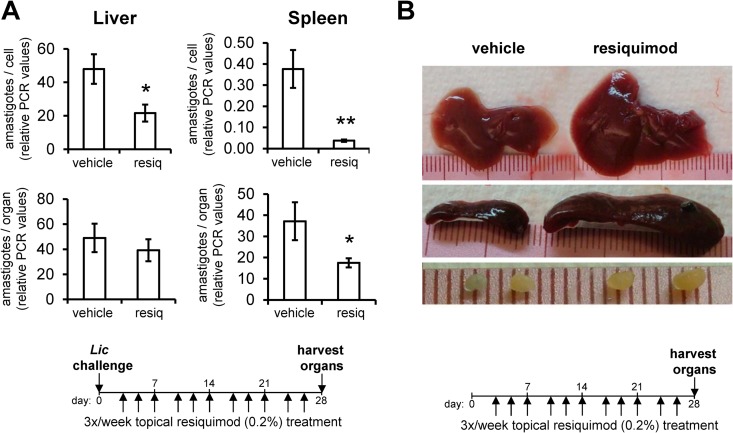
To determine whether the antileishmanial effects of resiquimod were dose concentration dependent, we next tested the therapeutic efficacy of a less concentrated (0.2%) formulation of resiquimod than has previously been tested in healthy adults (35). As a more sensitive assessment of parasite burden, we utilized qPCR to measure the amount of parasite DNA in organ homogenates, a method which generates similar quantitative results as liver section microscopy and allows for the quantitation of parasite loads in the spleen (which are difficult to assess by microscopy). Topical 0.2% resiquimod treatment over 4 weeks led to a significant parasite reduction in both the liver and spleen on a per-cell basis, as measured by PCR (Fig. 3A). Overall parasite burdens were also significantly reduced in the spleens of resiquimod-treated mice, but not in the liver. As in prior experiments, we observed that resiquimod-treated mice displayed significant increases in organ sizes. Since both Leishmania infection and TLR agonist treatment are known to cause organ enlargement (22, 36), we verified that the organ enlargements were primarily due to resiquimod, by topically treating uninfected mice with 0.2% resiquimod as before. Enlargement of livers and spleens were consistently observed in naive, resiquimod-treated mice compared to vehicle-treated mice (Fig. 3B), with an average liver weight of 1.86 g versus 1.13 g (P = 3.2 × 10−8) and an average spleen weight of 480 mg versus 95 mg (P = 3.5 × 10−15). Giemsa-stained liver and spleen sections displayed no differences in gross architecture, despite their enlargement following resiquimod treatment. Due to the resiquimod-induced increased size of the liver, the total numbers of parasites in the liver were not significantly different from controls, even though the density of parasites was reduced by resiquimod (Fig. 3A). Overall, these data demonstrate that topical resiquimod treatment impacts both organ size and parasite density in both the liver and spleen in mice with established infections.
FIG 3.
Topical resiquimod treatment leads to organomegaly and reduction of parasite density in liver and spleen. (A) Mice were intravenously infected with L. infantum chagasi. After 4 days, vehicle gel or resiquimod (0.2%) was applied to the shaved dorsal areas of mice three times a week for 4 weeks. Mice were euthanized 28 days after infection, and the parasite burdens in the liver and spleen were measured by qPCR. Parasite loads are expressed in relative PCR units as either parasites per cell or parasites per organ (n = 8 to 10 mice per group). The results are representative of two independent experiments. Error bars denote the SEM; *, P < 0.05; **, P < 0.005. (B) Livers, spleens, and lymph nodes from uninfected mice that were treated with resiquimod (0.2% gel, or vehicle gel) for 4 weeks were harvested and weighed. Displayed are representative livers (top), spleens (middle), and a pair of lymph nodes (bottom) from each group. Average weights were calculated from n = 8 animals per group.
Resiquimod has limited effects on Leishmania infantum chagasi promastigotes in vitro.
