Abstract
To monitor and evaluate the safety of the influenza A(H1N1) vaccine in pregnant women and its influence on the fetus and neonate, we performed a prospective study in which 122 pregnant Chinese women who received the influenza A(H1N1) vaccine and 104 pregnant women who did not receive any vaccine (serving as controls) were observed. The results indicated that the seroconversion rate in the vaccinated group was 90.4% (95% confidence interval [CI], 82.6% to 95.5%). The rate of adverse events following immunization in the pregnant women who received the influenza A(H1N1) vaccine was 3.3%. The spontaneous abortion rates in the vaccinated group and the unvaccinated group were 0.8% and 1.9%, respectively (exact probability test, P = 0.470), the prolonged-pregnancy rates were 8.2% and 4.8%, respectively (χ2 = 1.041, P = 0.308), the low-birth-weight rates were 1.6% and 0.95%, respectively (exact probability test, P = 1.000), and the spontaneous-labor rates were 70.5% and 75%, respectively (χ2 = 0.573, P = 0.449). All newborns who have an Apgar score of ≥7 are considered healthy; Apgar scores of ≥9 were observed in 38.5% and 57.7% of newborns in the vaccinated group and the unvaccinated group, respectively (χ2 = 8.274, P = 0.004). From these results, we conclude that the influenza A(H1N1) vaccine is safe for pregnant women and has no observed adverse effects on fetal growth. (This study has been registered at ClinicalTrials.gov under registration no. NCT01842997.)
INTRODUCTION
Pregnant women have a higher risk for serious complications from influenza than nonpregnant women of reproductive age (1–3). Since 1997, the Advisory Committee on Immunization Practices (ACIP) has recommended that pregnant women receive the inactivated influenza vaccine (4). Furthermore, in 2004, the recommendation was expanded to cover all trimesters of pregnancy (5). In April 2009, the pandemic influenza A(H1N1) virus infection was identified in China. Some studies showed that pregnant women had a higher rate of complications from the 2009 H1N1 virus infection than other populations (5–7). In September 2009, the China Food and Drug Administration licensed the first 2009 influenza A(H1N1) vaccines, which were inactivated split-virus vaccines (8).
We conducted a prospective cohort study to monitor and evaluate the safety of pregnant women who have been inoculated with the influenza A(H1N1) vaccine; our primary purposes were to study the impact of the vaccine on the fetus and newborn and to report the results.
MATERIALS AND METHODS
Study design.
The objectives of this study were to monitor and evaluate the safety of the influenza A(H1N1) vaccine in pregnant women and to determine the vaccine's influence on the fetus and neonate. Healthy pregnant women with no history of novel influenza H1N1 virus infection or novel influenza H1N1 vaccination were recruited. The recruited women resided in 4 adjacent villages or communities of Xiangshui County in Jiangsu Province. In the process of recruitment, all of the pregnant women voluntarily participated in the study and were told that approximately half of the participants would be administered 1 dose of influenza A(H1N1) vaccine; however, the unvaccinated participants were allowed to request 1 dose of the influenza A(H1N1) vaccine at the end of the study, if appropriate. The pregnant women who received 1 dose of the influenza A(H1N1) vaccine comprised the vaccinated group. Those in the control group (unvaccinated) were defined as pregnant women who resided in the same or adjacent village/community, had an age difference of ≤3 years compared to the women in the vaccinated group, had a gestational age of <3 weeks, and had the same numbers of pregnancies as those in the vaccinated group. The pregnant women in the control group were not offered any vaccines. Blood samples of the pregnant women in the vaccinated group were collected once before vaccination and once 28 days after vaccination. The pregnant women in the vaccinated group were also required to record any adverse experiences using a vaccination report card (see Fig. 1 for a flow chart of the study).
FIG 1.

Flow chart of the cohort study on safety of the influenza A(H1N1) vaccine in pregnant Chinese women.
We used active observation and passive reporting. Active observation indicates that during the observation period of 30 min after the vaccination, doctors at the vaccination sites took the initiative to ask the vaccinated pregnant women about their reactions to the vaccination; the doctors also provided follow-up care at 72 h and 15 days. Passive reporting indicates that before the vaccination, each participant signed an informed consent form that included the vaccine varieties, function, and contraindications, the possibility of adverse events following immunization (AEFI), and precautions. The participants were also told to report to the doctors at the vaccination sites in a timely manner when fever, local swelling and induration, rash, or other symptoms occurred. The observation time for the pregnancy outcomes lasted 28 days postpartum. The AEFI and influenza-like illness were observed for 6 months after the vaccination.
