Abstract
In this phase III, open-label, multicenter, and descriptive study in India, children primed with 3 doses (at ages 6, 10, and 14 weeks) of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) were randomized (1:1) to receive a booster dose at 9 to 12 (early booster) or 15 to 18 months old (late booster) in order to evaluate impact of age at booster. We also evaluated a 2-dose catch-up vaccination plus an experimental booster dose in unprimed children age 12 to 18 months. The early booster, late booster, and catch-up vaccinations were administered to 74, 95, and 87 children, respectively; 66, 71, and 81 children, respectively, were included in the immunogenicity according-to-protocol cohort. One month postbooster, for each PHiD-CV serotype, ≥95.2% (early booster) and ≥93.8% (late booster) of the children had antibody concentrations of ≥0.2 μg/ml; ≥96.7% and ≥93.0%, respectively, had opsonophagocytic activity (OPA) titers of ≥8. The postbooster antibody geometric mean concentrations (GMCs) were in similar ranges for early and late boosters; the OPA titers appeared to be lower for most PHiD-CV serotypes (except 6B and 19F) after the early booster. After dose 2 and postbooster, for each PHiD-CV serotype, ≥88.6% and ≥96.3%, respectively, of the catch-up immunogenicity according-to-protocol cohort had antibody concentrations of ≥0.2 μg/ml; ≥71.4% and ≥90.6%, respectively, had OPA titers of ≥8. At least 1 serious adverse event was reported by 2 children in the early booster (skin infection and gastroenteritis) and 1 child in the catch-up group (febrile convulsion and urinary tract infection); all were resolved, and none were considered by the investigators to be vaccine related. PHiD-CV induced robust immune responses regardless of age at booster. Booster vaccination following 2 catch-up doses induced robust immune responses indicative of effective priming and immunological memory. (These studies have been registered at www.clinicaltrials.gov under registration no. NCT01030822 and NCT00814710; a protocol summary is available at www.gsk-clinicalstudyregister.com [study ID 112909]).
INTRODUCTION
Streptococcus pneumoniae is the leading cause of pneumonia, meningitis, and septicemia. Worldwide, pneumococcal infections are estimated to have been responsible for 541,000 deaths in 2008 in children <5 years of age, with a high burden in Southeast Asia (108,000 deaths in 2008 in children <5 years) (http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/).
The 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) has been shown to be immunogenic and well tolerated in infants in India (1). Moreover, recent double-blind randomized controlled trials demonstrated that infant vaccination with PHiD-CV was effective in preventing vaccine-type invasive pneumococcal disease (2, 3), community-acquired pneumonia (4, 5), and acute otitis media (6).
PHiD-CV is typically given as a 2- or 3-dose primary series in infants, with a booster dose administered in their second year of life. However, in some countries, vaccination visits beyond the first year are not routine; thus, vaccination ends at the age of ≤6 months, without a booster dose being administered. Developing countries generally opt for a 3-dose primary schedule for PHiD-CV, without a booster dose. Moreover, compliance with vaccination decreases as children get older (7). Nevertheless, epidemiological and clinical evidence with other pneumococcal conjugate vaccine (PCV) formulations suggest that booster vaccination may be of high value (8). Recently, the World Health Organization (WHO) has also endorsed a 2-dose schedule starting at 6 weeks of age, followed by a third dose at 9 to 15 months of age (9).
The current study compared the immunogenicity and safety of PHiD-CV booster doses given at 9 to 12 months and at 15 to 18 months old. Antibody persistence in both groups was assessed up to the age of 24 months (9 to 15 months after booster vaccination). In addition, this study assessed the immunogenicity and safety of PHiD-CV administered as a 2-dose catch-up schedule in unprimed children in their second year of life, followed by an experimental booster dose.
MATERIALS AND METHODS
Study objectives.
The primary objective was to assess the immune responses following vaccination with a PHiD-CV booster dose in children age 9 to 12 months (early booster group) or 15 to 18 months (late booster group) who were previously vaccinated with 3 PHiD-CV doses at 6, 10, and 14 weeks of age (1). The secondary objectives comprised an assessment of antibody persistence following primary vaccination and booster vaccination up to approximately 24 months of age, as well as an assessment of the safety and reactogenicity of the booster dose. Additionally, we assessed the immunogenicity and safety of PHiD-CV when administered as a 2-dose catch-up immunization during the second year of life (12 to 18 months of age at the time of first vaccination), followed by an experimental booster dose at 18 to 24 months of age.
Study design.
This was a phase III randomized study (ClinicalTrials.gov registration no. NCT01030822) with 3 parallel groups (early booster, late booster, and catch-up) conducted in 4 centers in India between April 2010 and August 2011 (ClinicalTrials.gov registration no. NCT00814710). The study was conducted in an open manner, as the participants from the different groups received the study vaccine according to different vaccination schedules.
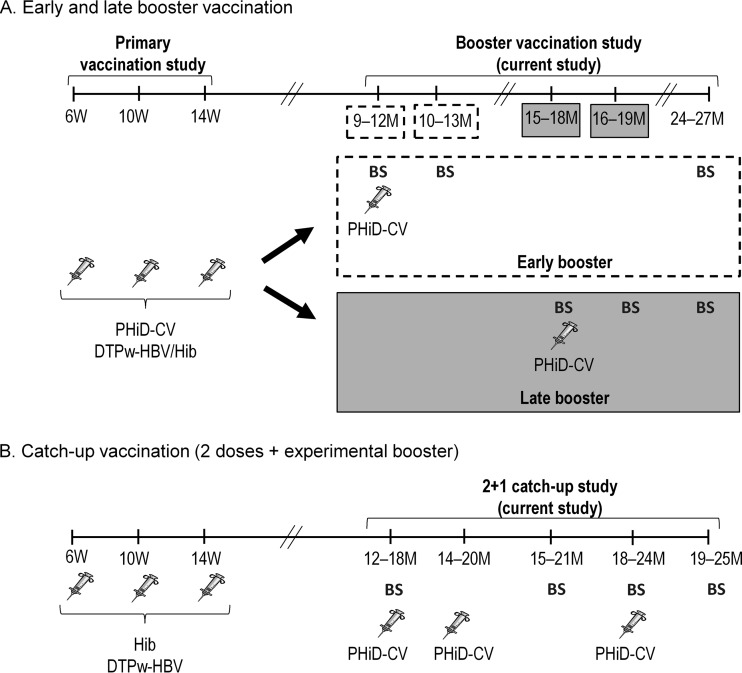
For the early and late booster groups, the study comprised 3 visits at month 0 (booster vaccination), month 1, and month 9 (late booster group) or 15 (early booster group). For the catch-up group, the study comprised 5 visits at month 0 (dose 1), month 2 (dose 2), month 3, month 6 (booster), and month 7 (Fig. 1).
FIG 1.
Study design for the early and late booster vaccination (A) and catch-up vaccination (B). W, weeks; M, months; syringe, vaccination; BS, blood sampling. Due to a delay in the study start, the age range for receiving the booster dose in the 9- to 12-month group was extended to 9 to 18 months of age, but only the results for the 9- to 12-month subgroup were used for the assessment of the early booster.