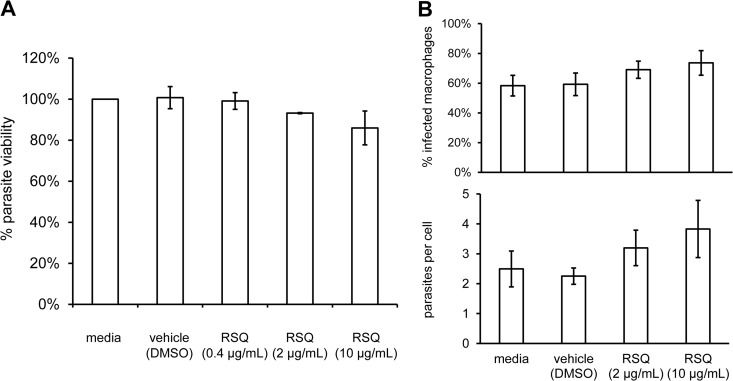
We next assessed the direct in vitro effects of soluble resiquimod on L. infantum chagasi parasites to determine whether the in vivo protection could be explained by direct leishmanicidal activity. Log-phase promastigotes were exposed to various concentrations of soluble resiquimod for 48 h, and viability was measured by using an MTT assay. Exposure of promastigotes to resiquimod at various concentrations in vitro had no detectable effect on promastigote growth compared to controls (Fig. 4A). Since Leishmania parasites predominantly exist in the intracellular amastigote stage during their life cycle following infection, we next determined whether resiquimod could impact amastigote survival within macrophages. RAW264.7 macrophages were infected with L. infantum chagasi promastigotes and then treated with increasing doses of resiquimod. Resiquimod treatment for 24 h did not lead to a reduction in the percentage of infected macrophages or the numbers of parasites per cell, with moderate increases (not statistically significant) observed for both measurements (Fig. 4B). We therefore did not detect any significant parasiticidal activity of soluble resiquimod on Leishmania growth in vitro, either as axenic promastigotes or intracellular amastigotes within macrophages.
FIG 4.
Soluble resiquimod does not impact growth of promastigotes or intracellular amastigotes in vitro. (A) Log-phase promastigotes were incubated with increasing concentrations of soluble resiquimod, and metabolic activities (normalized to media alone control) were assessed after 48 h with an MTT assay. Error bars denote the SEM of three combined independent experiments; each experiment was performed in triplicate. (B) RAW264.7 macrophages harboring L. infantum chagasi amastigotes were treated with increasing concentrations of soluble resiquimod, and infected cells were assessed by microscopy after 24 h to quantitate the percentage of cells infected and the average number of parasites per cell. Error bars denote the SEM of four independently infected coverslips.
Topical resiquimod alters cellular immune profiles in target organs.
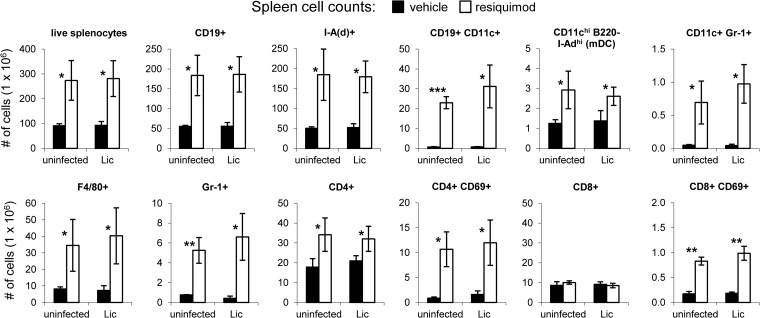
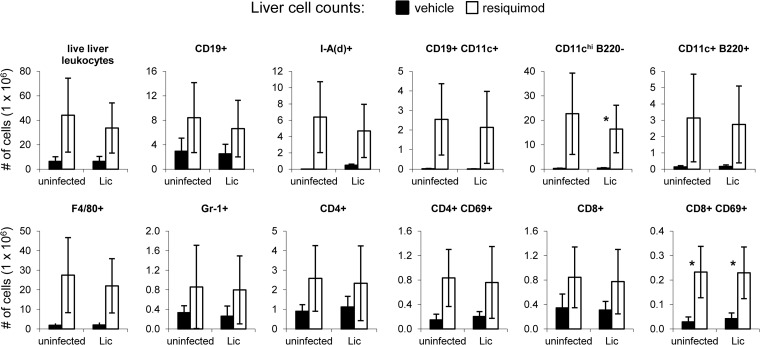
Toll-like receptor agonist molecules, including imidazoquinolines, alter cell migration (21), induce cytokine production (20), and modulate the distribution of leukocyte subsets (22, 36). To determine the immunological impact of topical resiquimod in the VL model, we assessed the cellular content of livers and spleens from both infected and uninfected mice after 4 weeks of treatment with 0.2% resiquimod. Consistent with the significant increases in organ sizes observed after resiquimod treatment, there were higher overall numbers of leukocytes in both the livers and the spleens of resiquimod-treated mice compared to mice treated with vehicle gel. Approximately 100 × 106 live splenocytes were recovered from vehicle mice compared to an average of 280 × 106 splenocytes from resiquimod-treated mice, regardless of infection status (Fig. 5). Homogenized liver preparations yielded 6 × 106 live leukocytes compared to an average of approximately 40 × 106 leukocytes from vehicle- and resiquimod-treated mice, respectively, whether naive or L. infantum chagasi infected (Fig. 6).