Throat swab specimens from the pregnant women with influenza-like illnesses were collected and sent to the influenza virus lab of the Jiangsu Provincial Center for Disease Control and Prevention. Influenza A(H1N1) virus reverse transcription (RT)-PCR detection kits obtained from the national influenza virus lab were used for the purification and detection of the influenza A(H1N1) virus. The kits contain two parts, (i) an influenza A(H1N1) virus RNA purification kit and (ii) a ready-to-use detection kit for the rapid and accurate detection of influenza A(H1N1) virus by endpoint RT-PCR (9). Through the use of the kits, trained laboratory staff completed the rapid extraction and quantitative detection of the H1 hemagglutinin viral RNA transcript of the influenza A(H1N1) virus in the throat swab specimens from the participants.
Approval for the study was obtained from the Medical Ethics Committee of the Xiangshui County Center for Disease Control and Prevention. Each participant signed a written informed consent form.
Vaccine.
A split-virion nonadjuvanted influenza A(H1N1) vaccine (lot 200909008) produced by the Shanghai Institute of Biological Products was used. Each pregnant woman in the vaccinated group was administered 1 dose (15 μg) of the H1N1 vaccine.
Safety observations. (i) AEFI.
Any local reactions, such as redness, pain, and induration at the injection site, systemic reactions, such as fever, headache, fatigue, nausea, vomiting, and diarrhea, and suspected adverse reactions, such as anaphylactic shock, angioedema, urticaria, maculopapular rash, laryngeal edema, thrombocytopenic purpura, allergic purpura, allergic local necrosis (Arthus) reaction, hot-water seizures, epilepsy, multiple neuritis, and Guillain-Barré syndrome, were reported.
(ii) Pregnancy complications and outcomes.
Pregnancy complications include gestational hypertension disease, anemia, placenta previa, placental abruption, preeclampsia and eclampsia, and liver disease. Adverse pregnancy outcomes include miscarriage, premature birth, stillbirth, low birth weight, and birth defects. Any such complications were recorded by the maternity and child health care organizations or midwifery agencies included in this study, according to routine prenatal and delivery services in the pregnant women's health records. Each of these organizations and agencies filled out a unified form on complications during pregnancy and pregnancy outcome.
(iii) Report of influenza-like illness.
Influenza-like illness was defined according to WHO guidelines, which include documented fever (≥38.0°C) and cough or sore throat. All of the participants were told to contact the local vaccination site or the Xiangshui County Center for Disease Control and Prevention in a timely manner once influenza-like symptoms appeared. The local maternal and child health care organizations or midwifery organizations reported the influenza-like cases in a timely manner to the Xiangshui County Center for Disease Control and Prevention. After receiving the reports, the Xiangshui County Center for Disease Control and Prevention was responsible for epidemiology surveys and specimen collection.
Seroconversion.
The seroconversion rate was defined as either a prevaccination hemagglutination inhibition (HAI) titer of <1:10 and a postvaccination titer of ≥1:40 or a prevaccination titer of ≥1:10 and a minimum 4-fold increase at 28 days postvaccination.
Statistical analysis.
The following indicators were calculated when the observation was completed: the incidence of AEFI, the incidence of spontaneous abortion, the incidence of induced abortion, the incidence of preterm pregnancy, the incidence of postterm pregnancy, the proportion of underweight babies, and the incidence of influenza-like illness. A chi-square test and an exact probability method were used to detect the differences between the 2 groups when appropriate. A Wilcoxon rank sum test was used to compare the median duration of disease between the 2 groups. A P value of <0.05 was considered statistically significant, and all of the tests were conducted using the R 3.0.0 statistical software (10).
RESULTS
Baseline of pregnant women observed.
