Abstract
Hepatitis B antibody persistence was assessed in individuals who had previously received a vaccine booster. We measured hepatitis B surface antigen antibody (anti-HBs) levels 7 to 9 years post-hepatitis B booster in individuals with primary vaccination at birth. While 95 (91.3%) of 104 participants had detectable anti-HBs (minimum, 0.1 mIU/ml; maximum, 1,029 mIU/ml), only 43 (41%) had protective levels of ≥10 mIU/ml. Pre- and week 4 postbooster anti-HBs levels were significant predictors of hepatitis B immunity at follow-up (P < 0.001). Almost all participants had detectable anti-HBs 7 to 9 years after the hepatitis B vaccine booster, but less than half had levels ≥10 mIU/ml.
INTRODUCTION
Routine infant hepatitis B vaccination has substantially reduced the incidence of acute hepatitis B virus (HBV) infection and the prevalence of chronic infection (1, 2). However, the duration of protection following infant vaccination is unknown (3, 4). In studies from a variety of settings conducted up to 22 years after a primary vaccine series, 23% to 66% of vaccinated infants had low or undetectable concentrations of hepatitis B virus surface antibody (anti-HBs), the serologic marker of immunologic protection (5–7). Although antibody levels in many participants were below the level considered protective, most individuals likely remained immune, as shown by the infrequent serologic evidence of hepatitis B virus infection. Furthermore, individuals with evidence of breakthrough infection (the presence of antibody to hepatitis B virus core antigen [anti-HBc]) did not develop acute symptomatic or chronic HBV infection if they responded to the primary vaccine series (5).
Individuals with undetectable levels of anti-HBs may retain HBV-specific immune memory. Humoral evidence of immune memory in previously vaccinated persons is demonstrable by administering a single challenge dose of vaccine and measuring the anti-HBs response. A rapid increase in anti-HBs titers represents an anamnestic response and indicates the presence of HBV-specific immune memory (3, 4, 8). We previously reported anti-HBs responses after administration of a booster dose of hepatitis B vaccine among children and adolescents aged 7 to 14 years who were born to HBsAg-negative women in Anchorage, Alaska, were vaccinated starting at birth, and had no HBsAg-positive persons living in their household at that time (9). Although the majority of participants had no serologic evidence of protective immunity at baseline, the absence of symptomatic or chronic infections and the presence of anamnestic responses to a challenge dose indicated that immune memory remained intact for most participants (9). Our aim was to determine the persistence of markers of HBV immunity in these individuals 7 to 9 years after they received a hepatitis B vaccine booster.
MATERIALS AND METHODS
Participants.
All participants were part of the original “Youth Hepatitis B Protection Study” consisting of 389 children of HBsAg-negative mothers who received three doses of hepatitis B vaccine starting at birth followed by a 5-μg recombinant vaccine (Recombivax) booster dose during the study (9). From this study population, we recruited a convenience sample of three types of participants distinguished by primary vaccine type and age at booster dose as follows: adolescent booster following plasma primary vaccine (AS group); adolescent booster following recombinant primary vaccine (AR group); and child booster following recombinant primary vaccine (CR group). All participants received a birth dose (administered within the first 7 days of life) and completed the three-dose primary vaccine series at no later than 10 months of age with appropriate dosages and intervals. Anti-HBs levels were not tested following the primary vaccination series. The AS group included adults whose primary vaccine series was 3 doses of 10 μg of Heptavax followed by a 5-μg Recombivax booster at age 10 to 13 years. The AR group included adolescents whose initial vaccine series was 3 doses of Recombivax (any of the licensed dosages [2.5, 5, or 10 μg] at that time) followed by a 5 μg Recombivax booster at 10 to 13 years. The CR group included adults whose initial vaccine series was 3 doses of Recombivax (either of the licensed dosages [5 or 10 μg] at that time) followed by a 5 μg Recombivax booster at age 5 to 7 years. Only participants who responded to the booster dose, defined as anti-HBs at ≥10 mIU/ml at 4 to 6 weeks, were eligible. This represented 89% of the original cohort. We excluded individuals if they had evidence of immunosuppression, had ever received cancer chemotherapy, or had received corticosteroids for more than 6 months.