FIG 5.
Topical resiquimod treatment leads to increased leukocyte numbers and activation status in spleens of both uninfected and Leishmania-infected mice. Mice were intravenously injected with either saline (uninfected) or L. infantum chagasi. After 4 days, vehicle gel (black bars) or resiquimod (0.2%; white bars) was applied to the shaved dorsal areas of mice three times a week for 4 weeks (as in Fig. 3). Mice were euthanized 28 days after infection. Single cell suspensions of splenocytes were counted, stained with antibodies against various cell surface markers, and analyzed by flow cytometry. The data are presented in millions of total cells. Error bars represent the SEM (n = 3 mice per group). *, P < 0.05; **, P < 0.005; ***, P < 0.0005. Vehicle was compared to resiquimod for each comparison.
FIG 6.
Topical resiquimod treatment leads to increased leukocyte numbers and activation status in livers of both uninfected and Leishmania-infected mice. Mice were intravenously injected with either saline (uninfected) or L. infantum chagasi. After 4 days, vehicle gel (black bars) or resiquimod (0.2%; white bars) was applied to the shaved dorsal areas of mice three times a week for 4 weeks. Mice were euthanized 28 days after infection. Single cell suspensions of livers were counted, stained with antibodies against various cell surface markers, and analyzed by flow cytometry. The data are presented in millions of total cells. Error bars represent the SEM (n = 3 mice per group). *, P < 0.05. Vehicle was compared to resiquimod for each comparison.
To determine changes in the numbers of specific cellular subsets after 4 weeks of resiquimod treatment, we used flow cytometry and various antibodies to detect cellular markers. Resiquimod-treated mice had higher numbers of CD19+ B cells and cells expressing major histocompatibility complex (MHC) class II on their surface [I-A(d)+] in the spleen (Fig. 5). A significant subset of the resiquimod-induced CD19+ cells were also CD11c+, cells that were not detected in untreated mice. Non-B cell CD11c+ cells that were B220− but expressed high levels of MHC class II [I-A(d)+] were likely myeloid DCs and were moderately increased in spleens from resiquimod-treated mice. A small population of CD11c+ Gr-1+ pDCs was detected in resiquimod-treated mice (but not untreated mice) as well. Resiquimod treatment also led to significant increases in the numbers of neutrophils (Gr-1+ cells) and macrophages (F4/80+), but not NK cells (data not shown). Resiquimod led to increased expression of the early activation marker CD69 on CD4+ and CD8+ T cells and to higher overall numbers of CD4+ T cells in the spleen. However, the total number of CD8+ cells was not significantly different compared to controls. All resiquimod-induced cellular changes observed were similar in both L. infantum chagasi-infected and in uninfected animals. In the liver, resiquimod-induced cellular changes similar to those in the spleen were observed. However, most of these differences were not statistically significant due to high variability in the number of live leukocytes isolated from livers. The numbers of CD19+ B cells, CD11c+ DCs, Gr-1+ neutrophils, F4/80+ macrophages, and activated CD4+ and CD8+ T cells all trended higher in resiquimod-treated livers (Fig. 6) compared to vehicle treatment, similar to the spleen. Unlike the spleen, we were unable to detect any CD11cint Gr-1+ pDCs in the liver. Large increases were seen overall in the number of APCs expressing MHC class II molecules [I-A(d)+]. In sum, these data demonstrate significant increases in specific subsets of lymphocytes, granulocytes, macrophages, and APCs in the liver and spleen, the major organs infected by visceralizing species of Leishmania.