A total of 226 healthy pregnant women 18 to 35 years old who had no history of novel influenza H1N1 virus infection or novel influenza H1N1 vaccination participated in the study. The pregnancies ranged from 5 weeks' to 32 weeks' gestation. The subjects were divided into 1 of 2 groups, vaccinated (n = 122) and unvaccinated (n = 104) patients. The subjects in the vaccinated group were administered 1 dose (15 μg) of H1N1 vaccine. The subjects in the unvaccinated group received no vaccine and were considered controls for the vaccinated group. Each group was divided into 4 age stratifications, 18 to 20 years, 21 to 25 years, 26 to 30 years, and ≥30 years.
The number of gestational weeks in the vaccinated group and the unvaccinated group were stratified by patient age, as shown in Table 1. There were no statistically significant differences in the number of gestational weeks between the vaccinated group and the unvaccinated group (χ2 = 2.898, P = 0.408).
TABLE 1.
Gestational weeks in vaccinated and unvaccinated study groups, stratified by patient age
| Patient age (yr) | Vaccinated group |
Unvaccinated groupa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | % of patients at a gestational age (wk) of: |
No. of patients | % of patients at a gestational age (wk) of: |
|||||||
| 5–9 | 10–19 | 20–29 | ≥30 | 5–9 | 10–19 | 20–29 | ≥30 | |||
| 18–20 | 4 | 0.0 | 25.0 | 50.0 | 25.0 | 3 | 0.0 | 33.3 | 0.0 | 66.7 |
| 21–25 | 81 | 16.0 | 30.9 | 9.9 | 43.2 | 63 | 23.8 | 31.7 | 11.2 | 33.3 |
| 26–30 | 28 | 10.7 | 32.1 | 10.7 | 46.4 | 33 | 30.3 | 24.2 | 9.1 | 36.4 |
| ≥30 | 9 | 33.3 | 11.1 | 0.0 | 55.6 | 5 | 0.0 | 20.0 | 20.0 | 60.0 |
| Total | 122 | 15.6 | 29.5 | 10.7 | 44.3 | 104 | 24.0 | 28.8 | 10.6 | 36.6 |
χ2 = 2.898, P = 0.408.
The numbers of gravidities in the vaccinated group and the unvaccinated group were stratified by age, as shown in Table 2. There were no statistically significant differences in the number of gravidities between the vaccinated group and the unvaccinated group (exact probability, P = 0.327).
TABLE 2.
Gravidities in vaccinated and unvaccinated study groups, stratified by patient age
| Patient age (yr) | Vaccinated group |
Unvaccinated groupa |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | % of patients with the indicated no. of gravidities |
No. of patients | % of patients with the indicated no. of gravidities |
|||||
| 1 | 2 | ≥3 | 1 | 2 | ≥3 | |||
| 18–20 | 4 | 100.0 | 0.0 | 0.0 | 3 | 66.7 | 33.3 | 0.0 |
| 21–25 | 81 | 85.2 | 12.4 | 2.4 | 63 | 84.2 | 7.9 | 7.9 |
| 26–30 | 28 | 50.0 | 28.6 | 21.4 | 33 | 60.6 | 30.3 | 9.1 |
| ≥30 | 9 | 44.4 | 44.4 | 11.2 | 5 | 0.0 | 80.0 | 20.0 |
| Total | 122 | 74.6 | 18.0 | 7.4 | 104 | 72.1 | 19.2 | 8.7 |
Exact probability, P = 0.327.
AEFI in the vaccinated group.
Five suspected AEFI were reported: 2 fever reactions (1.6%; 95% confidence interval [CI], 0.2% to 5.8%), 1 complaint of dizziness and nausea (0.8%; 95% CI, 0.1% to 4.5%), 1 complaint of abdominal pain (0.8%; 95% CI, 0.1% to 4.5%), and 1 incident of sudden deafness (this subject recovered and was diagnosed with coupling). Therefore, the incidence rate of AEFI was 3.3% (4/122; 95% CI, 0.9% to 8.8%).
Pregnancy outcomes.
In the vaccinated group, 1 spontaneous abortion (0.8%) occurred. This pregnant woman was 28 years old and had a history of 2 prior pregnancies; she received the influenza vaccine during the 10th week of pregnancy. Fetal hydrocephalus and stillbirth were detected in the 37th week of pregnancy. While the Apgar scores in the vaccinated group were significantly lower than those in the unvaccinated group, there were no statistically significant differences in any of the other indicators between the vaccinated group and the unvaccinated group (Table 3).