We obtained informed consent from each participant ≥18 years old and consent from a parent or guardian plus assent from participants who were <18 years old at the time of the blood draw. Adult participants or the parent/guardian of minor participants were notified via phone or mailing of the blood test and the aggregate study results within 12 weeks of analysis. The Alaska Area Native Health Service, Indian Health Service, and Centers for Disease Control and Prevention institutional review boards and two Alaska Native health organizations, the Southcentral Foundation and Alaska Native Tribal Health Consortium, approved this study.
Laboratory testing.
The Arctic Investigations Program (AIP) laboratory quantitatively tested all the follow-up sera for anti-HBs (ETI-AB-AUK Plus kit and ABAU STD set; DiaSorin, Stillwater, MN). Anti-HBs concentrations are given in milli-international units (mIU) per milliliter (ml), and the lower limit of detection of the assay for anti-HBs was ≥0.1 mIU/ml.
Data analysis.
We log-transformed anti-HBs levels and calculated antibody geometric mean concentrations (GMCs). For univariable analysis, we used the χ2 test, a randomization test, or a test for trend across ordered groups and used analysis of variance (ANOVA) to evaluate the proportion of persons with anti-HBs of ≥10 mIU/ml and GMCs, respectively. We used logistic regression to model the proportion of persons with anti-HBs at ≥10 mIU/ml according to age, gender, vaccine schedule, initial response to the HBV vaccine series, and peak anti-HBs level following the booster dose. All P values were determined by two-sided exact tests when necessitated by the sample size, and we used a threshold of <0.05 for statistical significance. STATA version 10 (StataCorp, College Station, TX) was used to calculate the statistics.
RESULTS
We recruited 104 (27%) of 389 participants from a previous study for this follow-up. Among the groups (see Materials and Methods for definitions), we enrolled 17 AS participants, 38 AR participants, and 49 CR participants, a group distribution similar to that used in the original study (P = 0.913). The follow-up participants did not differ significantly from other responders who were not included with respect to anti-HBs level, booster time from third dose, or sex (data not shown). The average time since hepatitis B booster immunization was 8.4 years (P = 0.005 for differences across groups) (Table 1). The average ages at follow-up were 22.6 years (AS), 19.9 years (AR), and 14.3 years (CR). Fifty-three percent of participants were female.
TABLE 1.
Demographic characteristics and anti-HBs levels in hepatitis B vaccine booster study participants at year 7 to 9 follow-upa
| Characteristic | Value for vaccine group |
Total value (n = 104) | P value comparing groups | ||
|---|---|---|---|---|---|
| AS (n = 17) | AR (n = 38) | CR (n = 49) | |||
| Demographic | |||||
| No. (%) of males | 7 (41) | 14 (37) | 28 (57) | 55 (53) | 0.148 |
| Mean (SD) age at booster, yrs | 13.8 (0.59) | 11.7 (1.25) | 5.9 (0.73) | 9.3 (3.44) | <0.001 |
| Mean (SD) age at follow-up, yrs | 22.6 (0.55) | 19.9 (1.09) | 14.3 (0.84) | 17.7 (3.48) | <0.001 |
| Mean (SD) elapsed time since booster, yrs | 8.7 (0.33) | 8.3 (0.58) | 8.38 (0.48) | 8.39 (0.52) | 0.005 |
| Anti-HBs levels (mIU/ml) | |||||
| Prebooster GMC (95% CI) | 2.13 (0.7, 6.5) | 1.53 (0.92, 2.54) | 3.28 (1.94, 5.54) | 2.31 (1.61, 3.32) | 0.156 |
| Minimum–maximum | 0–213 | 0–133 | 0–309 | 0–309 | |
| Week 2 postbooster GMC (95% CI) | 59 (67.4, 994) | 618 (344, 1,112) | 1,274 (736, 2,206) | 754 (498, 1,141) | 0.018 |
| Minimum–maximum | 0–36,947 | 20.2–28,561 | 10.6–64,676 | 0–64,676 | |
| Week 4 postbooster GMC (95% CI) | 246 (71.4, 847) | 472 (273, 817) | 804 (485, 1,335) | 544 (373, 794) | 0.074 |
| Minimum–maximum | 18.4–34,533 | 34.1–28,561 | 10.6–22,463 | 10.6–34,533 | |
| Years 7–9 postbooster GMC (95% CI) | 11.0 (3.7, 32.4) | 5.6 (3.0, 10.5) | 5.6 (3.4, 9.3) | 6.3 (4.3, 9.1) | 0.417 |
| Minimum–maximum | 0–1,029 | 0–215 | 0–238 | 0–1,029 | |
CI, confidence interval. Bolded values represent statistical significance.