The study was conducted according to the principles of Good Clinical Practice and the Declaration of Helsinki, as well as with the approval of an independent ethics committee. When deviations were detected, corrective actions were implemented where feasible, and the appropriate ethics committees and regulatory authorities were notified. Written informed consent was obtained from the parents or a legally acceptable representative for each child before enrollment. For 10 out of 287 enrolled subjects, informed consent (IC) was obtained using the initial version of the IC form instead of an updated IC form version. However, the nature of the changes introduced in the IC update are considered not to have affected the validity of the informed consent of these subjects; thus, their data were included in the analyses.
Study participants and vaccines.
Healthy children were previously randomized in the primary vaccination study to receive PHiD-CV (Synflorix; GlaxoSmithKline, Belgium) coadministered with diphtheria-tetanus-whole-cell pertussis-hepatitis B virus/Hib (DTPw-HBV/Hib) (Tritanrix HepB/Hib; GlaxoSmithKline) or a control vaccine (Hib vaccine [Hiberix; GlaxoSmithKline] coadministered with DTPw-HBV [Tritanrix HepB, GlaxoSmithKline]) (1); this treatment allocation was kept in the current study. Children previously primed with PHiD-CV at 6, 10, and 14 weeks of age (1) were randomized (1:1) using a central internet randomization system (SBIR) to receive a PHiD-CV booster vaccination at 9 to 12 months of age (early booster group) or at 15 to 18 months of age (late booster group). Due to a delay in the study start, the age range for receiving the booster dose in the 9- to 12-month group was extended to 9 to 18 months of age. In order to allow for an evaluation of the booster vaccination at 9 to 12 months of age (primary objective), this group was divided into 2 subgroups (9 to 12 and 13 to 18 months) for analysis. The results for the 9- to 12-month subgroup are presented here (early booster group); the results for the entire 9- to 18-month group can be found in Tables S1 to S3 in the supplemental material.
For the catch-up group, healthy unprimed children from the control group of the primary vaccination study (1) were enrolled to receive the first catch-up dose at 12 to 18 months of age.
During the study, children may have been vaccinated with a measles vaccine or with the diphtheria-tetanus-acellular pertussis (DTPa)/Hib vaccine (Infanrix/Hiberix; GlaxoSmithKline Vaccines) according to the recommendations of the Indian Academy of Pediatrics (IAP). PHiD-CV was administered intramuscularly in the right or left thigh; concomitantly administered vaccines were administered in the opposite anterolateral thigh.
Exclusion criteria.
Children were excluded from participation if they had used other investigational or nonregistered products within 30 days before vaccination or had planned their use during the study period, had received immune-modifying drugs for >14 days within 6 months before vaccination, or had received any pneumococcal vaccine since the end of the primary vaccination course. Children who had any confirmed or suspected immunosuppressive or immunodeficient condition, a history of allergic disease or reactions likely to be exacerbated by any component of the vaccine, major congenital defects or a serious chronic illness, or a history of neurologic disorders or seizures were also excluded from participation. The participants were not to have received any blood products within 3 months before vaccination or during the study period.
Assessment of antibody responses.
In the early and late booster groups, blood samples were taken before and 1 month after vaccination and at approximately 24 months of age. In the catch-up group, blood samples were taken before vaccination, 1 month after dose 2, prebooster, and 1 month after booster vaccination.
Pneumococcal serotype-specific IgG antibodies against PHiD-CV serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F, as well as against cross-reactive serotypes 6A and 19A, were quantified using an enzyme-linked immunosorbent assay developed by GlaxoSmithKline Vaccines (22F-ELISA; assay cutoff, 0.05 μg/ml). The immune response was calculated in terms of the percentage of children with antibody concentrations of ≥0.2 μg/ml. An antibody concentration of 0.2 μg/ml measured by the 22F-ELISA is equivalent to the antibody concentration of 0.35 μg/ml measured by the non-22F ELISA of the WHO reference laboratory (10). Note that no serotype-specific immunological correlate of protection has yet been established for PCVs, and these percentages thus hold no protective meaning.
Opsonophagocytic activity (OPA) against the above-mentioned serotypes was measured by a pneumococcal killing assay using an HL-60 cell line, with an opsonic titer cutoff value of 8. The results are presented as the reciprocal of the dilution of serum (opsonic titer) able to sustain 50% killing of pneumococci under the assay conditions.
Anti-protein D antibodies were quantified using an ELISA developed by GlaxoSmithKline Vaccines, with a cutoff of 100 ELISA units (EL.U)/ml.
Safety assessment.
Solicited local and general symptoms were recorded within 4 days postvaccination, and unsolicited adverse events (AEs) were recorded within 31 days postvaccination. Fever was defined as a rectal temperature of ≥38°C or oral/axillary/tympanic temperature of ≥37.5°C; the preferred route for recording temperature in this study was axillary. Symptom intensity was graded on a scale of 1 (mild) to 3 (severe). Grade 3 symptoms were defined as follows: for redness and swelling, an injection site with a diameter of >30 mm; for pain, crying when the vaccinated limb was moved or spontaneous pain in the limb; for irritability, crying that could not be comforted or that prevented normal activity; for loss of appetite, not eating at all; for fever, oral/axillary/tympanic temperature of >39.5°C or rectal temperature of >40°C; for drowsiness, drowsiness that prevented normal activity.
Statistical analysis.
The numbers of children enrolled in the early and late booster groups were dependent on the number of children who had received a 3-dose primary vaccination course with PHiD-CV in the primary vaccination study; the number of children enrolled in the catch-up group was dependent on the number of unprimed children in the control group of the primary study (1).
Immunogenicity analyses were performed on the according-to-protocol (ATP) cohort for immunogenicity, comprising vaccinated children who met all eligibility criteria, complied with the protocol-defined procedures and intervals, and who had results available for at least one antibody assay.
Safety analyses were performed on the total vaccinated cohort (TVC), including all children with ≥1 vaccine dose documented.
ELISA antibody geometric mean concentrations (GMCs) and OPA geometric mean titers (GMTs) with 95% confidence intervals (CIs) and seropositivity rates with exact 95% confidence intervals (CIs) were determined for each vaccine serotype or antigen. The GMCs and GMTs were calculated by taking the anti-log10 of the mean of the log10 antibody concentration or titer transformations. Antibody concentrations/titers below the assay cutoffs were given an arbitrary value of half the cutoff for the purpose of GMC/GMT calculation. The statistical analyses were performed using the SAS Drug and Development (SDD) Web portal version 3.5 and SAS version 9.2. No confirmatory objectives with predefined success criteria were set up in this descriptive study; differences were assessed based on nonoverlapping 95% CIs. An exploratory inferential analysis was performed on ELISA GMC and OPA GMT ratios, in which the exclusion of 1 from the 95% CIs was used to highlight potential differences between the early and late booster groups.
RESULTS
Study population.