TABLE 3.
Pregnancy outcomes in vaccinated and unvaccinated study groups
| Outcome | Outcome (%) in: |
Statistic type (value) | P value | |
|---|---|---|---|---|
| Vaccinated group (n = 122) | Unvaccinated group (n = 104) | |||
| Spontaneous abortion | 0.8 | 1.9 | Exact probability | 0.327 |
| Artificial abortion | 4.9 | 5.8 | χ2 (0.081) | 0.776 |
| Postnatal death | 0.0 | 1.0 | Exact probability | 0.460 |
| Premature birth | 0.0 | 1.0 | Exact probability | 0.460 |
| Prolonged pregnancy | 8.2 | 4.8 | χ2 (1.041) | 0.308 |
| Low birth wt | 1.6 | 1.0 | Exact probability | 1.000 |
| Delivery mode | χ2 (0.573) | 0.449 | ||
| Eutocia | 70.5 | 75.0 | ||
| Cesarean delivery | 29.5 | 25.0 | ||
| Birth wt (g) | χ2 (2.252) | 0.133 | ||
| <3,500 | 46.7 | 56.7 | ||
| ≥3,500 | 53.3 | 43.2 | ||
| Apgar score at 1 min | χ2 (8.274) | 0.004 | ||
| 7–8 | 61.5 | 42.3 | ||
| ≥9 | 38.5 | 57.7 | ||
Incidence of influenza-like illness in the vaccinated and unvaccinated groups.
There were a total of 12 influenza-like illness cases found during the 6 months of the study. However, throat swab specimens indicated that the suspected cases were not infected by the influenza A(H1N1) virus. Although the H1N1 virus-negative specimens were not further tested for other influenza viruses, the clinical symptoms and the duration of the influenza-like illnesses indicated that the pregnant women most likely caught the seasonal influenza A(H3N2) virus, which was diagnosed with routine surveillance during the previous 3 years. However, there were significant differences in the incidence rates of influenza-like illness found between the 2 groups (Table 4).
TABLE 4.
Comparison of the incidence of influenza-like illness between vaccinated and nonvaccinated study groupsa
| Group | No. of patients | Influenza-like cases (%) |
|---|---|---|
| Vaccinated | 122 | 2.5 |
| Unvaccinated | 104 | 8.7 |
χ2 = 4.285, P = 0.038.
The differences in the adverse events, outcomes, and main clinical features (fever, sore throat, cough, rhinorrhea, fatigue, and the median duration of disease) of influenza-like illness between the vaccinated group and the unvaccinated group are shown in Table 5.
TABLE 5.
Adverse events, outcomes, and clinical features of influenza-like illness in vaccinated and unvaccinated study groups
| Clinical featurea | Data (%) for: |
P valuea | |
|---|---|---|---|
| Vaccinated (n = 3) | Unvaccinated (n = 9) | ||
| Spontaneous abortion | 0.0 | 0.0 | |
| Artificial abortion | 0.0 | 0.0 | |
| Postnatal deaths | 0.0 | 0.0 | |
| Premature birth | 0.0 | 0.0 | |
| Prolonged pregnancy | 0.0 | 0.0 | |
| Low birth wt | 0.0 | 0.0 | |
| Delivery mode | |||
| Eutocia | 100.0 | 100.0 | |
| Cesarean delivery | 0.0 | 0.0 | |
| Birth wt | 0.750 | ||
| <3,500 g | 33.3 | 55.6 | |
| ≥3,500 g | 66.7 | 44.4 | |
| Apgar score | 0.127 | ||
| 7–8 | 66.7 | 11.1 | |
| ≥9 | 33.3 | 88.9 | |
| Main clinical feature | |||
| Fever | 100 | 88.9 | 1.000 |
| Sore throat | 33.3 | 88.9 | 0.127 |
| Cough | 100 | 88.9 | 1.000 |
| Rhinorrhea | 100 | 77.8 | 1.000 |
| Fatigue | 0 | 11.1 | 1.000 |
| Median duration of disease (days) | 5 | 6 | 0.196b |
Values are exact probabilities unless noted otherwise.
Wilcoxon rank sum test (z = −1.293).
Seroconversion in the vaccinated group.