DISCUSSION
We initially set out to explore the adjuvant properties of resiquimod compared to imiquimod, hypothesizing that it would enhance prophylactic vaccination in a murine model of VL. Resiquimod applied topically during a 4-week course of subcutaneous vaccination did indeed result in significantly reduced liver parasite burdens following systemic challenge, to a greater extent than imiquimod. Surprisingly, however, resiquimod was directly effective, even in the absence of vaccination. Even when administered up to 8 weeks prior to parasite challenge, resiquimod treatment alone generated significant prophylactic protection compared to controls. The long-lasting nature of this protection was striking and suggested that resiquimod stimulates protective mechanisms that do not require prior priming of Leishmania antigen-specific T cells. We hypothesized that other cellular changes resulting from resiquimod-induced cytokine and chemokine production might be potential drivers of the observed protection. The cellular changes in both the liver and the spleen that we report here are a first step in determining the mechanistic basis for these cellular increases and their relationship to the observed long-lasting resiquimod-induced protection against VL.
Because imidazoquinolines are poorly soluble in water, administration of these compounds has focused primarily on delivery via topical formulations. Human trials of topical imiquimod in CL patients, in combination with pentavalent antimony, demonstrated modest improvements over antimony treatment alone (15–18). These studies have focused exclusively on cutaneous disease to which imiquimod could be directly applied, however. Our data in mice suggest that topical administration of the more potent resiquimod has beneficial immunomodulatory effects in cases of systemic, organ-infecting VL. New therapies are needed for these infections, and the ability to apply a topical agent relatively infrequently (e.g., once per week) would be of substantial advantage in deploying the therapy in underdeveloped countries where VL predominates.
Use of a 0.2% resiquimod gel on a small area of shaved mouse skin was sufficient to induce significant cellular changes in the organs and reduction of existing parasite burden, without causing any severe adverse effects either locally on the skin or systemically. Mild wrinkling and crusting on the skin of mice was observed, and only mild skin irritation has been reported after topical application in humans (35). Other groups have recently delivered resiquimod parenterally to mice by utilizing liposomal (37) or microparticle (38) encapsulation and demonstrated reduced parasite loads of L. donovani in vivo. Although the pharmacokinetics of these various forms of delivery will be distinct in humans compared to mouse models, the imidazoquinolines are in general associated with low degrees of toxicity in humans when applied topically, in contrast to many of the currently approved chemotherapies for human VL (35).
In humans, resiquimod stimulates both TLR7 and TLR8, whereas imiquimod binds only TLR7. However, murine TLR8 does not appear to respond to resiquimod (39, 40). The unique ability of resiquimod, but not imiquimod, to generate prophylactic antileishmanial protection in our mouse model of VL infection is therefore probably not attributable to the stimulation of distinct TLR pathways but is perhaps due to differences in the degree of stimulation by the two molecules. Resiquimod induces cytokine production from monocytes and macrophages at doses approximately 10- to 100-fold less than imiquimod (7, 19, 20, 41, 42). Resiquimod, but not imiquimod, is efficient at inducing IgM synthesis and class switching in B cells (43). Further dose-response and mechanistic experiments will be necessary to determine whether the protective efficacy of resiquimod alone against VL is due to its increased potency or to distinct characteristics of the molecule compared to imiquimod.