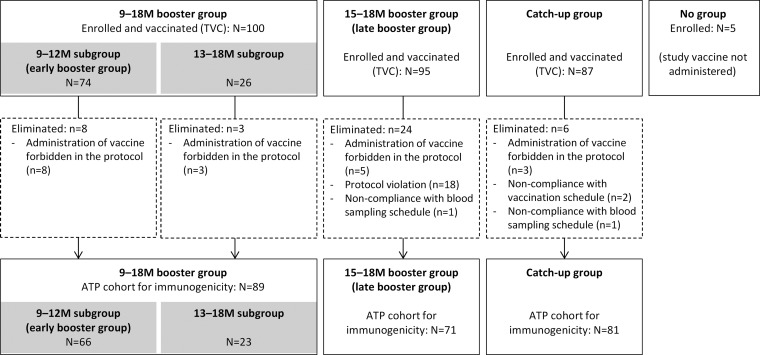
A total of 287 children were enrolled in the study (Fig. 2). As the 9- to 18-month group was split into 9- to 12-month and 13- to 18-month subgroups, a smaller number of children was included in the early booster group than in the late booster group (74 and 95, respectively). Sixty-six children from the early booster group and 71 children from the late booster group were included in the ATP cohort for immunogenicity. Nine of 74 (12.2%) and 2/95 (2.1%) children in the early and late booster groups, respectively, received measles vaccination concomitantly with PHiD-CV, and 2/95 (2.1%) children in the late booster group received DTPa/Hib vaccination concomitantly with PHiD-CV.
FIG 2.
Participant flow diagram. M, month; TVC, total vaccinated cohort; ATP, according-to-protocol cohort; N, number of children in the specified group; n, number of children with the specified characteristics.
The TVC of the catch-up group comprised 87 children, 81 of whom were included in the ATP cohort for immunogenicity (Fig. 2).
The ages of the participants in the various groups can be found in Table 1. The proportion of females was slightly lower in the early booster group than in the late booster group (Table 1).
TABLE 1.
Summary of demographic characteristics (ATP and TVC cohorts)
| Characteristic | Data by group (n)a |
|||||
|---|---|---|---|---|---|---|
| Early booster |
Late booster |
Catch-up |
||||
| TVC (74) | ATP (66) | TVC (95) | ATP (71) | TVC (87) | ATP (81) | |
| Age (mean ± SD) (mo) at: | ||||||
| Booster dose | 10.9 ± 0.37 | 10.9 ± 0.39 | 15.6 ± 1.27 | 15.9 ± 1.07 | NA | NA |
| Dose 1 | NA | NA | NA | NA | 16.1 ± 1.18 | 16.1 ± 1.15 |
| Dose 2 | NA | NA | NA | NA | 18.4 ± 1.38 | 18.4 ± 1.37 |
| Catch-up booster dose | NA | NA | NA | NA | 22.6 ± 1.63 | 22.6 ± 1.65 |
| No. (%) of females | 30 (40.5) | 26 (39.4) | 50 (52.6) | 35 (49.3) | 48 (55.2) | 43 (53.1) |
| Ethnicity (no. [%]) | ||||||
| Central/South Asia | 74 (100) | 66 (100) | 94 (98.9) | 70 (98.6) | 87 (100) | 81 (100) |
| American Indian or Alaskan Native | 0 (0) | 0 (0) | 1 (1.1) | 1 (1.4) | 0 (0) | 0 (0) |
TVC, total vaccinated cohort; ATP, according-to-protocol; NA, not applicable.
Antibody responses in the early (9 to 12 months) and late (15 to 18 months) booster groups.
For serotypes 4, 18C, and 19F, the prebooster antibody GMCs were lower in the late booster than in the early booster group (Table 2). For each serotype, the percentages of children with antibody concentrations of ≥0.2 μg/ml were in similar ranges in a comparison of the early and late booster group (see Table S4 in the supplemental material). Additionally, for each serotype, the prebooster OPA GMTs were in similar ranges in the early and late booster group, except for 9V, for which the GMTs were lower in the early booster group (Table 2).
TABLE 2.
Antibody GMCs and OPA GMTs at different time points (ATP cohort for immunogenicity) for the early and late booster groups
| Serotype | Vaccination timea | Antibody responses by group (n) by testb: |
|||
|---|---|---|---|---|---|
| ELISA GMC (95% CI) |
OPA GMT (95% CI) |
||||
| Early booster (66) | Late booster (71) | Early booster (66) | Late booster (71) | ||
| Vaccine serotypes | |||||
| 1 | Postprimary | 3.38 (2.73–4.18) | 3.51 (2.78–4.43) | 171.6 (102.5–287.3) | 151.7 (90.3–254.7) |
| Prebooster | 0.45 (0.34–0.60) | 0.31 (0.23–0.41) | 18.1 (11.5–28.6) | 14.3 (9.3–22.1) | |
| Postbooster | 4.92 (4.01–6.05) | 5.98 (4.54–7.90) | 1,053.0 (724.4–1,530.7) | 2,096.7 (1,464.8–3,001.1) | |
| Age 24 mo | 0.84 (0.56–1.26) | 1.37 (1.00–1.87) | 52.6 (26.7–103.6) | 173.8 (105.5–286.5) | |
| 4 | Postprimary | 3.60 (2.74–4.74) | 4.25 (3.31–5.47) | 738.8 (462.3–1,180.7) | 861.8 (531.3–1,398.1) |
| Prebooster | 1.03 (0.79–1.34) | 0.58 (0.44–0.75) | 80.5 (48.4–133.7) | 101.6 (62.6–164.9) | |
| Postbooster | 7.22 (5.34–9.76) | 11.56 (7.96–16.79) | 2,803.0 (2,121.2–3,703.9) | 7,202.1 (5,336.1–9,720.6) | |
| Age 24 mo | 1.09 (0.75–1.59) | 2.29 (1.53–3.42) | 325.2 (131.4–804.4) | 2,525.6 (1,474.5–4,325.9) | |
| 5 | Postprimary | 4.42 (3.56–5.48) | 4.03 (3.20–5.07) | 106.3 (69.0–164.0) | 100.1 (66.2–151.4) |
| Prebooster | 0.60 (0.47–0.77) | 0.38 (0.29–0.48) | 13.7 (9.7–19.3) | 9.7 (7.2–13.1) | |
| Postbooster | 6.06 (4.91–7.48) | 7.20 (5.25–9.86) | 434.8 (328.2–576.1) | 834.6 (610.9–1,140.2) | |
| Age 24 mo | 1.07 (0.74–1.55) | 2.03 (1.43–2.86) | 34.2 (20.1–58.1) | 90.6 (54.8–149.8) | |