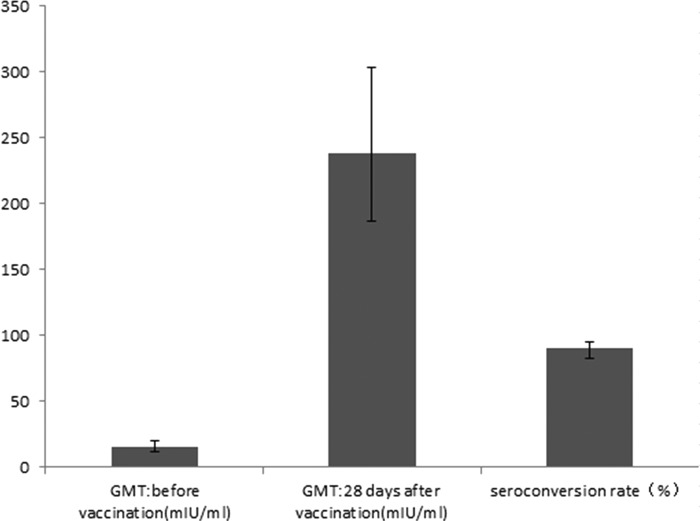
A total of 107 and 94 serum samples were collected before vaccination and 28 days after vaccination, respectively. The seroconversion rate was 90.4% (85/94; 95% CI, 82.6% to 95.5%). Figure 2 shows the geometric mean titers (GMTs) and seroconversion rates before vaccination and 28 days after vaccination.
FIG 2.
Geometric mean titers (GMTs) and seroconversion before vaccination and 28 days after vaccination.
DISCUSSION
The populations at the highest risk for influenza A(H1N1) virus infection are the elderly, young children, pregnant women, and people with an original underlying disease (11–13). In particular, pregnant women who are infected with the influenza A(H1N1) virus can more easily suffer from dyspnea, hypoxemia, and acute respiratory distress syndrome, which may lead to miscarriage, premature delivery, fetal distress, or fetal intrauterine death (14, 15). Viruses can induce an increase in the symptoms of underlying diseases, and in severe cases, these underlying diseases can lead to death. For example, influenza A(H1N1) virus infection in a pregnant woman with an underlying disease can aggravate that underlying disease, which may result in death (13).
Administering the influenza vaccine during pregnancy is the most effective way of preventing influenza and its complications, and its administration is recommended by the World Health Organization (WHO) (16–18). However, the influenza A(H1N1) vaccine is a relatively newly licensed vaccine in China. Although its immunogenicity has been effective (8, 16, 19, 20), there are very few prospective reports addressing the safety of the vaccine in pregnant women in China. Our study indicated that the seroconversion rate in the vaccinated pregnant women was 90.4% (95% CI, 82.6% to 95.5%) and was not significantly different from that in adults 18 to 60 years old in 2 other studies carried out in China (8, 21).
As for the adverse events, the 2 incidences of fever and 1 incident of dizziness and nausea can be classified as non-pregnancy-related maternal outcomes, with an incidence rate of 2.5% (95% CI, 0.5% to 7.2%), which is consistent with the results of a study by Zhu et al. (8) conducted in adults 18 to 60 years of age (incidence rate, 6.7%; 95% CI, 2.7% to 11.3%). The 1 report of abdominal pain can be classified as a pregnancy-specific adverse event or outcome, with an incidence rate of 0.8% (95% CI, 0.1% to 4.5%), which was significantly lower than those of other clinical trials on pregnant women (5, 6, 22). This result should not be considered evidence of the safety of the different H1N1 vaccines due to the relatively small sample size and the lack of a control study including different H1N1 vaccines.
Furthermore, reactions, such as fever, dizziness, nausea, and abdominal pain, had little impact on pregnancy, and the symptoms were mild and resolved within several days without requiring any treatment or hospitalization. No report was found regarding the impact of the influenza A(H1N1) vaccine on fetal growth. There were no statistically significant differences between the vaccinated group and the unvaccinated group in spontaneous abortion, artificial abortion, postnatal death, premature birth, prolonged pregnancy, or low birth weight. These results suggest that influenza A(H1N1) vaccination during pregnancy is safe. These results were similar to those from the other studies conducted with pregnant women (12, 18, 22).