The cytokine expression profile induced by imidazoquinolines has been well characterized (44). In general, imidazoquinolines induce type I interferons (IFN-α and -β) in pDCs that play an important role in the clearance of many viral infections (45). These cytokines can stimulate cell activation via modulation of the IFN-γ pathway (46, 47), which triggers activation of NO production by macrophages and is generally associated with a protective response in CL (48–50). Early in vitro studies of the effects of resiquimod on cytokine expression by immune cells demonstrated high expression levels at concentrations of 1 μg/ml (20). Our in vitro results using a macrophage cell line demonstrated that resiquimod concentrations up to 10 μg/ml did not induce macrophage killing of L. infantum chagasi intracellular parasites. Soluble resiquimod was previously shown to directly enhance leishmanicidal activity in L. donovani-infected macrophages in vitro, via induction of iNOS and enhanced release of NO (10). The reasons for this discrepancy are unclear but may reflect different parasite species or different doses of resiquimod used.
This study demonstrates that topical resiquimod treatment leads to systemic immunomodulation, distinct cellular changes in the liver and spleen, and significant protection against experimental murine VL. These results are unique in that topical administration demonstrates efficacy against a systemic (nonlocal) infection and, in the case of prophylactic application, protection occurs in the absence of specific antigen-stimulation prior to challenge. These data support the idea that imidazoquinolines have value for use in direct therapy, as vaccine adjuvants, and/or in combination with conventional chemotherapies, to confer protective or therapeutic responses against protozoan infections (51). Future studies of resiquimod will be necessary to determine the exact mechanisms of this protection and to determine whether these effects are specific to leishmaniasis or whether they can be expanded to other pathogens. Finally, these results demonstrate the potential translational prophylactic and therapeutic benefit of a simple, topical regimen that could be deployed widely even in underdeveloped areas to combat deadly VL infections.
ACKNOWLEDGMENTS
This study was supported in part by the National Institutes of Health (grant number AI078431 to N.C.) and the Los Angeles Biomedical Research Institute (seed grants to R.B. and K.W.B.).
We thank Mary Wilson from the University of Iowa and her lab members for assistance with establishing the models of visceral leishmaniasis in our lab. We thank Brad Spellberg for critical reading of the manuscript. We thank T. C. Meng, Robert Babilon, and Mark Tomai for critical reading of the manuscript and assistance in obtaining the imiquimod, resiquimod, and placebo vehicle agents. We thank 3M Pharmaceuticals and Graceway Pharmaceuticals for providing the imiquimod, resiquimod, and placebo vehicles used in these studies.
Footnotes
Published ahead of print 16 July 2014
REFERENCES
- 1.Desjeux P. 2001. The increase in risk factors for leishmaniasis worldwide. Trans. R. Soc. Trop. Med. Hyg. 95:239–243. 10.1016/S0035-9203(01)90223-8 [DOI] [PubMed] [Google Scholar]
- 2.Shaw J. 2007. The leishmaniases—survival and expansion in a changing world: a minireview. Mem. Inst. Oswaldo Cruz 102:541–547. 10.1590/S0074-02762007000500001 [DOI] [PubMed] [Google Scholar]
- 3.Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366:1561–1577. 10.1016/S0140-6736(05)67629-5 [DOI] [PubMed] [Google Scholar]
- 4.van GJ, Balasegaram M, Meheus F, Alvar J, Lynen L, Boelaert M. 2010. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 10:184–194. 10.1016/S1473-3099(10)70011-6 [DOI] [PubMed] [Google Scholar]