| 6B | Postprimary | 0.75 (0.51–1.10) | 0.68 (0.48–0.97) | 364.0 (159.1–833.0) | 478.3 (229.7–995.8) |
| Prebooster | 0.64 (0.49–0.84) | 0.49 (0.37–0.64) | 48.1 (27.5–83.9) | 60.0 (32.9–109.3) | |
| Postbooster | 2.78 (2.03–3.80) | 2.98 (2.05–4.32) | 1,459.4 (936.2–2,274.8) | 1,781.2 (1,042.5–3,043.6) | |
| Age 24 mo | 0.68 (0.45–1.03) | 0.90 (0.61–1.32) | 242.4 (110.1–533.7) | 287.5 (149.0–555.0) | |
| 7F | Postprimary | 3.61 (2.95–4.42) | 3.61 (2.89–4.52) | 1,690.5 (1,259.0–2,269.9) | 1,621.7 (1,112.7–2,363.7) |
| Prebooster | 1.30 (1.02–1.65) | 0.93 (0.73–1.19) | 1,050.0 (796.1–1,385.0) | 1,539.1 (1,262.9–1,875.6) | |
| Postbooster | 6.52 (4.87–8.73) | 7.89 (5.91–10.54) | 7,229.8 (5,338.9–9,790.5) | 11,064.4 (8,182.9–14,960.5) | |
| Age 24 mo | 1.14 (0.82–1.60) | 1.98 (1.43–2.75) | 2,399.1 (1,462.6–3,935.1) | 5,371.9 (3,712.4–7,773.4) | |
| 9V | Postprimary | 4.36 (3.49–5.44) | 4.24 (3.30–5.45) | 1,398.6 (886.3–2,207.1) | 1,350.0 (848.6–2,147.7) |
| Prebooster | 1.32 (1.04–1.68) | 0.96 (0.74–1.25) | 373.3 (261.3–533.4) | 704.9 (533.7–931.1) | |
| Postbooster | 8.64 (6.53–11.43) | 9.76 (7.08–13.44) | 3,755.9 (2,710.5–5,204.4) | 7,870.3 (5,634.4–10,993.4) | |
| Age 24 mo | 1.52 (1.02–2.26) | 2.27 (1.64–3.14) | 1,384.3 (844.8–2,268.3) | 3,504.5 (2,501.0–4,910.6) | |
| 14 | Postprimary | 5.22 (3.99–6.83) | 5.44 (4.18–7.10) | 800.3 (400.3–1,600.1) | 1,159.8 (675.8–1,990.4) |
| Prebooster | 2.09 (1.33–3.30) | 1.52 (1.08–2.15) | 196.6 (115.7–334.2) | 184.0 (109.8–308.2) | |
| Postbooster | 10.49 (7.52–14.63) | 11.85 (8.15–17.22) | 1,970.8 (1,452.1–2,674.8) | 4,407.5 (3,193.2–6,083.5) | |
| Age 24 mo | 2.70 (1.83–3.98) | 4.11 (2.98–5.67) | 517.4 (277.2–965.8) | 1,641.2 (944.2–2,852.9) | |
| 18C | Postprimary | 15.37 (11.96–19.75) | 13.35 (9.65–18.46) | 1,023.7 (762.5–1,374.4) | 774.4 (461.9–1,298.5) |
| Prebooster | 3.09 (2.35–4.07) | 1.55 (1.19–2.03) | 37.9 (25.4–56.5) | 22.2 (14.1–35.0) | |
| Postbooster | 35.86 (27.01–47.61) | 42.43 (31.48–57.20) | 1,565.0 (1,131.9–2,163.8) | 3,643.2 (2,572.2–5,160.2) | |
| Age 24 mo | 4.67 (3.15–6.91) | 10.04 (6.55–15.37) | 123.1 (68.7–220.6) | 1,155.0 (662.0–2,015.0) | |
| 19F | Postprimary | 11.85 (9.08–15.45) | 9.67 (7.15–13.07) | 890.2 (600.1–1,320.4) | 670.0 (401.1–1,119.2) |
| Prebooster | 2.19 (1.60–3.01) | 1.23 (0.97–1.57) | 50.2 (33.2–75.9) | 34.7 (23.4–51.6) | |
| Postbooster | 14.17 (10.12–19.83) | 13.64 (9.42–19.76) | 1,645.0 (1,020.1–2,652.8) | 2,404.4 (1,583.1–3,651.6) | |
| Age 24 mo | 2.33 (1.53–3.55) | 4.15 (2.88–5.98) | 73.7 (36.5–148.6) | 357.9 (218.9–585.0) | |
| 23F | Postprimary | 1.25 (0.86–1.81) | 1.01 (0.71–1.43) | 1,611.5 (965.1–2,691.0) | 1,356.3 (818.3–2,247.9) |
| Prebooster | 0.85 (0.61–1.19) | 0.65 (0.48–0.89) | 193.2 (96.0–388.6) | 498.2 (231.4–1,072.9) | |
| Postbooster | 5.94 (4.21–8.39) | 6.48 (4.69–8.96) | 2,828.0 (1,829.7–4,370.9) | 5,937.2 (3,587.6–9,825.5) | |
| Age 24 mo | 1.18 (0.72–1.92) | 1.38 (0.96–1.98) | 1,015.3 (354.7–2,906.3) | 3,018.5 (1,389.0–6,559.6) | |
| Cross-reactive serotypes | |||||
| 6A | Postprimary | 0.16 (0.12–0.22) | 0.15 (0.12–0.20) | 24.7 (12.0–50.8) | 19.0 (10.0–36.0) |
| Prebooster | 0.18 (0.13–0.26) | 0.19 (0.14–0.27) | 22.1 (13.1–37.0) | 27.1 (15.7–47.0) | |
| Postbooster | 0.86 (0.57–1.29) | 0.95 (0.63–1.44) | 164.2 (85.3–316.4) | 262.5 (135.4–509.0) | |
| Age 24 mo | 0.33 (0.20–0.56) | 0.36 (0.22–0.59) | 67.4 (31.6–143.5) | 96.6 (45.4–205.6) | |
| 19A | Postprimary | 0.32 (0.23–0.46) | 0.26 (0.19–0.35) | 10.6 (5.8–19.4) | 10.7 (5.7–19.9) |
| Prebooster | 0.32 (0.20–0.50) | 0.30 (0.22–0.41) | 8.9 (6.0–13.3) | 7.0 (5.3–9.3) | |
| Postbooster | 2.29 (1.36–3.86) | 2.87 (1.78–4.61) | 115.2 (61.9–214.4) | 385.6 (223.4–665.5) | |
| Age 24 mo | 0.62 (0.35–1.10) | 1.33 (0.85–2.08) | 11.7 (6.1–22.5) | 60.0 (33.0–109.2) | |
The measurement time points are 1 month after primary vaccination (postdose 3), before and 1 month after vaccination (pre- and post-early/late booster), and 9 to 15 months after booster vaccination (age 24 months).
OPA, opsonophagocytic activity; ATP, according-to-protocol. The group numbers represent the maximum number of children with available results.
At 1 month postbooster, for each PHiD-CV serotype, ≥95.2% of the children in the early booster group and ≥93.8% of the children in the late booster group had antibody concentrations of ≥0.2 μg/ml. For each PHiD-CV serotype, ≥96.7% of the children in the early booster group and ≥93.0% of the children in the late booster group had OPA titers of ≥8 (see Table S4 in the supplemental material).