The Apgar score is an important index by which to judge the severity of hypoxia in newborns (22). The Apgar scores of all the newborn infants in the vaccinated group and the unvaccinated group were >7. However, the proportion of newborns with an Apgar score of >9 in the vaccinated group was significantly lower than that of newborns in the unvaccinated group. This is an interesting phenomenon that other relevant studies have not described. However, we do not consider this result evidence that the vaccine is unsafe. First, a healthy newborn's Apgar score is generally between 7 and 10; a score of <7 is considered mild asphyxia, and a score of <4 is considered severe asphyxia, as other clinical trials on pregnant women have shown (5, 9, 12). In this study, a baby with a score of 7 to 8 at 1 min was reevaluated at 5 min. All of the babies scored a 10 at the second evaluation. Second, there are many risk factors that influence the Apgar score, including mother's age, forceps delivery, gestational hypertension, birth complications, low birth weight, and birth deformities (23–25). Among the 75 vaccinated pregnant women whose babies had an Apgar score between 7 and 8, 1 baby had a low birth weight, 6 were prolonged pregnancies, and 18 were cesarean deliveries. The other pregnancies were normal and involved none of the aforementioned risk factors. Although the significant difference between the vaccinated group and the unvaccinated group (61.5% versus 42.3%) regarding the proportion of Apgar scores between 7 and 8 may not be a safety concern of the vaccine, the potential impact of the vaccine on pregnancy requires further study.
Although no case of influenza A(H1N1) virus infection was found in either of the two groups, the incidence of influenza-like illness in the vaccinated group was significantly lower than that in the unvaccinated group (χ2 = 4.285, P = 0.038), suggesting cross-protection after influenza A(H1N1) vaccination (26). Although no significant differences were found in adverse events and outcomes from influenza-like illness between the vaccinated group and the unvaccinated group, there still may be benefits from the vaccine. For example, the median duration of the influenza-like illness in the vaccinated group was relatively short. Therefore, our results reveal that the influenza A(H1N1) vaccine is safe for pregnant women, has no adverse effects on fetal growth, and may prevent influenza-like illnesses, as indicated by the seroconversion rate in the vaccinated group.
This study had some limitations. The sample size was small, and the H1N1 virus-negative specimens were not tested for other influenza viruses, such as H3N2. However, we have provided evidence regarding the safety of the H1N1 vaccines produced in China, where few reports have been found. New evidence demonstrating the relationship between Apgar scores and the vaccines should be explored further.
ACKNOWLEDGMENTS
We are very appreciative of the field work conducted by the Yancheng Center for Disease Control and Prevention (CDC) and the Xiangshui CDC and the laboratory work conducted by the Jiangsu provincial CDC.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 2 July 2014
REFERENCES
- 1.Anderson BL, Rouse DJ, Fitzsimmons C. 2011. Clinical characteristics of pregnant women with influenza-like illness during the 2009 H1N1 pandemic and use of a standardized management algorithm. Am. J. Obstet. Gynecol. 204(Suppl 1):S31–S37. 10.1016/j.ajog.2011.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson LC, Allen SH, Konamme SP, Chestnut J, Wilson P. 2010. The successful use of extra-corporeal membrane oxygenation in the management of a pregnant woman with severe H1N1 2009 influenza complicated by pneumonitis and adult respiratory distress syndrome. Int. J. Obstet. Anesth. 19:443–447. 10.1016/j.ijoa.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahloul M, Chaari A, Algia NB, Chtara K, Dammak H, Hamida CB, Kallel H, Chelly H, Bouaziz M. 2011. A literature review of respiratory failure following influenza A (H1N1) virus infection in pregnant and postpartum women. Trends Anaesth Crit. Care 1:252–256. 10.1016/j.tacc.2011.08.006 [DOI] [Google Scholar]