- 5.Alvar J, Croft S, Olliaro P. 2006. Chemotherapy in the treatment and control of leishmaniasis. Adv. Parasitol. 61:223–274. 10.1016/S0065-308X(05)61006-8 [DOI] [PubMed] [Google Scholar]
- 6.Miller RL, Meng TC, Tomai MA. 2008. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect. 21:69–87. 10.1358/dnp.2008.21.2.1188193 [DOI] [PubMed] [Google Scholar]
- 7.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP. 2002. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell. Immunol. 218:74–86. 10.1016/S0008-8749(02)00517-8 [DOI] [PubMed] [Google Scholar]
- 8.Wagstaff AJ, Perry CM. 2007. Topical imiquimod: a review of its use in the management of anogenital warts, actinic keratoses, basal cell carcinoma, and other skin lesions. Drugs 67:2187–2210. 10.2165/00003495-200767150-00006 [DOI] [PubMed] [Google Scholar]
- 9.Reynolds KA, Loughlin WA, Young DJ. 2013. Quinolines as chemotherapeutic agents for leishmaniasis. Mini. Rev. Med. Chem. 13:730–743. 10.2174/1389557511313050010 [DOI] [PubMed] [Google Scholar]
- 10.Buates S, Matlashewski G. 1999. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J. Infect. Dis. 179:1485–1494. 10.1086/314782 [DOI] [PubMed] [Google Scholar]
- 11.Seeberger J, Daoud S, Pammer J. 2003. Transient effect of topical treatment of cutaneous leishmaniasis with imiquimod. Int. J. Dermatol. 42:576–579. 10.1046/j.1365-4362.2003.01955.x [DOI] [PubMed] [Google Scholar]
- 12.Firooz A, Khamesipour A, Ghoorchi MH, Nassiri-Kashani M, Eskandari SE, Khatami A, Hooshmand B, Gorouhi F, Rashighi-Firoozabadi M, Dowlati Y. 2006. Imiquimod in combination with meglumine antimoniate for cutaneous leishmaniasis: a randomized assessor-blind controlled trial. Arch. Dermatol. 142:1575–1579. 10.1001/archderm.142.12.1575 [DOI] [PubMed] [Google Scholar]
- 13.Al-Mutairi N, Alshiltawy M, El KM, Joshi A, Eassa BI, Manchanda Y, Gomaa S, Darwish I, Rijhwani M. 2009. Tropical medicine rounds: treatment of Old World cutaneous leishmaniasis with dapsone, itraconazole, cryotherapy, and imiquimod, alone and in combination. Int. J. Dermatol. 48:862–869. 10.1111/j.1365-4632.2008.04010.x [DOI] [PubMed] [Google Scholar]
- 14.El-On J, Bazarsky E, Sneir R. 2007. Leishmania major: in vitro and in vivo anti-leishmanial activity of paromomycin ointment (Leshcutan) combined with the immunomodulator Imiquimod. Exp. Parasitol. 116:156–162. 10.1016/j.exppara.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 15.Arevalo I, Ward B, Miller R, Meng TC, Najar E, Alvarez E, Matlashewski G, Llanos-Cuentas A. 2001. Successful treatment of drug-resistant cutaneous leishmaniasis in humans by use of imiquimod, an immunomodulator. Clin. Infect. Dis. 33:1847–1851. 10.1086/324161 [DOI] [PubMed] [Google Scholar]
- 16.Miranda-Verastegui C, Llanos-Cuentas A, Arevalo I, Ward BJ, Matlashewski G. 2005. Randomized, double-blind clinical trial of topical imiquimod 5% with parenteral meglumine antimoniate in the treatment of cutaneous leishmaniasis in Peru. Clin. Infect. Dis. 40:1395–1403. 10.1086/429238 [DOI] [PubMed] [Google Scholar]
- 17.Arevalo I, Tulliano G, Quispe A, Spaeth G, Matlashewski G, Llanos-Cuentas A, Pollack H. 2007. Role of imiquimod and parenteral meglumine antimoniate in the initial treatment of cutaneous leishmaniasis. Clin. Infect. Dis. 44:1549–1554. 10.1086/518172 [DOI] [PubMed] [Google Scholar]
- 18.Miranda-Verastegui C, Tulliano G, Gyorkos TW, Calderon W, Rahme E, Ward B, Cruz M, Llanos-Cuentas A, Matlashewski G. 2009. First-line therapy for human cutaneous leishmaniasis in Peru using the TLR7 agonist imiquimod in combination with pentavalent antimony. PLoS Negl. Trop. Dis. 3:e491. 10.1371/journal.pntd.0000491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomai MA, Gibson SJ, Imbertson LM, Miller RL, Myhre PE, Reiter MJ, Wagner TL, Tamulinas CB, Beaurline JM, Gerster JF. 1995. Immunomodulating and antiviral activities of the imidazoquinoline S-28463. Antivir. Res. 28:253–264. 10.1016/0166-3542(95)00054-P [DOI] [PubMed] [Google Scholar]