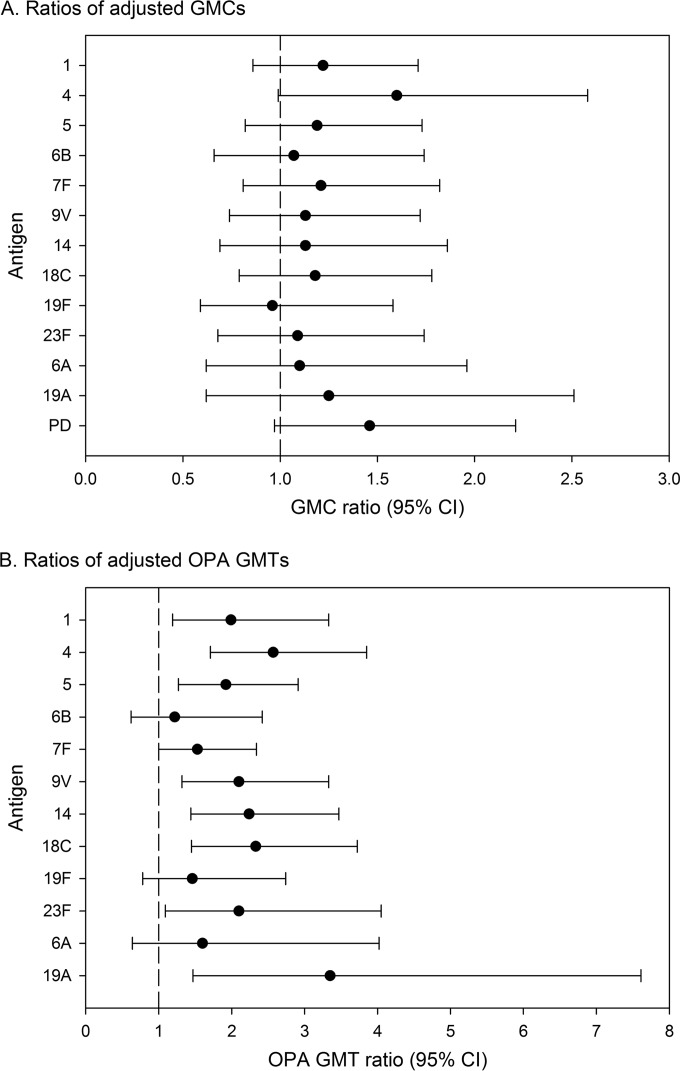
In the exploratory inferential analysis, the postbooster antibody GMCs were within similar ranges for the early and late booster groups for each of the PHiD-CV serotypes and cross-reactive serotypes 6A and 19A (Fig. 3A). However, the postbooster OPA titers were higher in the late booster group than in the early booster group, except for serotypes 6B and 19F, cross-reactive serotype 6A (95% CI included 1), and serotype 7F (95% CI lower limit, 1) (Fig. 3B).
FIG 3.
Ratios of adjusted GMCs (A) and adjusted OPA GMTs (B) at 1 month after booster between the early and late booster groups for antibodies against vaccine serotypes and protein D (ATP cohort for immunogenicity). The exclusion of 1 from the 95% CIs highlights potential differences between the early and late booster groups. No adjustment for multiplicity of comparisons was performed. GMC, geometric mean concentration; OPA, opsonophagocytic activity; GMT, geometric mean titer; ATP, according-to-protocol; PD, protein D.
At 1 month postbooster, 98.4% of the children in the early booster group and all children in the late booster group were seropositive for antibodies against protein D. The anti-protein D antibody GMC was 3,625.6 EL.U/ml (95% CI, 2,709.9 to 4,850.7) in the early booster group and 5,297.6 EL.U/ml (95% CI, 3,934.0 to 7,133.8) in the late booster group. The exploratory analysis suggested that the postbooster anti-protein D antibody levels were in a similar range for the early and late booster groups (Fig. 3A).
At the age of 24 months (9 to 15 months after booster vaccination), for each of the PHiD-CV serotypes, ≥90.0% of the children in each group had antibody concentrations of ≥0.2 μg/ml, except for serotype 6B in the early booster group (80.0%). At least 76.3% of the children in the early booster group and 83.6% of the late booster group had OPA titers of ≥8, except for serotype 1 in the early booster group (68.4%) (see Table S4 in the supplemental material). The antibody GMC point estimates were higher in the late booster group than those in the early booster group for most tested serotypes, including 19A, but the CIs overlapped. The OPA GMTs remained higher in the late booster group than in the early booster group at the age of 24 months, with overlapping CIs for serotypes 5, 6B, 7F, 14, 23F, and 6A (Table 2).
A total of 97.5% of the children in the early booster group and 98.1% of the children in the late booster group were seropositive for antibodies against protein D at the age of 24 months. The anti-protein D antibody GMCs were 1,161.4 EL.U/ml (95% CI, 782.2 to 1724.6) in the early booster group and 2,191.9 EL.U/ml (95% CI, 1,582.5 to 3,036.0) in the late booster group.
Immunogenicity in the catch-up group.
One month after dose 2, for each PHiD-CV serotype, ≥94.4% of the children in the catch-up group had antibody concentrations of ≥0.2 μg/ml, except for serotypes 6B (88.6%) and 23F (90.1%). For cross-reactive serotypes 6A and 19A, 65.7% and 94.4% of the children, respectively, had antibody concentrations of ≥0.2 μg/ml (see Table S5 in the supplemental material). The postdose 2 antibody GMCs in the catch-up group were in a similar range as the postdose 3 antibody GMCs of the early and late booster groups for all investigated serotypes except 18C, 6A, and 19A (higher in the catch-up group), and 9V (lower in the catch-up group) (Table 3). For each PHiD-CV serotype, ≥90.6% of the children had OPA titers of ≥8, except for serotypes 6B (71.4%) and 1 (79.4%). For the cross-reactive serotypes 6A and 19A, 78.5% and 87.5%, respectively, had OPA titers of ≥8 (see Table S5 in the supplemental material).
TABLE 3.