- 4.Vargas-Parada L. 2009. H1N1: a Mexican perspective. Cell 139:1203–1205. 10.1016/j.cell.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 5.Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, Guh A, Haber P, Destefano F, Vellozzi C. 2011. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am. J. Obstet. Gynecol. 204:146.e141–e147. 10.1016/j.ajog.2010.08.050 [DOI] [PubMed] [Google Scholar]
- 6.Lim S-H, Lee J-H, Kim B-C, Jung S-U, Park Y-B, Lee C-S. 2010. Adverse reaction of influenza A (H1N1) 2009 virus vaccination in pregnant women and its effect on newborns. Vaccine 28:7455–7456. 10.1016/j.vaccine.2010.08.087 [DOI] [PubMed] [Google Scholar]
- 7.Carcione D, Blyth CC, Richmond PC, Mak DB, Effler PV. 2013. Safety surveillance of influenza vaccine in pregnant women. Aust. N. Z. J. Obstet. Gynaecol. 53:98–99. 10.1111/ajo.12034 [DOI] [PubMed] [Google Scholar]
- 8.Zhu F-C, Wang H, Fang H-H, Yang JG, Lin XJ, Liang X-F, Zhang X-F, Pan H-X, Meng F-Y, Hu YM, Liu W-D, Li C-G, Li W, Zhang X, Hu JM, Peng WB, Yang BP, Xi P, Wang H-Q, Zheng J-S. 2009. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 361:2414–2423. 10.1056/NEJMoa0908535 [DOI] [PubMed] [Google Scholar]
- 9.Sokolov DN, Zarubaev VV, Shtro AA, Polovinka MP, Luzina OA, Komarova NI, Salakhutdinov NF, Kiselev OI. 2012. Anti-viral activity of (−)- and (+)-usnic acids and their derivatives against influenza virus A (H1N1)2009. Bioorg. Med. Chem. Lett. 22:7060–7064. 10.1016/j.bmcl.2012.09.084 [DOI] [PubMed] [Google Scholar]
- 10.R Development Core Team. 2009. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 11.Abdelaty NM. 2013. Risk factors and prognostic criteria in 230 patients with influenza A (H1N1) infection. Egypt. J. Chest Dis. Tuber. 62:1–8. 10.1016/j.ejcdt.2013.02.006 [DOI] [Google Scholar]
- 12.Rodríguez-Baño J, Paño-Pardo JR, Múñez Rubio E, Segura Porta F. 2012. Pregnancy, obesity and other risk factors for complications in influenza A(H1N1) pdm09 infection. Enferm. Infecc. Microbiol. Clín. 30(Suppl 4):32–37. 10.1016/S0213-005X(12)70102-7 [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni PS, Manjunath K, Agarkhedkar S. 2012. Safety and immunogenicity of an adjuvanted whole virion, inactivated A(H1N1) 2009 influenza vaccine in young and elderly adults, and children. Vaccine 31:20–22. 10.1016/j.vaccine.2012.10.081 [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez A, Álvarez-Rocha L, Sirvent JM, Zaragoza R, Nieto M, Arenzana A, Luque P, Socías L, Martín M, Navarro D, Camarena J, Lorente L, Trefler S, Vidaur L, Solé-Violán J, Barcenilla F, Pobo A, Vallés J, Ferri C, Martín-Loeches I, Díaz E, López D, López-Pueyo MJ, Gordo F, del Nogal F, Marqués A, Tormo S, Fuset MP, Pérez F, Bonastre J, Suberviola B, Navas E, León C. 2012. Recommendations of the Infectious Diseases Work Group (GTEI) of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) and the Infections in Critically Ill Patients Study Group (GEIPC) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) for the diagnosis and treatment of influenza A/H1N1 in seriously ill adults admitted to the intensive care unit. Med. Intensiva 36:103–137. 10.1016/j.medine.2012.03.002(In Spanish.) [DOI] [PubMed] [Google Scholar]
- 15.Renner B, Reuter T. 2012. Predicting vaccination using numerical and affective risk perceptions: the case of A/H1N1 influenza. Vaccine 30:7019–7026. 10.1016/j.vaccine.2012.09.064 [DOI] [PubMed] [Google Scholar]