At follow-up, 44 (42%) of 104 of participants had anti-HBs levels of ≥10 mIU/ml, ranging from 10.05 mIU/ml to as high as 1,029 mIU/ml (Table 1). The proportions did not vary significantly by group (P = 0.831): for AS, 8 (47%) of 17; for AR, 16 (42%) of 38; and for CR, 19 (39%) of 49. The proportions of participants with anti-HBs of ≥10 mIU/ml pre- and postbooster (2 and 4 weeks) did not significantly vary between groups (P = 0.071).
The proportions of persons with no detectable anti-HBs (<0.1 mIU/ml) on follow-up were 1 (6%) of 17 for AS, 2 (5%) of 38 for AR, and 6 (12%) of 49 for CR, and these proportions did not differ significantly across groups (P = 0.582). The proportions of persons with anti-HBs of <10 mIU/ml but ≥0.1 mIU/ml were 47% (n = 8) for AS, 53% (n = 20) for AR, and 62% (n = 24) for CR, and these proportions also did not differ statistically across groups (P = 0.694). The presence of any detectable anti-HBs trended with higher prebooster anti-HBs levels, but this was not statistically significant (P = 0.185). However, the presence of detectable anti-HBs was significantly associated with higher 2-week postbooster anti-HBs levels (P = 0.004).
The geometric mean concentrations (GMC) of anti-HBs at follow-up in the AS (11.0 mIU/ml), CR (5.6 mIU/ml), and AR (5.6 mIU/ml) groups were not statistically different (P = 0.494). The pre- and postbooster GMCs were highest in the CR group (Fig. 1).
FIG 1.
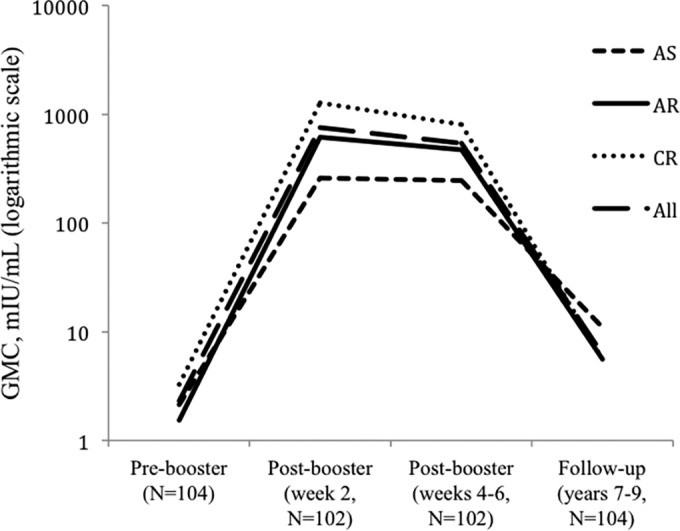
Geometric mean concentrations (GMC) of anti-HBs for the three vaccine groups, AS (adolescent plasma), AR (adolescent recombinant), and CR (child recombinant), and a combined total for each of the time periods evaluated.
Higher anti-HBs levels at previous time points were significantly associated with anti-HBs levels of ≥10 mIU/ml at 7 to 9 years postbooster (Table 2). Antibody levels at 4 to 6 weeks postbooster accounted for the greatest variations in booster response, followed by week 2 postbooster and prebooster antibody levels. After controlling for week 4 to week 6 postbooster antibody levels, antibody levels at the other time points were found not to be statistically significant predictors of vaccine booster response.
TABLE 2.