Antibody GMCs and OPA GMTs at different time points (ATP cohort for immunogenicity) for the catch-up group
| Serotype | Vaccination timea | Antibody responses by group by test (n = 81 each)b: |
|
|---|---|---|---|
| ELISA GMC (95% CI) | OPA GMT (95% CI) | ||
| Vaccine serotypes | |||
| 1 | Prevaccination | 0.04 (0.03–0.04) | 4.3 (3.9–4.8) |
| Postprimary | 2.50 (1.93–3.24) | 76.1 (49.0–118.2) | |
| Prebooster | 1.03 (0.82–1.29) | 18.1 (11.5–28.4) | |
| Postbooster | 3.32 (2.69–4.10) | 309.8 (203.0–472.9) | |
| 4 | Prevaccination | 0.04 (0.03–0.05) | 5.8 (4.0–8.6) |
| Postprimary | 5.89 (4.23–8.22) | 2,256.3 (1,840.0–2,766.8) | |
| Prebooster | 2.23 (1.85–2.69) | 959.7 (727.2–1,266.6) | |
| Postbooster | 8.22 (5.92–11.42) | 2,946.2 (2,097.9–4,137.4) | |
| 5 | Prevaccination | 0.05 (0.04–0.06) | 4.2 (3.8–4.7) |
| Postprimary | 2.81 (2.20–3.58) | 76.3 (53.3–109.3) | |
| Prebooster | 1.39 (1.13–1.70) | 24.6 (17.0–35.6) | |
| Postbooster | 5.32 (4.21–6.72) | 212.0 (148.9–301.7) | |
| 6B | Prevaccination | 0.03 (0.03–0.04) | 5.3 (3.9–7.2) |
| Postprimary | 0.71 (0.53–0.95) | 348.2 (165.7–731.6) | |
| Prebooster | 0.61 (0.47–0.78) | 201.5 (98.2–413.5) | |
| Postbooster | 1.40 (1.04–1.88) | 740.5 (419.6–1,306.8) | |
| 7F | Prevaccination | 0.06 (0.05–0.08) | 1,106.8 (540.7–2,265.9) |
| Postprimary | 4.63 (3.38–6.34) | 7,462.5 (5,653.9–9,849.6) | |
| Prebooster | 2.72 (2.28–3.24) | 6,295.9 (4,545.0–8,721.4) | |
| Postbooster | 7.41 (5.87–9.34) | 10,104.0 (7,377.8–13,837.4) | |
| 9V | Prevaccination | 0.04 (0.03–0.05) | 205.9 (98.7–429.4) |
| Postprimary | 2.09 (1.53–2.87) | 5,792.5 (4,586.3–7,315.9) | |
| Prebooster | 1.74 (1.40–2.17) | 3,463.4 (2,716.3–4,416.1) | |
| Postbooster | 4.88 (3.73–6.37) | 7,000.0 (5,265.1–9,306.6) | |
| 14 | Prevaccination | 0.06 (0.05–0.08) | 12.4 (7.1–21.6) |
| Postprimary | 5.01 (3.75–6.69) | 2,359.8 (1,576.1–3,533.2) | |
| Prebooster | 2.84 (2.25–3.57) | 1,293.6 (886.8–1,887.2) | |
| Postbooster | 7.59 (5.87–9.80) | 3,709.4 (2,463.5–5,585.2) | |
| 18C | Prevaccination | 0.04 (0.03–0.05) | 4.8 (3.8–6.1) |
| Postprimary | 29.10 (19.86–42.64) | 2,487.5 (1,541.2–4,014.8) | |
| Prebooster | 12.44 (9.16–16.91) | 2,546.4 (1,755.0–3,694.7) | |
| Postbooster | 75.19 (57.69–98.01) | 8,814.6 (6,810.9–11,407.7) | |
| 19F | Prevaccination | 0.07 (0.05–0.10) | 4.2 (3.9–4.6) |
| Postprimary | 16.39 (11.15–24.10) | 1,768.6 (1,120.3–2,792.0) | |
| Prebooster | 7.74 (5.85–10.24) | 753.8 (497.3–1,142.5) | |
| Postbooster | 30.71 (23.76–39.68) | 3,808.8 (2,689.5–5,393.9) | |
| 23F | Prevaccination | 0.04 (0.03–0.05) | 38.7 (17.1–87.5) |
| Postprimary | 1.13 (0.82–1.57) | 3,378.1 (2,014.8–5,664.0) | |
| Prebooster | 0.85 (0.67–1.09) | 1,868.2 (956.9–3,647.5) | |
| Postbooster | 2.15 (1.69–2.75) | 4,357.3 (2,246.9–8,449.8) | |
| Cross-reactive serotypes | |||
| 6A | Prevaccination | 0.03 (0.03–0.04) | 10.2 (6.1–17.1) |
| Postprimary | 0.35 (0.25–0.50) | 324.9 (176.1–599.5) | |
| Prebooster | 0.33 (0.24–0.45) | 329.0 (181.0–598.2) | |
| Postbooster | 0.76 (0.53–1.09) | 616.2 (369.2–1,028.5) | |
| 19A | Prevaccination | 0.06 (0.04–0.08) | 4.8 (4.0–5.8) |
| Postprimary | 2.49 (1.77–3.51) | 506.6 (305.5–840.1) | |
| Prebooster | 1.94 (1.44–2.61) | 402.1 (248.9–649.5) | |
| Postbooster | 7.91 (5.62–11.13) | 1,770.9 (1,190.6–2,634.0) | |
The measurement time points are before vaccination (prevaccination), 1 month after primary vaccination (postdose 2), before booster vaccination (prebooster), and 1 month after booster vaccination (age 24 months).
OPA, opsonophagocytic activity; ATP, according-to-protocol. The group numbers represent the maximum number of children with available results.
At 1 month postbooster, for each of the PHiD-CV serotypes, ≥96.3% of the children had antibody concentrations of ≥0.2 μg/ml, and ≥90.6% had OPA titers of ≥8. For the cross-reactive serotypes 6A and 19A, 87.0% and 100.0% of the children, respectively, had antibody concentrations of ≥0.2 μg/ml; 92.3% and 98.1%, respectively, had OPA titers of ≥8. All children had measurable antibodies against protein D (≥100 EL.U/ml). The postbooster anti-protein D antibody GMC was 1,727.2 (95% CI, 1,306.6 to 2,283.3).
Reactogenicity and safety in the early (9 to 12 months) and late (15 to 18 months) booster groups.
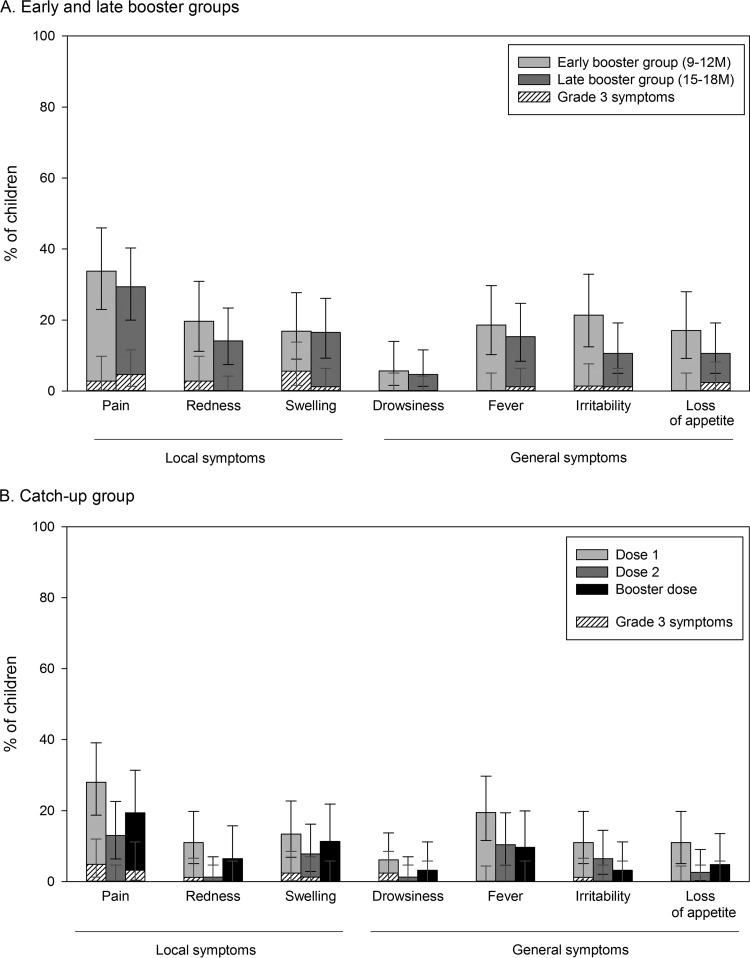
The most commonly reported solicited local symptom was pain at the injection site for both the early and late booster groups (33.8% and 29.4% of children, respectively; Fig. 4A). A large swelling reaction (diameter, 60 mm) at the PHiD-CV injection site was reported for 1 child in the early booster group on day 1 following booster vaccination. The swelling resolved without sequelae within 1 day after onset.