- 16.Hatz C, von Sonnenburg F, Casula D, Lattanzi M, Leroux-Roels G. 2012. A randomized clinical trial to identify the optimal antigen and MF59 adjuvant dose of a monovalent A/H1N1 pandemic influenza vaccine in healthy adult and elderly subjects. Vaccine 30:3470–3477. 10.1016/j.vaccine.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 17.Hatz C, Cramer JP, Vertruyen A, Schwarz TF, von Sonnenburg F, Borkowski A, Lattanzi M, Hilbert AK, Cioppa GD, Leroux-Roels G. 2012. A randomised, single-blind, dose-range study to assess the immunogenicity and safety of a cell-culture-derived A/H1N1 influenza vaccine in adult and elderly populations. Vaccine 30:4820–4827. 10.1016/j.vaccine.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 18.Fukushima W, Ohfuji S, Deguchi M, Kawabata K, Hatayama H, Yoshida H, Maeda A, Hirota Y. 2012. Effectiveness of an influenza A (H1N1) 2009 monovalent vaccine among Japanese pregnant women: a prospective observational study assessing antibody efficacy. Vaccine 30:7630–7636. 10.1016/j.vaccine.2012.10.027 [DOI] [PubMed] [Google Scholar]
- 19.Kung H-C, Huang K-C, Kao T-M, Lee Y-C, Chang F-Y, Wang N-C, Liu Y-C, Lee W-S, Liu H-J, Chen C-I, Chen C-H, Huang L-M, Hsieh S-M. 2010. A clinical study to assess the immunogenicity and safety of a monovalent 2009 influenza A (H1N1) vaccine in an area with low-level epidemics of pandemic influenza. Vaccine 28:7337–7343. 10.1016/j.vaccine.2010.08.073 20817013 [DOI] [PubMed] [Google Scholar]
- 20.Nicholson KG, Tyrrell DAJ, Harrison P, Potter CW, Jennings R, Clark A, Schild GC, Wood JM, Yetts R, Seagroatt V, Huggins A, Anderson SG. 1979. Clinical studies of monovalent inactivated whole virus and subunit A/USSR/77 (H1N1) vaccine: serological responses and clinical reactions. J. Biol. Stand. 7:123–136. 10.1016/S0092-1157(79)80044-X [DOI] [PubMed] [Google Scholar]
- 21.Liang X-F, Wang H-Q, Wang J-Z, Fang H-H, Wu J, Zhu F-C, Li R-C, Xia S-L, Zhao Y-L, Li F-J, Yan S-H, Yin W-D, An K, Feng D-J, Cui X-L, Qi F-C, Ju C-J, Zhang Y-H, Guo Z-J, Chen P-Y, Chen Z, Yan K-M, Wang Y. 2010. Safety and immunogenicity of pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 375:56–66. 10.1016/S0140-6736(09)62003-1 [DOI] [PubMed] [Google Scholar]
- 22.Xie HY, Yasseen AS III, Xie RH, Fell DB, Sprague AE, Liu N, Smith GN, Walker MC, Wen SW. 2013. Infant outcomes among pregnant women who used oseltamivir for treatment of influenza during the H1N1 epidemic. Am. J. Obstet. Gynecol. 208:293.e291–293.e297. 10.1016/j.ajog.2013.01.015 [DOI] [PubMed] [Google Scholar]
- 23.Cotte L, Voirin N, Richard C, Brochier C, Schlienger I, Lack P, Lina B, Vanhems P, Zoulim F. 2011. Factors associated with pandemic influenza A/H1N1 vaccine coverage in a French cohort of HIV-infected patients. Vaccine 29:5638–5644. 10.1016/j.vaccine.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 24.Wong LP, Sam IC. 2010. Factors influencing the uptake of 2009 H1N1 influenza vaccine in a multiethnic Asian population. Vaccine 28:4499–4505. 10.1016/j.vaccine.2010.04.043 [DOI] [PubMed] [Google Scholar]
- 25.Lau JTF, Yeung NCY, Choi KC, Cheng MYM, Tsui HY, Griffiths S. 2010. Factors in association with acceptability of A/H1N1 vaccination during the influenza A/H1N1 pandemic phase in the Hong Kong general population. Vaccine 28:4632–4637. 10.1016/j.vaccine.2010.04.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin JK, Chow MYK, Khandaker G, King C, Richmond P, Heron L, Booy R. 2012. Impacts on influenza A(H1N1)pdm09 infection from cross-protection of seasonal trivalent influenza vaccines and A(H1N1)pdm09 vaccines: systematic review and meta-analyses. Vaccine 30:3209–3222. 10.1016/j.vaccine.2012.02.048 [DOI] [PubMed] [Google Scholar]