- 20.Wagner TL, Ahonen CL, Couture AM, Gibson SJ, Miller RL, Smith RM, Reiter MJ, Vasilakos JP, Tomai MA. 1999. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell. Immunol. 191:10–19. 10.1006/cimm.1998.1406 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Tomai MA, Miller RL, Sauder DN. 2000. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J. Invest. Dermatol. 114:135–141. 10.1046/j.1523-1747.2000.00833.x [DOI] [PubMed] [Google Scholar]
- 22.Craft N, Bruhn KW, Nguyen BD, Prins R, Lin JW, Liau LM, Miller JF. 2005. The TLR7 agonist imiquimod enhances the anti-melanoma effects of a recombinant Listeria monocytogenes vaccine. J. Immunol. 175:1983–1990. 10.4049/jimmunol.175.3.1983 [DOI] [PubMed] [Google Scholar]
- 23.Weeratna RD, Makinen SR, McCluskie MJ, Davis HL. 2005. TLR agonists as vaccine adjuvants: comparison of CpG ODN and resiquimod (R-848). Vaccine 23:5263–5270. 10.1016/j.vaccine.2005.06.024 [DOI] [PubMed] [Google Scholar]
- 24.Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP. 2007. Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev. Vaccines 6:835–847. 10.1586/14760584.6.5.835 [DOI] [PubMed] [Google Scholar]
- 25.Othoro C, Johnston D, Lee R, Soverow J, Bystryn JC, Nardin E. 2009. Enhanced immunogenicity of Plasmodium falciparum peptide vaccines using a topical adjuvant containing a potent synthetic Toll-like receptor 7 agonist, imiquimod. Infect. Immun. 77:739–748. 10.1128/IAI.00974-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton GC, Vedvick TS, Reed SG, Coler RN. 2009. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine 27:3063–3071. 10.1016/j.vaccine.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang WW, Matlashewski G. 2008. Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infect. Immun. 76:3777–3783. 10.1128/IAI.01527-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mark KE, Spruance S, Kinghorn GR, Sacks SL, Slade HB, Meng TC, Selke S, Magaret A, Wald A. 7 April 2014. Three phase III randomized controlled trials of topical resiquimod 0.01% gel to reduce anogenital herpes recurrences. Antimicrob. Agents Chemother. 10.1128/AAC.00077-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauricio IL, Stothard JR, Miles MA. 2000. The strange case of Leishmania chagasi. Parasitol. Today 16:188–189. 10.1016/S0169-4758(00)01637-9 [DOI] [PubMed] [Google Scholar]
- 30.Berens RL, Brun R, Krassner SM. 1976. A simple monophasic medium for axenic culture of hemoflagellates. J. Parasitol. 62:360–365. 10.2307/3279142 [DOI] [PubMed] [Google Scholar]
- 31.Bradley DJ, Kirkley J. 1977. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin. Exp. Immunol. 30:119–129 [PMC free article] [PubMed] [Google Scholar]
- 32.Bruhn KW, Marathe C, Maretti-Mira AC, Nguyen H, Haskell J, Tran TA, Vanchinathan V, Gaur U, Wilson ME, Tontonoz P, Craft N. 2010. LXR deficiency confers increased protection against visceral Leishmania infection in mice. PLoS Negl. Trop. Dis. 4:e886. 10.1371/journal.pntd.0000886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasilakos JP, Tomai MA. 2013. The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev. Vaccines 12:809–819. 10.1586/14760584.2013.811208 [DOI] [PubMed] [Google Scholar]
- 34.Streit JA, Recker TJ, Filho FG, Beverley SM, Wilson ME. 2001. Protective immunity against the protozoan Leishmania chagasi is induced by subclinical cutaneous infection with virulent but not avirulent organisms. J. Immunol. 166:1921–1929. 10.4049/jimmunol.166.3.1921 [DOI] [PubMed] [Google Scholar]