FIG 4.
Incidence of local and general solicited symptoms in the early and late booster groups (A) and in the catch-up group (B) (total vaccinated cohort). Grade 3 symptoms were defined as follows: for redness and swelling, an injection site with a diameter of >30 mm; for pain, crying when the vaccinated limb was moved or spontaneous pain in the limb; for irritability, crying that could not be comforted or that prevented normal activity; for loss of appetite, not eating at all; for fever, oral/axillary/tympanic temperature of >39.5°C or rectal temperature of >40°C; for drowsiness, drowsiness that prevented normal activity. The error bars indicate 95% confidence intervals.
The most common solicited general symptoms were irritability in the early booster group (21.4%) and fever in the late booster group (15.3%). The most frequently reported grade 3 solicited symptoms were swelling at the injection site (5.6% of children) in the early booster group and loss of appetite (2.4% of children) in the late booster group (Fig. 4A).
At least 1 unsolicited symptom was reported for 5.4% of the children after early booster and 1.1% of the children after late booster vaccination. The symptoms reported for the early booster group were diarrhea, gastroenteritis, nasopharyngitis, pustular rash, and cough (each reported by 1 child); for the late booster group, only cough was reported (1 case).
In the early booster group, 2 children each reported 1 serious adverse effect (SAE) (skin infection and gastroenteritis); both resolved, and neither of them was considered by the investigator to be vaccine related. No fatal SAEs were reported during the entire study period.
Reactogenicity and safety in the catch-up group.
In the catch-up group, pain at the injection site was the most common solicited local symptom after each dose and was most frequently reported after dose 1 (28%) (Fig. 4B).
Fever, reported for up to 19.5% of the children, was the most frequently reported solicited general symptom (Fig. 4B).
Of the 3 catch-up doses, 2.5% were followed by ≥1 unsolicited AE, none of which were considered by the investigator to be vaccine related. No grade 3 unsolicited AEs were reported.
One of 87 children in the catch-up group had 2 SAEs (febrile convulsion and urinary tract infection), which resolved without sequelae and were not considered by the investigator to be vaccine related.
DISCUSSION
The PHiD-CV booster dose after 3-dose priming is usually administered in the second year of life. This was the first study to investigate the safety and immunogenicity of a PHiD-CV booster dose administered at the age of 9 to 12 months. This would allow the PHiD-CV booster to be administered at the same time as routine measles vaccination, which is scheduled at the age of 9 months in most developing countries, and it may potentially facilitate the operational administration and enhanced coverage of such booster doses.
The PHiD-CV booster vaccinations were generally well tolerated, regardless of the age at administration. The immune responses observed 1 month after booster vaccination in this study were in line with those observed in other Asian countries (11, 12). Our findings indicate that the antibody response is comparable for children receiving a PHiD-CV booster dose at 9 to 12 months of age and those receiving the booster at 15 to 18 months of age. However, an exploratory inferential analysis suggested that for the majority of serotypes, the postbooster OPA titers were lower in children who had received the booster dose at 9 to 12 months of age than in those who had received the booster at 15 to 18 months of age. Also, at 24 months of age, the OPA titers were lower in the early booster group than in the late booster group for vaccine serotypes 1, 4, 9V, 18C, 19F, and the cross-reactive serotype 19A. From an immunological perspective, postponing the booster dose to a later age may thus be beneficial for the functional antibody responses to the booster. This difference has to be interpreted with caution, as no adjustment was made for multiplicity of comparisons; moreover, the clinical relevance of such a difference in the OPA titers is not known.
Other factors also have to be taken into account when deciding on the age of booster dose administration in national immunization programs, such as the burden of pneumococcal disease in that country, the circulating serotypes, and the fact that most of the disease burden occurs early in life. Compliance (and therefore coverage) may also be higher when the booster dose is given at an infant visit foreseen in the immunization program than when administration depends on an additional visit being scheduled at an older age.
A robust immune response was also seen following a 2-dose catch-up immunization in children during their second year of life. PHiD-CV was also generally well tolerated in this catch-up group and had an acceptable safety profile. The immune response in terms of ELISA GMCs and OPA GMTs after the 2 catch-up doses was in the same range as that observed after a 3-dose primary vaccination course at 6, 10, and 14 weeks of age (1). Booster vaccination following the 2 catch-up doses induced robust immune responses against the vaccine and cross-reactive serotypes indicative of effective priming and immunological memory. Moreover, in a previous large-scale cluster-randomized double-blind trial assessing PHiD-CV effectiveness against invasive pneumococcal disease (IPD), 5 IPD cases occurred in the control group and none in the children who received a 2-dose PHiD-CV catch-up vaccination at 12 to 18 months of age (vaccine effectiveness, 100% [95% CI, 79 to 100%]) (2). These effectiveness results thus show that the 2-dose catch-up schedule is sufficient to provide protection against IPD. In the same trial, the 2-dose catch-up schedule also provided protection against hospital-diagnosed pneumonia (vaccine effectiveness, 27.1% [95% CI, 8.8 to 41.8]) and hospital-treated primary pneumonia (vaccine effectiveness, 33.5% [95% CI, 5.3 to 53.3]) (13). These findings together with our study results suggest that a booster dose may not be needed after a 2-dose catch-up schedule in the second year of life.
The findings presented here should be interpreted with caution, as no confirmatory analyses were performed, and no adjustment for multiplicity of comparisons was performed for the exploratory analyses. Moreover, the sample size was limited, and in the early (9 to 12 month) booster group, the age range had to be enlarged due to a delay in the study start. Additionally, only 9 children (12.2%) received coadministration of the measles vaccine with the PHiD-CV booster dose at 9 to 12 months of age; this number was too low to assess the impact of the coadministration of these 2 vaccines on their safety and immunogenicity.
In conclusion, in children primed with PHiD-CV at 6, 10, and 14 weeks of age, PHiD-CV vaccination showed robust immune responses 1 month after both early (9 to 12 months) and late (15 to 18 months) booster vaccination and was generally well tolerated regardless of age. Additionally, 2-dose catch-up immunization with PHiD-CV in the second year of life was well tolerated and showed robust immune responses. Considering the evidence provided by a PHiD-CV effectiveness trial (2), this suggests that a 2-dose catch-up schedule without a booster dose might be sufficient to provide protection against pneumococcal diseases.