Demographic and immune predictors of hepatitis B immunity 7 to 9 years following hepatitis B booster vaccinationa
| Characteristic | Category | Odds ratio (95% CI) |
|---|---|---|
| Vaccine group | AS | 1.0 (reference) |
| AR | 0.82 (0.26, 2.58) | |
| CR | 0.71 (0.23, 2.17) | |
| Sex | Female | 1.0 (reference) |
| Male | 0.82 (0.37, 1.79) | |
| Prebooster anti-HBs level (log mIU/ml) | 1.78 (1.37, 2.31) | |
| Prebooster antibody level | Anti-HBs < 10 mIU/ml | 1.0 (reference) |
| Anti-HBs ≥ 10 mIU/ml | 6.71 (2.49, 18.04) | |
| <0.1 mIU/ml | 1.0 (reference) | |
| 0.1–9.9 mIU/ml | 2.01 (0.75, 5.41) | |
| ≥10 mIU/ml | 9.09 (3.04, 27.20) | |
| Age at vaccine booster, yrs | 1.04 (0.92, 1.16) | |
| Postbooster anti-HBs (log mIU/ml), week 2 | 2.13 (1.55, 2.92) | |
| Booster antibody response | <1,000 mIU/ml | 1.0 (reference) |
| 1,000–1,999.9 mIU/ml | 9.78 (2.69, 35.60) | |
| ≥2,000 mIU/ml | 9.78 (3.61, 26.50) | |
| Postbooster anti-HBs (log mIU/ml), wks 4–6 | 2.28 (1.64, 3.19) | |
| Postbooster response,b wks 4–6 | Not anamnestic | 1.0 (reference) |
| Anamnestic | 2.22 (0.22, 22.12) | |
| Follow-up time, yrs | 0.96 (0.46, 2.64) | |
| Follow-up age, yrs | 1.03 (0.92, 1.15) |
HBV immunity is defined as anti-HBs values of ≥10 mIU/ml. Bolded values represent statistical significance at P <0.05.
For those with baseline anti-HBs levels of ≥10 mIU/ml, an anamnestic response was defined as a 4-fold or greater rise in the anti-HBs concentration. For those with baseline anti-HBs levels of <10 mIU/ml, an anamnestic response was defined as an increase in the anti-HBs concentration to ≥10 mIU/ml.
DISCUSSION
By study design, all of our participants had responded to a booster dose of hepatitis B vaccine (anti-HBs of ≥10 mIU/ml); however, most (59%) had anti-HBs levels of <10 mIU/ml when tested an average of 8 years later. We reported similar findings from a study in Alaska health care workers (10). All of these health care workers responded to an initial vaccination series (anti-HBs of ≥10 mIU/ml) as adults but had anti-HBs levels that approached the prebooster level value when tested 1 year after a booster vaccine dose. The results of our current study and those done previously suggest that the combination of giving booster doses during childhood and following anti-HBs levels is not an accurate way of monitoring long-term protection; however, additional studies are needed to confirm these findings.
We previously reported a significantly lower booster response in children whose primary vaccine series was plasma derived (71%) versus recombinant (88% and 99% for child and adolescent booster groups) (9). In this follow-up study, there were no significant differences across the three groups in anti-HBs GMC values or in the proportion with anti-HBs of ≥10 mIU/ml. This may be due to the follow-up study, which included only individuals who had previously responded to a booster dose.
The results of this study, along with those of two others performed in Alaska (10, 11), show that a booster dose results in an immunologic response regardless of immunization age in persons responsive to a primary immunization series. These data suggest that individuals remain protected against hepatitis B virus infection because of this anamnestic response, a conclusion supported by the absence of hepatitis B virus infection in a cohort followed for 30 years after their primary immunization series (11). We observed rapid decay of anti-HBs antibodies, and ensuring measurable levels of anti-HBs would require frequent anti-HBs testing and repeated booster doses for those with levels of <10 mIU/ml. This approach was found not to be cost-effective by the CDC, with an incremental cost per quality-adjusted life year (QALY) of over 3 million dollars, and is therefore not recommended (12).
In conclusion, over 91% of persons who received a hepatitis B vaccine in infancy and responded to a booster dose during childhood still had measurable anti-HBs levels 7 to 9 years later; however, for most, the levels were <10 mIU/ml. Additional data are needed to determine the duration of protection afforded by hepatitis B vaccination, particularly among persons in exposure-prone environments (e.g., health care) who were vaccinated during infancy. In this respect, because cellular immunity likely outlasts demonstrable humoral immunity, studies examining cell-mediated immunity should be undertaken (13).