- 35.Sauder DN, Smith MH, Senta-McMillian T, Soria I, Meng TC. 2003. Randomized, single-blind, placebo-controlled study of topical application of the immune response modulator resiquimod in healthy adults. Antimicrob. Agents Chemother. 47:3846–3852. 10.1128/AAC.47.12.3846-3852.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baenziger S, Heikenwalder M, Johansen P, Schlaepfer E, Hofer U, Miller RC, Diemand S, Honda K, Kundig TM, Aguzzi A, Speck RF. 2009. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood 113:377–388. 10.1182/blood-2008-04-151712 [DOI] [PubMed] [Google Scholar]
- 37.Peine KJ, Gupta G, Brackman DJ, Papenfuss TL, Ainslie KM, Satoskar AR, Bachelder EM. 2014. Liposomal resiquimod for the treatment of Leishmania donovani infection. J. Antimicrob. Chemother. 69:168–175. 10.1093/jac/dkt320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duong AD, Sharma S, Peine KJ, Gupta G, Satoskar AR, Bachelder EM, Wyslouzil BE, Ainslie KM. 2013. Electrospray encapsulation of Toll-like receptor agonist resiquimod in polymer microparticles for the treatment of visceral leishmaniasis. Mol. Pharm. 10:1045–1055. 10.1021/mp3005098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200. 10.1038/ni758 [DOI] [PubMed] [Google Scholar]
- 40.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. 2002. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3:499. 10.1038/ni0602-499 [DOI] [PubMed] [Google Scholar]
- 41.Fujisawa H, Shivji GM, Kondo S, Wang B, Tomai MA, Miller RL, Sauder DN. 1996. Effect of a novel topical immunomodulator, S-28463, on keratinocyte cytokine gene expression and production. J. Interferon Cytokine Res. 16:555–559. 10.1089/jir.1996.16.555 [DOI] [PubMed] [Google Scholar]
- 42.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S. 2002. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195:1507–1512. 10.1084/jem.20020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomai MA, Imbertson LM, Stanczak TL, Tygrett LT, Waldschmidt TJ. 2000. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell. Immunol. 203:55–65. 10.1006/cimm.2000.1673 [DOI] [PubMed] [Google Scholar]
- 44.Stanley MA. 2002. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin. Exp. Dermatol. 27:571–577. 10.1046/j.1365-2230.2002.01151.x [DOI] [PubMed] [Google Scholar]
- 45.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921. 10.1126/science.8009221 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O'Shea JJ, Biron CA. 2002. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 297:2063–2066. 10.1126/science.1074900 [DOI] [PubMed] [Google Scholar]
- 47.Freudenberg MA, Merlin T, Kalis C, Chvatchko Y, Stubig H, Galanos C. 2002. Cutting edge: a murine, IL-12-independent pathway of IFN-gamma induction by gram-negative bacteria based on STAT4 activation by type I IFN and IL-18 signaling. J. Immunol. 169:1665–1668. 10.4049/jimmunol.169.4.1665 [DOI] [PubMed] [Google Scholar]
- 48.Shankar AH, Morin P, Titus RG. 1996. Leishmania major: differential resistance to infection in C57BL/6 (high interferon-alpha/beta) and congenic B6.C-H-28c (low interferon-alpha/beta) mice. Exp. Parasitol. 84:136–143. 10.1006/expr.1996.0099 [DOI] [PubMed] [Google Scholar]
- 49.Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Rollinghoff M, Gresser I, Bogdan C. 1998. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity 8:77–87. 10.1016/S1074-7613(00)80460-4 [DOI] [PubMed] [Google Scholar]
- 50.Mattner J, Wandersee-Steinhauser A, Pahl A, Rollinghoff M, Majeau GR, Hochman PS, Bogdan C. 2004. Protection against progressive leishmaniasis by IFN-β. J. Immunol. 172:7574–7582. 10.4049/jimmunol.172.12.7574 [DOI] [PubMed] [Google Scholar]
- 51.Dalton JE, Kaye PM. 2010. Immunomodulators: use in combined therapy against leishmaniasis. Expert Rev. Anti Infect. Ther. 8:739–742. 10.1586/eri.10.64 [DOI] [PubMed] [Google Scholar]