Supplementary Material
ACKNOWLEDGMENTS
We thank the children who participated in this study and their parents. We also thank F. Shafi (GlaxoSmithKline Vaccines) for his contribution to the statistical analysis, A. Habib (GlaxoSmithKline Vaccines) for critically reviewing the manuscript, L. Manciu (GlaxoSmithKline Vaccines) for study report development, J. Vandewalle (XPE Pharma & Science on behalf of GlaxoSmithKline Vaccines) for drafting the manuscript, and B. van Heertum (XPE Pharma & Science on behalf of GlaxoSmithKline Vaccines) for manuscript coordination.
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the manuscript.
S.M., N.F., S.R., and D.B. are employees of the GlaxoSmithKline group of companies. D.B. owns restricted shares/stock option of the GlaxoSmithKline group of companies. A.St. is an employee of XPE Pharma & Science, contractor for GlaxoSmithKline Vaccines. A.Si., S.L., J.C., and S.C. have no conflicts of interest to declare.
Synflorix, Tritanrix, Hiberix, and Infanrix are trademarks of the GlaxoSmithKline group of companies.
J.C. and S.M. coordinated the clinical aspects of the study. J.C., S.C., and A.Si. collected data. D.B., S.M., S.L., and N.F. planned and designed the study. D.B., S.C., J.C., N.F., S.M., A.St., and S.R. interpreted the results. S.R. and N.F. did the statistical analyses. All authors critically reviewed the different drafts of the manuscript and approved the final version.
Footnotes
Published ahead of print 9 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00068-14.
REFERENCES
- 1.Lalwani S, Chatterjee S, Chhatwal J, Verghese VP, Mehta S, Shafi F, Borys D, Moreira M, Schuerman L. 2012. Immunogenicity, safety, and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with the DTPw-HBV/Hib vaccine in Indian infants: a single-blind, randomized, controlled study. Hum. Vaccin. Immunother. 8:612–622. 10.4161/hv.19287 [DOI] [PubMed] [Google Scholar]
- 2.Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, Puumalainen T, Lommel P, Hezareh M, Moreira M, Schuerman L, Kilpi TM. 2013. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 381:214–222. 10.1016/S0140-6736(12)61854-6 [DOI] [PubMed] [Google Scholar]
- 3.Tregnaghi MW, SÁez-Llorens X, López P, Abate H, Smith E, Pósleman A, Cortes-Barbosa C, Ceballos A, Tregnaghi M, Sierra A, MÁrquez V, Carabajal C, Falaschi A, Calvo A, Wong D, Caicedo Y, Castrejón MM, Lepetic A, Lommel P, Hausdorff WP, Borys D, Ruiz Guiñazú J, Ortega-Barría E, YarzÁbal JP, Schuerman L. 2013. Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) against invasive pneumococcal disease in Latin America, abstr. 348 Abstr. 9th Int. Sympos. Antimicrob. Agents Resist. (ISAAR), Kuala Lumpur, Malaysia, 13 to 15 March 2013 [Google Scholar]
- 4.Tregnaghi M, SÁez-Llorens X, López P, Abate H, Smith E, Pósleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, Tregnaghi M. 2011. Evaluating the efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein-D conjugate vaccine (PHiD-CV) against community-acquired pneumonia in Latin America, abstr. 411 Abstr. 29th Ann. Meet. Eur. Soc. Paediatr. Infect. Dis. (ESPID), The Hague, The Netherlands, 7 to 11 June 2011 [Google Scholar]
- 5.Tregnaghi MW, SÁez-Llorens X, López P, Abate H, Smith E, Pósleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, Tregnaghi M, Sierra A, MÁrquez V, Troitiño M, Castrejón MM, Lepetic A, Lommel P, Hausdorff WP, Borys D, Guiñazú JR, Ortega-Barría E, YarzÁbal JP, Schuerman L. 2013. Eficacia de la vacuna neumocócica 10-valente conjugada a la proteína D del Haemophilus influenzae no tipificable (PHiD-CV) contra neumonía adquirida en la comunidad en niños de Latinoamérica: un estudio controlado aleatorizado, abstr. OR-006 Abstr. 15th Congreso Latinoamericano de Infectiología PediÁtrica (SLIPE), São Paulo, Brazil, 26 to 29 June 2013 [Google Scholar]
- 6.SÁez-Llorens X, Castrejón MM, Rowley S, Wong D, Calvo A, Rodriguez M, Troitiño M, Lommel P, Hausdorff WP, Borys D, Ruiz Guiñazú J, Ortega-Barría E, YarzÁbal JP, Schuerman L. 2013. Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) against acute otitis media in children in Panama, abstr. 342 Abstr. 9th Int. Symp. Antimicrob. Agents Resist. (ISAAR), Kuala Lumpur, Malaysia, 13 to 15 March 2013 [Google Scholar]
- 7.Falagas ME, Zarkadoulia E. 2008. Factors associated with suboptimal compliance to vaccinations in children in developed countries: a systematic review. Curr. Med. Res. Opin. 24:1719–1741. 10.1185/03007990802085692 [DOI] [PubMed] [Google Scholar]
- 8.Klugman KP, Madhi SA, Adegbola RA, Cutts F, Greenwood B, Hausdorff WP. 2011. Timing of serotype 1 pneumococcal disease suggests the need for evaluation of a booster dose. Vaccine 29:3372–3373. 10.1016/j.vaccine.2011.02.089 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. 2012. Pneumococcal vaccines WHO position paper–2012. Wkly. Epidemiol. Rec. 87:129–144 http://www.who.int/wer/2012/wer8714.pdf [PubMed] [Google Scholar]
- 10.Poolman JT, Frasch CE, Käyhty H, Lestrate P, Madhi SA, Henckaerts I. 2010. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin. Vaccine Immunol. 17:134–142. 10.1128/CVI.00289-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CH, Kim JS, Cha SH, Kim KN, Kim JD, Lee KY, Kim HM, Kim JH, Hyuk S, Hong JY, Park SE, Kim YK, Kim NH, Fanic A, Borys D, Ruiz-Guiñazù J, Moreira M, Schuerman L, Kim KH. 2011. Response to primary and booster vaccination with 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine in Korean infants. Pediatr. Infect. Dis. J. 30:e235–e243. 10.1097/INF.0b013e31822a8541 [DOI] [PubMed] [Google Scholar]
- 12.Lim FS, Chan PC, Chong CY, Yehudi YWS, Shafi F, Swinnen K, Hezareh M, Borys D. 2012. Antibody persistence after primary vaccination and immunogenicity, safety and reactogenicity of booster vaccination with 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine in children in Singapore, abstr. PP26. Abstr. 14th Asia Pac. Congr. Pediatr. (APCP), Sarawak, Malaysia, 8 to 12 September 2012 [Google Scholar]
- 13.Kilpi T, Palmu AA, Puumalainen T, Nieminen H, Ruokokoski E, Rinta-Kokko H, Moreira M, Hezareh M, Borys D, Schuerman L, Jokinen J. 2013. Effectiveness of the 10-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against hospital-diagnosed pneumonia in infants–FinIP Trial, abstr. 175 Abstr. 31st Ann. Meet. Eur. Soc. Paediatr. Infect. Dis. (ESPID), Milan, Italy, 28 May to 1 June 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.