ACKNOWLEDGMENT
The findings and conclusions in this report are ours and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 23 July 2014
REFERENCES
- 1.Bonanni P, Pesavento G, Bechini A, Tiscione E, Mannelli F, Benucci C, Nostro AL. 2003. Impact of universal vaccination programmes on the epidemiology of hepatitis B: 10 years of experience in Italy. Vaccine 21:685–691. 10.1016/S0264-410X(02)00580-7 [DOI] [PubMed] [Google Scholar]
- 2.Harpaz R, McMahon BJ, Margolis HS, Shapiro CN, Havron D, Carpenter G, Bulkow LR, Wainwright RB. 2000. Elimination of new chronic hepatitis B virus infections: results of the Alaska immunization program. J. Infect. Dis. 181:413–418. 10.1086/315259 [DOI] [PubMed] [Google Scholar]
- 3.Fitzsimons D, Francois G, Hall A, McMahon B, Meheus A, Zanetti A, Duval B, Jilg W, Bocher WO, Lu SN, Akarca U, Lavanchy D, Goldstein S, Banatvala J, Damme PV. 2005. Long-term efficacy of hepatitis B vaccine, booster policy, and impact of hepatitis B virus mutants. Vaccine 23:4158–4166. 10.1016/j.vaccine.2005.03.017 [DOI] [PubMed] [Google Scholar]
- 4.European Consensus Group on Hepatitis Immunity B. 2000. Are booster immunisations needed for lifelong hepatitis B immunity? Lancet 355:561–565. 10.1016/S0140-6736(99)07239-6 [DOI] [PubMed] [Google Scholar]
- 5.McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. 2009. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J. Infect. Dis. 200:1390–1396. 10.1086/606119 [DOI] [PubMed] [Google Scholar]
- 6.But DY-K, Lai C-L, Lim W-L, Fung J, Wong DK-H, Yuen M-F. 2008. Twenty-two years follow-up of a prospective randomized trial of hepatitis B vaccines without booster dose in children: final report. Vaccine 26:6587–6591. 10.1016/j.vaccine.2008.09.034 [DOI] [PubMed] [Google Scholar]
- 7.van der Sande MAB, Waight PA, Mendy M, Zaman S, Kaye S, Sam O, Kahn A, Jeffries D, Akum AA, Hall AJ, Bah E, McConkey SJ, Hainaut P, Whittle HC. 2007. Long-term protection against HBV chronic carriage of Gambian adolescents vaccinated in infancy and immune response in HBV booster trial in adolescence. PLoS One 2:e753. 10.1371/journal.pone.0000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banatvala J, Van Damme P, Oehen S. 2000. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine 19:877–885. 10.1016/S0264-410X(00)00224-3 [DOI] [PubMed] [Google Scholar]
- 9.Samandari T, Fiore AE, Negus S, Williams JL, Kuhnert W, McMahon BJ, Bell BP. 2007. Differences in response to a hepatitis B vaccine booster dose among Alaskan children and adolescents vaccinated during infancy. Pediatrics 120:e373–e381. 10.1542/peds.2007-0131 [DOI] [PubMed] [Google Scholar]
- 10.Williams JL, Christensen CJ, McMahon BJ, Bulkow LR, Cagle HH, Mayers JS, Zanis CL, Parkinson AJ, Margolis HS. 2001. Evaluation of the response to a booster dose of hepatitis B vaccine in previously immunized healthcare workers. Vaccine 19:4081–4085. 10.1016/S0264-410X(01)00112-8 [DOI] [PubMed] [Google Scholar]
- 11.Bruce MG, Bruden DL, Hurlburt D, Zanis CL, Thompson GC, Rea LD, Toomey M, Townshend-Bulson LJ, Rudolph K, Bulkow LR, Spradling P, Baum R, Hennessy TW, McMahon BJ. 2013. Antibody levels and protection after hepatitis B vaccine: results of a 30 year follow-up study and response to a booster dose, abstr 187, p 300A 64th Annu. Meet. Am. Assoc. Study Liver Dis. Liver Meet. 2013. 10.1002/hep.26822 [DOI] [PubMed] [Google Scholar]
- 12.Schillie S, Murphy TV, Sawyer M, Ly K, Hughes E, Jiles R, de Perio MA, Reilly M, Byrd K, Ward JW; Centers for Disease Control and Prevention (CDC). 2013. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm. Rep. 62(RR-10):1–19 [PubMed] [Google Scholar]
- 13.Bauer T, Jilg W. 2006. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine 24:572–577. 10.1016/j.vaccine.2005.08.058 [DOI] [PubMed] [Google Scholar]


